Abstract
The genetic basis for susceptibility to malaria has been studied widely in African populations but less is known of the contribution of specific genetic variants in Asian populations. We genotyped 67 SNPs in 1030 severe malaria cases and 2840 controls from Vietnam. After data quality control, genotyping data of 956 cases and 2350 controls were analysed for 65 SNPs (3 gender confirmation, 62 positioned in/near 42 malarial candidate genes). 14 SNPs were monomorphic and 2 (rs8078340 and rs33950507) were not in HWE in controls (P<0.01). 7/46 SNPs in 6 genes (ICAM1, IL1A, IL17RC, IL13, LTA and TNF) were associated with severe malaria, with 3/7 SNPs in the TNFA/LTA region . Genotype phenotype correlations between SNPs and clinical parameters revealed that genotypes of rs708567 (IL17RC) correlate with parasitemia (P=0.028, r2=0.0086), with GG homozygotes having the lowest parasite burden. Additionally, rs708567 GG homozygotes had a decreased risk of severe malaria [P=0.007, OR=0.78 (95% CI; 0.65-0.93)] and death [P=0.028, OR=0.58 (95% CI; 0.37-0.93)] than those with AA and AG genotypes. In summary, variants in 6 genes encoding adhesion and pro-inflammatory molecules are associated with severe malaria in the Vietnamese. Further replicative studies in independent populations will be necessary to confirm these findings.
Keywords: severe malaria, SNP, genetic association, ICAM-1, TNF, IL-17RC
Introduction
Around half of the world’s population is at risk of malaria, with 243 million people infected and nearly 1 million deaths in 2008 1. Vietnam has made great achievements in controlling malaria during the last decade with the number of cases reduced from 187,994 in 1991 to 54,297 in 2010 and the number of deaths reduced from 4,646 deaths in 1991 to 21 deaths in 2010 2. This success is attributable to targeting interventions to high risk areas and balancing case management with prevention, i.e. combining impregnated bednets, insecticide spraying, and early diagnosis with the availability of effective treatment, namely artemisinin derivatives 3, 4. Despite this success in prevention malaria still persists in Vietnam. Additionally, a study by Erhart et al 5 demonstrated that the health information system in Vietnam greatly underestimates malaria burden. Malaria cases are generally found in ethnic minority areas of the central region that tend to be impoverished, less accessible to effective health systems and provide the forested areas that support the common mosquito vectors that transmit malaria, Anopheles dirus and An minimus 6. These regions, many of which border Cambodia, are also at risk of acquiring drug resistant Plasmodium falciparum. Recent reports suggest that the effectiveness of artemisinin-based combination therapy (ACT) and artesunate monotherapy has declined in western Cambodia 7. Although there have been many successes in malaria control in Vietnam, the emergence of reduced in vivo sensitivity of P. falciparum to artemisinin derivatives reminds us that challenges still lie ahead.
Malaria control in endemic countries would benefit greatly from an effective vaccine. Recently a number of pre-erythrocytic stage vaccines have been developed and tested in clinical trials 8-11. Even though it is likely that these vaccines, or ones similar, are heading towards licensure they are not yet able to provide sterilizing and life-long protection against P.falciparum infection. Blood stage vaccines may have a higher potential for protective immunity, but unfortunately have shown less success in clinical studies to date 12. To develop an efficacious multi-stage malaria vaccine that can provide protection in an endemic setting it is paramount to have a complete understanding of the molecular mechanisms of protective immunity. Studies of the human genome can teach us about resistance to malaria as populations have evolved different genetic variants to protect against malaria.
The MalariaGEN Consortium (www.malariagen.net) is a global research network which utilizes a genetics approach to identify new mechanisms of protective immunity against malaria which may lead to novel vaccine development 13. MalariaGEN has recruited cases of severe malaria and controls from 15 malaria endemic countries for genetic studies. As part of the MalariaGEN consortium, here we report genotyping data from 65 SNPs in 42 malarial candidate genes in 956 severe malaria cases and 2350 controls from Vietnam. Variants in 6 genes (ICAM1, IL1A, IL17RC, IL13, LTA and TNF) were associated with severe malaria in this population.
Results
Clinical characteristics of cohort
The baseline clinical and demographic characteristics of the severe malaria patients and population controls are shown in table 2. 956 adults meeting the clinical definition of severe malaria were recruited. The ethnic majority of malaria patients and controls was Vietnamese Kinh (87% in cases and 93% in controls), with more males in the malaria group than controls (71% in cases and 54% in controls). Our patients were adults with a median (interquartile range [IQR]) age of 28 (20-40) years and a medium (IQR) parasite count of 61,860 (7,756-254,200). The patients were rarely severely anaemic (>20% haematocrit) and their mortality rate was 10.4%
Table 2. Baseline characteristics and outcome of severe malaria patients and control subjects.
