Abstract
Vα14 natural killer T (iNKT) cells activated by alpha-galactosylceramide (GalCer) secrete a large amount of cytokines. Toll-like receptors (TLRs) play a critical role in the innate immune responses via the recognition of pathological antigen. Previously we demonstrated that the iNKT cells activated by GalCer augmented LPS-induced NO production in peritoneal cells. In this study, we examined the effect of GalCer and TLR agonists by IFN-γ production from splenocytes. Splenocytes pretreated with GalCer induced TLR3, 4, 7/8, and 9 agonists in vitro, resulting in the enhancement of IFN-γ mRNA expression. In particular, IFN-γ stimulated by GalCer and LPS was increased in NK cells and CD8 T cells, and inhibited by a neutralizing anti-IL-12 antibody. Pretreatment with GalCer enhanced the phosphorylation of IκB-α induced by LPS stimulation. The present study showed that co-stimulation of GalCer and TLR agonists powerfully induced the production of IFN-γ from splenocytes.
α-Galactosylceramide (GalCer) is a glycolipid that has been identified as a ligand recognized by Vα14 natural killer T (iNKT) cells1. A key feature of iNKT cells is the expression of a single invariant Vα14 antigen receptor that recognizes glycolipid antigens in mice. iNKT cells can recognize GalCer presented by CD1d molecules on antigen presenting cells. Although their precise physiological functions remain unknown, iNKT cells have been implicated in the immune system regulation. GalCer can strongly activate iNKT cells to produce immunoregulatory cytokines, including interferon (IFN)-γ, interleukin (IL)- 4, and IL-17, and thereby exert a variety of subsequent effects on other cells in the immune system2. Recent studies have shown the mechanism of GalCer-induced iNKT cell activation in immune responses to tumors and microbes, and in the suppression of autoimmune diseases3,4,5,6.
Previously, we established the murine endotoxin shock model induced by the administration of GalCer and lipopolysaccharide (LPS) in vivo7. In this model, tumor necrosis factor (TNF)-α and nitric oxide (NO) production was intensely enhanced by the administration of LPS to GalCer-sensitized mice in vivo. Pretreatment with GalCer enhanced the systemic inflammation induced by LPS injection in mice. In this endotoxin shock model, IFN-γ production was significantly increased, and IFN-γ played a critical role in the establishment of this endotoxin shock model. Moreover, it was previously shown that the administration of GalCer and LPS could enhance the production of nitric oxide (NO) in peritoneal cells and intrahepatic mononuclear cells in vitro8,9. In these models, IFN-γ contributed to the enhancement of NO production in peritoneal and intrahepatic mononuclear cells stimulated with GalCer and LPS. Thus, stimulation by GalCer and LPS enhanced the production of IFN-γ in immune cells and induced a robust immunological response. However, the mechanism of enhancement of IFN-γ production induced by GalCer and LPS administration is not well known. Furthermore, it is unclear whether other Toll-like receptor (TLR) agonists can enhance the production of IFN-γ in immune cells sensitized by the administration of GalCer.
In the present study, we examined the effect of GalCer and various TLR agonists on the IFN-γ production in murine splenocytes in vitro and defined the mechanism of enhancement of IFN-γ production.
