Abstract
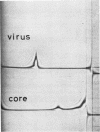
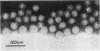
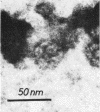
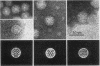
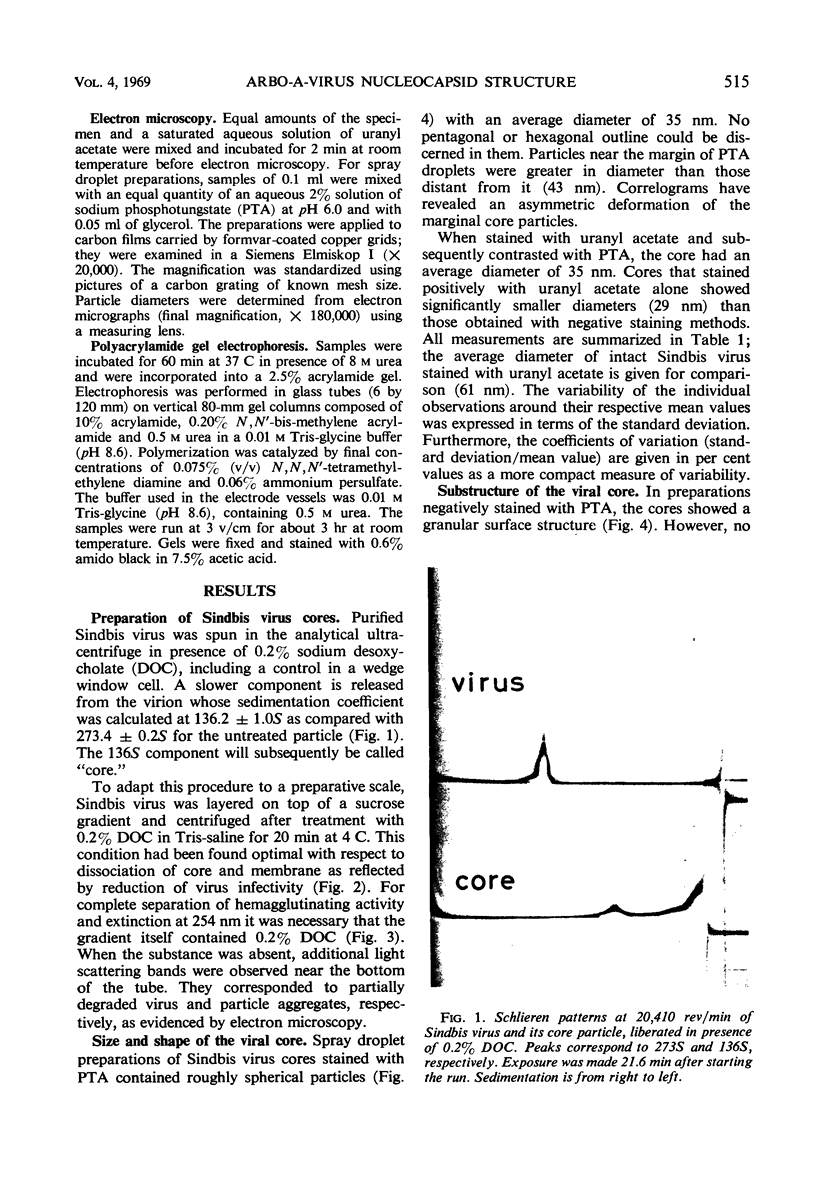
When Sindbis virus (273S) was treated with sodium desoxycholate, a nonhemagglutinating 136S particle was liberated from the virion, representing the viral nucleocapsid (core). Electron microscopically it appeared as a spherical particle 35 nm in diameter, showing ringlike morphological units 12 to 14 nm in diameter on its surface. When the one- and two-sided images of core particles were correlated, their structure could be demonstrated to have the T = 3 arrangement of 32 hexamer-pentamer morphological units within a symmetrical surface lattice. The core contained a further spherical structure (12 to 16 nm in diameter) which was designated as the central core component. Two proteins were found associated with the core, a third viral protein belonged to the hemagglutinating surface structures. The significance of these findings for virus classification is discussed.
