Abstract
Context:
Radiation therapy is the prime treatment modality against various cancers. However, its use is limited due to the effects of radiation on normal tissues.
Aims:
In view of this, present study was carried out to evaluate the radioprotective potential of Rutin (RUT) and Quercetin (QRT) in Swiss Albino mice exposed to the whole body gamma radiation. To gain insight into the mechanism of action, RUT and QRT were tested for its antioxidant levels in mice.
Settings and Designs:
Optimum protective dose of RUT and QRT against radiation induced animal mortality was selected by administration of various doses of the RUT and QRT before 10 Gy gamma irradiation.
Materials and Methods:
Swiss Albino mice were used for the assessment of radiation induced sickness along with the survival analysis and anti-oxidative properties of RUT and QRT.
Statistical Analysis Used:
Survival studies were determined using the Kaplan-Meier survival curves.
Results:
The maximum survival was observed with 10 mg/kg. b. wt. and 20 mg/kg. b. wt. of RUT and QRT respectively, this dose was considered as an optimal dose for radioprotection. Treatment of mice with RUT and QRT before irradiation delayed the onset of mortality as compared with the untreated irradiated controls. The oral administration of RUT and QRT resulted in an increase in the radiation tolerance and the dose reduction factor was found to be 1.15 and 1.11 respectively. RUT and QRT pre-treatment significantly (P < 0.01) elevated levels of reduced glutathione, glutathione-S-transferase, catalase, Superoxide dismutase, and a decreased lipid peroxidation in mouse liver homogenate at 24 h after exposure to 4.5 Gy.
Conclusions:
Present findings demonstrate the potential of RUT and QRT in mitigating radiation-induced mortality, which may be attributed to the elevation in the antioxidant status, anti-lipid peroxidative potential.
Keywords: Dose reduction factor, Quercetin, radioprotection, Rutin
INTRODUCTION
Ionizing radiation generates free radicals that damage Deoxyribonucleic acid (DNA) and kill cells. Most of the genetic damage is mediated by reactive oxygen species (ROS) and reactive nitrogen species.[1] Ionizing radiation, employed in radiotherapy for various cancers, this result not only in killing of cancer cells, but also damage the normal cells. Though, living cells have an endogenous antioxidant defense mechanism that includes glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), and GSH peroxidase, in the event of an increased production of ROS, the host antioxidant defense mechanisms can become overwhelmed, resulting in the oxidative damage of cellular components.[2] In this regard, numerous chemicals have been tried, which possess dependable defenses against radiation-induced damage in animals and cultured cells studies. However, their clinical efficacy is limited by drug toxicity with repeated administration. Search for the effective and non-toxic radioprotective agents that are able to protect human beings from the ionizing radiation is of considerable interest for radiation medicine, space flights, and nuclear emergencies.[3]
The uses of medicinal plants in traditional medicine are extensive and they serve for the development of the novel pharmacological agents.[4,5,6] A number of dietary antioxidants and medicinal plants have been reported for their hepatoprotective, neuroprotective, anti-inflammatory and also antioxidant or radical scavenging properties.[3,7,8] It may be therefore logical to expect that extracts and bioactive compounds with antioxidant potential derived from plants may render radioprotection to normal tissues as evidenced from earlier reports.[7,9,10,11,12,13,14,15]
Flavonoids are polyphenolic compounds containing an unique C6-C3-C6 structure (diphenyl propane structure). More than 4,000 varieties of flavonoids have been found in herbs, vegetables, fruits, and beverages.[16] Recently, flavonoids have attracted attention because of their beneficial biological activities to human health. Rutin's (RUT's) anti-inflammatory potential has been demonstrated in a number of animal studies.[16,17] In experimentally induced colitis, both pre- and post-induction treatment with the RUT conferred significant preventive and healing effects.[18] Quercetin (QRT) also has demonstrated significant anti-inflammatory activity because of direct inhibition of several initial processes of inflammation. For example, it inhibits both the production and release of histamine and other allergic/inflammatory mediators.[16] In addition, it exerts potent antioxidant activity and vitamin-C sparing action.[19,20] On the basis of this data, the present study was undertaken to evaluate the radioprotective potential of selected bioflavonoids-RUT and QRT in Swiss albino mice as an in vivo model system.
MATERIALS AND METHODS
Animals
Four to six weeks old inbred mice of Swiss Albino strain of either sex weighing 25-30 g were selected, and kept in well ventilated polypropylene cages under standard conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and light (10 and 14 h of light and dark, respectively). Animals were allowed food and water ad libitum. The animal experiments were carried with the prior approval from the institutional animal ethics committee. Animal care and handling was carried out according to the guidelines issued by the World Health Organization, Geneva, Switzerland and the Indian National Science Academy, New Delhi, India.
