Abstract
The absorption of light by bound or diffusible chromophores causes conformational rearrangements in natural and artificial photoreceptor proteins. These rearrangements are coupled to the opening or closing of ion transport pathways, the association or dissociation of binding partners, the enhancement or suppression of catalytic activity, or the transcription or repression of genetic information. Illumination of cells, tissues, or organisms engineered genetically to express photoreceptor proteins can thus be used to perturb biochemical and electrical signaling with exquisite cellular and molecular specificity. First demonstrated in 2002, this principle of optogenetic control has had a profound impact on neuroscience, where it provides a direct and stringent means of probing the organization of neural circuits and of identifying the neural substrates of behavior. The impact of optogenetic control is also beginning to be felt in other areas of cell and organismal biology.
Keywords: neuron, membrane potential, ion channel, photostimulation, signal transduction, behavior, optical methods
INTRODUCTION
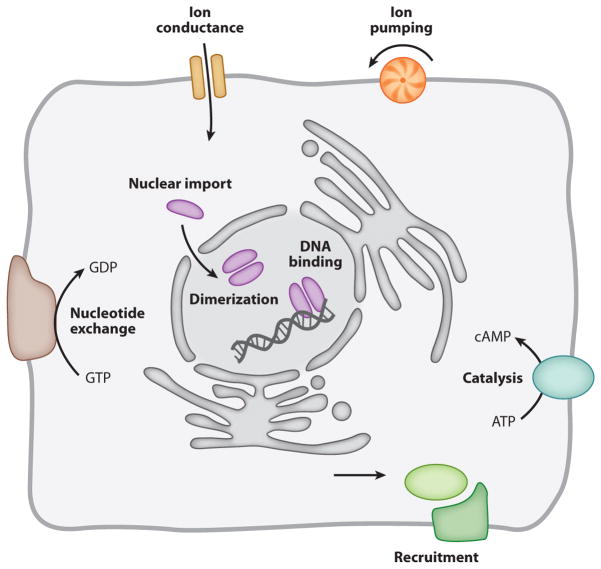
Energy is transferred to (and work can be performed on) biological matter when photons of light are absorbed, scattered, or reflected. Optical methods, so often thought of as purely observational, are also useful for intervention: Light can generate mechanical forces and switch electrical currents; it can regulate the transcription of genetic information and control the flow of substrates and signals in biochemical pathways (Figure 1).
Figure 1.
Cell biological processes that have been controlled with the help of optogenetic actuators.
Optical forces are miniscule on macroscopic scales. Photons raining down on a surface exert what Maxwell christened “radiation pressure.” Sunlight, for example, applies a pressure of approximately 10−5 Pa at the surface of the earth. This is a negligible force, amounting to less than 1/10,000,000,000 of the atmospheric air pressure at sea level. Although extraordinarily feeble, the effects of optical radiation become noticeable even in classical systems when the forces that dominate our everyday experience are outweighed. In interstellar space, radiation pressure is responsible for bending the tails of comets away from photon-emitting stars. And in the laboratory, the radiation pressure exerted by intense, focused laser beams is sufficient to accelerate or arrest small objects such as micrometer-sized latex beads (Ashkin 1970). In these optical traps or tweezers, illumination powers in the milliwatt range produce forces on a piconewton scale.
Unsurprisingly, the relative impact of being struck by a photon is much larger for a molecule than for a comet or a latex bead. Quanta of visible to UV light carry energies of 150 to 400 kJ mol−1, as determined by the product of Planck’s constant h and the optical frequency ν. These energies are of the same order of magnitude as the strengths of (weak) covalent single bonds and the π orbitals of some double bonds. The absorption of a photon can therefore provide the activation energy to break single bonds (photodissociation or photolysis) or the π components of double bonds, thus creating an opportunity for rotation about what is essentially a single bond in the excited state (photoisomerization). Photon absorption can also promote an electron from a lower to a higher orbital, which facilitates its subsequent removal or the refilling of the low-lying vacancy with another electron (charge transfers: photooxidation and photoreduction).
In cells, photochemical reactions are sequestered within proteins or protein complexes that funnel the absorbed optical energy into directed molecular motions. These optically responsive proteins can be classified, in analogy to the traditional division of electrical machinery, into power and communications devices: The primary role of some systems is the conversion of energy (photosynthetic reaction centers and light-driven ion pumps), whereas that of others is the transmission of information (photoreceptors). As in electrical engineering, the distinction between the two groups is not absolute, as energy is inevitably transferred during communication, and the amount of energy converted by power devices is informative.
Taken out of their natural context, optically responsive proteins make ideal experimental agents for probing the functional organization of cells and circuits: They can be called upon at precise times and in defined locations and be instructed remotely and noninvasively to apply exquisitely specific perturbations. Because they are encodable in DNA, their distribution can be restricted genetically to a particular subset of cells in an organism or a particular subcellular site in a cell, thus adding cellular to molecular specificity of action. The past decade has seen the realization of these advantages, as natural chromophore-containing photoreceptor proteins (Boyden et al. 2005, Li et al. 2005, Shimizu-Sato et al. 2002, Zemelman et al. 2002) and artificial proteins that mimic them (Banghart et al. 2004, Zemelman et al. 2003) have been developed as photochemical actuators and deployed in cells and whole animals (Figure 1). The resulting field, which forms part of what has come to be called optogenetics (Deisseroth et al. 2006, Miller 2006), is the topic of this and several complementary reviews (Fiala et al. 2010; Gorostiza & Isacoff 2008b; Gradinaru et al. 2007; Knöpfel et al. 2010; Miesenböck 2004, 2008, 2009; Miesenböck & Kevrekidis 2005; Scanziani & Häusser 2009; Sjulson & Miesenböck 2008; Szobota & Isacoff 2010; Zemelman & Miesenböck 2001; Zhang et al. 2006, 2007a).
PHOTOCHEMICAL ACTUATORS MAKE LIGHT WORK
For a quantum of light to have a photochemical effect, it must be absorbed. Intuitively, an absorption event can be viewed as a collision between a photon and a target molecule; the frequency with which the target is hit depends on its size (the absorption cross section) and the number of photons passing through a unit cross section of sample per unit time (the photon flux density or irradiance). When a good microscope objective focuses 1 mW of blue light into a Gaussian spot of 0.5-μm diameter, the irradiance at the focus is approximately 5,000 W mm−2, or 1022 photons mm−2 s−1. Under these conditions, which are common in confocal microscopy, a light-sensitive molecule with a typical absorption cross section on the order of 10 Å2 will be excited roughly once every 10 ns, which is near or at the saturation limit. Wide-field illumination of biological tissues and organisms by lasers and light-emitting diodes, in the power ranges of 0.5–50 mW mm−2 that are characteristic of photostimulation experiments in vivo (Aravanis et al. 2007, Claridge-Chang et al. 2009, Lima & Miesenböck 2005, Szobota et al. 2007), produces photon flux densities of 1015–1017 photons mm−2 s−1, which excite a typical chromophore once every 1–100 ms.
Following absorption of a photon, the fate of the excited molecule is to lose or use the absorbed energy, either through photophysical processes (fluorescence emission or nonradiative energy transfer, ionization, or physical quenching) or photochemical channels that comprise dissociation, charge transfer, and isomerization reactions. The likelihood that an excited molecule decays via a particular process is measured by an efficiency parameter termed the primary quantum yield φ. The effectiveness of a photochemical actuator—the rate at which light can drive the desired reaction—is therefore the product of the reaction’s primary quantum yield and the photon absorption rate, which in turn can be factored (up to the saturation limit) into the product of the absorption cross section and photon flux density. The chromophores of efficient actuators have large absorption cross sections and high primary quantum yields, which ensure that photons are captured readily and the absorbed energy is channeled efficiently into the desired photochemical process.
The occurrence of secondary reactions can make the overall quantum yield of a photochemical process far exceed the primary quantum yield of the reaction that ignites it. One of the most striking natural examples of such secondary amplification underlies the photon-counting ability of visual photoreceptors (Bialek 1987). Electrophysiological recordings from vertebrates and invertebrates have shown that photoreceptor cells generate significant electrical currents after absorbing a single photon; psychophysical experiments in humans argue that these currents—whose proximate cause is the light-driven conformational change of a single rhodopsin molecule—are perceived as faint flashes of light (Bialek 1987). Rhodopsin owes its remarkable strength as a photochemical actuator to a cascade of secondary reactions that result in altered gating of many ion channels, each controlling the flow of many ions, thus increasing the overall quantum yield dramatically (Baylor 1996).
It is instructive to consider the case of the photoreceptor cell not only in kinetic but also in energetic terms. A light-adapted fly photoreceptor cell undergoing approximately 5 × 105 photoconversions per second (de Ruyter van Steveninck & Laughlin 1996) gains, from photon absorption, the energetic equivalent of hydrolyzing 106 molecules of ATP. Transmitting information, however, also incurs a metabolic cost, which has been estimated as the equivalent of hydrolyzing 7.5 × 109 molecules of ATP per second (Laughlin et al. 1998). Despite some quantitative uncertainties attached to the argument, it is clear from the magnitude of this disparity that the light the receptor cell absorbs cannot power phototransduction. Rather, the energetic cost of communication must be met from the cell’s own metabolism. In other words, the actuator, rhodopsin, functions akin to an electromechanical relay: It allows control of a high-power device (the synaptic output of the photoreceptor cell) with a low-power signal (a photon of light).
Most light-controlled actuators operate according to the same principle: The photo-chemical reaction sets the rate of a biological process, but it does not power the process itself. A simple illustration of this separation of control and power is an optically gated ion channel. Once light opens the gate of the channel pore, ions flood through passively, down their electrochemical gradient. True to its definition as a “device that causes some other device to move or operate without providing the motive power for it to do so,” the actuator taps a local energy supply to make light work.
A light-driven ion pump, in contrast, draws its power directly from photon absorption; here, the distinction between control and power lines collapses. The amount of optical energy that can be pumped into such a power device directly determines—and limits—the amount of work performed because the gate controlled by photon absorption must be driven through a cycle of conformations simply to transport one ion.
