Abstract
Spinal tuberculosis (TB) or Pott's spine is the commonest extrapulmonary manifestation of TB. It spreads through hematogenous route. Clinically, it presents with constitutional symptoms, back pain, tenderness, paraplegia or paraparesis, and kyphotic or scoliotic deformities. Pott's spine accounts for 2% of all cases of TB, 15% of extrapulmonary, and 50% of skeletal TB. The paradiscal, central, anterior subligamentous, and neural arch are the common vertebral lesions. Thoracic vertebrae are commonly affected followed by lumbar and cervical vertebrae. Plain radiographs are usually the initial investigation in spinal TB. For a radiolucent lesion to be apparent on a plain radiograph there should be 30% of bone mineral loss. Computed tomographic scanning provides much better bony detail of irregular lytic lesions, sclerosis, disc collapse, and disruption of bone circumference than plain radiograph. Magnetic resonance imaging (MRI) is the best diagnostic modality for Pott's spine and is more sensitive than other modalities. MRI frequently demonstrates disc collapse/destruction, cold abscess, vertebral wedging/collapse, marrow edema, and spinal deformities. Ultrasound and computed tomographic guided needle aspiration or biopsy is the technique for early histopathological diagnosis. Recently, the coexistence of human immunodeficiency virus infections and TB has been increased globally. In recent years, diffusion-weighted MRI (DW-MRI) and apparent diffusion coefficient values in combination with MRI are used to some extent in the diagnosis of spinal TB. We have reviewed related literature through internet. The terms searched on Google scholar and PubMed are TB, extrapulmonary TB, skeletal TB, spinal TB, Pott's spine, Pott's paraplegia, MRI, and computed tomography (CT).
Keywords: Computed tomography, Diffusion weighted magnetic resonance imaging, Magnetic resonance imaging, Pott's spine, Radiography, Tuberculosis
Introduction
Percival Pott was the first person to present the classic description of spinal tuberculosis (TB) in 1779; hence, spinal TB was called ‘Pott's Disease’.[1,2,3,4] TB of the spine is one of the oldest demonstrated diseases of mankind and is the common extrapulmonary form of TB. The morbidity and mortality rate due to spinal TB is higher than other infections in developing countries with dense population. Since the advent of antituberculous drugs and improved public health measures, spinal TB has become uncommon in industrialized countries, although it is still a significant cause of disease in developing countries. Spinal TB has the potential for serious morbidity, including permanent neurologic deficits and severe deformity.[1,2,3,4] World Health Organization (WHO) estimates that the largest number of new pulmonary TB cases in 2008 occurred in the southeast Asia Region, which accounted for 35% of incident cases globally. However, the estimated incidence rate in sub-Saharan Africa is nearly twice that of the southeast Asia Region with over 350 cases per 100,000 populations. An estimated 1.7 million people died from pulmonary TB in 2009.[2,3] The estimated per capita incidence of tubercular infection was declined in all six WHO regions in year 2008; but this slow decline is compensated by increased population growth rate. Hence, in every year the occurrence of new cases are still increasing worldwide; Africa, the eastern Mediterranean and southeast Asia.[2,3]
Spinal TB accounts for 2% of all cases of TB, 15% of the cases of extrapulmonary TB and 50% of the cases of skeletal TB.[1] Spinal TB is usually secondary to lung or abdominal involvement and may also be the first manifestation of TB. Usually two continuous vertebrae are involved but several vertebrae may be affected, skip lesions, and solitary vertebral involvement may also be seen. The so called skip lesion or a second lesion not contiguous with the more obvious lesion is seen in 4-10% of cases.[4,5,6,7,8,9] Lower thoracic and lumbar vertebrae are the most common sites of spinal TB followed by middle thoracic and cervical vertebrae.[7,10,11,12,13,14,15] The second cervical to seventh cervical region is reportedly involved in 3-5% of cases and atlantoaxial articulation is less than 1% cases. In TB, involvement of posterior elements due to TB is not so uncommon. The lamina was most commonly involved followed by pedicles, articular processes, spinous processes, and transverse processes.[4,5,6,7,11,12,16]
This review article focuses on various aspects of spinal TB with emphasis on various imaging modalities and recent advances in technology. We have reviewed related literature through internet. The terms searched on Google Scholar and PubMed are tuberculosis, extrapulmonary tuberculosis, skeletal tuberculosis, spinal tuberculosis, Pott's spine, Pott's paraplegia, magnetic resonance imaging (MRI), and computed tomography (CT).