Drug preparation and mode of administration
RUT and QRT was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. RUT powder was suspended in water using 0.5% w/v carboxy methyl cellulose (CMC) and was given once daily (5 ml/kg body weight), various doses of RUT and QRT 10-100 mg/kg body weight orally once a day for 5 consecutive days. Radiation exposure was performed 1 h after the last dose of RUT and QRT administration.
Radiation exposure
Unanesthetized mice were restrained in a specially designed well-ventilated acrylic box and exposed to whole-body radiation from 60Co gamma tele-therapy facility (Theratron Atomic Energy Agency, Canada) at the Shirdi Sai Baba Cancer Hospital, Manipal, at a dose rate of 1.33 Gy/min and source to surface distance of 61 cm.
Selection of optimum dose of RUT and QRT against radiation
To study the optimum dose of RUT and QRT for the radiation protection, animals were divided into following groups (n = 12, per group). The dose of 10 Gy was selected to calculate the LD50(30) as it kills 50% of the animals within 30 days both by gastrointestinal and bone marrow (BM) syndrome. The animals were divided into the following groups:
Radiation alone group
One hour after oral administration of 0.1 ml/kg. b. wt. of 0.5% w/v CMC on the 5th day, the animals of this group were whole body exposed to 10 Gy of gamma radiation.
RUT and QRT + radiation group
The animals of this group were administered orally with 5, 10, 15, 20 mg/kg. b. wt. of RUT and QRT consecutively for 5 days and after the last dose, i. e., on the 5th day, animals were exposed to 10 Gy of gamma radiation.
The animals were monitored daily for the development of symptoms of radiation sickness, such as a reduction in the food and water intake, irritability, watering of eyes, epilation, weight loss, emaciation, lethargy, diarrhea, facial edema etc., morbidity, behavioral toxicity, and mortality were recorded. The actuarial survival curves were drawn by Kaplan-Meier method.[21] The optimum dose thus obtained was used for further investigation.
Estimation of radiation dose reduction factor (DRF)
To determine the protective role of RUT and QRT against lethal gamma irradiation, the DRF was calculated. For this, animals were divided into following groups (n = 12, per group).
Radiation alone group
Animals of this group were orally administered. 1 ml/kg. b. wt. of 0.5% w/v CMC orally for 5 consecutive days. One hour after of the last administration animals were exposed to 7, 8, 9, 10 or 11 Gy of gamma radiation respectively.
RUT and QRT + radiation group
Animals of this group were treated with the optimal dose 10 mg/kg. b. wt. and 20 mg/kg. b. wt. of RUT and QRT once daily for 5 consecutive days and after the last dose, i.e., on the 5th day, animals were exposed to 7, 8, 9, 10 or 11 Gy of gamma radiation respectively.
The animals of both groups were observed daily for up to 30 days post-irradiation for signs of radiation sickness and mortality. The DRF was calculated according the method of Miller and Tainter[22] as follows:
![]()
Biochemical estimations
To understand the mechanism of radioprotection, biochemical estimations were carried out. Animals were divided into groups of six animals each as:
Untreated control group
The animals of this group were orally administered with 0.1 ml/kg. b. wt. of 0.5% w/v CMC as a single dose.
RUT and QRT treated group
The animals of this group were administered optimum dose of RUT (10 mg/kg. b. wt.) and QRT (20 mg/kg. b. wt.) orally for 5 consecutive days.
Radiation alone group
These animals were administered with 0.1 ml/kg. b. wt. of 0.5% w/v CMC orally once daily for 5 consecutive days. One hour after the last administration on the 5th day animals were exposed to 4.5 Gy gamma radiations.
RUT and QRT + radiation group
Here, the animals were administered with optimum dose of 10 mg/kg. b. wt. and 20 mg/kg. b. wt. of RUT and QRT once daily for 5 consecutive days and after the last dose, i.e., on the 5th day, animals were exposed to 4.5 Gy of gamma radiation.
All the animals were euthanized at 24 h post-irradiation time intervals and their livers were perfused with ice-cold saline and 10% homogenate was prepared with ice-cold PBS pH 8.0 using a homogenizer (Yamato LSC LH-21, Japan).