Results
Up-regulation of IFN-γ in splenocytes stimulated with TLR agonist and GalCer
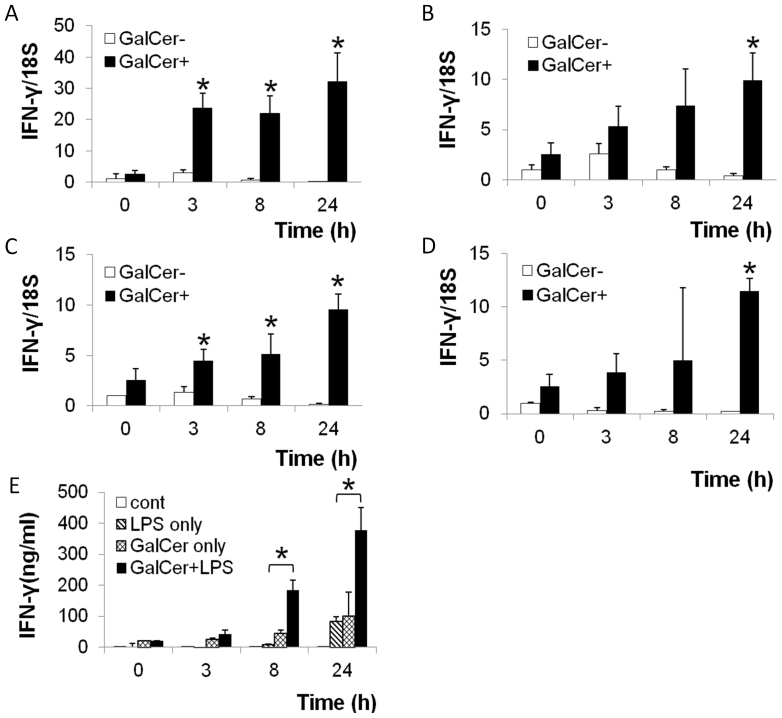
Splenocytes at 1 × 106 cells/ml were cultured with GalCer for 18 h in vitro. GalCer-treated splenocytes were stimulated with various TLR agonists (LPS, poly-I:C, IMQ, or CpG ODN). These cells were harvested at 3, 8, and 24 h after stimulation with TLR agonists, and the expression of IFN-γ mRNA in these cells was measured by real-time RT-PCR. LPS (TLR4 agonist), poly-I:C (TLR3 agonist), IMQ (TLR7 agonist), and CpG ODN (TLR9 agonist) stimulation strongly enhanced the expression of IFN-γ mRNA in GalCer-pretreated splenocytes in vitro (Figs. 1A–D). The production of IFN-γ in splenocytes stimulated with both LPS and GalCer was higher than that in splenocytes stimulated with LPS or GalCer alone (Fig. 1E).
Figure 1. Effect of GalCer and TLR agonists on IFN-γ production in mice splenocytes.
Splenocytes (1 × 106 cells/ml) were cultured with or without GalCer (100 ng/ml) for 18 h. IFN-γ mRNA expression in GalCer-pretreated splenocytes stimulated with LPS (0.01 μg/ml) (A), Poly-I:C (1 μg/ml) (B), IMQ (0.3 μg/ml) (C), or CpG ODN (0.3 μg/ml) (D) was measured by real-time RT-PCR and was determined on the basis of 18S rRNA expression. The concentration of culture supernatant IFN-γ was determined at 0, 3, 8, and 24 h after LPS (1 μg/ml) administration with or without pretreatment with GalCer (100 ng/ml) (E). The data are presented as the means ± SD of the results of three samples from each group. *P < 0.05.