Natural Course of the Disease
Spinal involvement is usually a result of hematogenous spread of Mycobacterium tuberculosis into the dense vasculature of cancellous bone of the vertebral bodies. The primary infection site is either a pulmonary focus or other extraosseous foci such as lymph nodes, gastrointestinal, or any other viscera which may be active or quiescent.[17,18] Predisposing factors for spinal TB include poverty, overcrowding, illiteracy, malnutrition, alcoholism, drug abuse, diabetes mellitus, immunosuppressive treatment, chronic peritoneal dialysis, previous tuberculous infection, and HIV infection.[19]
Spread occurs either via the arterial or venous route. Anterior and posterior spinal arteries in the subchondral region of each vertebra facilitate hematogenous spread of the infection in paradiscal regions. Batson's paravertebral venous plexus in the vertebra is a valve-less system that allows free flow of blood in both directions depending upon the pressure generated by intraabdominal and intrathoracic cavities following strenuous activities like coughing. Spread of the infection via the intraosseous venous system may be responsible for central vertebral body lesions. Spinal TB is initially apparent in the anterior inferior portion of the vertebral body. Later on, it spreads into the central part of the body or disc. Paradiscal, anterior, central, and neural arch lesions are the common types of vertebral involvement.
In the paradiscal lesion, infection spreads through arterial plexus. The infection begins from the anterior vertebral body adjacent to the end plate, involving the disc and resulting in disc destruction. With further progression, anterior wedging of vertebra occurs with resultant kyphosis. Intraosseous and extraosseous abscess formation are often found in this type of lesions which is major risk of cord damage due to pressure effect by the abscess, displaced bone, or ischemia from spinal artery thrombosis.[20,21]
In the central lesion, infection starts in the center of the body and spreads along with Batson's venous plexus. Initially there is expansion of the vertebral body and later concentric collapse occurs. Disc is not involved, and collapse of the vertebral body produces vertebra plana. Vertebra plana indicates complete compression of the vertebral body.
In younger patients, the disc is primarily involved because it is more vascularized. In old age, the disc is not primarily involved because of its age-related avascularity. In spinal TB, there is involvement of more than one vertebra because its segmental arteries bifurcate to supply two adjacent vertebrae. Spread of the disease beneath the anterior or posterior longitudinal ligaments involves multiple contiguous vertebrae. A lack of proteolytic enzymes in mycobacterial infections (as compared with pyogenic infections) has been suggested as the cause of the of the subligamentous spread of infection.[20,21,22,23] In spinal TB, characteristically, there is destruction of the intervertebral disc space and the adjacent vertebral bodies, collapse of the spinal elements, and anterior wedging leading to the characteristic angulation and gibbus formation.
Paraplegia is the most devastating complication of spinal TB. It has been divided into two groups: early onset paraplegia and late onset paraplegia. Early onset paraplegia develops in the active stage of spinal TB and requires active treatment. This type of paraplegia has a better prognosis and is frequently seen in adults with Pott's spine. In these patients, paraplegia is caused by formation of debris, pus, and granulation tissue due to destruction of bone and intervertebral disk. Destruction of the anterior vertebral column leads to subluxation and subsequent dislocation of the spine. Late onset paraplegia is a neurological complication that develops after a variable period in a patient with healed TB. Late onset paraplegia may develop 2-3 decades after active infection. It is often associated with marked spinal deformities.
Clinical Presentation
In Pott's spine, the onset of symptoms is usually insidious and disease progression is slow. Duration of symptoms prior to diagnosis ranges from 2 weeks to several years. Historically, this interval was at least 12 months on average, decreasing to between 3 and 6 months in the recent era. Presentation depends upon stage of the disease, site of the disease, presence of complications such as neurologic deficit, abscesses or sinus tracts, and constitutional symptoms such as weakness, loss of appetite, loss of weight, evening rise of temperature, and night sweats generally occur before the symptoms related to the spine manifest.[24] Clinical findings included back pain, paraparesis, kyphosis, sensory disturbance, and bowel and bladder dysfunction.[24] Subclinical and early stage of spinal TB may not present clinically; however, there may be spinal involvement of TB.
There may be evidences of associated extraskeletal TB like cough, expectoration, lymphadenopathy, diarrhea, and abdominal distension. Back pain is the earliest and most common symptom in Pott's spine. This pain may worsen with activity. Relaxation of muscles during sleep permits movements which are very painful. As the infection progresses, pain increases and paraspinal muscle spasm occurs. Muscle spasm obliterates the normal spinal curves and all spinal movements become restricted and painful.