| Parameter | Sample size | no. (%) or median (IQR) | min-max |
|---|---|---|---|
| SEVERE MALARIA CASES | |||
| Genotyped Sex (%) - male / female | 956 | 684 (71.5) / 272 (28.5) | |
| Ethnicity (%) - Kinh | 956 | 828 (86.61) | |
| - S’tieng | 956 | 48 (5.02) | |
| - others | 956 | 43 (4.50) | |
| - unknown | 956 | 37 (3.87) | |
| Age (yrs), median (IQR) | 942 | 28 (20-40) | 0.66-79 |
| Fever within past 48 hours (%) | 475 | 473 (99.6) | |
| Administered antimalarials prior admission (%) | 248 | 43 (17.3) | |
| GCS, median (IQR) | 849 | 11 (8-15) | 3-15 |
| BCS, median (IQR) | 43 | 5 (3-5) | 1-5 |
| Convulsions after admission (%) | 837 | 69 (8.2) | |
| Parasite count (/ul), median (IQR) | 828 | 61,860 (7,756-254,200) | 0-3,534,000 |
| Haematocrit (%), median (IQR) | 894 | 30.6 (24.5-36.0) | 6-60 |
| Haemoglobin (g/dL), median (1QR) | 121 | 10.2 (8.1-11.7) | 2.5-14.9 |
| blood transfusion received (%) | 698 | 213 (30.5) | |
| respiratory rate (/min), median (IQR) | 832 | 28 (24-32) | 14-68 |
| Lactate (mmol/L), median (IQR) | 747 | 3.3 (2-5.05) | 0.022-28 |
| Glucose (mmmol/l), median (IQR) | 711 | 5.5 (4.1-7.3) | 0.5-31.3 |
| administered glucose (%) | 941 | 214 (23) | |
| Mortality (%) | 914 | 96 (10.4) | |
| CONTROL SUBJECTS | |||
| Genotyped Sex (%) - male / female | 2221 | 1195 (53.80) / 1026 (46.20) | |
| Ethnicity (%) - Kinh | 2351 | 2194 (93.32) | |
| - S’tieng | 2351 | 96 (4.08) | |
| - others | 2351 | 52 (2.21) | |
| - unknown | 2351 | 9 (0.38) | |
| Age (yrs), median (IQR) - cord blood control | 1811 | 0 | 0 |
| - community control | 534 | 23 (10-34) | 0.66-73 |
Note: IQR is interquartile range.
Malaria candidate SNPs are associated with severe malaria in Vietnam
In total we genotyped 67 SNPs in 1030 severe malaria cases and 2840 population controls. Subjects were removed from the dataset if they had greater than 10% of their total genotype data missing. SNPs were removed from the dataset if greater than 15% of the subjects genotyped for that SNP had missing genotype data. After the removal of sample and SNP missingness, we analysed data for 65 SNPs genotyped in 956 severe malaria cases and 2350 populations. The 65 SNPs lie in or near 42 genes. Data from 3 SNPs in the amelogenin gene (AMELX) was used for gender confirmation. The remaining 62 SNPs are found near or within 41 malarial candidate genes. Two SNPs (rs33950507, rs8078340) were not in HWE (controls; P<0.01) and 14 SNPs were monomorphic (Supplementary table 1). This was not unexpected as 4 SNPs included in our study were of known African origin and as such were present as the ancestral allele in our population [HbS (rs334), G6PD+376 (rs1050829), G6PD+202 (rs1050828) and Duffy (rs1803632)].
Table 3 shows that 7/46 SNPs in 6 malarial candidate genes are associated with severe malaria in this Vietnamese cohort; rs5498 (ICAMI), rs17561 (IL1A), rs708567 (IL17RC), rs20541 (IL13), rs909253 (LTA+252), rs1799964 (TNFA-1031) and rs1800629 (TNFA-308). P values representing allelic and genotypic association are shown in table 3. Data for all 62 SNPs is provided in supplementary table 1. All association analysis of the cases and controls was adjusted by ethnic group. We performed an analysis within the Vietnamese Kinh ethnic group alone and found only 5 out of the 7 SNPs above were associated with severe malaria (rs909253 and rs1799964 in LTA and TNF respectively, did not remain significantly associated). Due to the significantly smaller sample sizes of ethnic groups other than Vietnamese Kinh we were unable to perform a sufficiently powered analysis stratified by ethnic group. As the population controls were a mixture of newborn (cord blood controls) and adults (community controls) we used the allele frequencies of the 46 SNPs to calculate the Fst of the two groups. A mean and median Fst of 0.000595 and 0.000479 respectively is evidence to suggest both control groups are very similar.