Identification of IFN-γ producing cells following GalCer and LPS stimulation
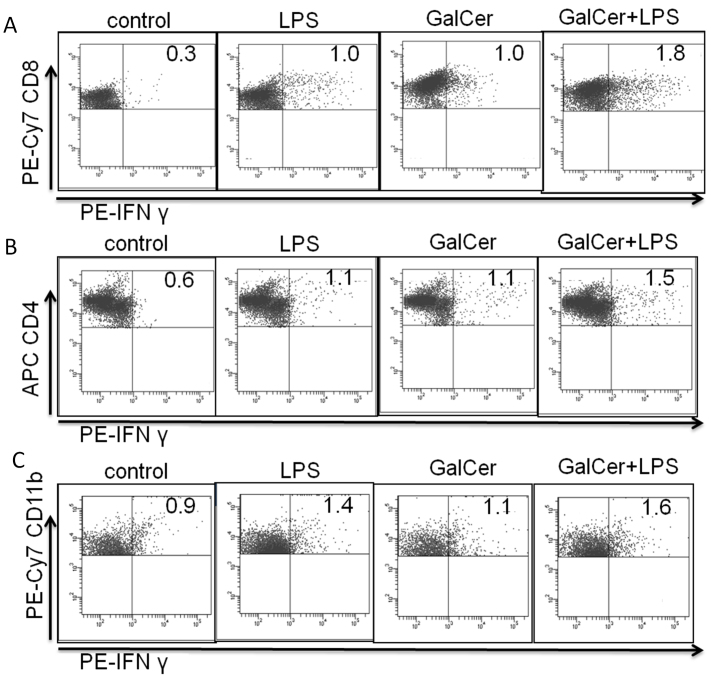
Next, to indentify the IFN-γ producing cell type following GalCer and LPS stimulation in vitro, we measured the production of IFN-γ in splenocytes by intracellular cytokine staining. As shown in Figure 2, intracellular cytokine staining analysis indicated that IFN-γ production in CD8-positive cells was enhanced by co-stimulation with LPS and GalCer. Co-administration of LPS and GalCer mildly increased the production of IFN-γ in CD4- and CD11b-positive cells compared to stimulation with LPS or GalCer alone. Administration of LPS and GalCer did not enhance IFN-γ production in CD19- positive cells (data not shown).
Figure 2. Identification of IFN-γ producing cells after GalCer and LPS stimulation.
Splenocytes (2.5 × 106 cells/ml) were stimulated with GalCer (100 ng/ml) and LPS (1 μg/ml). Protein Transport Inhibitor was added to each well 5 h before harvesting. Cells were harvested for fixation and permeabilization with cytofix/cytoperm. After fixation and permeabilization of splenocytes, these cells were stained with anti IFN-γ, CD8 (A), CD4 (B), and CD11b (C) antibodies. The stained cells were analyzed using flow cytometry. The data are representative of at least 2 independent experiments with similar results.
Enhancement of IL-12 production in splenocytes following GalCer and LPS stimulation
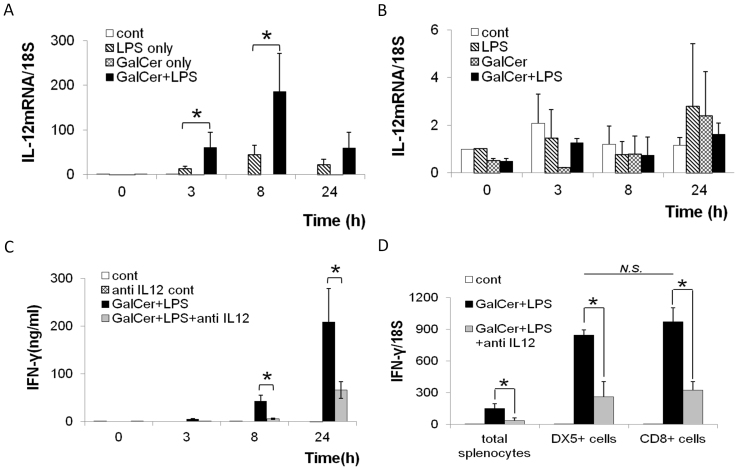
In general, IFN-γ production in immune cells is strongly enhanced by IL-12 stimulation. IL-12 is mainly produced by dendritic cells, macrophages, and B cells. Therefore, we next measured the expression of IL-12 mRNA in adherent and non-adherent cells from splenocytes after stimulation with GalCer and LPS (Figs. 3A and B). Although co-stimulation by GalCer and LPS did not increase the expression of IL-12 mRNA in non-adherent cells, the expression of IL-12 mRNA in the adherent cells was synergistically enhanced by GalCer and LPS stimulation in vitro. Further, we tested the effect of neutralizing antibody against IL-12 on the enhancement of IFN-γ production after stimulation with GalCer and LPS. As shown in Fig. 3C, the IFN-γ production after the co-stimulation of GalCer and LPS in splenocytes was significantly reduced by the administration of neutralizing antibody against murine IL-12. Previous study demonstrated that natural killer (NK) cells were the major IFN-γ source after the administration of GalCer10. Therefore, we measured the expression of IFN-γ mRNA in NK cells and CD8-positive cells after the administration with GalCer and LPS in vitro (Fig. 3D). The co-stimulation of GalCer and LPS markedly enhanced the expression of IFN-γ mRNA in both NK cells and CD8-positive cells. The enhancement of IFN-γ mRNA expression was reduced by the administration of anti-IL-12 antibody.