Physical examination of the spine reveals localized tenderness, soft tissue swelling, paravertebral muscle spasm, kyphotic or scoliotic deformities due to collapse and anterior wedging of vertebral bodies, varying degrees of weakness, nerve root compression, and sensory involvement. Late onset paraplegia occurs in patients in whom a marked kyphotic deformity has developed and who have had prolonged anterior impingement on the cord by a sharp osseous kyphosis or possibly from constriction caused by fibrosis around the neural elements.[6] Tuberculous necrotic material from the dorsolumbar spine may lead to cold abscess in the rectus sheath and lower abdominal wall along the intercostal, ilioinguinal, and iliohypogastric nerves; in the thigh along the psoas sheath; in the back along the posterior spinal nerves; in the buttock along the superior gluteal nerve; in the Petit's triangle along the flat muscles of abdominal wall or in the ischiorectal fossa along the internal pudendal nerve.[1] Sometimes only psoas abscess is presented clinically with flexion deformity of hip joint without any spinal symptoms, however possibility of spinal involvement cannot be ruled out. So imaging helps to determine the spinal involvement. Involvement of upper cervical spine though less common, can cause dangerous and rapidly progressive symptoms. The retropharyngeal abscess may track down the mediastinum to enter trachea, esophagus or pleura; may spread to sternocleidomastoid muscle. The tubercular abscess or granulation tissue can directly compress the neuraxis leading to symptoms of cranial nerve involvement or spinal nerve root compression. Clumsiness in walking and spontaneous twitching of muscles is early signs of neurological involvement which can progress to single nerve palsy, to hemiplegia or paraplegia with spasticity, sensory impairment, and bladder/bowel involvement.[6,7,11,16,25,26,27] The inflammatory exudates in cerebrospinal fluid can cause clumping of the nerve root leading to arachnoiditis.
Diagnosis
Diagnosis of spinal TB is made on the basis of typical clinical presentation along with systemic constitutional manifestation, evidence of past exposure to TB or concomitant visceral TB, and neuroimaging modalities.[20] Skin test and hematological investigations like complete blood count (CBC), erythrocyte sedimentation rate (ESR), Montoux test, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR) are also done in diagnosing spinal TB.[28] Bone tissue or abscess samples are obtained to stain for acid-fast bacilli (AFB) and isolate organisms for culture, antibiotic sensitivity, and histopathology; the method widely used is CT guided or ultrasonography (USG) guided needle biopsy and/or aspiration or surgical biopsy.[6,7]
Imaging
Plain radiographs
Plain radiographs are usually the initial investigation in patients with spinal TB. For a radiolucent lesion to be apparent on a plain radiograph, there must be 30% of bone mineral loss.[1,2] In paradiscal type of lesion, the earliest radiological features are narrowing of the joint space and indistinct paradiscal margin of vertebral bodies. The disc space narrows due to either atrophy or prolapse into the vertebral body of the disc tissue. With further progression, anterior wedging or collapse occurs, resulting in varying degree of kyphosis.[6] In anterior type of lesion, the collection of tuberculous granulation tissue and necrotic material leads formation of paravertebral abscess. In the region of thoracic spine, it is visible on plain radiographs as a fusiform or globular radiodense shadow called the bird nest appearance. Long standing abscesses may produce concave erosions around the anterior margins of the vertebral bodies producing a scalloped appearance called the aneurysmal phenomenon. Anterior type is more common in the pediatric dorsal spine.[6] Central type of lesion presents as destruction, ballooning of vertebral bodies, and concentric collapse. In appendiceal or neural arch type of lesion, there is involvement of the posterior arches (spinous process, lamina, pedicle, and transverse process as well as lateral masses of the atlas), pedicular or laminar destruction, erosion of the adjacent ribs in the thoracic region or posterior cortex of the vertebral body with relative sparing of the intervertebral discs, and a large paraspinal mass.[4,11,12]
Computed tomography
Computed tomographicscanning provides much better bony detail of irregular lytic lesions, sclerosis, disc collapse, and disruption of bone circumference than plain radiograph. CT is more effective for defining the shape and calcification of soft tissue abscesses. However, CT is less accurate in defining the epidural extension of the disease and its effect on neural structures. The pattern of bone destruction (fragmentary, osteolytic, sclerotic, and subperiosteal) can be seen well on CT. It is ideal for guiding a percutaneous diagnostic needle in potentially hazardous or relatively inaccessible sites. The presence of calcification within the abscess is virtually diagnostic of spinal TB.[1] In the past, CT myelography was one of the modality used for spinal TB for assessing cord compression, but it has been replaced by MRI nowadays.
Magnetic resonance imaging
MRI is done by taking noncontrast T1-weighted (T1W), T2-weighted (T2W), and short tau inversion recovery (STIR) sequences in axial, sagittal, and coronal planes followed by contrast-enhanced T1W sequences after intravenous administration of gadolinium contrast agent. MRI features of Pott's spine are abnormal signal intensities appearing hypointense on T1W and hyperintense on T2W sequences with heterogeneous enhancement of the vertebral body. STIR sequences are helpful in differentiating fluid from fatty component in non-contrast sequences. Characteristic findings included destruction of two adjacent vertebral bodies and opposing end plates; destruction of intervening disc; vertebral body edema; and occurrence of prevertebral, paravertebral, and epidural abscesses [Figures 1-3].[10,11,12,13,15] MRI plays an important role in the diagnosis of spinal TB with a high specificity and sensitivity.[20,22,29]
Figure 1.