Table 3. SNPs associated with severe malaria in Vietnam adjusted by ethnic group.
| case allele |
control allele |
HWE 10 control |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs number | gene | Chr | 1 | 2 | freq 16 | freq 1 | P (add7) | OR (95% CI)8 | P (gen9) | P |
| rs54981 | ICAM1 | 19 | A | G | 0.80 | 0.78 | 0.039 | 0.87 (0.76-0.99) | 0.008 | 0.53 |
| rs17561 | IL1A | 2 | G | T | 0.94 | 0.95 | 0.30 | 1.14 (0.90-1.44) | 0.005 | 0.02 |
| rs708567 | IL17RC | 3 | A | G | 0.14 | 0.11 | 0.004 | 0.79 (0.67-0.93) | 0.016 | 0.37 |
| rs205412 | IL13 | 5 | C | T | 0.62 | 0.58 | 0.007 | 0.86 (0.77-0.96) | 0.023 | 0.90 |
| rs9092533 | LTA | 6 | C | T | 0.46 | 0.49 | 0.035 | 1.12 (1.01-1.25) | 0.079 | 0.36 |
| rs17999644 | TNF | 6 | C | T | 0.25 | 0.23 | 0.023 | 0.86 (0.76-0.98) | 0.072 | 0.87 |
| rs18006295 | TNF | 6 | A | G | 0.06 | 0.07 | 0.054 | 1.24 (0.99-1.54) | 0.027 | 0.58 |
ICAM1 codon469,
IL-13 46457,
LTA Nco1,
TNFa-1031,
TNF -308,
frequency,
the additive model,
odds ratio (95% confidence interval),
general,
Hardy Weinberg Equilibrium
Malaria candidate SNPs are associated with death from severe malaria in Vietnam
Two SNPs in 2 malarial candidate genes were associated with death from severe malaria [rs7935564 (TRIM) and rs708567 (IL17RC)] when fatal severe malaria cases were compared with cases that recovered from the disease (table 4).
Table 4. SNPs associated with death from severe malaria in Vietnam adjusted by ethnic group.
| rs number | gene | Chr | 1 | 2 | fatal cases allele freq 11 |
recovered cases allele freq 1 |
P (add2) | OR (95% CI)3 | P (gen4) | HWE5 control P |
|---|---|---|---|---|---|---|---|---|---|---|
| rs7935564 | TRIM | 11 | A | G | 0.125 | 0.188 | 0.049 | 1.55 (0.98-2.44) | 0.138 | 0.25 |
| rs708567 | IL17RC | 3 | A | G | 0.176 | 0.136 | 0.073 | 0.68 (0.45-1.02) | 0.047 | 0.13 |
frequency,
the additive model,
odds ratio (95% confidence interval),
General,
Hardy Weinberg Equilibrium
IL-17RC genotypes are associated with parasitemia in severe malaria patients
Genotype phenotype correlations between our SNP genotype data and clinical parameters of the severe malaria patients were tested. We compared the mean parasite count/μl of blood from 832 patients with the AA, AG and GG genotypes of rs708567 by anova (linear model) and found that genotypes of this SNP correlated with parasitemia (P=0.028, r2=0.0086; figure 1). rs708567 in IL17RC was associated with severe malaria, and the recessive model shows a protective effect of GG homozygotes [i.e. the risk of severe malaria is lower in GG homozygotes; P= 0.007, OR=0.78 (95% CI; 0.65-0.93)]. In addition, rs708567 in IL17RC was associated with death from severe malaria (genotypic P value =0.047, table 4), and the recessive model also shows a protective effect of GG homozygotes [i.e. the risk of death from severe malaria is lower in GG homozygotes; P= 0.028, OR=0.58 (95% CI; 0.37-0.93)]. Interestingly, the parasite burden was lowest in GG homozygotes (figure 1).
Figure 1. Parasite count by rs708567 genotype.
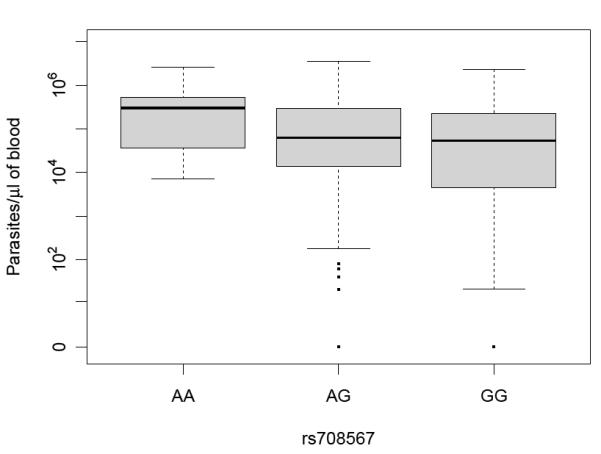
Parasite counts of 832 severe malaria patients were grouped by genotype of IL17RC SNP rs708567. To test for genotype phenotype correlations we compared the mean parasitemia from patients with the AA, AG and GG genotypes of rs708567 by anova (linear model; P=0.028, r2=0.0086). Data for parasites /μl of blood are plotted with the median and IQR represented by the horizontal bar and light grey box.
Discussion
As malaria is a complex disease it is expected that the overall disease risk of an individual will be determined by modest contributions of multiple genes. By genotyping SNPs in a number of known malarial candidate genes we found disease associations with genes involved in a variety of critical events in malaria pathogenesis. Genes encoding molecules involved in adhesion (ICAM1) as well as the pro-inflammatory response (TNFA, LTA, IL-1A) were associated with severe malaria and as expected, all variants modestly contributed to the overall disease risk.