DESIGN PRINCIPLES OF PHOTOCHEMICAL ACTUATORS
Photochemical actuators combine the functions of light sensor and biological effector. These functions reside in different chemical entities. The role of light sensor is played by a chromophore of natural or synthetic origin. Chromophores contain extended conjugated π electron systems that tune them to light in particular spectral bands; as a rule of thumb, the larger the possible extent of electron delocalization is, the longer the wavelength of light the chromophore will absorb. Part of the absorbed energy is channeled into photochemical processes, which generate biologically active products or conformers with typical primary quantum yields of 0.1 to 0.9. Protein effectors detect the appearance of these photochemical reaction products and respond with conformational changes that create or expose binding surfaces, active sites, cellular localization signals, or ion conduction pathways (Figure 1).
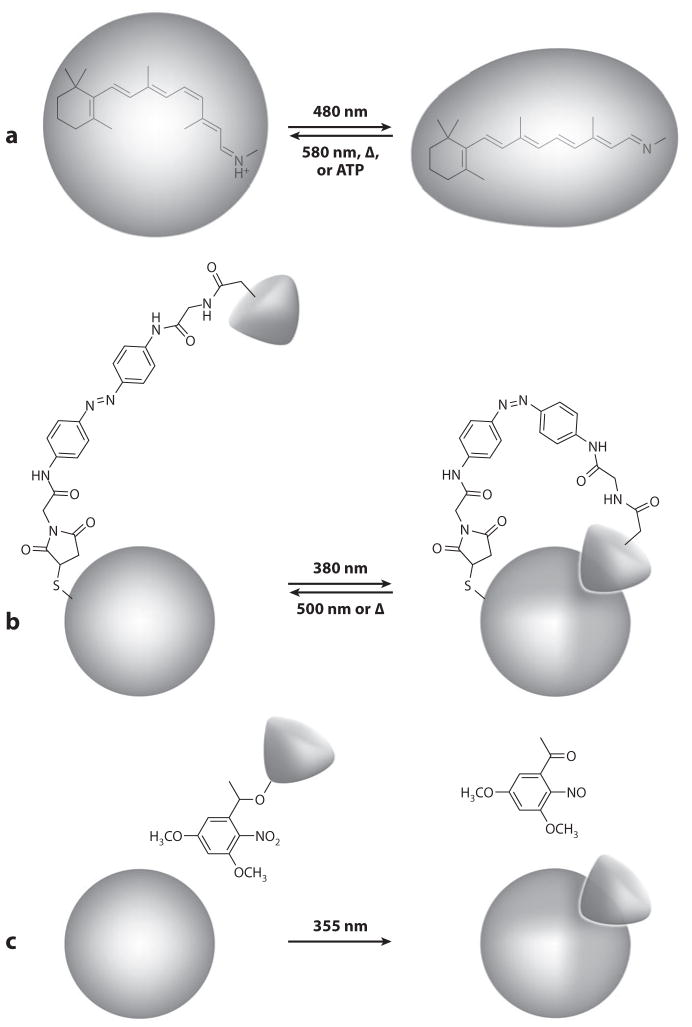
In many actuators, the chromophore is physically linked to the effector protein to form a light-sensitive prosthetic group (Figure 2). All natural photoreceptor proteins of plants, animals, and microbes (Hegemann 2008, Möglich et al. 2010, Spudich et al. 2000, Zoltowski & Gardner 2011), as well as engineered proteins bearing photoisomerizable tethered ligands (PTLs) (Banghart et al. 2004, Bartels et al. 1971, Lester et al. 1980, Volgraf et al. 2006), belong to this category (Figure 2a,b). In other actuators, the functions of light sensor and effector are physically uncoupled (Figure 2c). In these systems, all known examples of which are engineered, the chromophore is a photocleavable or photo-isomerizable protecting group that blocks the biological activity of the compound to which it is attached, figuratively trapping the molecule in a light-sensitive cage (Adams & Tsien 1993, Bartels et al. 1971, Ellis-Davies 2007, Kaplan et al. 1978, Lester & Nerbonne 1982, Mayer & Heckel 2006). Light-induced cleavage or isomerization of the chromophore liberates or “uncages” the biologically active species, and native or foreign protein effectors once again respond to the appearance of a photochemical reaction product with conformational changes. Specificity of action in these diffusible chromophore-effector pairs arises from the chemical affinity of the photochemical reaction product for a particular effector protein, not its physical proximity. This specificity can be heightened by engineering a private, orthogonal chemical interaction between the photochemical reaction product and its intended effector protein.
Figure 2.
Optical control of three types of photoreceptor proteins. (a) Natural photoreceptor protein with bound chromophore. In the example depicted, absorption of a photon of 480-nm light causes isomerization of a retinal chromophore, which triggers a conformational change in a retinylidene photoreceptor. The lit state reverts to the ground state via absorption of a photon of a different color (580 nm), thermal relaxation (Δ), or enzymatic back conversion that requires ATP hydrolysis. (b) Artificial photoreceptor protein with a photoisomerizable tethered ligand (PTL). Absorption of a photon of 380-nm light causes trans-to-cis isomerization of an azobenzene tether bearing an allosteric effector. Isomerization brings the effector in contact with its binding site on the receptor, which changes conformation. The lit state reverts to the ground state via thermal relaxation (Δ) or absorption of a 500-nm photon. (c) Artificial photoreceptor protein and caged effector. In the example depicted, attachment of a dimethyl-nitrobenzyl group blocks the biological activity of an effector. Absorption of a 355-nm photon causes photolysis of the caging group and release of the biologically active effector. Binding of the effector to its cognate receptor induces a conformational change in the receptor protein. Photolysis is irreversible.
PHOTORECEPTOR PROTEINS: NATURE’S PHOTOCHEMICAL ACTUATORS
Optical signals orchestrate plant development, from germination to stem elongation, leaf expansion, flowering, and senescence; they entrain rhythms of gene expression with consequences for circadian physiology and behavior; they activate DNA repair enzymes and the intracellular transport of pigment-containing organelles; they orient the self-propelled motions of microbes and simple animals; and they provide the visual information that higher animals use to build internal representations of the surrounding world. All this is possible because cells contain a variety of photoreceptors, which are families of light-responsive proteins with distinct structures, chromophores, evolutionary origins, spectral ranges, kinetics, biophysical mechanisms, subcellular locations, and physiological roles.
The idea that photoreceptors could be taken out of their normal context and used as experimental tools to exert genetically targeted optical control of normally light-insensitive processes can be traced to two reports published in 2002. The first showed that rhodopsin could serve as an optically controlled regulator of electrical current and drive light-evoked action potentials in neurons (Zemelman et al. 2002). The second demonstrated the use of a phytochrome photoswitch for optical control of gene expression in yeast (Shimizu-Sato et al. 2002).
Thus far, six broad classes of photoreceptor proteins have been characterized: rhodopsins, phototropins, BLUF proteins (blue-light sensors utilizing flavin adenine dinucleotide), cryptochromes, phytochromes, and photoactive yellow proteins (Hegemann 2008, Möglich et al. 2010, Spudich et al. 2000, Zoltowski & Gardner 2011). It is probably safe to assume that many unidentified members of existing families, as well as whole new classes of optically responsive proteins, are still lurking in the dark. The following subsections describe the known photoreceptor classes in more detail.
Rhodopsins
Rhodopsins are integral membrane proteins that regulate or drive ionic currents as a function of light intensity. All rhodopsins detect light via a retinal chromophore (or a hydroxylated retinal derivative) that isomerizes after photon absorption. The structural change of the chromophore is transmitted to the effector protein opsin, whose seven transmembrane domains line the retinal-binding cavity.
A comparison of opsin sequences indicates the existence of two unrelated receptor families (Henderson & Schertler 1990, Spudich et al. 2000)—type 1 (microbial) and type 2 (visual or G protein–coupled) rhodopsins—whose prototypical representatives are bacteriorhodopsin (Lanyi 1995) and bovine rod rhodopsin (Palczewski 2006), respectively. In both types of photoreceptors, the retinal chromophore forms a protonated Schiff base with a conserved lysine residue in the seventh transmembrane domain (Palczewski 2006, Spudich et al. 2000). The chromophores of different rhodopsin classes assume different conformations in the dark and cycle through different intermediates upon illumination: Type 1 rhodopsins contain all-trans retinal in the dark state, which photoisomerizes to 13-cis retinal; most type 2 rhodopsins, in contrast, contain 11-cis retinal in the dark state, which photoisomerizes to all-trans retinal. Despite differences in the structures of their photocycles, the immediate consequence of absorbing a photon is the same for all retinal chromophores: The acidity of the Schiff base—its propensity to donate a proton—increases sharply (Spudich et al. 2000). The knock-on effect of this photochemical core event, however, differs in different classes of rhodopsin.
In light-driven proton pumps, exemplified by bacteriorhodopsin, the Schiff base ejects a proton to the extracellular side of the membrane. Reprotonation occurs after a conformational switch that renders the Schiff base accessible only from the intracellular side; the cycle therefore results in net outward transport of one proton (Lanyi 1995). Chloride pumps, such as halorhodopsin, operate according to the same fundamental mechanism (Kolbe et al. 2000). However, in these proteins a tightly bound chloride counterion, which occupies the place of the extracellular proton acceptor site in bacteriorhodopsin, prevents the deprotonation of the Schiff base. As the chromophore isomerizes, the N–H dipole vector of the Schiff base flips from an extracellular to a cytoplasmic orientation; in halorhodopsin, this reorientation drags the electrostatically bound chloride ion to a holding position nearer the cytoplasmic side. From there, the ion is discharged when a chloride conduction path opens to the cytoplasm (Kolbe et al. 2000). The result is net inward transport of one chloride ion.
The photocycles of passively conducting type 1 rhodopsins, such as channelrhodopsin-2 (ChR2), are thought also to resemble that of bacteriorhodopsin, in that de- and reprotonation of the chromophore at opposite sides of the membrane result in active proton transport (Feldbauer et al. 2009). Following reprotonation of the Schiff base, however, these pumps remain transiently permeable to cations; they form passive leak conductances (Ernst et al. 2008; Nagel et al. 2002, 2003; Zhang et al. 2008). Neither the number, nor the identity, nor the structural basis of these conductances is fully understood. In ChR2, the major leak conductance is generally identified with the spectroscopic P520 intermediate, but the rise and decay kinetics of P520 and photocurrent do not match precisely, which hints at the existence of additional passively conducting states (Bamann et al. 2008, 2010; Ernst et al. 2008; Ritter et al. 2008).
