Abstract
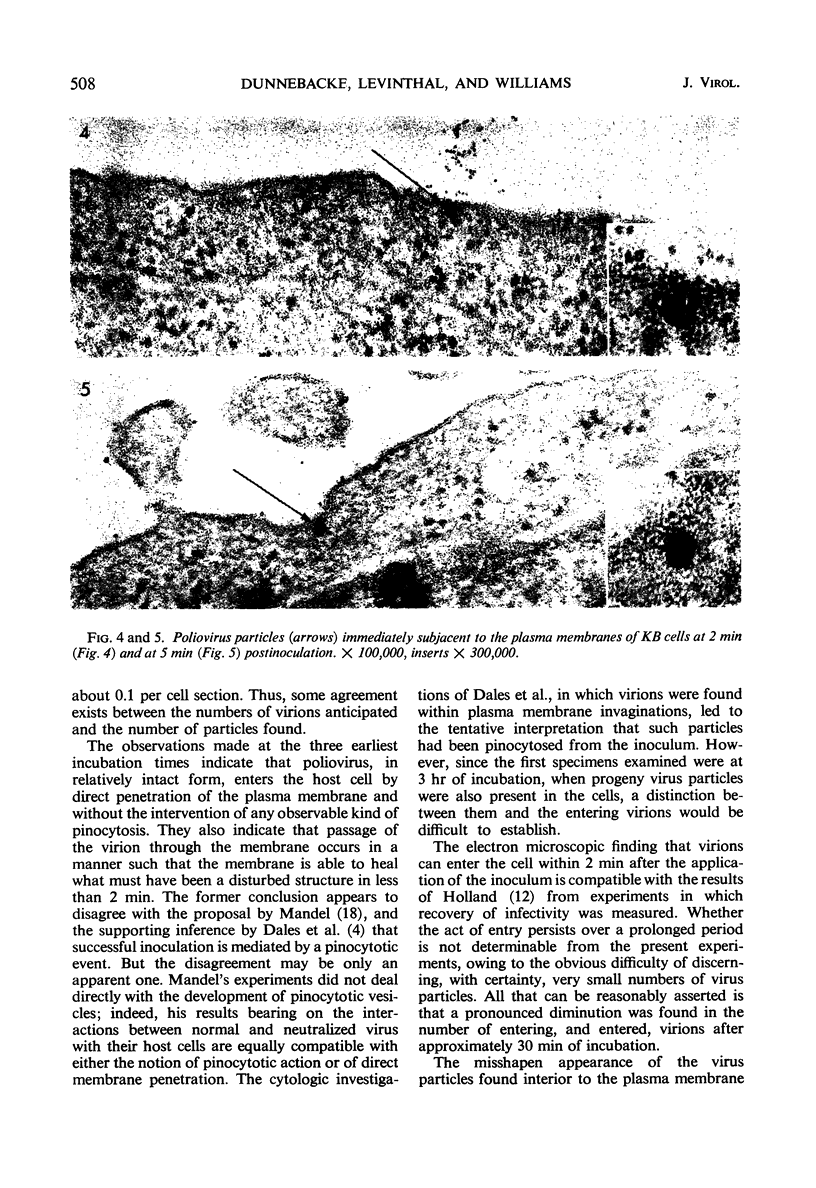
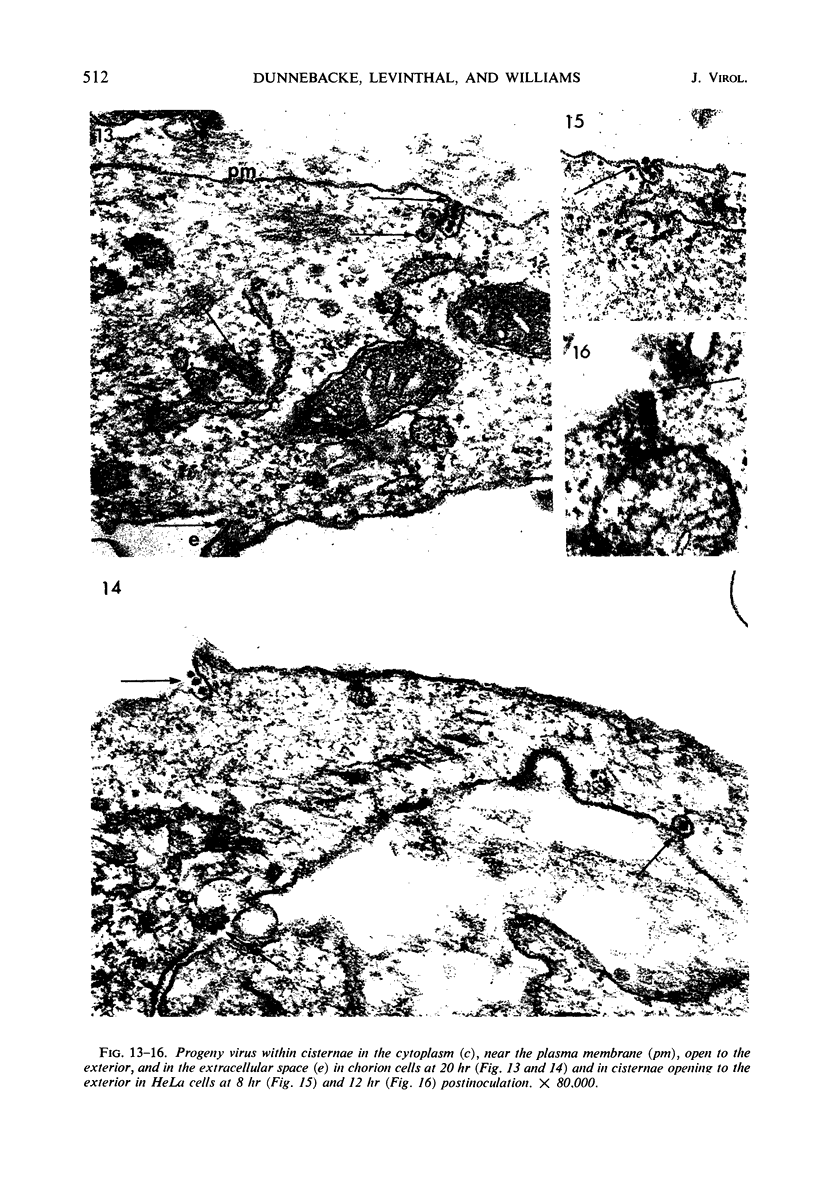
Cells of differing culture types were inoculated with poliovirus at 37 C, sampled at intervals during the replicative cycle, and examined in thin sections by electron microscopy. The earliest samples, taken at 2 and 5 min postinoculation, showed virus particles adjacent to the exterior of the plasma membrane and others that had apparently penetrated it directly; later samples showed fewer such particles or none. Particles lying in the peripheral cytoplasm frequently appeared swollen and distorted in shape. No sign of virus entry by a pinocytotic process was found at any time. At 3 hr, and subsequently during the replication cycle, particles of progeny virus appeared in the cytoplasm. They were found free in the cytoplasmic matrix, aligned along the elements of filamentous complexes, and enclosed within vesicles. Some of the vesicles were found to be open to the extracellular space, indicating a likely mechanism of virus release.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANG F. B., GEY G. O. Viruses and cells--a study in tissue culture applications. Trans N Y Acad Sci. 1951 Jun;13(8):324–327. doi: 10.1111/j.2164-0947.1951.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Blinzinger K., Simon J., Magrath D., Boulger L. Poliovirus crystals within the endoplasmic reticulum of endothelial and mononuclear cells in the monkey spinal cord. Science. 1969 Mar 21;163(3873):1336–1337. doi: 10.1126/science.163.3873.1336. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNNEBACKE T. H., WILLIAMS R. C. The maturation and release of infectious polio and Coxsackie viruses in individual tissue cultured cells. Arch Gesamte Virusforsch. 1962;11:583–591. doi: 10.1007/BF01241308. [DOI] [PubMed] [Google Scholar]