Figure 3. Effect of IL-12 on IFN-γ production in splenocytes stimulated with GalCer and LPS.
Splenocytes (1 × 106 cells/ml) were cultured with or without GalCer (100 ng/ml) for 18 h, and IL-12 mRNA expression in adherent cells (A) and non-adherent cells (B) at 0, 3, 8, and 24 h after LPS (1 μg/ml) treatment was determined using real-time RT-PCR. Splenocytes (1 × 106 cells/ml) were cultured with GalCer (100 ng/ml) and/or anti-IL-12 antibody (1 μg/ml). LPS (1 μg/ml) was added to the culture medium after stimulation with GalCer. The concentration of IFN-γ in culture supernatant was determined at 0, 3, 8, and 24 h after LPS stimulation using ELISA (C). The expression of IFN-γ mRNA in isolated DX5- or CD8-possitive splenocytes was measured after the stimulation with GalCer (100 ng/ml), LPS (1 μg/ml) in the presence or absence of anti-IL-12 antibody (1 μg/ml) (D). The data are represented as the means ± SD of the results of 3 samples from each group. *P < 0.05.
Up-regulation of IFN-γ production via NFkB pathway in vivo after the administration of LPS and GalCer
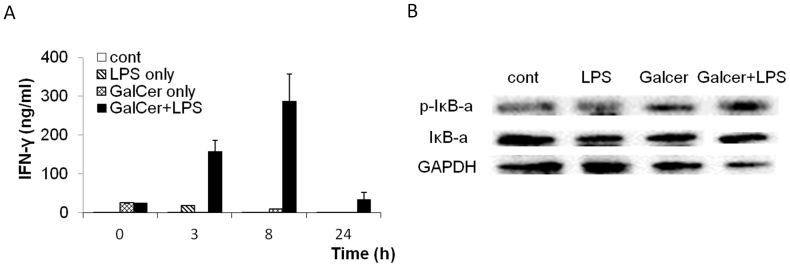
Next, we examined the production of IFN-γ in vivo after the administration with GalCer and LPS. The concentration of serum IFN-γ was determined at 0, 3, 8, and 24 h after LPS (1 μg) injection with or without pretreatment by GalCer (1 μg) (Fig. 4A). The administration of LPS and GalCer strongly enhanced the production of IFN-γ in vivo. The translocation of NFκB to the nucleus is preceded by the phosphorylation and proteolytic degradation of IκB-α. The phosphorylated levels of IκBα protein were examined by western blot analysis (Fig. 4B). After the stimulation with LPS and GalCer, the level of phosphorylated IκB-α protein was significantly increased in spleen compared to those in the spleen from the mice treated with LPS alone or GalCer alone.
Figure 4. Effect of GalCer and LPS on the activation of NFκB signaling.
IFN-γ concentrations in serum were measured at 0, 3, 8 and 24 h after LPS injection on the mice treated with GalCer (1 μg/mice) and LPS (1 μg/mice) (A). The mice treated with GalCer (1 μg/mice) and LPS (1 μg/mice) and the spleen was obtained 1 h after the administration of LPS (B). The expression of the phosphorylated-IκB-α protein was examined by western blot analysis and based on the expression of the GAPDH protein. To determine equivalent loading of samples the blots were stripped and reprobed with anti-GAPDH antibody. The gels have been run under the same experimental condition. The blot image was cropped around the region of interest. These experiments were repeated twice and produced the same results.