In type 2 rhodopsins, which serve as the photoreceptors of vertebrate and invertebrate eyes (Baylor 1996, Hardie & Raghu 2001, Palczewski 2006, Spudich et al. 2000, Wald 1968), the protonated Schiff base forms an intramolecular salt bridge with a nearby glutamate side chain (Kim et al. 2004). De-protonation breaks this salt bridge and thereby unlocks the receptor for subsequent conformational rearrangements, which culminate in the unmasking of a latent guanine nucleotide exchange factor (GEF) activity toward the cognate heterotrimeric G protein (Palczewski 2006). The rhodopsins of vertebrate and invertebrate photoreceptors act as GEFs for different classes of G proteins and thus indirectly modulate different sets of conductances. The visual rhodopsins of vertebrate photoreceptor cells signal through Gt, or transducin, which activates cyclic GMP (cGMP) phosphodiesterase. Illumination of a vertebrate photoreceptor thus leads to the consumption of cGMP; the resulting concentration drop closes cGMP-gated sodium channels, which carry a depolarizing dark current, and hyperpolarizes the cell (Baylor 1996). Invertebrate visual rhodopsins, in contrast, couple to Gq/11, which leads, through a poorly understood mechanism, to the activation of transient receptor potential (TRP) channels and cell depolarization (Hardie & Raghu 2001). Vertebrate visual opsins expel the all-trans retinal chromophore after photon absorption and thus require a constant supply of 11-cis retinal (Wald 1968). Invertebrate visual opsins instead retain all-trans retinal and use the energy of a second photon at a shifted wavelength to convert the all-trans isomer back to the 11-cis isomer (Hardie & Raghu 2001).
Phototropins, Blue-Light Sensors Utilizing Flavin Adenine Dinucleotide (BLUF Proteins), and Cryptochromes
Plants, microbes, fungi, and animals contain light-controlled regulatory proteins that measure irradiance at the blue and red ends of the visible spectrum. With the exception of photoactive yellow protein, all known blue-sensitive photoswitches—phototropins, BLUF proteins, and cryptochromes—carry flavin chromophores (Möglich et al. 2010, Zoltowski & Gardner 2011). Phototropins and cryptochromes are distributed widely, whereas BLUF proteins have been found only in bacteria and some lower eukaryotes.
Phototropins and BLUF proteins possess modular structures with distinct and easily separable light-sensing units, termed LOV (light, oxygen, and voltage) and BLUF domains, respectively (Hegemann 2008, Möglich et al. 2010, Zoltowski & Gardner 2011). One or more of these sensor domains often precede, within a single reading frame, a sequence encoding an enzymatic effector domain, such as a kinase in the case of phototropins, or an adenylyl/guanylyl cyclase or phosphodiesterase in some BLUF proteins. The modular organization of these photoreceptors immediately suggests that they are optically controlled enzymes and that the mechanism of control might be made portable by grafting the light-sensor domains onto different protein structures.
The LOV domains of phototropins adopt the typical fold of the larger PAS domain family of environmental sensors, of which they are members (Crosson & Moffat 2001). In the dark state, four α-helices hold the flavin mononucleotide (FMN) chromophore noncovalently against a shelf of five β-strands. The shelf sits on top of a fifth, C-terminal helix, which is designated Jα. Blue illumination disrupts the β scaffold and causes the Jα helix to undock from the shelf, resulting in activation (or disinhibition) of enzymatic activity (Crosson & Moffat 2002, Harper et al. 2003). Although the structural and mechanistic details differ, light activation of BLUF domains also appears to involve rupture of a structural anchor between a C-terminal helical cap and a core domain containing the flavin adenine dinucleotide (FAD) chromophore (Gauden et al. 2006, Sadeghian et al. 2008, Wu & Gardner 2009, Zoltowski & Gardner 2011).
Members of the cryptochrome family constitute a third class of flavin-containing blue-light receptors (Möglich et al. 2010, Zoltowski & Gardner 2011). The family is large and diverse; it includes not only the cryptochrome photoreceptors of plants and animals but also light-independent transcription factors with roles in the circadian clock (the type 2 cryptochromes of animals) and light-induced DNA repair enzymes (photolyases). Despite significant sequence divergence, the light sensor domains of cryptochrome photoreceptors and photolyases adopt virtually the same topology, perhaps reflecting the sensitivity to distance and orientation of the charge transfer reactions for which these domains are specialized. Both protein subfamilies incorporate FAD as the chromophore, albeit at different redox potentials (Balland et al. 2009). The biological activity of cryptochromes depends on largely disordered C-terminal extensions that are untucked from the light-sensor domain in the lit state (Partch et al. 2005, Yang et al. 2000) and expose buried recognition elements for other proteins. How photoreduction of FAD drives this conformational switch is unknown.
Phytochromes
Phytochromes provide plants and microbes with a primitive form of color vision in the red by switching between a red-sensitive Pr and a far-red-sensitive Pfr conformer (Bae & Choi 2008, Möglich et al. 2010). A three-domain core sensory module located at the N terminus cradles the tetrapyrrole chromophore whose isomerization controls the photoswitch; the C terminus harbors a histidine kinase–related domain whose enzymatic activity and light regulation have been verified in prokaryotes but not in plants. In plants, the Pfr conformer translocates into the nucleus (Chen et al. 2005, Yamaguchi et al. 1999), where it binds to constitutively active transcription factors termed phytochrome-interacting factors (PIFs) (Ni et al. 1999) and induces their phosphorylation and subsequent destruction.
ARTIFICIAL PHOTORECEPTORS
The vast majority of biochemical reactions that govern the behavior of cells and organisms are controlled by regulatory proteins that respond, not to fluctuations in light intensity, but to changes in the concentrations of metabolites, signaling molecules, or ions; to the binding of other proteins; or to enzymatic modifications. These regulatory proteins also can be turned into photochemical actuators, provided that synthetic chromophores are harnessed to deliver the small-molecule ligands or ions necessary for activity, to grant access to protein interaction surfaces or active sites, or to permit posttranslational modifications (Figure 2b,c). When built according to the principle of orthogonality, these artificial photoreceptors—consisting of synthetic chromophores and foreign effector proteins—achieve the same specificity of action as naturally light-responsive proteins. Of course, encoding the effector protein in DNA affords the genetic resolution of cell types that is the hallmark of optogenetics.
Because the construction of artificial photoreceptors can draw on a potentially larger pool of biological and synthetic raw material, some of the limitations that constrain the performance of natural photoreceptors may not apply in these artificial systems. For example, the known natural photoconductors, such as ChR2, have tiny conductances (Feldbauer et al. 2009) that are not always adequate for driving neurons to action potential threshold (Figure 3b,d); the conductances of artificial light-gated ion channels are up to 1,000-fold larger (Zemelman et al. 2003) (Figure 3c,e). Injecting current into neurons with the help of a diffusely localized photo-conductor distorts synaptic integration; activating an artificial, optically gated neurotransmitter receptor (Volgraf et al. 2006), in contrast, can mimic a localized synaptic input. The dynamic ranges of photoswitches are bounded by free energy differences between the dark and lit states. LOV domains, for example, operate with an energy difference of approximately 16 kJ mol−1, which translates to a light-driven shift in the occupancy of the lit state from 1.6% to 91% (Yao et al. 2008). Engineered photo-switches can achieve potentially larger dynamic ranges, and therefore cleaner control, by exploiting larger activation energies.
Figure 3.
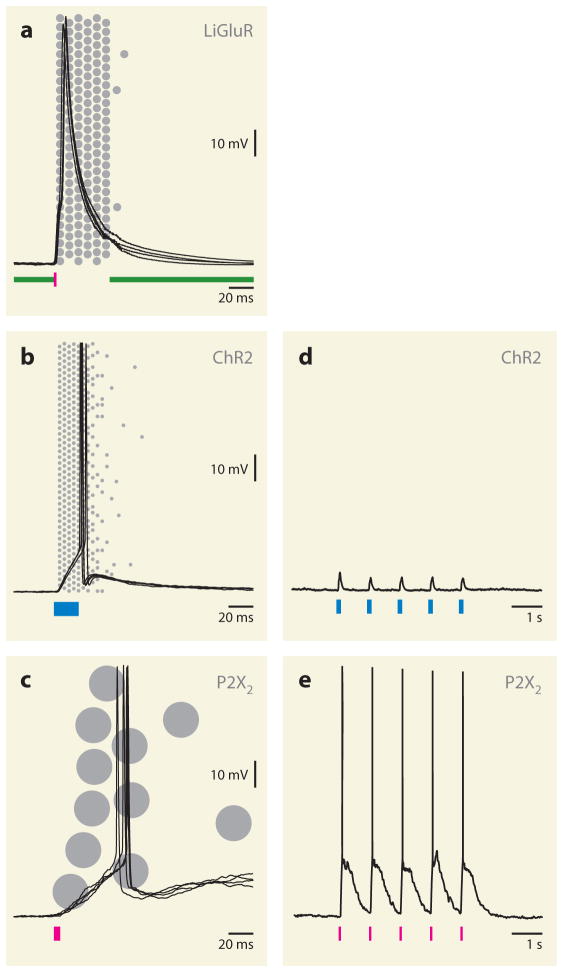
Control of electrical signals. (a–c) Pulse illumination of mammalian neurons expressing the optically gated channels LiGluR (a), ChR2 (b), and P2X2 (c) elicits single action potentials. Membrane potential changes recorded during four repetitions of an optical stimulus at 1 Hz are overlaid; traces are aligned to the onset of illumination. Optical stimulation regimes are indicated by colored bars at the bottom of the traces. (a) Continuous green (500-nm) illumination is paused, and a 1-ms pulse of 380-nm light is applied to gate open LiGluR. (b) A 20-ms pulse of 473-nm light activates ChR2. (c) A 5-ms pulse of 355-nm light uncages ATP to activate P2X2. Shaded circles in the background symbolize the single-channel conductances of the actuator molecules and the approximate openings of a population of channels in response to light. Recordings in panel a were obtained from transfected hippocampal neurons in dissociated culture (courtesy of S. Szobota and E. Isacoff). Recordings in panels b and c were obtained from inhibitory neurons in neocortical slices, which were harvested from knock-in mice carrying otherwise identical cassettes for expression of the respective actuators at their GT(ROSA)26Sor loci (Kätzel et al. 2011). (d,e) Pulse illumination of pyramidal neurons in neocortical slices obtained from knock-in mice carrying otherwise identical cassettes for expression of the respective actuators at their GT(ROSA)26Sor loci (D. Kätzel & G. Miesenböck, unpublished data). Both ChR2 and P2X2 are effective in driving spiking in inhibitory neurons (b,c), but ChR2 causes only subthreshold depolarizations in pyramidal cells (d), presumably because of its small single-channel conductance (b). P2X2, whose single-channel conductance is two to three orders of magnitude larger than that of ChR2 (c), also drives action potentials in cortical pyramidal neurons (e).