Cytoadherence is a critical event in malaria pathogenesis and is thought to be an immune evasion strategy. Parasitised erythrocytes are sequestered in small blood vessels 14 allowing the parasites to remain within the vascular compartment and avoid circulation through the spleen. Sequestration occurs through binding of parasitized erythrocytes to a range of receptors, one of these being intercellular adhesion molecule 1 (ICAM1; CD54). ICAM1, a cell surface glycoprotein, functions as an endothelial- and immune cell adhesion receptor for integrin-expressing leukocytes. Genetic associations of ICAM1 variants with severe malaria have often been contradictory. Early studies in Kenya 15 and Gabon 16 identified associations between rs5491 (ICAM-1Kilifi) and malaria, however these initial observations were not replicated 17-19, including a large, well-powered study combining 3 populations (Gambia, Kenya and Malawi) with familial and case control association analysis 20. In the Vietnamese cohort rs5491 was excluded due to high genotyping failure rate, however we found an association with rs5498 and severe malaria. rs5498, a non-synonomous SNP in the exon 6 region encoding Ig-like domain 5 of the extracellular portion of ICAM1, is also associated with an increased risk of severe malaria in Nigeria 21 and India 22.
Many reports on the human immunological response to malaria exist, however it is still unclear which of these provide protective immunity against malaria, and what is their underlying mechanism. Studying the genetic interaction between malaria and the immune system may provide further opportunities to uncover these mechanisms, therefore variants of pro-inflammatory cytokine genes have been assessed in multiple populations. The TNFA gene region has been a candidate for malaria since a promoter polymorphism (TNFA -308) was first found associated with cerebral malaria in 1994 23, however subsequent studies have not always replicated this finding. A recent large study involving >10,000 individuals from 3 African populations sought to clarify these inconsistencies, and found some evidence of TNF haplotypes associated with severe malaria in the Gambia, but not in the closely related Kenya and Malawi populations 24. The TNFA SNPs associated with severe malaria in the Gambia (TNF-308, TNF-1031) were also associated in the Vietnamese Kinh, along with LTA+252. Explanations for inconsistencies in data across different populations with sufficiently powered sample sizes, are the haplotypic diversity between populations, as well as phenotypic differences in patients. The issue of haplotypic diversity between populations is particularly relevant when the causal variant responsible for the disease association is unknown and surrogate markers are being genotyped. This may be the case here as it has been suggested that the causal variants may be located some distance downstream of TNF and LTA 25, 26.
Interleukin 1 (IL-1) exists in 2 forms, IL-1α and IL-1β, and both have a prominent role in the acute phase response by inducing expression of multiple cytokines and inflammatory molecules, leading to activation of T cells and monocytes and upregulation of adhesion molecules. Variants in this gene region have been associated with various diseases 27-30 and specifically to malaria 31, 32. Walley, et al 31 reported the only association between IL1A +4845 G-T (rs17561) and mild malaria. In the Vietnamese rs17561 is associated with severe malaria, a variant that causes a non-conservative amino acid change (Ala 114 Ser) of the IL-1 α protein. Different case definitions or demographics of the malarial populations could explain why rs17561 is associated with mild malaria in the Gambia and severe malaria in the Vietnamese. In Vietnam, an area of low transmission, malaria is generally a disease of non-immune adults as opposed to areas of high transmission in Africa where it is generally a disease of children.
For our analysis we have classified different clinical phenotypes together as severe malaria, even though the genetic etiology underlying each sub-phenotype may be different. In particular, the cerebral form of malaria may have a different genetic determinant compared to hemocratic-forms of malaria. When the association analysis was restricted only to the patients affected by the cerebral form of malaria (GCS <9), we observed a suggestive genotypic association between cerebral malaria onset with rs17561 (IL1A; p-value = 0.039; data not shown). A polymorphism in the TLR4 gene, rs4986790, also had a suggestive association between cerebral malaria onset (rs4986790, p-value = 0.044, OR = 2.85 [1.14 - 7.17]) however both cerebral malaria association were not significant after the most conservative Bonferroni adjustment.
IL-17A and IL-17F are inflammatory cytokines expressed by Th17 cells, a unique lineage of CD4+ helper T cells which promote innate effector mechanisms of inflammation 33. The IL-17 receptor complex composed of IL-17RA and IL-17RC is essential for the biological activity of IL-17 34, and this complex induces the activation of the NF-κB and MAPK signaling pathways. Appropriate regulation of the IL-17 signaling axis plays an integral role in host defense against extracellular bacteria and fungi, however there is limited evidence regarding its role in the host defense against protozoa 33, 35. Previously, rs708567 in IL17RC was associated with two subsets of malaria patients within an ethnically diverse epidemiological cohort from the Sudan 36 and based on data available for rs708567, Fumagalli et al 37 determined that IL17RC is a target of pathogen-driven selection along with 44 other interleukin genes. For the Vietnamese, having the GG genotype of rs708567 makes one 22% less likely to get severe malaria and affords 42% protection from death if one happens to get it. Interestingly, the GG homozygotes (Ser 111 Leu), which are the most frequent in the Vietnamese, have a lower parasite burden than AA or AG patients. Carrying the A allele of rs708567 is detrimental in terms of malaria, however it is yet to be determined whether this is due to interference in mediating the IL-17 signaling axis or from an IL-17RC -specific function.