Full text
PDF
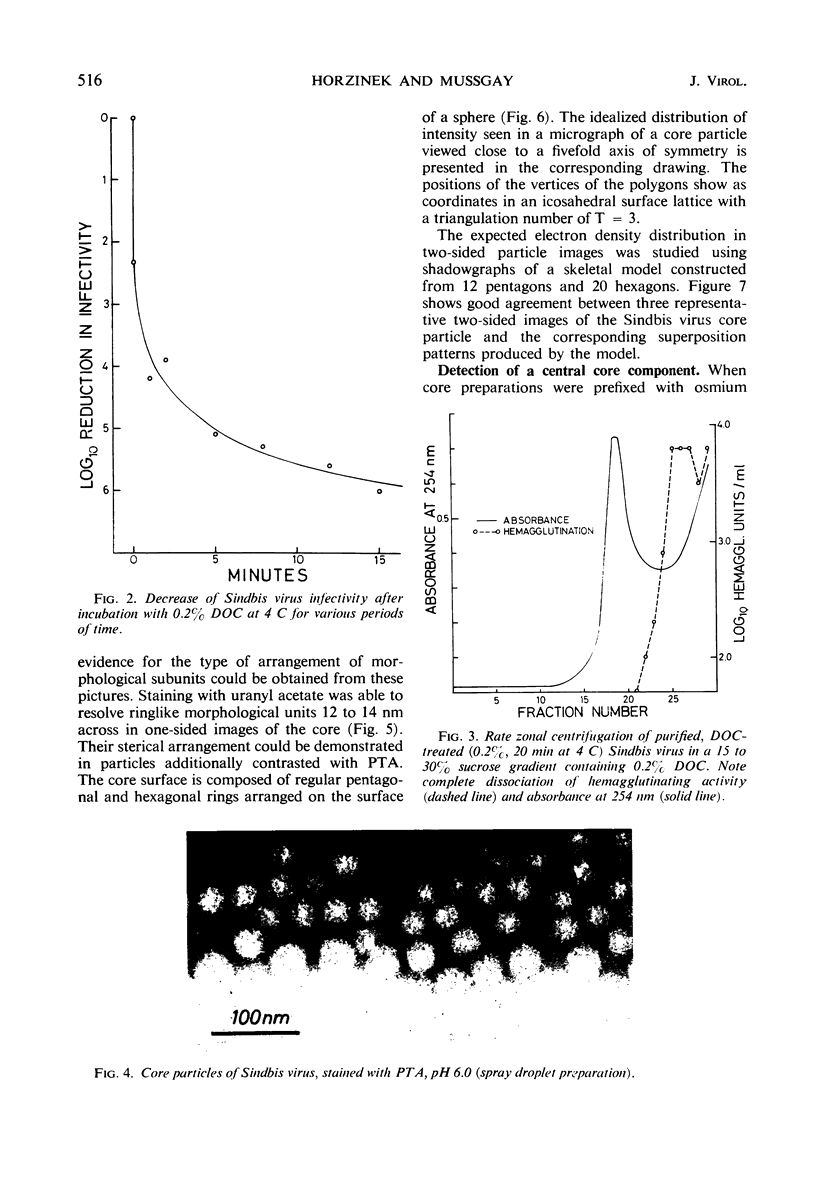
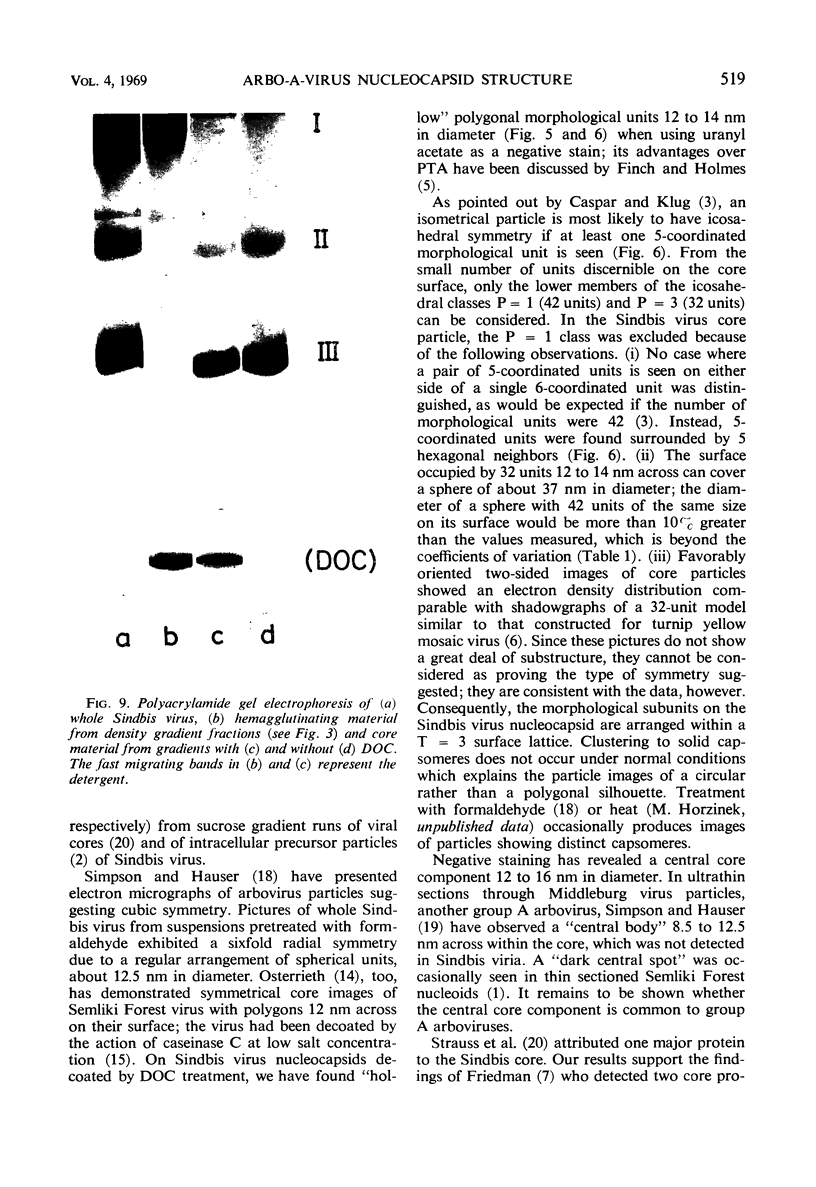

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
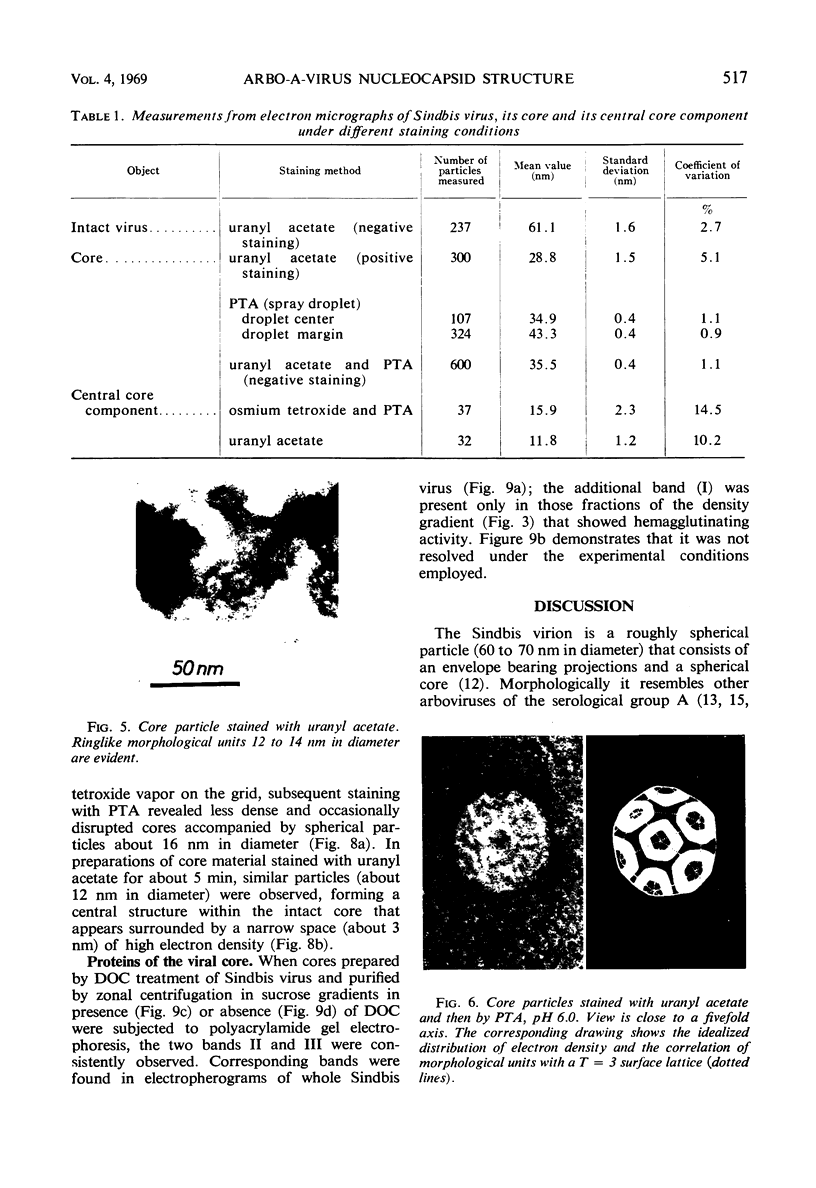
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ishai Z., Goldblum N., Becker Y. The intracellular site and sequence of Sindbis virus replication. J Gen Virol. 1968 May;2(3):365–375. doi: 10.1099/0022-1317-2-3-365. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Arrangement of protein subunits and the distribution of nucleic acid in turnip yellow mosaic virus. II. Electron microscopic studies. J Mol Biol. 1966 Jan;15(1):344–364. doi: 10.1016/s0022-2836(66)80231-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Structural and nonstructural proteins of an arbovirus. J Virol. 1968 Oct;2(10):1076–1080. doi: 10.1128/jvi.2.10.1076-1080.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimenko S. M., Yershov F. I., Gofman Y. P., Nabatnikov A. P., Zhdanov V. M. Architecture of Venezuelan equine encephalomyelitis virus. Virology. 1965 Oct;27(2):125–128. doi: 10.1016/0042-6822(65)90152-2. [DOI] [PubMed] [Google Scholar]