Discussion
In the present study, we showed that the administration of various TLR agonists strongly enhanced the production of IFN-γ in splenocytes pretreated with GalCer in vitro. In particular, CD8- positive cells can strongly secrete IFN-γ by co-stimulation with GalCer and LPS. This augmentation of IFN-γ production depended on the enhancement of IL-12 production in splenic adherent cells after GalCer and LPS stimulation. Moreover, our finding showed that the splenocytes pretreated with GalCer enhanced the TLR signaling induced by LPS.
Our previous studies have indicated that GalCer and TLR agonists can induce severe in vitro and in vivo immunological responses7,8,9,11. GalCer and LPS can synergistically enhance NO production in murine peritoneal cells in vitro8. Intrahepatic mononuclear cells from mice also strongly produced NO upon co-stimulation with GalCer and LPS9. These enhancements of NO production following the administration of GalCer and LPS were dependent on IFN-γ production. Although IFN-γ production in peritoneal cells, including many macrophages, was enhanced by the co-administration of GalCer and LPS, it is unknown whether the enhancement of IFN-γ production after stimulation by GalCer and LPS is observed in lymphoid tissues, or whether other TLR agonists can enhance IFN-γ production in splenocytes pretreated with GalCer. As shown in Figure 1A, various TLR (TLR3, TLR4, TLR7, and TLR9) agonists and GalCer synergistically enhanced the expression of IFN-γ mRNA in splenocytes in vitro. In particular, stimulation with LPS and GalCer strongly enhanced IFN-γ production in splenocytes (Figs. 1A and E). The concentration of serum IFN-γ significantly increased after the administration with LPS and GalCer in mice (Fig. 4A). Furthermore, IFN-γ production was increased by the stimulation with GalCer and LPS from CD8- positive cells. In addition, the production of IFN-γ in CD4- and CD11b-positive cells was up-regulated similar to CD8-positive cells (Fig. 2). Previous report indicated that the administration of GalCer mainly increased the IFN-γ production in hepatic NK cells in vivo10. In the present study, the co-stimulation of GalCer and LPS enhanced the expression of IFN-γ mRNA in both NK cells and CD8 T cells in vitro (Fig. 3D). Thus, stimulation with GalCer and TLR agonist can increase the production of IFN-γ in various types of lymphocytes (in particular CD8 T cells and NK cells) in the spleen. These TLR agonists can activate inflammatory immune responses through the MyD88-dependent pathway. Pretreatment with GalCer to splenocytes may affect the TLR signaling induced by TLR agonists via MyD88. Indeed, pretreatment with GalCer enhanced the phosphorylation of IκB-α induced by LPS in vivo (Fig. 4B).
IFN-γ production in splenocytes is up-regulated by the stimulation with various cytokines. The cytokines IL-2, IL-12 and IL18 are known to induce Th1 cells and increase the IFN-γ production in T cells and NK cells12,13. IL-12 is secreted by monocyte/macrophages and dendritic cells after stimulation by some TLR agonists14,15. As shown in Figure 3A, the administration of LPS up-regulated the expression of IL-12 mRNA in adherent cells but not in non-adherent cells. Moreover, these data indicated that adherent cells pretreated with GalCer secreted more IL-12 than did non-pretreated adherent cells after LPS administration. This enhancement of IL-12 mRNA expression in adherent cells can lead to the increase of IFN-γ production in splenocytes after the stimulation of GalCer and LPS. IL-2 and IL-18 mRNA expressions were not enhanced by the administration of GalCer and LPS (data not shown). On the other hand, the experiment using neutralizing antibody against mouse IL-12 showed that the production of IFN-γ induced by GalCer and LPS in splenocytes was inhibited by the addition of anti-IL-12 antibody (Fig. 3C). The co-stimulation of GalCer and LPS markedly enhanced the expression of IFN-γ mRNA in both of NK cells and CD8 T cells, and anti-IL-12 antibody could reduce the enhancement of IFN-γ expression (Fig. 3D). Thus, IL-12 is critical for the enhancement of IFN-γ production after GalCer and LPS stimulation in this model.