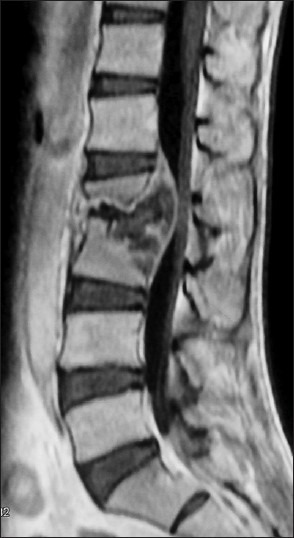
Contrast-enhanced T1-weighted sagittal magnetic resonance image showing destruction of L2 and L3 vertebral bodies with intraosseous and epidural abscess resulting in spinal canal stenosis
Figure 3.
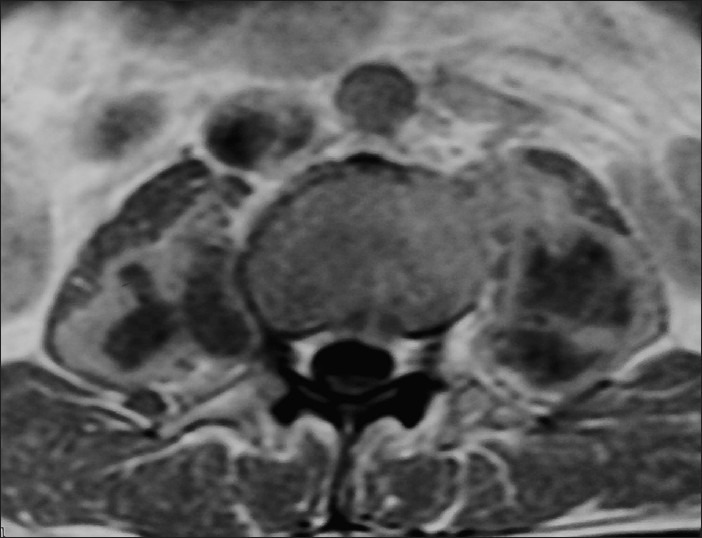
Contrast-enhanced T1-weighted axial MR image showing bilateral psoas abscesses
Figure 2.
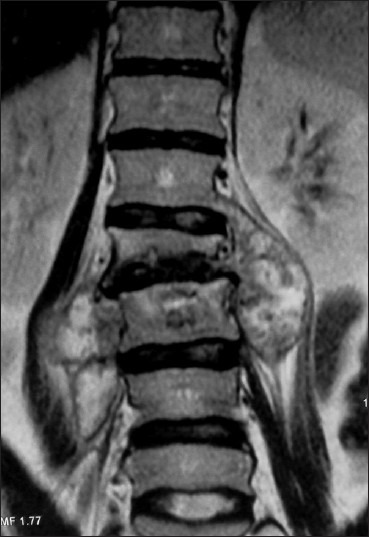
T2-weighted coronal MR image showing collapse of L1 vertebral body with irregularity of superior end plate of L2 along with bilateral psoas abscesses
In contrast to most imaging methods, MRI has the advantages of improved contrast resolution for bone and soft tissues along with versatility of direct imaging in multiple planes. With the aid of intravenous administration of magnetic resonance contrast agents, MRI was highly accurate in distinguishing granulation tissue from cold abscess.[30,31,32] MRI can reveal more extensive involvement than the plain films.[25,33,34] MRI provided more exact anatomic localization of vertebral and paravertebral abscesses in multiple planes not previously available with more conventional diagnostic methods in the patients with suspected TB spondylitis.[14,26,35,36] MRI clearly demonstrated the extent of soft tissue disease and its effect on the theca, cord, and foramen in cases with doubtful CT findings.[8,33] Both CT and MRI are extremely helpful for diagnosis, and tissue aspirate is a good confirmatory method.[20,31,37,38]
Administration of gadolinium with diethylenetri-aminepentaacetic acid (Gd-DTPA) is useful to assess the extent of soft tissue mass and to differentiate postoperative spondylitis from a normal postoperative course, by showing disc enhancement. Disc enhancement occurs infrequently in the normal postoperative course. If it is associated with adjacent vertebral bone marrow changes, it should be considered as postoperative spondylitis. Differential diagnosis between pyogenic, tuberculous, fungal, and postoperative spondylitis is difficult; although the pattern of enhancement in TB spondylitis was different from the other cases of spondylitis. MRI imaging is a very useful technique for differentiation of TB spondylitis from pyogenic spondylitis.[22,29,39]
Kyphosis and cord compressions were the most common complications.[40] The neurological involvement is relatively benign if urgent decompression is performed at the onset of the disease.[14,41] Late onset paraplegia is a neurological complication that develops after a variable period in a patient with healed TB of spine.[42] MRI is extremely useful in diagnosing the difficult and rare sites of disease like the craniovertebral junction. It detects the marrow changes, exudative and granulation types, extra- and intradural disease, and radiological response to treatment in the early follow-up period around 6-8 weeks.[43]
Increase in the prevertebral soft tissue shadow on radiograph is a useful guide to resort to CT scan/MRI to diagnose TB of cervical spine. The anterior convexity and forward displacement of tracheal shadow of more than 8 mm from the vertebral bodies in a lateral view of plain X-ray and widening of superior mediastinum in an anteroposterior X-ray are useful indicators of tuberculous involvement at cervicodorsal region.[44]
Sacroiliac joint TB is rare; its coexistence with vertebral TB is even rarer, with only a few such patients reported in the literature. MRI is the most sensitive and specific imaging modality for diagnosing sacroiliitis at its early stage. Sacroiliac joint TB can reach advanced stages with extensive joint destruction and periarticular abscesses if diagnosis and treatment are delayed. The addition of a coronal STIR T2-weighted sequence to the routine MRI evaluation of patients studied for lumbar disc disease may be useful for recognizing sacroiliac joint pathology at an earlier stage.[45]
Treatment