Using a genome-wide approach to identify disease genes using a human model with natural conditions of infection can potentially reveal genes encoding important protective immune responses that are currently unknown. GWA studies are therefore a crucial step in determining the contribution of all known and unknown genes involved in an unbiased manner. We are currently preparing a GWAS of severe malaria in the Vietnamese Kinh using the case and control cohorts introduced here within the MalariaGEN consortium, who have already published a GWAS of severe malaria in the Gambians 38. Meta-analysis of multiple GWAS from ethnically diverse populations will ultimately be able to identify all contributing mechanisms necessary for protective immunity against malaria.
Subjects and methods
Human subjects and study design
The Hospital for Tropical Diseases (HTD) and Oxford University Clinical Research Unit (OUCRU) in Vietnam have recruited severe malaria patients into research studies between 1991-2009 (table 1). Severe malaria patients were recruited specifically into randomized controlled clinical trials 39, 40 at HTD, a tertiary referral hospital in Ho Chi Minh City, or into an epidemiological study conducted at HTD and two provincial hospitals in Binh Phuoc province, Phuoc Long and Dong Xoai district hospitals. Binh Phuoc province is a low highland region in south central Vietnam. It has low seasonal transmission of Plasmodium falciparum and P. vivax mainly in the forested rural areas, with very low entomological inoculation rates (in most areas <1). A collection of severe malaria patients primarily for genetics studies is currently ongoing at HTD.
Table 1. Sample collection of severe malaria cases and controls.
| Collection Year | Study description | Location | Sample no. |
|---|---|---|---|
| SEVERE MALARIA |
|||
| 1991-1995 | Randomised controlled trial40 | Hospital for Tropical Diseases (HTD), Ho Chi Minh City (HCMC) | 420 |
| 1996-2001 | Randomised controlled trial 39 | HTD, HCMC | 249 |
| 2000-2005 | Epidemiological study | HTD, HCMC | 300 |
| Phuoc Long and Dong Xoai District Hospitals, BinhPhuoc Province | |||
| 2005- ongoing | MalariaGEN collection | HTD, HCMC | 132 |
| CONTROLS | |||
| 2000-2005 | Epidemiological study | community controls, Phuoc Long and Dong Xoai District Hospitals | 600 |
| 2000-2005 | Epidemiological study | family controls, Phuoc Long and Dong Xoai District Hospitals | 132 (66 trios) |
| 2003-2006 | Birth cohort | cord blood controls, Hung Vuong hospital, HCMC | 1000 |
| 2006-2007 | Birth cohort | cord blood controls, Hung Vuong hospital, HCMC | 1250 |
Severe malaria cases were defined as those who had asexual forms of P. falciparum in their peripheral blood smear and had at least 1 of the following; impaired consciousness (Glasgow coma score <11 or Blantyre coma score <5), pulmonary oedema, acute renal failure (oliguria and serum creatinine >265 μmol/L), jaundice (serum bilirubin >51 μmol/L with parasite count >100,000/μL or with serum creatinine >250 μmol/L), hypoglycaemia (blood glucose <2.2 mmol/L), anaemia (haematocrit <20% with parasite count >100,000/μL), hyperparasitaemia (parasite count >500,000/μL), hyperlactataemia (plasma lactate > 4 mmol/L), metabolic acidosis (standard base excess > - 5 mmol/L, base deficit <10 mmol/L), pigmented neutrophil count (>4/100) and shock (SBP< 80mmHg with cool extremities); or had parasitemia ≥ 5% but none of the above features. Patients with cerebral malaria were defined as those with a Glasgow coma score <9.
The population control individuals (N=2840) were either cord blood controls or community controls. Cord blood control samples (N=2270) were collected from babies born in 2003 and between 2006-2007 at Hung Vuong Obstetric Hospital in Ho Chi Minh City (HCMC) and from babies born in 2003 at Dong Thap Hospital in Dong Thap province. In addition community controls (N=570) were recruited as part of the epidemiological study which were individually matched to a subset of the severe malaria cases by age (0-73 yrs), gender, ethnicity and location. Potential community controls were questioned about any possible history of severe malaria or time spent in hospital. Any candidates who had spent more than 48 hours in hospital other than for an operation, injury or known non-malaria diagnosis were excluded.