IFN-γ is critical for innate and adaptive immunity against viral and intracellular bacterial infection and for tumor control16,17. TLR agonists can induce IFN-γ production in immune cells, and they are used as therapeutic tools for infectious diseases and cancer18,19. A study of human papillomavirus (HPV) infection in rhesus macaques showed that the co-administration of HPV antigen and TLR3 agonist increased IFN-γ and enhanced HPV-specific T-cell responses20. Further, the effects of various TLR4 agonists on viral infectious diseases and cancer wrer examined in numerous clinical studies21,22,23,24. Pre-clinical studies indicated that the vaccination of TLR7/8 agonists with the HIV-1 Gag protein enhanced Ag-specific IgG and cytotoxic T lymphocyte (CTL) responses25. In addition, TLR7/8 agonists have been used as adjuvants in combination with several cancer vaccines26,27. TLR9 agonists were used as adjuvants in vaccines against some viral infectious diseases and cancer28,29. However, TLR agonist therapy alone is considered not become fully effective for viral infection and cancer. On the other hand, GalCer is a well-known, powerful reagent to induce IFN-γ. GalCer has been also tested to infectious disease and cancer in some pre-clinical studies30,31. Although GalCer was used as an adjuvant in combination with the HBV and HCV antigens, the effect of GalCer in the elimination of these hepatitis viruses was limited32. Pre-clinical studies have shown clinical safety and efficacy of GalCer therapy in cancer patients31. Although GalCer monotherapy for cancer did not show a marked effect, the patient group with increased numbers of IFN-γ producing cells showed significantly prolonged overall survival. The enhancement of IFN-γ production in GalCer therapy in cancer patients may lead to improvements in the disease prognosis.
Although, TLR agonists and GalCer have been used in several infectious diseases and cancer, mono-therapy of the TLR agonist or GalCer is insufficient to treat various infections and cancer. Our finding that the co-administration of TLR agonist and GalCer can powerfully induce the production of IFN-γ in splenocytes may lead to a new treatment strategy for various infectious diseases and cancer.
Methods
Reagents
Synthesized GalCer was obtained from Funakoshi co., Ltd. (Tokyo, Japan). LPS from Escherichia coli O111:B4 was purchased from Sigma-Aldrich (St Louis, MO, USA). Imiquimod-R837 (IMQ) and Poly-I:C were purchased from InvivoGen (San Diego, CA, USA). CpG ODN (5″-GCTAGACGTTAGCGT-3″-) was synthesized at Hokkaido System Science (Sapporo, Japan). Anti-mouse IL-12 neutralization antibody (AB-419-NA) was obtained from R&D Systems (Minneapolis, MN, USA). Mouse IFN-γ antibody was purchased from eBioscience (San Diego, CA, USA). Phospho-IκB-α antibody and GAPDH antibody were obtained from Cell Signaling Technology (Danvers, MA, USA).
Mice
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the guidelines for care and use of animals established by the Animal Ethics Committee of Gifu University (approval number: 24-16). Male C57BL/6 mice at the age of approximately 8 weeks were obtained from Japan SLC (Hamamatsu, Japan).
Cell culture
Splenocytes (1 × 106 cells/ml) were incubated with or without GalCer (100 ng/ml) for 18 h in vitro and TLR agonists were added into the culture medium. Splenocytes were obtained and suspended in RPMI 1640 medium (Wako Pure Chemical Industries, Osaka, Japan) containing 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cultured at 37°C in a 5% CO2 atmosphere.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IFN-γ in culture supernatant and serum from the mice treated with GalCer and LPS were measured using ELISA. Ninety-six-well Immulon 2HB plates (Thermo Lab Systems, OH, USA) were coated with purified IFN-γ-specific antibody and then blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Culture supernatants were applied in each well on the ELISA plate and bound cytokine was detected by the addition of biotin-labeled secondary IFN-γ-specific antibody (all antibodies were obtained from eBioscience), followed by phosphatase-conjugated avidin and a phosphatase-specific colorimetric substrate (Sigma-Aldrich). Standard curves were generated using recombinant cytokines purchased from R&D Systems.
Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Real-time RT-PCR was used to quantify the levels of IFN-γ and IL-12 mRNA expression in splenocytes. Total RNA was isolated using an RNeasy mini kit (Qiagen GmbH, Hilden, Germany) and transcribed to cDNA using the High Capacity cDNA transcription kit (Applied Biosystems, Foster City, CA, USA). Purified cDNA was used as the template for real-time PCR conducted using pre-designed primer/probe sets for IFN-γ, IL-12 and 18S rRNA (Applied Biosystems), according to the manufacturer's instruction. 18S rRNA was used as an internal control. Real-time PCR was carried out using a Light-Cycler Rapid Thermal Cycler System (Roche Diagnostic Systems, Indianapolis, IN, USA).
Intracellular cytokine analysis
Splenocytes (2.5 × 106 cells/ml) pretreated with or without GalCer (100 ng/ml) were incubated with LPS (1 μg/ml). Protein Transport Inhibitor (GolgiPlug: [BD Biosciences]) was added to each well 5 h before harvesting. Cells were harvested for fixation and permeabilization with cytofix/cytoperm (BD Biosciences). These cells were stained with phycoerythrin (PE)-Cy7 conjugated anti-mouse CD8 (clone 53–6.7), PE-Cy7 conjugated anti-mouse CD11b (clone M1/70), allophycocyanin (APC)-conjugated anti-mouse CD4 (clone RM4-5), and PE-conjugated anti-mouse IFN-γ (clone XMG1.2) (eBioscience,). The stained cells were analyzed using a FACSCanto II instrument (BD Biosciences).
Cell isolation
DX5- and CD8-possitive cells were isolated from total splenocytes after the stimulation with GalCer, LPS in vitro using anti-DX5 and anti-CD8 conjugated magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The magnetically labeled cells were purified using the QuadroMACS separation unit attached to a MACS multi-stand and LS columns (Miltenyi Biotec GmbH).
Western blot analysis
The spleen was obtained from the mice treated with GalCer (1 μg/mice) and LPS (1 μg/mice). Protein (40 μg) from the spleen lysate was subjected to sodium dodecyl sulfate –polyacrylamide gel electrophoresis (SDS –PAGE) and transferred to a PVDF membrane (GE Healthcare, Uppsala). The membrane was blocked with 0.1% TBST with 1% BSA, and incubated with antidobies against –phosphorylated -IκB-α at 4°C overnight. The membrane was thereafter washed with 0.1% TBST and incubated with peroxidase-labeled anti-mouse IgG antibody for 60 min at room temperature. Immuno-reactive protein bands were visualized using an enhanced chemiluminescence (ECL) system (Wako, Osaka, Japan). To determine equivalent loading of samples the blots were stripped and reprobed with anti-GAPDH antibody.
Statistical analysis
In each experiment, the results were expressed as the mean ± SD. The statistical significance of the difference in mean values was determined by Student's t test or one-way analysis of variance followed by Scheffe's test. P values less than 0.05 were considered significant.
Author Contributions
Conceived and designed the experiments: H.I. and T.A. Performed the experiments: T.A. and H.O. Analyzed the data: H.I., T.A. & M.S. Wrote the paper: H.I., T.A. and M.S.