The principal drawback of artificial photoreceptors is their dependency on synthetic chromophores. Although no photochemical actuator is fully encodable, many of the small-molecule cofactors that serve as chromophores in natural photoreceptors, particularly retinal and flavins, are widely bioavailable. In many heterologous expression systems, functional protein thus can be reconstituted from endogenous sources. The synthetic chromophores of artificial photoreceptors, in contrast, invariably must be supplied exogenously. In addition, natural photoreceptors recycle their chromophores through many rounds of excitation, returning them to the ground state via thermal relaxation or optical or enzymatic backconversion (Figure 2a). Although some artificial photoreceptors also operate closed photocycles based on reversible photoisomerization reactions (Figure 2b), other synthetic chromophores undergo photolysis (Figure 2c). These chromophores are, of course, irreversibly consumed in a single reaction and need to be replenished for sustained actuation.
Synthetic chromophores are generally smaller than their natural counterparts and absorb in the UV. The reason is convenience rather than a minimal energy requirement for photochemistry: Red-shifting the absorption maxima would require the synthesis of more complex, extended molecules prone to disintegration in ambient light (Adams & Tsien 1993). Caging chromophores, such as o-nitrobenzyls, coumarins, hydroxyphenacyl, and cinnamate, undergo irreversible photolysis within micro-to milliseconds (Ellis-Davies 2007); reversible photoswitches, such as azobenzenes, photoisomerize within picoseconds (Gorostiza & Isacoff 2008a). Because isomerization changes not only the conformation but also the absorption spectra of these bistable chromophores, they can be toggled back and forth with different colors of light (Figures 2b, 3a). Integral photoswitches have been used to alter the fit of soluble ligands to binding sites (Bartels et al. 1971, Volgraf et al. 2007) or to inflate and contract the volume sampled by PTLs (Banghart et al. 2004; Gorostiza & Isacoff 2008a,b; Volgraf et al. 2006). Although reversibly caged soluble molecules tend to retain enough activity in the obstructed state to limit their utility, the additional geometric constraints imposed by attachment to a protein surface make PTLs effective chromophores for regulating active and allosteric sites.
CONTROL OF GENE EXPRESSION
Binding of activator and repressor proteins to regulatory DNA sequences controls gene expression. The activity of transcription factors, in turn, is regulated at several levels: nuclear import, oligomerization, and DNA binding. Building a photoswitch into any of these steps has the potential to render gene expression light responsive (Figure 1).
The native phytochrome-PIF system is an example of transcriptional control by nuclear import and subsequent dimerization. Photo-conversion to the Pfr form exposes a cryptic nuclear localization signal at the C terminus of the phytochrome (Chen et al. 2005, Yamaguchi et al. 1999), which leads to its nuclear import and association with PIFs (Ni et al. 1999). In a stripped-down engineered phytochrome-PIF system (Shimizu-Sato et al. 2002), only the interacting modules of phytochrome and PIF are retained, and these are fused to the DNA-binding and activating domains, respectively, of the yeast transcription factor GAL4. Optical switching of the phytochrome domain between the Pr and Pfr conformers prevents or allows reconstitution of GAL4 activity from the two hybrid protein fragments. The system achieves fast, tunable induction of gene expression by up to several hundred-fold in yeast (by titrating the Pr/Pfr ratio optically) on top of reportedly minimal dark activity, but it requires an exogenous source of the tetrapyrrole chromophore phycocyanobilin (Shimizu-Sato et al. 2002).
The same principle of light-induced dimerization underlies the reconstitution of split proteins bearing, respectively, the sensor domain of plant cryptochrome 2 and its interacting partner CIB1 (cryptochrome-interacting basic helix-loop-helix protein 1) (Liu et al. 2008). Blue illumination of mammalian cells expressing complementary fragments of GAL4 or Cre recombinase, tagged with cryptochrome and CIB1 domains, causes modest increases in basal transcription levels or recombination frequencies, respectively (Kennedy et al. 2010).
The third mechanism of transcriptional control—optical regulation of DNA binding—is realized in an engineered transcription factor called LovTAP (Strickland et al. 2008). The protein, an end-to-end fusion between the LOV domain of a phototropin and the Escherichia coli trp repressor, binds DNA only in the lit state, after the Jα helix has undocked from the LOV domain. The photoswitching dynamic range of LovTAP and similar proteins is, at only two- to tenfold (Strickland et al. 2008, Wu et al. 2009), much smaller than that of the parent photoreceptor (Yao et al. 2008). The appearance in the fusion protein of new forces that pull Jα away from its docking site and lead to elevated dark activity seems responsible for this compression of dynamic range, as dark activity is diminished and the dynamic range extended by mutations that stabilize helical docking (Strickland et al. 2010).
Although the design of optically responsive transcriptional control systems has, at its best, showcased the rational application of principles of allostery and recruitment, no study yet appears to have put light-controlled gene expression to practical use. Perhaps this is because, at transcriptional timescales, the advantages of rapid optical induction or repression of gene expression fade in comparison with more robust or better established pharmacological or thermal approaches. Still, the option of patterning gene expression arbitrarily in space, which only optical methods provide, would be expected to stir the imagination of developmental biologists. Alas, it seems not to have done so.
CONTROL OF BIOCHEMICAL SIGNALS
Biochemical information processing involves transient protein interactions whose lifetimes are often clocked against an internal standard set by GTP hydrolysis. Accordingly, attention has focused on optical means of controlling protein interactions (using the mechanisms and principles just described for transcription factors) or the nucleotide switches that determine their persistence (Figure 1).
The light-induced association of phytochrome and PIF domains can couple small GTPases, such as Cdc42, directly to effectors, overriding the nucleotide switch that normally controls the interaction (Leung et al. 2008). Alternatively, the switch itself can be operated by recruiting catalytically active GEFs to docking sites at the plasma membrane; spot illumination then drives localized guanine nucleotide exchange (Levskaya et al. 2009, Yazawa et al. 2009). Another means to a similar end, demonstrated for Rac1 and Cdc42, is the occlusion of effector binding by LOV domains and its relief by illumination (Wu et al. 2009). Finally, signaling proteins tagged with immunophilin modules can be optically dimerized by uncaging a rapamycin analog (Karginov et al. 2011).
Type 2 rhodopsins are naturally light-activated GEFs for a second broad class of signaling GTPases, heterotrimeric G proteins. Their specificity—for Gt in vertebrates and Gq/11 in invertebrates—has been redirected by stitching the intracellular loops and C-terminal tails of other G protein–coupled receptors into the rhodopsin frame (Airan et al. 2009). Depending on which intracellular recognition features are so created, the chimeric receptors, termed optoXRs, instruct the production of different small-molecule second messengers such as cAMP, diacylglycerol, and inositol-1,4,5-triphosphate (IP3). The photoactivatable adenylyl and guanylyl cyclases of BLUF proteins offer an alternative route to the light-controlled synthesis of cyclic nucleotides that sidesteps the heterotrimeric G protein altogether (Koon et al. 2011, Ryu et al. 2010, Schröder-Lang et al. 2007, Stierl et al. 2011).
CONTROL OF ELECTRICAL SIGNALS
The basis of electrical signaling by excitable cells is the diffusion of ions along electrochemical gradients. Because different ionic species have different mobilities, electrical potential differences inevitably appear at the boundaries of solutions whose electrolyte compositions differ, such as the intra- and extracellular compartments. Imagine that a solid partition between these compartments is suddenly removed and ions begin to diffuse down their concentration gradients. Differences in mobility give the faster ions an initial head start over their slower counterions. This microscopic charge separation establishes an electrical junction potential whose polarity and magnitude act precisely to balance differences in mobility. Diffusion would of course eventually equilibrate the concentration differences and extinguish the junction potential. The system can, however, be kept off equilibrium by restricting the movements of some ions. For instance, when the partition is made selectively permeable to some ions, the freely mobile ions strain in perpetuity against the electrostatic tether of their impermeant countercharges.
Cellular membrane potentials are, at their core, stabilized junction potentials. As formalized in the Goldman-Hodgkin-Katz model, the membrane potential approximates the average of the equilibrium potentials EX of all permeant ionic species, weighted by the relative conductances gX. In neurons, potassium, sodium, and chloride dominate; their typical equilibrium potentials are EK = −90 mV, ENa = 55 mV, and ECl = −75 mV, and their (very approximate) relative conductances at rest are gK :gNa:gCl ≈ 100:1:10.
The Goldman-Hodgkin-Katz model illustrates some important principles of electrical signal processing. First, increasing the transmembrane conductance of a particular ion pulls the membrane potential closer to the equilibrium potential of that ion: At the peak of an action potential, gNa increases as much as 500-fold, causing the membrane potential to flip polarity despite gK remaining virtually unchanged. Second, increasing any one conductance dilutes the relative impact of other conductances. Increasing gCl, for example, often has a minimal effect on membrane potential because ECl tends to be close to the resting membrane potential, but it curtails the voltage excursions caused by other conductances. Third, deceptively simple interactions between conductances generate rich membrane potential dynamics—the physical substrate of neural computation. Photochemical actuators work within the context of these dynamics, ideally and most directly by adding photosensitivity to a passive conductance. Two sets of conductances are the natural targets of such interventions: nonselective cation channels that push the membrane potential toward action potential threshold and potassium and chloride channels that oppose excitation.