Estimation of GSH in liver
GSH contents were measured as total non-protein sulfhydryl group using the method of Moron et al.[23] Briefly, proteins were precipitated with 25% trichloroacetic acid TCA, centrifuged, and the supernatant was collected, which was mixed with 0.2M sodium phosphate buffer pH 8.0, 0.06 mM 5,5-dithiobis-2-nitrobenzoic acid and incubated for 10 min at room temperature. The absorbance of the samples was read against the blank at 412 nm in a UV–vis. spectrophotometer (Shimadzu UV-260, Shimadzu Corp., Tokyo, Japan) and the GSH concentration was calculated from a standard curve.
Estimation of glutathione-S-transferase (GST) in liver
GST was determined according to the procedure of Habig et al.[24] The specific activity of GST is expressed as μmol of reduced 1-Chloro-2,4-Dinitrobenzene CDNB conjugate formed per minute per mg protein of the tissue. The absorbance was recorded against blank at 340 nm.
Estimation of CAT in liver
CAT activity was determined by catalytic reduction of hydrogen peroxide using a standard method.[25] Briefly, 30% hydrogen peroxide was added to the liver homogenate and was incubated at 37°C. The decomposition of hydrogen peroxide was monitored by recording the absorbance against blank at 240 nm.
Estimation of (SOD) activity in liver
SOD activity was assayed by the nitroblue tetrazolium (NBT) method.[26] NBT will be reduced to blue form by O2−, which has a strong absorbance at 560 nm. The presence of SOD inhibits this reaction. The assay mixture consisted of 0.05 M sodium carbonate buffer (pH 10.2) containing 3 m Mxanthine, 0.75 mM nitroblue tetrazolium (NBT), 3 mM Ethylene diamine tetra-acetic acid (EDTA), 1.5 mg/mL BSA and 50 μL of liver homogenate. The reaction was initiated by the addition of 50 μL of xanthine oxidase (0.1 mg/mL) and incubated for 30 min at room temperature. The reaction was stopped by the addition of 6 mM copper (II) chloride and centrifuged at 1500 rpm for 10 min. The absorbance of blue form in the supernatants was measured at 560 nm.
Estimation of lipid peroxidation (LPO) in liver
LPO was measured using the method of Buege and Aust.[27] Briefly, the tissue homogenate was mixed with TCA – thiobarbutyric acid TBA – HCl and the resultant mixture was heated for 15 min in a boiling water bath. After centrifugation, the absorbance was recorded at 535 nm using a UV – vis. spectrophotometer. LPO was expressed as nmol malondialdehyde mg−1 total protein.
Statistical analysis
All data are expressed as the mean ± standard error of mean (SEM). Survival studies were determined using Kaplan-Meier survival curves. For survival studies, statistical significances (P < 0.05) of the mean values between the treatment groups were analyzed using the Mantel – Cox Log Rank test although, the Student's t-test was used for all biochemical estimations.
RESULTS
Assessment of optimum protective dose of RUT and QRT against radiation
The administration of various doses of RUT (5, 10, 15, 20 mg/kg. b. wt.) for 5 consecutive days before 10 Gy gamma irradiation resulted in 60, 70, 50 and 50% animal survival respectively [Figure 1]. Survival was significantly (P < 0.05) increased in all the doses of RUT-pre-treated groups compared to the vehicle group. Since maximum survival was observed with 10 mg/kg. b. wt. of RUT, this dose was considered as an optimal dose for radioprotection and further experiments were performed using this dose.
Figure 1.
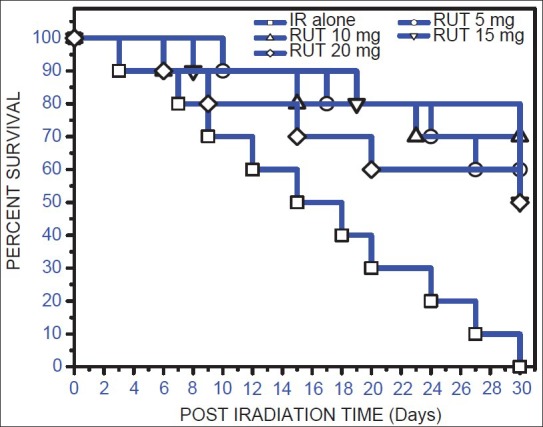
Kaplan-Meier's estimate of survival for mice treated with different doses of Rutin (5-20 mg/kg. b. wt.) administered orally for 5 consecutive days. One hour after the last administration, on the 5th day, animals were exposed to 10 Gy of gamma radiation. Values are mean ± standard error of mean
Animals exposed to 10 Gy of gamma radiation presented signs of radiation sickness such as a reduction in food and water intake, weight loss, diarrhea, lethargy, ruffled hair within 2-4 days of post irradiation [Figure 2]. The animals pre-treated with 5, 10, 15, 20 mg/kg. b. wt. of QRT showed 50, 50, 60, 70% survival respectively against 10 Gy gamma radiation. The optimum dose of QRT (20 mg/kg. b. wt.) administered for 5 consecutive days before exposure to 10 Gy gamma radiation rendered significant (P < 0.05) radiation protection as indicated by delayed onset of radiation sickness, its reduced severity and significantly increased animal survival in post irradiation days, this dose of QRT was used for further investigation.