Combined surgical and medical treatment gave excellent results.[14,16,35,46] Surgical treatment consisting of extensive posterior decompression/instrumented fusion and three-level posterior vertebral column resection, followed by anterior debridement/fusion with cage reconstruction. A patient with progressive Pott's paraplegia and severe kyphotic deformity, for whom medical treatment failed, posterior vertebral column resection, multiple level posterior decompression, and instrumented fusion, followed by an anterior interbody fusion with cage was used to decompress the spinal cord, restore sagittal alignment, and debride the infection.[14,26,35,36,47]
Currently, treatment of spinal tubercular infections requires a multidisciplinary team that includes infectious diseases experts, neuroradiologists, and spine surgeons. The key to successful management is early detection and timely and judicious surgical intervention, the decision of which needs to be taken in view of clinicoradiological compression of the spinal cord and nerve roots, age of the patient and responsiveness of antitubercular therapy (ATT).[24,29,48,49]
HIV and Tuberculosis
Recently the coexistence of human immunodeficiency virus infections (HIV) and TB has been increased globally.[23] Seventy-one percent of South African TB patients are estimated to be HIV positive.[50] Pulmonary complications of the HIV infection study demonstrated that respiratory symptoms are a common complaint among the HIV-infected individual and increasingly frequent as CD4 counts declined to less than 200 cells/mm3.[3] HIV infection increases the risk of developing active TB by 15-30 times.[51] According to National AIDS Control Organization (NACO), TB is the commonest opportunistic infection, both pulmonary and extrapulmonary (62.2%) in India, which can explain its high incidence in HIV-infected individuals in India as compared to western countries.[51] In HIV positive patients, the incidence of skeletal TB increases to 60%.[52,53,54] It has been reported that HIV positive patients are 12-20 times more susceptible to spinal TB as compared with HIV negative patients.[52,53,54] Destruction of vertebrae with resultant kyphosis is less in HIV positive patients than in HIV negative patients; however the site, pattern, and volume of abscess formation are same in both groups. There is greater epidural pus formation in the HIV positive group. In HIV patients the less vertebral body destruction is thought to be due to disruption of type 4 hypersensitivity reaction causing granulomatous inflammation.[52]
Differential Diagnosis
Common differential diagnosis includes pyogenic spondylitis, brucellar spondylitis, osteoporotic, metastasis, multiple myeloma, and lymphoma. Brucellar spondylitis is commonly found in middle-age group. Lumbar spine is frequently involved followed by thoracic and cervical spine. Disc involvement and small paraspinal soft tissue component can be seen; however gibbus formation is not found in cases of brucellar spondylitis.
Pyogenic spondylitis can be found at any age; usually lumbar and cervical spines are affected. Destruction of vertebral bodies, intervertebral disc, markedly enhancing lesion, and epidural abscesses can be seen.[55,56] In pyogenic spondylitis, there is sparing of posterior elements and usually no gibbus deformity seen. In osteoporosis, thoracic vertebrae are frequently involved with sparing of the pedicles. Apart from destruction of multiple vertebral bodies, reduced bone density is usually seen in osteoporotic vertebrae.
In metastatic disease, thoracic region is most commonly involved. Posterior wall of the vertebral body (60%), pedicles and lamina (50%) are involved in metastatic disease; however, intervertebral disc heights are preserved.[21] Intervertebral discs may be affected in lymphoma and multiple myeloma. In elderly patients with vertebral collapse, metastatic disease of the spine should always be considered.