The samples came from unrelated individuals whose ethnic background was assessed by questionnaire. The treating physician was responsible for obtaining the patient’s informed consent and recruiting patients into the study. Prior to study recruitment, patients were informed of the risks and benefits of being in these studies and patients could refuse to participate. Written informed consent was obtained from each volunteer, however in the cases where the patient was unable to consent, i.e. unconscious, the consent of the relatives was acceptable. For cord blood control samples, informed consent was obtained from the mother. Ethical approvals were granted by the scientific and ethical committees at either the Hospital for Tropical Diseases HCMC, Hung Vuong Hospital HCMC, Dong Thap Hospital Dong Thap Province, and the People’s Committee of Ho Chi Minh City, Department of Health. Protocols were also approved by the Oxford Tropical Research Ethics Committee, UK.
DNA extraction and quantification
Genomic DNA from severe malaria patients and community controls was extracted from between 1-5ml of venous blood collected in tubes containing EDTA anti-coagulant. DNA was extracted by using either the blood midi kit or maxi kit from Qiagen (Lewes, UK). For controls, genomic DNA was extracted from 10 ml of cord blood using the Qiagen blood maxi kit (Qiagen, Lewes, UK). DNA was shipped frozen to Oxford University, UK. After arrival, the sample manifest was confirmed and all samples were relabeled and recoded with new sample codes to a standardised format bearing no relationship to the original coding. Sample volumes were recorded and the DNA was quantified using the PicoGreen® reagent (Invitrogen, Paisley, UK) as per manufacturer’s instruction.
Primer-extension amplification (PEP)
gDNA was diluted to 1ng/ul in 96-well plates. A PCR reagent mixture [45ul comprising 2.2ul of 1:10 diluted N15 primers (Genetix Ltd, UK), 1.25ul 8mM pooled dNTP’s, 2.5ul 50mM MgCl2, 5ul of 10X buffer, 0.5ul 5U/ul Biotaq polymerase (Bioline, UK), 33.55ul MilliQ water] was added to each well. gDNA (5ul of 1ng/ul) was added to the PEP PCR mixture, the plates were sealed and thermocycled with the following programme: 94°C for 3 min; 50 cycles of (94 °C for 1min, 37 °C for 2 min with a 0.1 °C increase per cycle up to 55 °C; 55 °C for 4min); and a final extension of 72°C for 5 min. PEP DNA was stored at −20°C until used. Quality and performance of the PEP reaction and products was assessed by PCR using a primer pair selected from the genotyping assays described below and run out on 2% agarose gels to check band intensity and fidelity.
Selection of SNPs
The selection of SNPs for genotyping was undertaken by the MalariaGEN consortium. This provided a common set of SNPs typed on all samples within the MalariaGEN consortium as part of a process for quality control of samples included in the Consortium’s projects. These SNPs were selected by interrogation of the literature and ongoing Consortium experiments for evidence of association with severe malaria. The 2 Sequenom® iPLEX® reactions designed also included gender-typing SNPs. Full details can be found at www.malariagen.net and the manuscript is in preparation.
Genotyping
Genotyping of PEP DNA samples diluted 1:10 was performed using the SEQUENOM® iPLEX® Gold platform and were performed according to the manufacturers specifications (http://www.sequenom.com). All primers were purchased lyophilised from Metabion International AG (Martinsried, Germany).
Data Analysis
Genotypic deviations from Hardy-Weinberg equilibrium (HWE) were assessed using a chi-square statistical test. SNPs were excluded from analysis if they had at least 15% of genotype calls missing or there was significant deviation from HWE (p<0.01). Subjects were removed from the dataset if they had greater than 10% of their total genotype data missing, or their self-reported gender deviated from the gender–specific genetic markers (amelogenin gene, 3 SNPs). Univariate analysis was performed for categorical variables with Pearson’s χ2 test to assess associations between disease phenotypes and allele or genotype frequencies. All association analysis of the cases and controls was adjusted by ethnic group, where a categorical variable encoding the ethnicities is included as a covariate in the logistic regression. The logistic regression also allows the odds ratios for the genotypes to be estimated. In this approach we modeled the SNP of interest assuming several related genotypic mechanisms (additive, dominant, recessive, heterozygous advantage and general models) and report the minimum P-value from these correlated tests. More specifically, the general model was used to compare genotype frequencies. Briefly, the hypothesis of this model is that one genotype group (e.g. AA group) is the baseline, and there is no pattern at all between the odds ratios for the genotype groups AB and BB. Therefore, there are separate estimates for the odds ratio for genotype AB, and another odds ratio for genotype BB. This model which uses the 3 genotype classes makes no assumptions about the risk or mean for AB heterozygotes compared to AA and BB homozygotes. We calculated the Fst between the cord blood controls and the community controls using allele frequencies and the following formula (p1 − p2)^2/[(p1 + p2)(2 − p1 − p2)]. Continuous outcomes (e.g. parasite count/μl) were tested for association using a linear model. All analyses were performed using the R statistical package (http://www.r-project.org). Performing multiple statistical tests leads to inflation in the occurrence of false positives, therefore this study should be regarded as exploratory. To be able to use these associated variants for any translational purposes it is essential to replicate these SNP associations in independent cohorts. Therefore the data presented here is unadjusted, but given the number of SNPs tested (N=65) many SNPs would not remain significant after conservative adjustment (e.g. Bonferroni). However, given that all tests are not independent (related phenotypes, SNPs not independent due to linkage disequilibrium, etc.) Bonferroni adjustment may not be appropriate.