- DUNNEBACKE T. H., ZITCER E. M. Preparation and cultivation of primary human amnion cells. Cancer Res. 1957 Dec;17(11):1043–1046. [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- FOGH J., STUART D. C., Jr Intracellular crystals of polio-viruses in HeLa cells. Virology. 1960 May;11:308–311. doi: 10.1016/0042-6822(60)90074-x. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOWES D. W. The growth cycle of poliovirus in cultured cells. II. Maturation and release of virus in suspended cell populations. Virology. 1959 Sep;9:96–109. doi: 10.1016/0042-6822(59)90104-7. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Fusion of vesicular stomatitis virus with the cytoplasmic membrane of L cells. J Virol. 1969 Jun;3(6):619–622. doi: 10.1128/jvi.3.6.619-622.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C., Morgan C. Interactions between Sendai virus and human erythrocytes. J Virol. 1969 Jan;3(1):70–81. doi: 10.1128/jvi.3.1.70-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A., DULBECCO R., VOGT M., LWOFF M. Kinetics of the release of poliomyelitis virus from single cells. Virology. 1955 May;1(1):128–139. doi: 10.1016/0042-6822(55)90010-6. [DOI] [PubMed] [Google Scholar]
- Levinthal J. D., Wicker R., Cerottini J. C. Study of intracellular SV40 antigens by indirect immunoferritin technique. Virology. 1967 Mar;31(3):555–558. doi: 10.1016/0042-6822(67)90239-5. [DOI] [PubMed] [Google Scholar]
- MAYOR H. D., STINEBAUGH S. E., JAMISON R. M., JORDAN L. E., MELNICK J. L. Immunofluorescent, cytochemical, and microcytological studies on the growth of the simian vacuolating virus (SV-40) in tissue culture. Exp Mol Pathol. 1962 Oct;1:397–416. doi: 10.1016/0014-4800(62)90033-3. [DOI] [PubMed] [Google Scholar]
- Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967 Apr;31(4):702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Daniel W. A. Replication of poliovirus in HeLa cells: electron microscopic observations. Virology. 1965 Aug;26(4):646–663. doi: 10.1016/0042-6822(65)90328-4. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro L. S., Rose H. M., Morgan C., Hsu K. C. Electron microscopic study of the development of simian virus 40 by use of ferritin-labeled antibodies. J Virol. 1967 Apr;1(2):384–399. doi: 10.1128/jvi.1.2.384-399.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWERDT C. E., FOGH J. The ratio of physical particles per infectious unit observed for poliomyelitis viruses. Virology. 1957 Aug;4(1):41–52. doi: 10.1016/0042-6822(57)90042-9. [DOI] [PubMed] [Google Scholar]
- STUART D. C., Jr, FOGH J. Electron microscopic demonstration of intracellular poliovirus crystals. Exp Cell Res. 1959 Oct;18:378–381. doi: 10.1016/0014-4827(59)90019-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E., Dales S. Viropexis of vesicular stomatitis virus by L cells. Virology. 1969 Feb;37(2):285–290. doi: 10.1016/0042-6822(69)90209-8. [DOI] [PubMed] [Google Scholar]
- Zee Y. C., Hackett A. J., Talens L. T. Electron microscopic studies on the vesicular exanthema of swine virus. II. Morphogenesis of VESV type H54 in pig kidney cells. Virology. 1968 Apr;34(4):596–607. doi: 10.1016/0042-6822(68)90081-0. [DOI] [PubMed] [Google Scholar]
- Zee Y. C., Talens L., Hackett A. J. Localization of a small ribonucleic acid virus within cytoplasmic cisternae. J Virol. 1967 Dec;1(6):1271–1273. doi: 10.1128/jvi.1.6.1271-1273.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]