Advances in Technology
Recently, diffusion-weighted MRI (DW-MRI) and apparent diffusion coefficient (ADC) values are used in patients of spinal TB and is useful in differentiating tuberculous vertebral body involvement from metastatic lesions. DW-MRI provides information about the composition of tissues, physical properties, and the microstructure of the tissues.[57,58] ADC values are a measure of the diffusion ability of molecules which provides the composition of the given tissue.[57,58] A high ADC value means increased diffusion of molecules, that is, no restriction, suggesting less compact tissue microstructure. However in TB, an overlap of ADC values can be seen with those of metastatic disease. Hence, DW-MRI and ADC values should always be interpreted in association with clinical history and conventional MRI findings.[57,58]
Conclusion
MRI is the best diagnostic modality for spinal TB and is more sensitive than other modalities. It provides the diagnosis earlier than conventional methods, offering the benefits of earlier detection and treatment. MRI allows for rapid determination of the mechanism for neurologic compression and can distinguish between bone and soft tissue lesion. MRI with contrast is helpful in differentiating from noninfectious causes and delineating the extent of disease. Serial MRI can be used to assess the response to treatment and regression of the disease. In recent years, DW-MRI and apparent diffusion coefficient values in combination with MRI are used to some extent in the diagnosis of spinal TB.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Chauhan A, Gupta BB. Spinal tuberculosis. Indian Acad Clin Med. 2007;8:110–4. [Google Scholar]
- 2.World Health Organisation. The global tuberculosis control. November. 2010 [Google Scholar]
- 3.Padyana M, Bhat RV, Dinesha M, Nawaz A. HIV-Tuberculosis: A Study of Chest X-Ray Patterns in Relation to CD4 Count. N Am J Med Sci. 2012;4:221–5. doi: 10.4103/1947-2714.95904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore SL, Rafi M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39:329–42. doi: 10.1016/s0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Sawhney S, Berry M. Computed tomography of vertebral tuberculosis: Pattern of bone destruction. Clin Radiol. 1993;47:196–9. doi: 10.1016/s0009-9260(05)81162-6. [DOI] [PubMed] [Google Scholar]
- 6.Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg Am. 1996;78:288–98. doi: 10.2106/00004623-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Dass B, Puet TA, Watanakunakorn C. Tuberculosis of the spine (Pott's disease) presenting as ‘compression fractures’. Spinal Cord. 2002;40:604–8. doi: 10.1038/sj.sc.3101365. [DOI] [PubMed] [Google Scholar]
- 8.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. AJR Am J Roentgenol. 2002;179:979–83. doi: 10.2214/ajr.179.4.1790979. [DOI] [PubMed] [Google Scholar]
- 9.Kaila R, Malhi AM, Mahmood B, Saifuddin A. The incidence of multiple level noncontinguous vertebral tuberculosis detected using whole spine MRI. J Spinal Disord Tech. 2007;20:78–81. doi: 10.1097/01.bsd.0000211250.82823.0f. [DOI] [PubMed] [Google Scholar]
- 10.Liu GC, Chou MS, Tsai TC, Lin SY, Shen YS. MR evaluation of tuberculous spondylitis. Acta Radiol. 1993;34:554–8. [PubMed] [Google Scholar]
- 11.Narlawar RS, Shah JR, Pimple MK, Patkar DP, Patankar T, Castillo M. Isolated tuberculosis of posterior elements of spine: Magnetic resonance imaging findings in 33 patients. Spine (Phila Pa 1976) 2002;27:275–81. doi: 10.1097/00007632-200202010-00015. [DOI] [PubMed] [Google Scholar]
- 12.Yusof MI, Hassan E, Rahmat N, Yunus R. Spinal tuberculosis: The association between pedicle involvement and anterior column damage and kyphotic deformity. Spine (Phila Pa 1976) 2009;34:713–7. doi: 10.1097/BRS.0b013e31819b2159. [DOI] [PubMed] [Google Scholar]
- 13.Zaidi H, Akram MH, Wala MS. Frequency and magnetic resonance imaging patterns of tuberculous spondylitis lesions in adults. J Coll Physicians Surg Pak. 2010;20:303–6. [PubMed] [Google Scholar]
- 14.Mohammadreza E, Fariborz S, Gholamreza B. Pott's Disease: A review of 58 cases. Med J Islamic Republic Iran. 2010;23:200–6. [Google Scholar]