Nonselective Cation Conductances
In the original formulation of an encodable photoconductor, an unidentified endogenous conductance of hippocampal neurons (presumably a nonselective cation channel of the TRPC family) was regulated by a blue-sensitive type 2 rhodopsin borrowed from fly photoreceptor cells (Zemelman et al. 2002). The rhodopsin was functionally coupled to the channel via a co-expressed Gα subunit and chaperoned through its retinal cycle by a third exogenously supplied protein, arrestin-2. ChARGed neurons—so named to indicate the presence of a light-activated trigger and commemorate its three components—responded to light with action potentials.
Similar to phototransduction in the eye, chARGe in neurons benefits from secondary amplification of the primary photochemical reaction. In contrast to photoreceptor cells, which code light intensity fluctuations as analog changes in membrane potential, the voltage-gated channels of spiking neurons provide a further stage of electrical amplification. The typical membrane potential response of a chARGed neuron to sustained illumination thus consists of a depolarizing ramp to the activation threshold of voltage-gated sodium channels, followed by several action potentials (Zemelman et al. 2002). The slope of the ramp, and hence the latency of the first spike after the onset of the optical stimulus, varies, ranging from a few hundred milliseconds to tens of seconds. After cessation of the light stimulus, the settling time of the chARGed neuron again varies; not infrequently, a tail of low-level activity persists for several seconds. Loose coupling between photoreceptor and channel probably lies at the root of these phenomena.
Combining photoreceptor and conducting pore in a single molecule should eliminate much of the transduction noise and permit tighter control of action potential timing. The confirmation that type 1 opsin sequences of green algae (GenBank accession number AF461397; Hegemann et al. 2001, Sineshchekov et al. 2002, Suzuki et al. 2003) encode passive light-gated conductances (Nagel et al. 2002, 2003), as had been predicted on kinetic grounds (Braun & Hegemann 1999, Ehlenbeck et al. 2002), offered such a simplified photoconductor architecture (Boyden et al. 2005, Li et al. 2005). ChR2 is the best characterized and currently most widely used of a family of actuators that also includes ChR1 (Nagel et al. 2002) and homologs from a different alga (VChR1 and VChR2) (Ernst et al. 2008, Zhang et al. 2008). The conductance of ChR2 opens within <200 μs after exposure to blue light, peaks at ~130 fS, and partially inactivates, with a voltage-dependent time constant of ~10–40 ms, to a sustained plateau conductance of ~40 fS (Berndt et al. 2009, Feldbauer et al. 2009, Lin et al. 2009, Nagel et al. 2003); after illumination offset, the protein relaxes to a nonconducting dark state with a time constant of 10–45 ms and recovers slowly (τ ≈ 5–30 s) from inactivation (Boyden et al. 2005, Nagel et al. 2003, Petreanu et al. 2007). Although the actuator itself switches to the conducting state rapidly, the first light-evoked action potential is delayed by 4–25 ms following exposure to saturating illumination (Boyden et al. 2005, Kätzel et al. 2011, Lima et al. 2009, Wang et al. 2007) (Figure 3b). The length of this latency window reflects the time it takes the small ChR2 conductance to pass sufficient current to lift the membrane potential to firing threshold. Increasing the single-channel conductance or calcium permeability (via protein engineering; Kleinlogel et al. 2011, Nagel et al. 2005) or the density of photoconductors in the membrane (via massive overexpression) reduces this latency period, whereas opening non-ChR2 conductances prolongs it by dissipating light-evoked voltage changes. Fluctuations of endogenous conductance states probably underlie the variable spike latencies sometimes seen during repeated optical stimulation of the same neuron.
Photochemical actuators with overpoweringly large single-channel conductances should achieve more accurate control if they drive wide membrane potential excursions instantly and reproducibly. The largest unitary conductances (30 and 35 pS) among light-gated ion channels are found in artificial photoreceptors based on P2X2 and TRPV1, respectively (Brake et al. 1994, Caterina et al. 1997, Valera et al. 1994), which in many neurons function as orthogonal, nonnative pores (Arenkiel et al. 2008, Lima & Miesenböck 2005, Zemelman et al. 2003). The channels are operated optically by releasing extracellular ATP or capsaicin from caged precursors (Gilbert et al. 2007, Zemelman et al. 2003, Zhao et al. 2006). However, the theoretical advantage of large photocurrents for rapid spike initiation is offset in practice by the shot noise of asynchronous channel openings (Figure 3c), which arises from diffusion of the photochemical reaction products to their binding sites on the receptors. Although inferior to ChR2 in terms of temporal fidelity, the vastly larger photocurrents of P2X2 and TRPV1 allow them to drive spiking in situations in which ChR2 fails (Figure 3d,e).
The light-gated glutamate receptor LiGluR combines a sizeable, noninactivating conductance of ~250 fS (Howe 1996) with a reversible integral gating mechanism (Volgraf et al. 2006). This artificial photoreceptor is formed by affinity labeling an engineered cysteine a measured distance away from the glutamate-binding site of an ionotropic glutamate receptor (iGluR6); the affinity label consists of a glutamate analog that is linked via an azobenzene arm to a cysteine-reactive maleimide (Gorostiza et al. 2007, Volgraf et al. 2006) (Figure 2b). In response to a pulse of 380-nm light, the azobenzene arm flexes and increases the effective concentration of glutamate at the ligand-binding site, which activates the channel with a time constant of 120–220 μs (Szobota & Isacoff 2010, Szobota et al. 2007). Illumination at 500 nm extends the arm and decreases the effective glutamate concentration ~40-fold; this leaves the binding site virtually unoccupied and closes the channel (Figure 3a). PTLs with shortened azobenzene arms evoke inverted optical responses from the same attachment point; a bent geometry now prevents glutamate from reaching the binding site, whereas a stretched geometry allows access and opens the pore (Numano et al. 2009).
The ability to terminate the conducting state actively, with a pulse of light at a different wavelength, distinguishes LiGluR from the light-gated receptors P2X2 and TRPV1 and also from ChR2 and VChR1, whose slow spontaneous off kinetics limits their fidelity and range. In the case of ChR2, several mutations have been engineered that enhance the spontaneous rate of channel closure (Gunaydin et al. 2010, Lin et al. 2009). A different type of ChR2 mutation has the converse effect; it prolongs the half-life of the open state nearly 10,000-fold (to >1 min; Bamann et al. 2010, Berndt et al. 2009) and permits its active termination with a pulse of yellow light (Berndt et al. 2009). Activating a long-lived but small nonselective cation conductance, whether that of LiGluR (Szobota et al. 2007) or a step-function mutant of ChR2 (Bamann et al. 2010, Berndt et al. 2009), shifts the membrane potential of a neuron closer to spike threshold. Although the increase in conductance decreases excitability, teetering close to threshold appears to swamp the conductance effect—a situation reminiscent of a neuron’s heightened responsiveness during barrages of synaptic activity (Shu et al. 2003).
Potassium Conductances and Chloride and Proton Pumps
The main physiological mechanisms that decrease or inhibit neuronal responses are the opening of potassium and chloride conductances, which clamp the membrane potential near resting level and/or shunt depolarizing currents.
Two design principles already encountered in nonselective photoconductors have been adapted for the construction of light-gated potassium channels. The first is regulation of an endogenous conductance by a type 2 rhodopsin. Vertebrate RO4, transplanted from photoreceptor cells to neurons lacking transducin, signals instead through Gi/o and modulates G protein–coupled inwardly rectifying potassium (GIRK) channels (Li et al. 2005). The second strategy is the incorporation of a PTL into a potassium-selective derivative of LiGluR, which was engineered by swapping the nonselective iGluR6 pore for a potassium-selective prokaryotic homolog (Janovjak et al. 2010). Light pulses of different colors switch the resulting bistable chimera, termed HyLighter, between sustained conducting and nonconducting states, during which action potentials are suppressed or released. HyLighter supplants the light-blocked potassium channel SPARK (Banghart et al. 2004), which is constitutively open before PTL attachment and thus produces current leaks if derivatization is incomplete.
Hyperpolarizing currents of an entirely different nature result from active inward transport of chloride by halorhodopsin (Han & Boyden 2007, Zhang et al. 2007b) and outward transport of protons by members of the bacteriorhodopsin family (Chow et al. 2010). The fundamental distinction between these proteins and the photoconductors discussed so far is that pumps are light-fueled power devices: Each absorbed photon drives the translocation of at most one ion. Importantly, the driving force of passive potassium or chloride currents grows as the membrane potential moves away from the equilibrium potential of the permeant ion; this creates an elastic restorative force that is balanced to the magnitude of perturbation. Ion pumps, in contrast, lack this autoregulatory feature. Their insensitivity to voltage context can cause pumps to produce effects that are excessive at one moment and barely adequate at the next. Active chloride transport by halorhodopsin generates, at high irradiances and with protein modifications that enhance transport to the cell surface (Gradinaru et al. 2008, 2010), currents that drive deep hyperpolarizations in neurons with few open conductances but may still negate only modest depolarizations (Sohal et al. 2009). Even though proton concentrations in tissue are a millionfold lower than those of chloride, proton pumps named Arch and Mac (Chow et al. 2010, Han et al. 2011) achieve turnover numbers that equal, if not exceed, that of enhanced halorhodopsin, possibly because the extreme mobility of protons in aqueous solution permits near-instant refilling of vacated transport sites. Proton pumps may lack the rebound excitation observed after prolonged chloride transport (Arrenberg et al. 2009) because a cell-endogenous safety valve prevents excessive hyperpolarization (Chow et al. 2010).
CONTROL OF NEURAL CIRCUITS AND BEHAVIOR
When photoreceptors were first commandeered to transduce optical impulses into electrical signals, both in vitro (Zemelman et al. 2002) and in vivo (Lima & Miesenböck 2005), the principle of optogenetic control was established. The growing field has produced many refinements and extensions of this principle, as detailed in preceding sections. I conclude with a look at some of the biological insights that have resulted, revisiting the expectations that motivated the development of optogenetic control in the first place (Lima & Miesenböck 2005, Miesenböck 2004, Zemelman & Miesenböck 2001, Zemelman et al. 2002): a desire to identify the neural underpinnings of behavior through controlled induction of electrical activity; an ability to interfere directly with circuits deep in the brain, bypassing sensory input; and a capacity to drive neuronal populations in analytically accessible but deafferented settings, such as explanted brain tissues. Although all of my examples are drawn from neurobiology, the impact of optogenetics is beginning to be felt in other fields as well (Arrenberg et al. 2010, Bruegmann et al. 2010, Miesenböck 2009, Stroh et al. 2011).