- LWOFF A., HORNE R., TOURNIER P. A system of viruses. Cold Spring Harb Symp Quant Biol. 1962;27:51–55. doi: 10.1101/sqb.1962.027.001.008. [DOI] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M. GROWTH CYCLE OF ARBOVIRUSES IN VERTEBRATE AND ARTHROPOD CELLS. Prog Med Virol. 1964;6:193–267. [PubMed] [Google Scholar]
- MUSSGAY M. STUDIES ON THE STRUCTURE OF A HEMAGGLUTINATING COMPONENT OF A GROUP A ARBO VIRUS (SINDBIS). Virology. 1964 Aug;23:573–581. doi: 10.1016/0042-6822(64)90241-7. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic demonstration of purified Venezuelan equine encephalitis virus. Virology. 1963 Jan;19:109–112. doi: 10.1016/0042-6822(63)90032-1. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. M., Calberg-Bacq C. M. Changes in morphology, infectivity and haemagglutinating activity of Semliki Forest virus produced by the treatment with caseinase C from Streptomyces albus G. J Gen Microbiol. 1966 Apr;43(1):19–30. doi: 10.1099/00221287-43-1-19. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Basic structure of group A arbovirus strains Middelburg, Sindbis, and Semliki Forest examined by negative staining. Virology. 1968 Feb;34(2):358–361. doi: 10.1016/0042-6822(68)90248-1. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural differentiation of group A arboviruses based on nucleoid morphology in ultrathin sections. Virology. 1968 Mar;34(3):568–570. doi: 10.1016/0042-6822(68)90077-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Pfefferkorn E. R., Darnell J. E., Jr Identification of the membrane protein and "core" protein of Sindbis virus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):533–537. doi: 10.1073/pnas.59.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
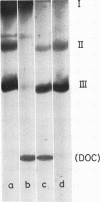
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WECKER E. The extraction of infectious virus nucleic acid with hot phenol. Virology. 1959 Feb;7(2):241–243. doi: 10.1016/0042-6822(59)90191-6. [DOI] [PubMed] [Google Scholar]