- 15.Maulin MS, Subir NJ, Tuli SM. Musculoskeletal tuberculosis in children Surgery in Africa. Monthly review. 2011 [Google Scholar]
- 16.Omari B, Robertson JM, Nelson RJ, Chiu LC. Pott's disease. A resurgent challenge to the thoracic surgeon. Chest. 1989;95:145–150. doi: 10.1378/chest.95.1.145. [DOI] [PubMed] [Google Scholar]
- 17.Boachie-Adjei O, Squillante RG. Tuberculosis of the spine. Orthop Clin North Am. 1996;27:95–103. [PubMed] [Google Scholar]
- 18.Schirmer P, Renault CA, Holodniy M. Is spinal tuberculosis contagious? Int J Infect Dis. 2010;14:e659–66. doi: 10.1016/j.ijid.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.McLain RF, Isada C. Spinal tuberculosis deserves a place on the radar screen. Cleve Clin J Med. 2004;71:543–9. doi: 10.3949/ccjm.71.7.537. [DOI] [PubMed] [Google Scholar]
- 20.Gautam MP, Karki P, Rijal S, Singh R. Pott's spine and paraplegia. JNMA J Nepal Med Assoc. 2005;44:106–15. [PubMed] [Google Scholar]
- 21.Gard RK, Somvanshi DS. Spinal tuberculosis: A review. J Spinal Cord Med. 2011;34:440–54. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–13. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 23.Jain AK, Dhammi IK. Tuberculosis of the spine: A review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318065b7c3. [DOI] [PubMed] [Google Scholar]
- 24.Nussbaum ES, Rockswold GL, Bergman TA, Erickson DL, Seljeskog EL. Spinal tuberculosis: A diagnostic and management challenge. J Neurosurg. 1995;83:243–7. doi: 10.3171/jns.1995.83.2.0243. [DOI] [PubMed] [Google Scholar]
- 25.Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153:399–405. doi: 10.2214/ajr.153.2.399. [DOI] [PubMed] [Google Scholar]
- 26.Bell GR, Stearns KL, Bonutti PM, Boumphrey FR. MRI diagnosis of tuberculous vertebral osteomyelitis. Spine (Phila Pa 1976) 1990;15:462–5. doi: 10.1097/00007632-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 27.R. Cranio-vertebral junction tuberculosis. Indian J Neurosurg. 2012;1:61–5. [Google Scholar]
- 28.Alli OA, Ogbolu OD, Alaka OO. Direct molecular detection of Mycobacterium tuberculosis complex from clinical samples-An adjunct to cultural method of laboratory diagnosis of tuberculosis. N Am J Med Sci. 2011;3:281–8. doi: 10.4297/najms.2011.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004;182:1405–10. doi: 10.2214/ajr.182.6.1821405. [DOI] [PubMed] [Google Scholar]
- 30.Kim NH, Lee HM, Suh JS. Magnetic resonance imaging for the diagnosis of tuberculous spondylitis. Spine (Phila Pa 1976) 1994;19:2451–5. doi: 10.1097/00007632-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Kotze DL, Erasmus J. MRI findings in proven Mycobacterium tuberculosis (TB) spondylitis. SA J Radiol. 2006;10:6–12. [Google Scholar]
- 32.Maron R, Levine D, Dobbs TE, Geisler WM. Two cases of Pott disease associated with bilateral psoas abscesses: Case report. Spine (Phila Pa 1976) 2006;31:E561–4. doi: 10.1097/01.brs.0000225998.99872.7f. [DOI] [PubMed] [Google Scholar]
- 33.Akman S, Sirvanci M, Talu U, Gogus A, Hamzaoglu A. Magnetic resonance imaging of tuberculous spondylitis. Orthopedics. 2003;26:69–73. doi: 10.3928/0147-7447-20030101-17. [DOI] [PubMed] [Google Scholar]
- 34.Teo EL, Peh WC. Imaging of tuberculosis of the spine. Singapore Med J. 2004;45:439–44. [PubMed] [Google Scholar]
- 35.Vidyasagar C, Murthy HK. Spinal tuberculosis with neurological deficits. Natl Med J India. 1996;9:25–7. [PubMed] [Google Scholar]
- 36.Parvin R, Haque MA, Islam MN, Shaha CK, Uddin SN, Sarkar S, et al. Pott's disease in a young child. Mymensingh Med J. 2008;17:206–9. [PubMed] [Google Scholar]
- 37.Alothman A, Memish ZA, Awada A, Al-Mahmood S, Al-Sadoon S, Rahman MM, et al. Tuberculous spondylitis: Analysis of 69 cases from Saudi Arabia. Spine (Phila Pa 1976) 2001;26:E565–70. doi: 10.1097/00007632-200112150-00020. [DOI] [PubMed] [Google Scholar]
- 38.Ousehal A, Gharbi A, Zamiati W, Saidi A, Kadiri R. Imaging findings in 122 cases of Pott's disease. Neurochirurgie. 2002;48:409–18. [PubMed] [Google Scholar]