Reconstitution of Animal Behavior
Before optogenetic control, the main strategy for uncovering the neural basis of behavior was the experimental disruption of function via anatomical, pharmacological, or genetic lesions. These interventions asked: Is activity in a particular group of neurons necessary? Optogenetic actuation allows one to pose the reciprocal question: Is activity in a particular group of neurons sufficient for function? By providing the means to reconstitute behavior through playback of controlled patterns of neural activity, optogenetics has raised neurobiology’s standards of causal inference to those of biochemistry and cell biology (Lima & Miesenböck 2005): The agents responsible for a process—neurons and their activity patterns in the neurobiologist’s case, molecules and their interactions in the biochemist’s—can now be pinpointed on the basis of their ability to replicate the process when supplied in pure form.
The first behaviors to be reconstituted in this manner were fixed action patterns, such as a fly’s escape movements (Lima & Miesenböck 2005); reflexive muscle contractions, such as a nematode’s touch response (Nagel et al. 2005); or elementary motor acts under cortical control, such as a rodent’s whisker deflections (Aravanis et al. 2007). From these beginnings, the reconstitution approach has spread to find applications in virtually every neural system (Figures 4 and 5): the homeostatic systems that regulate wakefulness, metabolism, respiration, and reproduction (Adamantidis et al. 2007, Aponte et al. 2011, Carter et al. 2010, Gourine et al. 2010, Pagliardini et al. 2011); the cognitive systems that construct internal models of the environment and choose actions on the basis of anticipated consequences (Cardin et al. 2009, Huber et al. 2008); the sensory systems that calibrate these models against current reality (Arenkiel et al. 2007, Cardin et al. 2009, Dhawale et al. 2010, Suh et al. 2007, Yonehara et al. 2011); the learning and memory systems that modify internal models from experience (Airan et al. 2009, Claridge-Chang et al. 2009, Schroll et al. 2006, Tsai et al. 2009); the motivational and emotional systems that integrate external incentives with internal states and learned goals (Ciocchi et al. 2010, Lin et al. 2011); and the motor systems that coordinate the execution of movement (Aravanis et al. 2007, Clyne & Miesenböck 2008, Gradinaru et al. 2009, Lima & Miesenböck 2005, Matyas et al. 2010, Schoonheim et al. 2010, Wyart et al. 2009). In fairness, not all of these studies have yielded insights of breathtaking originality; more often than not, optogenetic actuation of a group of neurons has simply confirmed—albeit more stringently and directly—a behavioral role strongly suspected, if not already established, from prior correlative evidence.
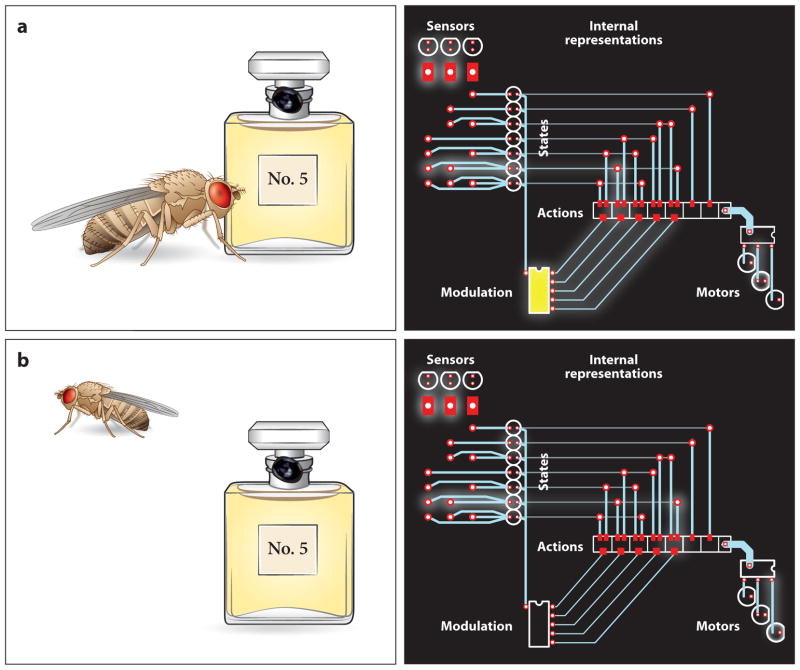
Figure 4.
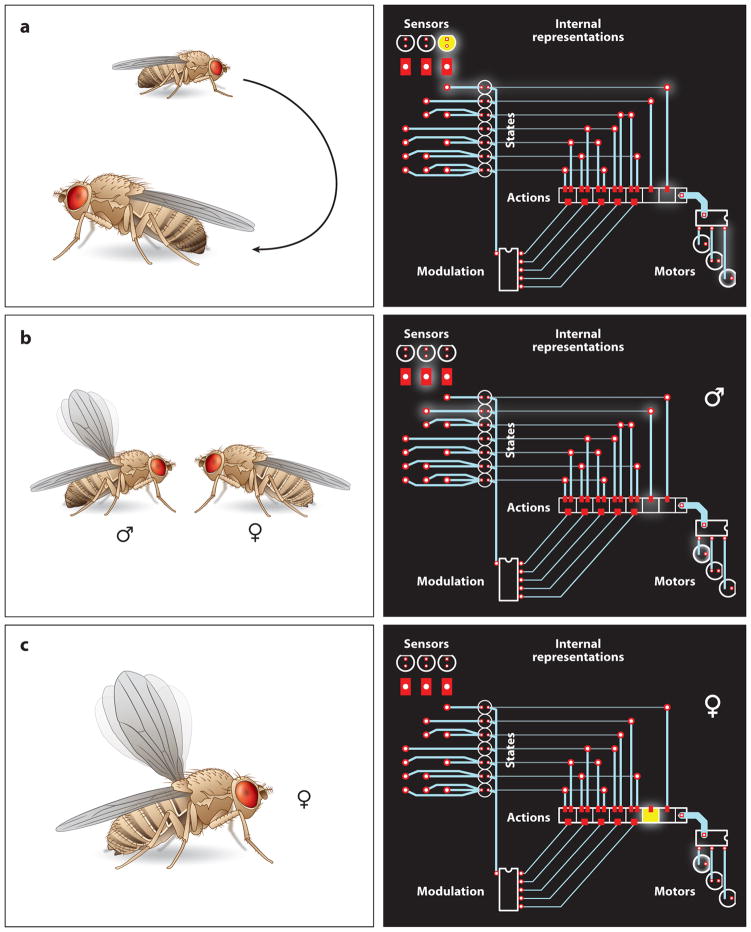
Control of behavior. The circuit diagrams on the right present an extremely simplified view of the brain, illustrating targets of optogenetic intervention (the circuit elements in yellow). Signals from external and internal sensors are used to construct internal representations of the animal’s state. Behavior is generated when states are mapped onto actions and the resulting action representations recruit motor systems. The probabilistic rules determining which states are mapped onto which actions are subject to short- and long-term modulation. (a) Flies sense ambient CO2 levels as an index of stressful overcrowding. Optogenetic activation of CO2-sensing neurons creates the illusion of crowded conditions and elicits avoidance behavior (Suh et al. 2007). (b) Pheromonal, gustatory, and visual signals indicate the presence of a potential mating partner. In the male brain, the state “presence of receptive female” is mapped onto the action “courtship,” which leads to the activation of a motor program involving a unilateral wing beat, the so-called courtship song. (c) In the female brain, the state “presence of receptive female” is not mapped onto the action “courtship.” However, the motor program for male-specific courtship is still present in the female, as it can be recruited optogenetically, bypassing sensory input altogether (Clyne & Miesenböck 2008).
Figure 5.
Reprogramming of behavior. The circuit diagrams on the right present an extremely simplified view of the brain, illustrating targets of optogenetic intervention (the circuit elements in yellow). Signals from external and internal sensors are used to construct internal representations of the animal’s state. Behavior is generated when states are mapped onto actions and the resulting action representations recruit motor systems. The probabilistic rules determining which states are mapped onto which actions are subject to short- and long-term modulation. (a) When a fly approaches odor No. 5, an attractant, dopaminergic modulatory neurons carrying aversive reinforcement signals are optogenetically activated. (b) This intervention results in a lasting remapping of the state “presence of odor No. 5” onto the action “avoidance”: An aversive olfactory memory has been programmed artificially (Claridge-Chang et al. 2009).
That said, the controlled induction of spikes is in many circumstances proving more informative than ablating or silencing neurons, because phenotypes do not depend on the disruption of activity that must be present spontaneously or evoked by sensory stimuli or behavioral tasks. The question about sufficiency also goes deeper, particularly if it asks which specific spatial or temporal aspects of an activity pattern are the relevant carriers of information (Cardin et al. 2009, Claridge-Chang et al. 2009, Gradinaru et al. 2009, Sohal et al. 2009). Silencing a neuron extinguishes all features of its activity, informative or not, and offers no clues as to which are which. We now know, for example, that the modulatory signals that instruct a fly to alter its olfactory choice behavior are emitted by a circumscribed minority of only 12 of the approximately 300 dopaminergic neurons in its brain (Figure 5) (Claridge-Chang et al. 2009). The axons of these 12 dopaminergic cells target synapses implicated in learned action choice, which suggests immediately that dopaminergic modulation of the strength of these synapses is the mechanism of behavioral adaptation. Timing of neural activity also clearly matters, to the extent that imposing different spike frequencies on the same neurons can have opposite behavioral consequences: High-frequency stimulation of cortical projections to the subthalamic nucleus ameliorates parkinsonian symptoms; low-frequency stimulation exacerbates them (Gradinaru et al. 2009).
Most dramatically, optogenetic actuation has been able to push neural circuits into functional states—and animals into behavioral regimes—that lie far outside their normal operating range. Despite—or because of—their unphysiological nature, such states can be especially revealing. As a case in point, an optogenetic push to the extreme margins of performance has shown that female flies harbor a dormant capacity for male-specific behavior (Clyne & Miesenböck 2008): Optical activation of neurons expressing the transcription factor fruitless, a principal sex determination factor (Yu & Dickson 2008), causes female flies to vibrate one wing, just as a male would when attempting to woo a female (Figure 4b,c). Why do females retain neural circuitry dedicated to a behavior they never express? One possible explanation is that the circuits involved in song execution are common to both sexes (perhaps because they are shared with other motor programs), and sex-specific control is exerted only at a hierarchically higher level, at which the organism’s behavioral repertoire is represented explicitly (Figure 4). In other words, setting a few high-level switches in an otherwise largely unisex system to male or female mode may suffice to generate the fundamental behavioral differences between the sexes. This would represent an elegant alternative to building each developing nervous system differently from the ground up, according to male or female plan (Clyne & Miesenböck 2008, Yu & Dickson 2008).