Supplementary Material
Acknowledgements
We would like to thank all the Vietnamese individuals who agreed to provide samples for this study. We acknowledge the work of the clinical staff from the Hospital of Tropical Diseases, HCMC and Phuoc Long and Dong Xoai District Hospitals in Binh Phuoc province, Viet Nam, who initially diagnosed and studied the patients with severe malaria. We would like to thank Dr. Nguyen Thi Hieu and his staff from Hung Vuong Obstetric Hospital for the collection of the cord blood controls. We thank the assistance of Susana Campino, Rachel Craik, Kate Rowlands, Angie Green and Christina Hubbart in the MalariaGEN resource centre in for the DNA handling and genotyping. We appreciate the contribution of the MalariaGEN ethics team (Oxford University, UK), especially Jantina de Vries. The clinical component of this study was funded through the Wellcome Trust Major Overseas Program in Vietnam (089276/Z/09/Z). The MalariaGEN Project is supported by the Wellcome Trust (WT077383/Z/05/Z) and the Bill and Melinda Gates Foundation through the Foundations of the National Institutes of Health (grant number 566) as part of the Grand Challenges in Global Health Initiative. This research was also supported by the Medical Research Council (G0600718; G0600230). Dominic Kwiatkowski receives support from the Medical Research Council (G19/9). The Wellcome Trust also provides core awards to the Wellcome Trust Centre for Human Genetics (075491/Z/04; 090532/Z/09/Z) and to the Wellcome Trust Sanger Institute (077012/Z/05/Z and 098051).
Footnotes
Conflict of interest statement: The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.World Health Organisation WH . World Malaria Report 2009. WHO; Geneva: 2009. [Google Scholar]
- 2.National Institute of Malariology Preventation and Epidemiology . Annual Report of Malaria and Parasite Prevention in 2010. Hanoi; Vietnam: 2010. [Google Scholar]
- 3.Barat LM. Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam. Am J Trop Med Hyg. 2006;74(1):12–6. [PubMed] [Google Scholar]
- 4.Hung le Q, Vries PJ, Giao PT, Nam NV, Binh TQ, Chong MT, et al. Control of malaria: a successful experience from Viet Nam. Bull World Health Organ. 2002;80(8):660–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Erhart A, Thang ND, Xa NX, Thieu NQ, Hung LX, Hung NQ, et al. Accuracy of the health information system on malaria surveillance in Vietnam. Trans R Soc Trop Med Hyg. 2007;101(3):216–25. doi: 10.1016/j.trstmh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Trung HD, Van Bortel W, Sochantha T, Keokenchanh K, Quang NT, Cong LD, et al. Malaria transmission and major malaria vectors in different geographical areas of Southeast Asia. Trop Med Int Health. 2004;9(2):230–7. doi: 10.1046/j.1365-3156.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bejon P, Lusingu J, Olotu A, Leach A, Lievens M, Vekemans J, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–32. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359(24):2533–44. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 10.Webster DP, Dunachie S, Vuola JM, Berthoud T, Keating S, Laidlaw SM, et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc Natl Acad Sci U S A. 2005;102(13):4836–41. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74(10):5933–42. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104(3):189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- 13.MalariaGEN Network A global network for investigating the genomic epidemiology of malaria. Nature. 2008;456(7223):732–7. doi: 10.1038/nature07632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10(2):143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Reyes D, Craig AG, Kyes SA, Peshu N, Snow RW, Berendt AR, et al. A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum Mol Genet. 1997;6(8):1357–60. doi: 10.1093/hmg/6.8.1357. [DOI] [PubMed] [Google Scholar]
- 16.Kun JF, Klabunde J, Lell B, Luckner D, Alpers M, May J, et al. Association of the ICAM-1Kilifi mutation with protection against severe malaria in Lambarene, Gabon. Am J Trop Med Hyg. 1999;61(5):776–9. doi: 10.4269/ajtmh.1999.61.776. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy R, Kwiatkowski D, Hill AV. Absence of an association between intercellular adhesion molecule 1, complement receptor 1 and interleukin 1 receptor antagonist gene polymorphisms and severe malaria in a West African population. Trans R Soc Trop Med Hyg. 1998;92(3):312–6. doi: 10.1016/s0035-9203(98)91026-4. [DOI] [PubMed] [Google Scholar]
- 18.Ndiaye R, Sakuntabhai A, Casademont I, Rogier C, Tall A, Trape JF, et al. Genetic study of ICAM1 in clinical malaria in Senegal. Tissue Antigens. 2005;65(5):474–80. doi: 10.1111/j.1399-0039.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Looareesuwan S, Tokunaga K. Absence of association between the allele coding methionine at position 29 in the N-terminal domain of ICAM-1 (ICAM-1(Kilifi)) and severe malaria in the northwest of Thailand. Jpn J Infect Dis. 2001;54(3):114–6. [PubMed] [Google Scholar]