- 39.Harada Y, Tokuda O, Matsunaga N. Magnetic resonance imaging characteristics of tuberculous spondylitis vs. pyogenic spondylitis. Clin Imaging. 2008;32:303–9. doi: 10.1016/j.clinimag.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol. 2002;32:798–805. doi: 10.1007/s00247-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 41.Turgut M. Spinal tuberculosis (Pott's disease): Its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurg Rev. 2001;24:8–13. doi: 10.1007/pl00011973. [DOI] [PubMed] [Google Scholar]
- 42.Rajeswari R, Ranjani R, Santha T, Sriram K, Prabhakar R. Late onset paraplegia: A sequelae to Pott's disease. A report on imaging, prevention and management. Int J Tuberc Lung Dis. 1997;1:468–73. [PubMed] [Google Scholar]
- 43.Kumar R. Spinal tuberculosis: With reference to the children of northern India. Childs Nerv Syst. 2005;21:19–26. doi: 10.1007/s00381-004-1029-9. [DOI] [PubMed] [Google Scholar]
- 44.Jain AK, Kumar S, Tuli SM. Tuberculosis of spine (C1 to D4) Spinal Cord. 1999;37:362–9. doi: 10.1038/sj.sc.3100833. [DOI] [PubMed] [Google Scholar]
- 45.Gelal F, Sabah D, Doğan R, Avci A. Multifocal skeletal tuberculosis involving the lumbar spine and a sacroiliac joint: MR imaging findings. Diagn Interv Radiol. 2006;12:139–41. [PubMed] [Google Scholar]
- 46.Pertuiset E, Beaudreuil J, Liote F, Horusitzky A, Kemiche F, Richette P, et al. Spinal tuberculosis in adults. A study of 103 cases in a developed country 1980-1994. Medicine (Baltimore) 1999;78:309–20. doi: 10.1097/00005792-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Pappou IP, Papadopoulos EC, Swanson AN, Mermer MJ, Fantini GA, Urban MK, et al. Pott's disease in the thoracolumbar spine with marked kyphosis and progressive paraplegia necessitating posterior vertebral column resection and anterior reconstruction with a cage. Spine (Phila Pa 1976) 2006;31:E123–7. doi: 10.1097/01.brs.0000199900.56446.ee. [DOI] [PubMed] [Google Scholar]
- 48.Kumar R, Srivastava AK, Tiwari RK. Surgical management of Pott's disease of the spine in pediatric patients: A single surgeon's experience of 8 years in a tertiary care center. J Pediatr Neurosci. 2011;6(Suppl 1):S101–8. doi: 10.4103/1817-1745.85726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain AK, Srivastava A, Saini NS, Dhammi IK, Sreenivasan R, Kumar S. Efficacy of extended DOTS category I chemotherapy in spinal tuberculosis based on MRI-based healed status. Indian J Orthop. 2012;46:633–9. doi: 10.4103/0019-5413.104191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Global tuberculosis control: A short update to the 2009 report. [Accessed June 15 2013]. at http://www.who.int/tb/publications/global_report/2009/update/en .
- 51.Sonkar SK, Gupta A, Atam V, Chaudhary SC, Tripathi AK, Sonkar GK. Clinical profile of neurological manifestation in Human Immunodeficiency Virus-positive patients. N Am J Med Sci. 2012;4:596–9. doi: 10.4103/1947-2714.103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anley CM, Brandt AD, Dunn R. Magnetic resonance imaging findings in spinal tuberculosis: Comparison of HIV positive and negative patients. Indian J Orthop. 2012;46:186–90. doi: 10.4103/0019-5413.93688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn R. The medical management of spinal tuberculosis. SAOJAutumn. 2010;9:37–41. [Google Scholar]
- 54.Moon MS. Development in the management of tuberculosis of the spine. Curr Orthop. 2006;20:132–40. [Google Scholar]
- 55.Yilmaz MH, Mete B, Kantarci F, Ozaras R, Ozer H, Mert A, et al. Tuberculous, brucellar and pyogenic spondylitis: Comparison of magnetic resonance imaging findings and assessment of its value. South Med J. 2007;100:613–4. doi: 10.1097/SMJ.0b013e3180600eaa. [DOI] [PubMed] [Google Scholar]
- 56.Chang MC, Wu HT, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine (Phila Pa 1976) 2006;31:782–8. doi: 10.1097/01.brs.0000206385.11684.d5. [DOI] [PubMed] [Google Scholar]
- 57.Palle L, Reddy B, Reddy KJ. Role of magnetic resonance diffusion imaging and apparent diffusion coefficient values in the evaluation of spinal tuberculosis in Indian patients. Indian J Radiol Imaging. 2010;20:279–83. doi: 10.4103/0971-3026.73544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basser PJ. Diffusion and diffusion tensor imaging. In: Atlas SW, editor. Magnetic Resonance Imaging of Brain and Spine. 3rd ed. Philadelphia: Lippincot Eilliams and Wilkins; 2002. pp. 197–212. [Google Scholar]