Neural Organization and Dynamics
Before optogenetic control, sensory systems provided the only portals through which neural circuits could be actively probed with distributed inputs. In a typical experiment, an animal would be exposed to visual, tactile, auditory, or chemical stimuli while neuronal responses were observed. Because sensory inputs are extensively reformatted as they propagate, the exact signals driving a neuron at some synaptic distance from the sensory surface are generally unknown. Indeed, the classical input/output analyses of systems neurophysiology forfeit biological realism altogether: Receptive fields and tuning curves, reverse correlation functions and spike-triggered averages are mathematical abstractions of a neuron’s response selectivity, but the biological origins of such selectivity remain firmly locked in a black box.
The ability to write artificial signals deep inside the brain has brought within direct reach of our laser beams and optical fibers circuits that are many synapses removed from sensory receptors. It is now possible to delineate sources of synaptic inputs to virtually any neuron and disentangle their functional contributions to the neuron’s activity or the animal’s behavior. Cell types are distinguished by genetic identity (using cell type–specific promoter/enhancer sequences; Lima & Miesenböck 2005), somatic location (using localized gene delivery; Gradinaru et al. 2007, Petreanu et al. 2007), projection pattern (using synaptic or transsynaptic regulation of gene expression; Lima et al. 2009, Gradinaru et al. 2010), or an intersection of these properties. Such studies have revealed degrees of order in the organization of neural circuits that are not readily apparent from morphological wiring diagrams alone. Excitatory inputs from subcortical nuclei, for example, appear segregated from cortical long- and short-range projections onto discrete dendritic domains of cortical pyramidal cells (Petreanu et al. 2009). Thalamocortical projections connect preferentially with distinct subclasses of cortical inhibitory interneurons (Cruikshank et al. 2010); local inhibitory interneurons, in turn, show striking selectivity for particular pyramidal neurons, assiduously avoiding their morphologically indistinguishable neighbors (Kätzel et al. 2011).
Optogenetic control makes it possible to quantify precisely the functional impact a particular class of input exerts on its postsynaptic targets. Mice, for example, can detect and report the artificial insertion of a single action potential into 300 cortical pyramidal neurons, or of 5 action potentials into 60 such neurons (Huber et al. 2008). Neuronal pools of 60–300 cells may thus represent the fundamental signaling units of cerebral cortex. This figure is remarkably close to an estimate derived from statistical first principles, which argues that information carried in firing rates can be decoded within a single interspike interval of 10–30 ms, provided that signals from 100 or so neurons are pooled (Shadlen & Newsome 1994). Ensemble representations, redundant connections, and noisy rate codes thus seem to go hand in hand. In contrast, the hair-trigger reflexes of some invertebrates and lower vertebrates operate with single neurons and the most minimal of temporal codes—a single action potential (Douglass et al. 2008, Lima & Miesenböck 2005).
An Inroad for Reductionism
Before optogenetic control, neural circuits could not be queried easily in accessible settings stripped of extraneous complexities, such as isolated brain tissues, because the possibility of sensory stimulation was eliminated. Encodable actuators allow one to connect explanted circuitry in physiologically meaningful ways to artificial inputs. In the simplest experimental setting (Figure 6), the artificial input is a small illumination spot that stimulates actuator-expressing neurons of a particular type. Scanning the illumination spot across neural tissue generates light-evoked synaptic currents in a recorded target cell whenever the spot falls on a connected partner, indicating its spatial location (Kätzel et al. 2011; Petreanu et al. 2007, 2009; Wang et al. 2007). Depending on where the recording electrode is placed, and depending on which genetic control strategy is used to drive expression of the actuator, the resulting images of synaptic currents depict the arrangements and strengths of connections between different classes of neurons (Figure 6) (Arenkiel et al. 2007; Cruikshank et al. 2010; Dhawale et al. 2010; Haubensak et al. 2010; Kätzel et al. 2011; Ren et al. 2011; Petreanu et al. 2007, 2009; Wang et al. 2007).
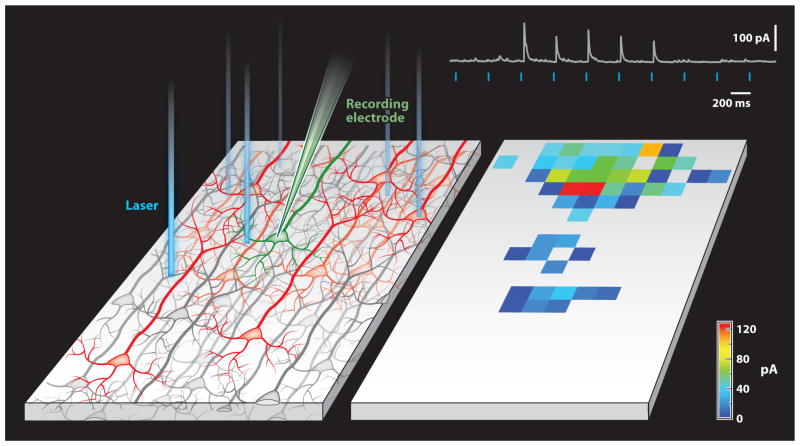
Figure 6.
Analysis of synaptic connectivity. (Left panel) One particular class of neuron (red) in the schematic tissue slice on the left is engineered to express an optogenetic actuator. Transmembrane currents in a potential target cell (green) are recorded while a laser beam scans the tissue slice. If an illuminated spot in the slice harbors an optogenetically activated cell that is connected to the recorded neuron, a synaptic current appears in the recording (top right). The magnitudes of the currents evoked at different locations are color coded to produce the input map (right panel).
Just as cell type–specific actuation of individual neurons is producing finely resolved maps of synaptic connectivity, control of extended neuronal populations facilitates the exploration of circuit dynamics (Miesenböck & Kevrekidis 2005). Temporally and/or spatially patterned illumination has been used to induce synaptic plasticity (Goold & Nicoll 2010, Zhang & Oertner 2007), discover unexpected cellular adaptations to changes in levels and patterns of electrical activity (Grubb & Burrone 2010), characterize the role of specific circuit motifs in the generation and dissolution of rhythms and synchrony (Cardin et al. 2009, Dhawale et al. 2010, Losonczy et al. 2010, Sohal et al. 2009), and delineate the firing rules used by feature detectors to integrate stimulus attributes carried separately in converging afferents (Arenkiel et al. 2007). It is not inconceivable that opto-genetic tools, applied with hard-nosed reductionism, will in the not too distant future strip even high-level problems, such as the persistent activity patterns thought to underlie working memory, or the attentional enhancement of neuronal responses, to their mechanistic core.
Acknowledgments
Work in my laboratory is or was supported by the Wellcome Trust, the Gatsby Charitable Foundation, the Medical Research Council, the National Institutes of Health, the Dana Foundation, the Office of Naval Research, the Human Frontiers Science Program, the Searle Scholars Program, and the Alfred P. Sloan, Beckman, Dana, Klingenstein and McKnight Foundations.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–24. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–84. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–29. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–55. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–56. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–18. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA. 2009;106:17968–73. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–74. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- Ashkin A. Acceleration and trapping of particles by radiation pressure. Phys Rev Lett. 1970;24:156–59. [Google Scholar]
- Bae G, Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- Balland V, Byrdin M, Eker AP, Ahmad M, Brettel K. What makes the difference between a cryptochrome and DNA photolyase? A spectroelectrochemical comparison of the flavin redox transitions. J Am Chem Soc. 2009;131:426–27. doi: 10.1021/ja806540j. [DOI] [PubMed] [Google Scholar]
- Bamann C, Gueta R, Kleinlogel S, Nagel G, Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49:267–78. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
- Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J Mol Biol. 2008;375:686–94. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–86. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E, Wassermann NH, Erlanger BF. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci USA. 1971;68:1820–23. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. How photons start vision. Proc Natl Acad Sci USA. 1996;93:560–65. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–34. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Bialek W. Physical limits to sensation and perception. Annu Rev Biophys Biophys Chem. 1987;16:455–78. doi: 10.1146/annurev.bb.16.060187.002323. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–68. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–23. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Braun FJ, Hegemann P. Two light-activated conductances in the eye of the green alga Volvox carteri. Biophys J. 1999;76:1668–78. doi: 10.1016/S0006-3495(99)77326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–67. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chen M, Tao Y, Lim J, Shaw A, Chory J. Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol. 2005;15:637–42. doi: 10.1016/j.cub.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–15. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–63. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Crosson S, Moffat K. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci USA. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson S, Moffat K. Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell. 2002;14:1067–75. doi: 10.1105/tpc.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–45. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter van Steveninck R, Laughlin SB. Light adaptation and reliability in blowfly photoreceptors. Int J Neural Syst. 1996;7:437–44. doi: 10.1142/s0129065796000415. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenböck G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–86. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci. 2010;13:1404–12. doi: 10.1038/nn.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 2008;18:1133–37. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenbeck S, Gradmann D, Braun FJ, Hegemann P. Evidence for a light-induced H+ conductance in the eye of the green alga Chlamydomonas reinhardtii. Biophys J. 2002;82:740–51. doi: 10.1016/S0006-3495(02)75436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–28. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst OP, Sánchez Murcia PA, Daldrop P, Tsunoda SP, Kateriya S, Hegemann P. Photoactivation of channelrhodopsin. J Biol Chem. 2008;283:1637–43. doi: 10.1074/jbc.M708039200. [DOI] [PubMed] [Google Scholar]
- Feldbauer K, Zimmermann D, Pintschovius V, Spitz J, Bamann C, Bamberg E. Channelrhodopsin-2 is a leaky proton pump. Proc Natl Acad Sci USA. 2009;106:12317–22. doi: 10.1073/pnas.0905852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A, Suska A, Schlüter OM. Optogenetic approaches in neuroscience. Curr Biol. 2010;20:R897–903. doi: 10.1016/j.cub.2010.08.053. [DOI] [PubMed] [Google Scholar]
- Gauden M, van Stokkum IH, Key JM, Luhrs D, van Grondelle R, et al. Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor. Proc Natl Acad Sci USA. 2006;103:10895–900. doi: 10.1073/pnas.0600720103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Funk K, Dekowski B, Lechler R, Keller S, et al. Caged capsaicins: new tools for the examination of TRPV1 channels in somatosensory neurons. ChemBioChem. 2007;8:89–97. doi: 10.1002/cbic.200600437. [DOI] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–28. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Isacoff EY. Nanoengineering ion channels for optical control. Physiology. 2008a;23:238–47. doi: 10.1152/physiol.00018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Isacoff EY. Optical switches for remote and noninvasive control of cell signaling. Science. 2008b;322:395–99. doi: 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D, Isacoff EY. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci USA. 2007;104:10865–70. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–75. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–59. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–39. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–38. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–74. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–92. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–93. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Harper SM, Neil LC, Gardner KH. Structural basis of a phototropin light switch. Science. 2003;301:1541–44. doi: 10.1126/science.1086810. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–76. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P. Algal sensory photoreceptors. Annu Rev Plant Biol. 2008;59:167–89. doi: 10.1146/annurev.arplant.59.032607.092847. [DOI] [PubMed] [Google Scholar]