- 20.Fry AE, Auburn S, Diakite M, Green A, Richardson A, Wilson J, et al. Variation in the ICAM1 gene is not associated with severe malaria phenotypes. Genes Immun. 2008;9(5):462–9. doi: 10.1038/gene.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amodu OK, Gbadegesin RA, Ralph SA, Adeyemo AA, Brenchley PE, Ayoola OO, et al. Plasmodium falciparum malaria in south-west Nigerian children: is the polymorphism of ICAM-1 and E-selectin genes contributing to the clinical severity of malaria? Acta Trop. 2005;95(3):248–55. doi: 10.1016/j.actatropica.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Sinha S, Qidwai T, Kanchan K, Anand P, Jha GN, Pati SS, et al. Variations in host genes encoding adhesion molecules and susceptibility to falciparum malaria in India. Malar J. 2008;7:250. doi: 10.1186/1475-2875-7-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire W, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371(6497):508–10. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 24.Clark TG, Diakite M, Auburn S, Campino S, Fry AE, Green A, et al. Tumor necrosis factor and lymphotoxin-alpha polymorphisms and severe malaria in African populations. J Infect Dis. 2009;199(4):569–75. doi: 10.1086/596320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman HC, Ribas G, Jallow M, Mott R, Neville M, Sisay-Joof F, et al. Complex haplotypic structure of the central MHC region flanking TNF in a West African population. Genes Immun. 2003;4(7):476–86. doi: 10.1038/sj.gene.6364008. [DOI] [PubMed] [Google Scholar]
- 26.Newton JL, Harney SM, Timms AE, Sims AM, Rockett K, Darke C, et al. Dissection of class III major histocompatibility complex haplotypes associated with rheumatoid arthritis. Arthritis Rheum. 2004;50(7):2122–9. doi: 10.1002/art.20358. [DOI] [PubMed] [Google Scholar]
- 27.Mfuna Endam L, Cormier C, Bosse Y, Filali-Mouhim A, Desrosiers M. Association of IL1A, IL1B, and TNF gene polymorphisms with chronic rhinosinusitis with and without nasal polyposis: A replication study. Arch Otolaryngol Head Neck Surg. 136(2):187–92. doi: 10.1001/archoto.2009.219. [DOI] [PubMed] [Google Scholar]
- 28.Karjalainen J, Hulkkonen J, Pessi T, Huhtala H, Nieminen MM, Aromaa A, et al. The IL1A genotype associates with atopy in nonasthmatic adults. J Allergy Clin Immunol. 2002;110(3):429–34. doi: 10.1067/mai.2002.126784. [DOI] [PubMed] [Google Scholar]
- 29.Sims AM, Timms AE, Bruges-Armas J, Burgos-Vargas R, Chou CT, Doan T, et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirms associations with ankylosing spondylitis. Ann Rheum Dis. 2008;67(9):1305–9. doi: 10.1136/ard.2007.081364. [DOI] [PubMed] [Google Scholar]
- 30.Han W, Kang SY, Kang D, Park SK, Lee JY, Kim H, et al. Multiplex genotyping of 1107 SNPs from 232 candidate genes identified an association between IL1A polymorphism and breast cancer risk. Oncol Rep. 23(3):763–9. [PubMed] [Google Scholar]
- 31.Walley AJ, Aucan C, Kwiatkowski D, Hill AV. Interleukin-1 gene cluster polymorphisms and susceptibility to clinical malaria in a Gambian case-control study. Eur J Hum Genet. 2004;12(2):132–8. doi: 10.1038/sj.ejhg.5201084. [DOI] [PubMed] [Google Scholar]
- 32.Gyan B, Goka B, Cvetkovic JT, Perlmann H, Lefvert AK, Akanmori B, et al. Polymorphisms in interleukin-1beta and interleukin-1 receptor antagonist genes and malaria in Ghanaian children. Scand J Immunol. 2002;56(6):619–22. doi: 10.1046/j.1365-3083.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- 33.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2010;32(1):33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177(1):36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 35.van de Veerdonk FL, Gresnigt MS, Kullberg BJ, van der Meer JW, Joosten LA, Netea MG. Th17 responses and host defense against microorganisms: an overview. BMB Rep. 2009;42(12):776–87. doi: 10.5483/bmbrep.2009.42.12.776. [DOI] [PubMed] [Google Scholar]
- 36.Eid NA, Hussein AA, Elzein AM, Mohamed HS, Rockett KA, Kwiatkowski DP, et al. Candidate malaria susceptibility/protective SNPs in hospital and population-based studies: the effect of sub-structuring. Malar J. 9:119. doi: 10.1186/1475-2875-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206(6):1395–408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009 doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phu NH, Tuan PQ, Day N, Mai NT, Chau TT, Chuong LV, et al. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 9:97. doi: 10.1186/1475-2875-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran TH, Day NP, Nguyen HP, Nguyen TH, Tran TH, Pham PL, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335(2):76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