References
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23, 877–900 (2005). [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Harada M., Kojo S., Nakayama T. & Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol 21, 483–513 (2003). [DOI] [PubMed] [Google Scholar]
- Mattarollo S. R. et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood 120, 3019–3029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly E. C. et al. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS One 7, e37991 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G. et al. High doses of alpha-galactosylceramide potentiate experimental autoimmune encephalomyelitis by directly enhancing Th17 response. Cell Res 20, 480–491 (2010). [DOI] [PubMed] [Google Scholar]
- Ito H. et al. Role of Valpha14+ NKT cells in the development of Hepatitis B virus-specific CTL: activation of Valpha14+ NKT cells promotes the breakage of CTL tolerance. Int Immunol 20, 869–879 (2008). [DOI] [PubMed] [Google Scholar]
- Ito H. et al. Lethal endotoxic shock using alpha-galactosylceramide sensitization as a new experimental model of septic shock. Lab Invest 86, 254–261 (2006). [DOI] [PubMed] [Google Scholar]
- Ito H. et al. Augmentation of lipopolysaccharide-induced nitric oxide production by alpha-galactosylceramide in mouse peritoneal cells. J Endotoxin Res 11, 213–219 (2005). [DOI] [PubMed] [Google Scholar]
- Ohtaki H. et al. Valpha14 NKT cells activated by alpha-galactosylceramide augment lipopolysaccharide-induced nitric oxide production in mouse intra-hepatic lymphocytes. Biochemical and biophysical research communications 378, 579–583 (2009). [DOI] [PubMed] [Google Scholar]
- Schmieg J., Yang G., Franck R. W. & Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. The Journal of experimental medicine 198, 1631–1641 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H. et al. High susceptibility to lipopolysaccharide-induced lethal shock in encephalomyocarditis virus-infected mice. Sci Rep 2, 367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J. T., Yi J. S. & Zajac A. J. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog 7, e1002273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Fonseca-Guimaraes F., Adib-Conquy M. & Cavaillon J. M. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med 18, 270–285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato M. et al. IL-10-induced microRNA-187 negatively regulates TNF-alpha, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proceedings of the National Academy of Sciences of the United States of America 109, E3101–3110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. et al. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol 186, 6287–6295 (2011). [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P. J., Ravasi T. & Hume D. A. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75, 163–189 (2004). [DOI] [PubMed] [Google Scholar]
- Zaidi M. R. & Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res 17, 6118–6124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen F., Kinjo T., Bode C. & Klinman D. M. TLR-based immune adjuvants. Vaccine 29, 3341–3355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska A., Lech-Maranda E. & Robak T. Toll-like receptors and their role in hematologic malignancies. Curr Mol Med 9, 324–335 (2009). [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C. et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog 5, e1000373 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland G. et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 23, 316–320 (2004). [DOI] [PubMed] [Google Scholar]
- Paavonen J. et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 374, 301–314 (2009). [DOI] [PubMed] [Google Scholar]
- Sondak V. K. et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol 20, 2058–2066 (2002). [DOI] [PubMed] [Google Scholar]
- Vantomme V. et al. Immunologic analysis of a phase I/II study of vaccination with MAGE-3 protein combined with the AS02B adjuvant in patients with MAGE-3-positive tumors. J Immunother 27, 124–135 (2004). [DOI] [PubMed] [Google Scholar]
- Wille-Reece U., Wu C. Y., Flynn B. J., Kedl R. M. & Seder R. A. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol 174, 7676–7683 (2005). [DOI] [PubMed] [Google Scholar]
- Adams S. et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol 181, 776–784 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyerabend S. et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 69, 917–927 (2009). [DOI] [PubMed] [Google Scholar]
- Halperin S. A. et al. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 24, 20–26 (2006). [DOI] [PubMed] [Google Scholar]
- Speiser D. E. et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 115, 739–746 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldt B. J. et al. Randomized placebo controlled phase I/II trial of alpha-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol 47, 356–365 (2007). [DOI] [PubMed] [Google Scholar]
- Fujii S. et al. Adjuvant activity mediated by iNKT cells. Semin Immunol 22, 97–102 (2010). [DOI] [PubMed] [Google Scholar]
- Woltman A. M. et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir Ther 14, 809–818 (2009). [DOI] [PubMed] [Google Scholar]