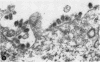Abstract
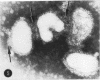
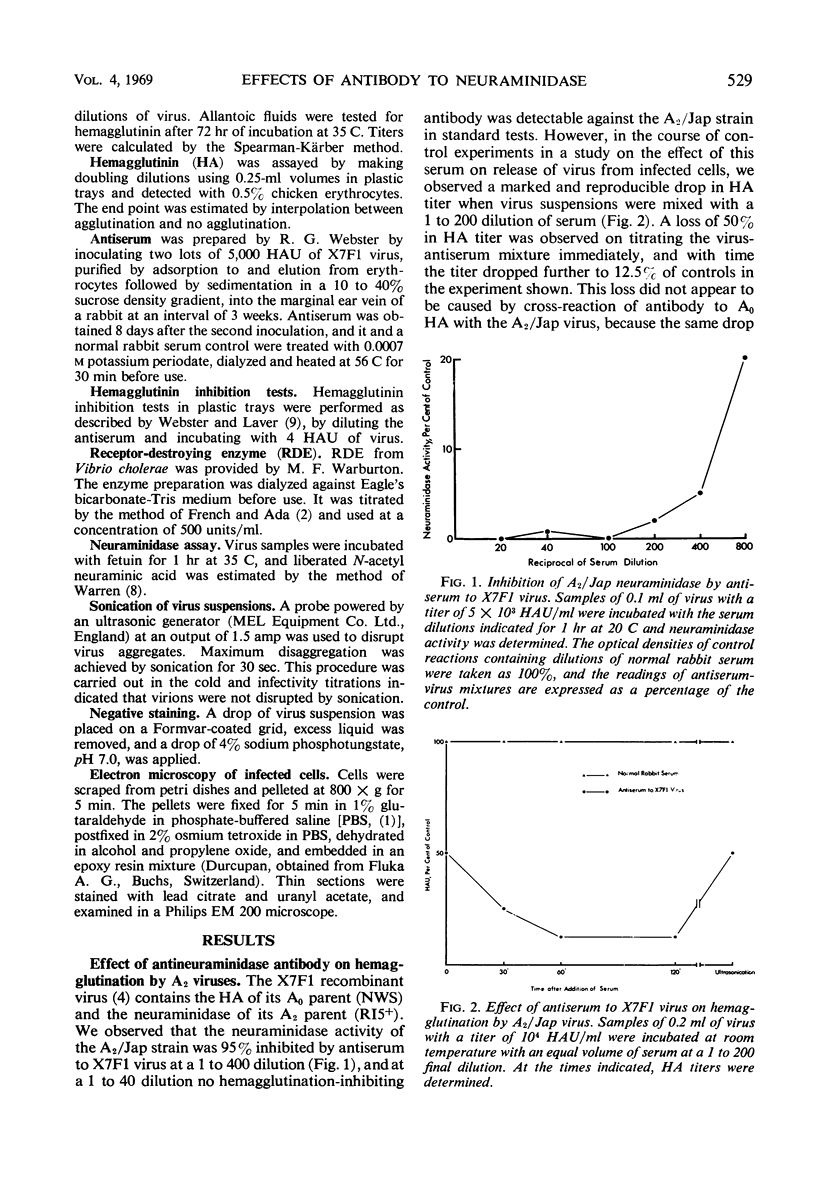
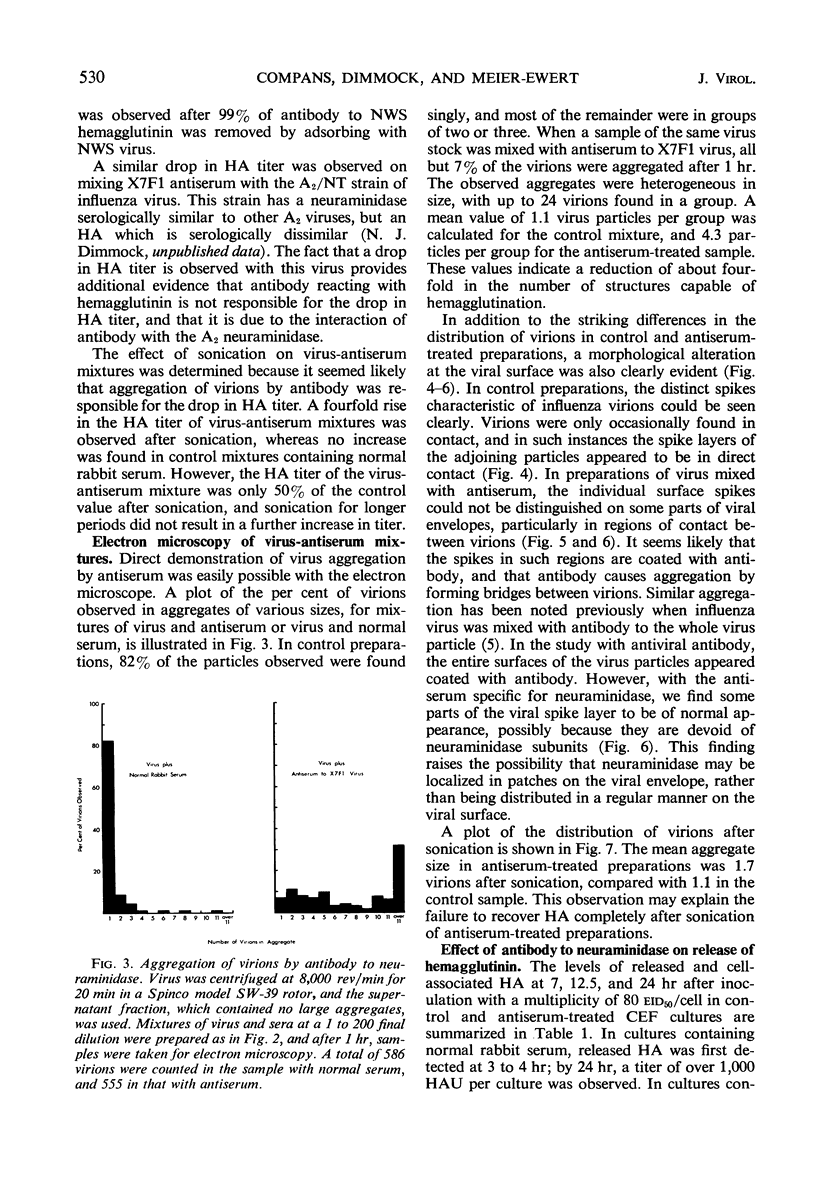
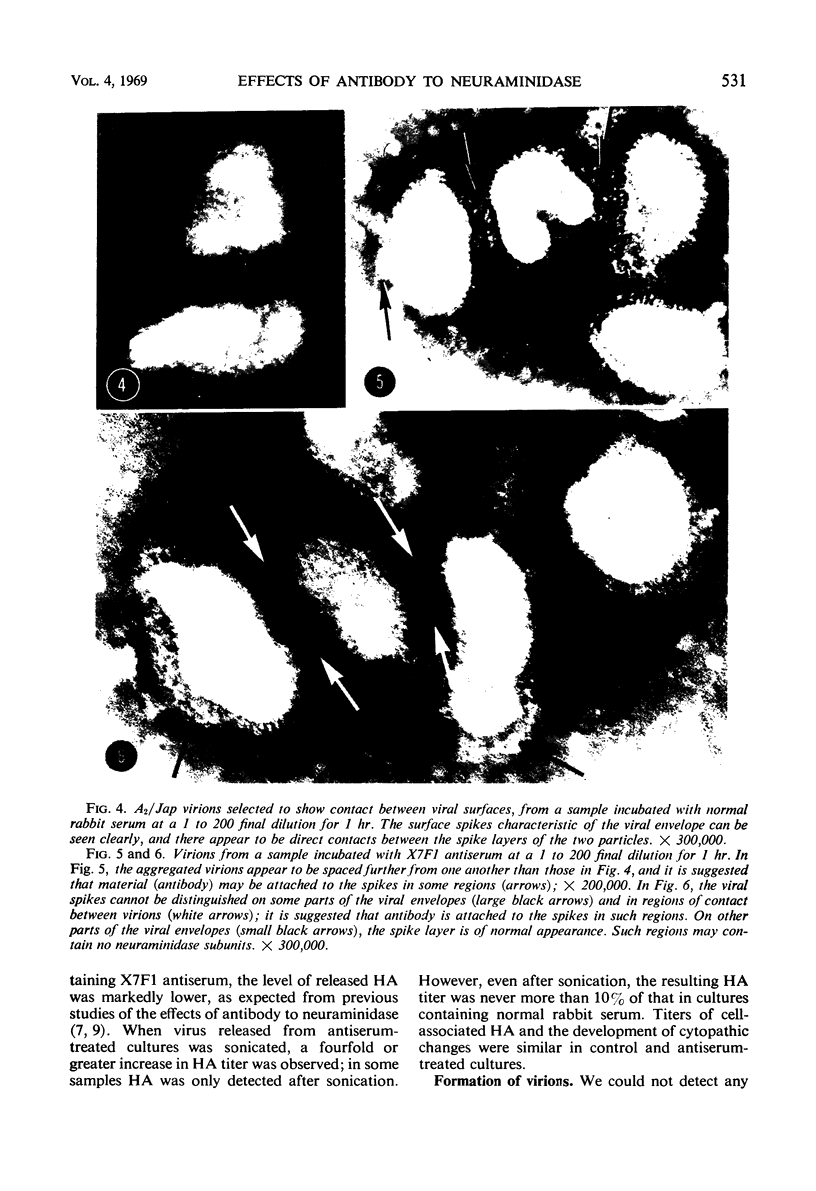
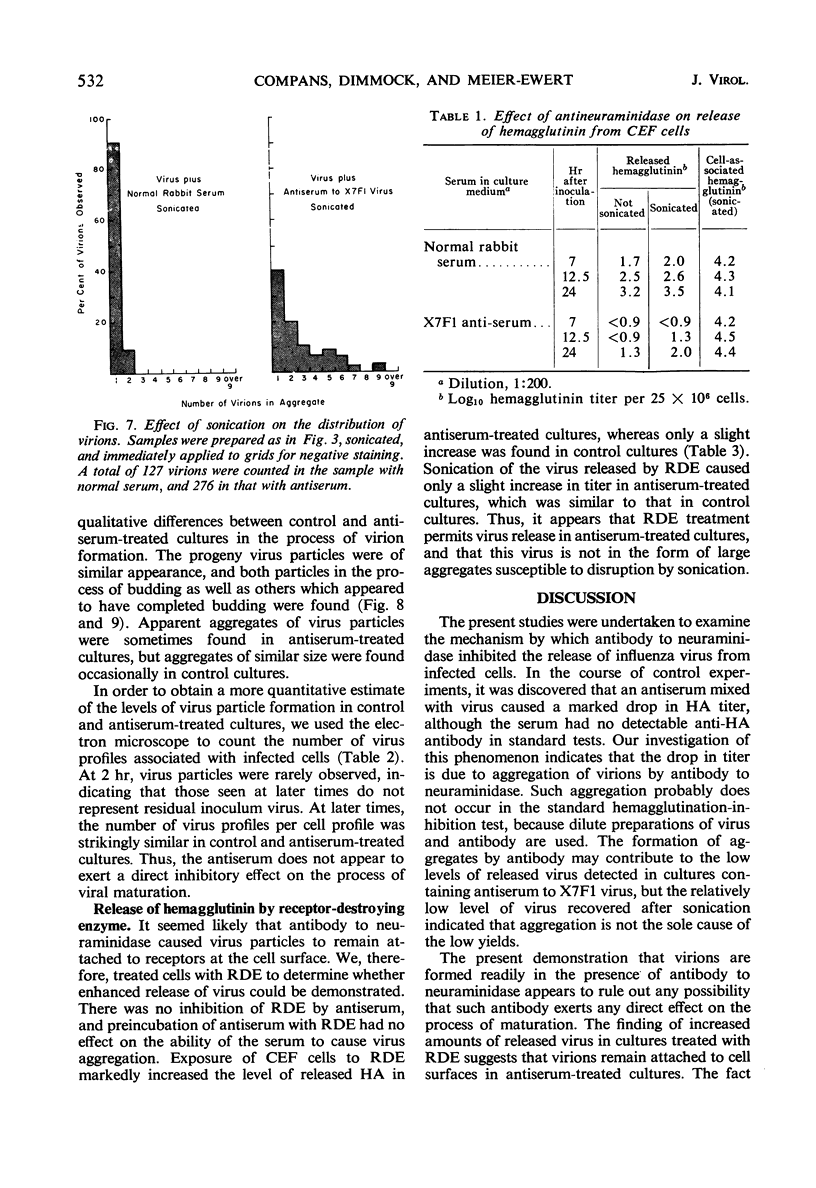
Antiserum to a recombinant between an Ao and an A2 influenza virus had no detectable antibody against an A2 virus in standard hemagglutination-inhibition tests, and inhibited 95% of viral neuraminidase activity at a 1 to 400 dilution. However, on mixing virus with antiserum, a drop of up to 90% in hemagglutinin titer was observed. The effects of ultrasonication and direct electron microscopic examination indicated that the antiserum caused aggregation of virus particles. When antiserum was added to A2 virus-infected chick embryo fibroblasts, release of virus appeared markedly inhibited. After ultrasonication to disrupt aggregates, an increase in released hemagglutinin was observed, but the resulting level was considerably lower than that in control cultures containing normal rabbit serum. In thin sections of infected cells, similar numbers of virus profiles were observed in control and antiserum-treated cultures. A marked increase in release of hemagglutinin was noted if receptor-destroying enzyme was added to antiserum-treated cultures. The results indicate that antibody to neuraminidase does not exert a direct effect on viral maturation, but inhibits the detachment of viral progeny from cell surface receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH E. L., ADA G. L. Stimulation of the production of neuraminidase in Vibrio cholerae cultures by N-acetylneuraminic acid and sialyl-lactose. J Gen Microbiol. 1959 Dec;21:550–560. doi: 10.1099/00221287-21-3-550. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Seto J. T., Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966 Dec;30(4):731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Preparation and properties of antibody directed specifically against the neuraminidase of influenza virus. J Immunol. 1967 Jul;99(1):49–55. [PubMed] [Google Scholar]