- Hegemann P, Fuhrmann M, Kateriya S. Algal sensory photoreceptors. J Phycol. 2001;37:668–76. [Google Scholar]
- Henderson R, Schertler GF. The structure of bacteriorhodopsin and its relevance to the visual opsins and other seven-helix G-protein coupled receptors. Philos Trans R Soc Lond B. 1990;326:379–89. doi: 10.1098/rstb.1990.0019. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–19. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY. A light-gated, potassium-selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci. 2010;13:1027–32. doi: 10.1038/nn.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Forbush B, III, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–35. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Karginov AV, Zou Y, Shirvanyants D, Kota P, Dokholyan NV, et al. Light regulation of protein dimerization and kinase activity in living cells using photocaged rapamycin and engineered FKBP. J Am Chem Soc. 2011;133:420–23. doi: 10.1021/ja109630v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kätzel D, Zemelman BV, Buetfering C, Wölfel M, Miesenböck G. The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci. 2011;14:100–7. doi: 10.1038/nn.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. 2010;7:973–75. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Altenbach C, Kono M, Oprian DD, Hubbell WL, Khorana HG. Structural origins of constitutive activation in rhodopsin: role of the K296/E113 salt bridge. Proc Natl Acad Sci USA. 2004;101:12508–13. doi: 10.1073/pnas.0404519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, et al. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14:513–18. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–5004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe M, Besir H, Essen LO, Oesterhelt D. Structure of the light-driven chloride pump halorhodopsin at 1.8 Å resolution. Science. 2000;288:1390–96. doi: 10.1126/science.288.5470.1390. [DOI] [PubMed] [Google Scholar]
- Koon AC, Ashley J, Barria R, DasGupta S, Brain R, et al. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat Neurosci. 2011;14:190–99. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi JK. Bacteriorhodopsin as a model for proton pumps. Nature. 1995;375:461–63. doi: 10.1038/375461a0. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF. A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol. 1980;75:207–32. doi: 10.1085/jgp.75.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Nerbonne JM. Physiological and pharmacological manipulations with light flashes. Annu Rev Biophys Bioeng. 1982;11:151–75. doi: 10.1146/annurev.bb.11.060182.001055. [DOI] [PubMed] [Google Scholar]
- Leung DW, Otomo C, Chory J, Rosen MK. Genetically encoded photoswitching of actin assembly through the Cdc42-WASP-Arp2/3 complex pathway. Proc Natl Acad Sci USA. 2008;105:12797–802. doi: 10.1073/pnas.0801232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–52. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–26. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–14. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yu X, Li K, Klejnot J, Yang H, et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science. 2008;322:1535–39. doi: 10.1126/science.1163927. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Zemelman BV, Vaziri A, Magee JC. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2010;13:967–72. doi: 10.1038/nn.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, et al. Motor control by sensory cortex. Science. 2010;330:1240–43. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem Int Ed Engl. 2006;45:4900–21. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- Miesenböck G. Genetic methods for illuminating the function of neural circuits. Curr Opin Neurobiol. 2004;14:395–402. doi: 10.1016/j.conb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Miesenböck G. Lighting up the brain. Sci Am. 2008;299:52–59. doi: 10.1038/scientificamerican1008-52. [DOI] [PubMed] [Google Scholar]
- Miesenböck G. The optogenetic catechism. Science. 2009;326:395–99. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu Rev Neurosci. 2005;28:533–63. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- Miller G. Optogenetics. Shining new light on neural circuits. Science. 2006;314:1674–76. doi: 10.1126/science.314.5806.1674. [DOI] [PubMed] [Google Scholar]
- Möglich A, Yang X, Ayers RA, Moffat K. Structure and function of plant photoreceptors. Annu Rev Plant Biol. 2010;61:21–47. doi: 10.1146/annurev-arplant-042809-112259. [DOI] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–84. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–98. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–45. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–84. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, et al. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci USA. 2009;106:6814–19. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K. G protein–coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–67. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Clarkson MW, Ozgur S, Lee AL, Sancar A. Role of structural plasticity in signal transduction by the cryptochrome blue-light photoreceptor. Biochemistry. 2005;44:3795–805. doi: 10.1021/bi047545g. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–68. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–45. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, et al. Habenula “cholinergic” neurons corelease glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–52. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Ritter E, Stehfest K, Berndt A, Hegemann P, Bartl FJ. Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy. J Biol Chem. 2008;283:35033–41. doi: 10.1074/jbc.M806353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem. 2010;285:41501–8. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghian K, Bocola M, Schutz M. A conclusive mechanism of the photoinduced reaction cascade in blue light using flavin photoreceptors. J Am Chem Soc. 2008;130:12501–13. doi: 10.1021/ja803726a. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Häusser M. Electrophysiology in the age of light. Nature. 2009;461:930–39. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H. Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci. 2010;30:7111–20. doi: 10.1523/JNEUROSCI.5193-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder-Lang S, Schwärzel M, Seifert R, Strunker T, Kateriya S, et al. Fast manipulation of cellular cAMP level by light in vivo. Nat Methods. 2007;4:39–42. doi: 10.1038/nmeth975. [DOI] [PubMed] [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Völler T, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–47. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Noise, neural codes and cortical organization. Curr Opin Neurobiol. 1994;4:569–79. doi: 10.1016/0959-4388(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nat Biotechnol. 2002;20:1041–44. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 2003;23:10388–401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2002;99:8689–94. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulson L, Miesenböck G. Photocontrol of neural activity: biophysical mechanisms and performance in vivo. Chem Rev. 2008;108:1588–602. doi: 10.1021/cr078221b. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JL, Yang CS, Jung KH, Spudich EN. Retinylidene proteins: structures and functions from Archaea to humans. Annu Rev Cell Dev Biol. 2000;16:365–92. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem. 2011;286:1181–88. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D, Moffat K, Sosnick TR. Light-activated DNA binding in a designed allosteric protein. Proc Natl Acad Sci USA. 2008;105:10709–14. doi: 10.1073/pnas.0709610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D, Yao X, Gawlak G, Rosen MK, Gardner KH, Sosnick TR. Rationally improving LOV domain–based photoswitches. Nat Methods. 2010;7:623–26. doi: 10.1038/nmeth.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh A, Tsai HC, Wang LP, Zhang F, Kressel J, et al. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2011;29:78–88. doi: 10.1002/stem.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–8. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamasaki K, Fujita S, Oda K, Iseki M, et al. Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem Biophys Res Commun. 2003;301:711–17. doi: 10.1016/s0006-291x(02)03079-6. [DOI] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–45. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Szobota S, Isacoff EY. Optical control of neuronal activity. Annu Rev Biophys. 2010;39:329–48. doi: 10.1146/annurev.biophys.093008.131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–84. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, et al. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–19. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc. 2007;129:260–61. doi: 10.1021/ja067269o. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–7. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci USA. 2007;104:8143–48. doi: 10.1073/pnas.0700384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Gardner KH. Structure and insight into blue light-induced changes in the BlrP1 BLUF domain. Biochemistry. 2009;48:2620–29. doi: 10.1021/bi802237r. [DOI] [PubMed] [Google Scholar]
- Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, et al. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–10. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol. 1999;145:437–45. doi: 10.1083/jcb.145.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell. 2000;103:815–27. doi: 10.1016/s0092-8674(00)00184-7. [DOI] [PubMed] [Google Scholar]
- Yao X, Rosen MK, Gardner KH. Estimation of the available free energy in a LOV2-Jα photoswitch. Nat Chem Biol. 2008;4:491–97. doi: 10.1038/nchembio.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE. Induction of protein-protein interactions in live cells using light. Nat Biotechnol. 2009;27:941–45. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–10. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- Yu JY, Dickson BJ. Hidden female talent. Nature. 2008;453:41–42. doi: 10.1038/453041a. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenböck G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Miesenböck G. Genetic schemes and schemata in neurophysiology. Curr Opin Neurobiol. 2001;11:409–14. doi: 10.1016/s0959-4388(00)00227-0. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Nesnas N, Lee GA, Miesenböck G. Photochemical gating of heterologous ion channels: remote control over genetically designated populations of neurons. Proc Natl Acad Sci USA. 2003;100:1352–57. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007a;8:577–81. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–33. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–92. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007b;446:633–39. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods. 2007;4:139–41. doi: 10.1038/nmeth988. [DOI] [PubMed] [Google Scholar]
- Zhao J, Gover TD, Muralidharan S, Auston DA, Weinreich D, Kao JP. Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation. Biochemistry. 2006;45:4915–26. doi: 10.1021/bi052082f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowski BD, Gardner KH. Tripping the light fantastic: blue-light photoreceptors as examples of environmentally modulated protein-protein interactions. Biochemistry. 2011;50:4–16. doi: 10.1021/bi101665s. [DOI] [PMC free article] [PubMed] [Google Scholar]