Figure 2.
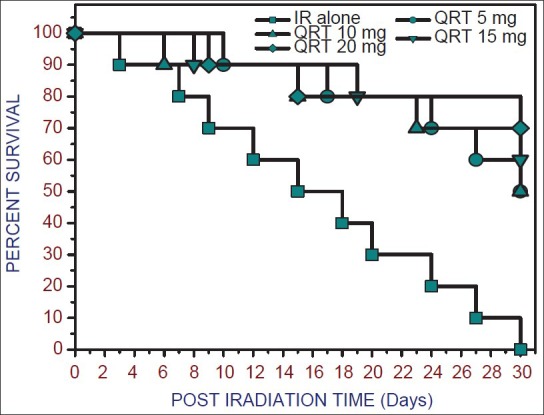
Kaplan-Meier survival curves of mice exposed to 10 Gy of gamma radiation following oral administration of Quercetin (5-20 mg/kg. b. wt.). Values are mean ± standard error of mean
Radiation DRF in mice treated with RUT and QRT
A dose-dependent increase in survival was observed in RUT treated and irradiated mice when compared to those treated with radiation alone [Figure 3]. The LD50/30 was found to be 9.1 Gy for radiation alone. Although, it was increased to 10.5 Gy after RUT treatment resulting in increased LD50/30 value by 1.4 Gy. The DRF of 1.15 was observed.
Figure 3.
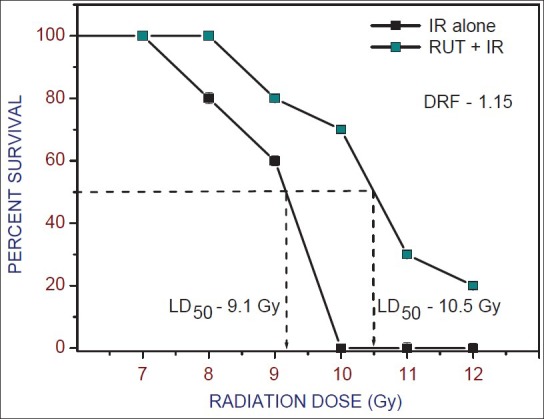
Radiation dose response curves for 30-day survival of mice after whole body gamma irradiation with or without Rutin (10 mg/kg b. wt.) before exposure to 7-12 Gy. The LD50(30) for radiation is 9.1 Gy and for Rutin + Irradiation group is 10.5 Gy (dose reduction factor: 1.15). Values are mean ± standard error of mean
QRT administered group also shown dose-dependent increase in survival of mice when compared with those treated with radiation alone [Figure 4]. The LD50/30 was found to be 9.3 Gy for radiation alone. Although, it was increased to 10.4 Gy after RUT treatment resulting in increased LD50/30 value by 1.1 Gy. The DRF of 1.11 was observed.
Figure 4.
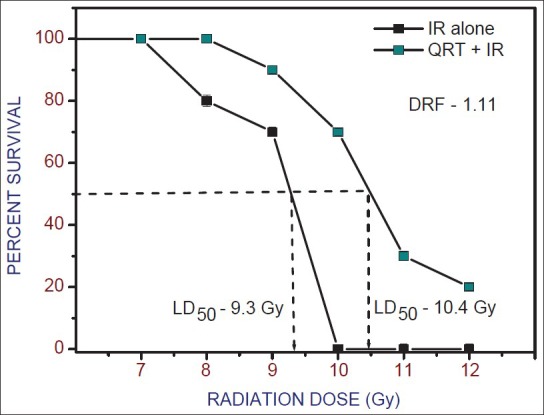
Radiation dose response curves for 30 day survival of mice after whole body gamma irradiation with or without Quercetin (20 mg/kg b. wt.) before exposure to 7-12 Gy. The LD50(30) for radiation is 9.3 Gy and for Quercetin + Irradiation group is 10.4 Gy (dose reduction factor: 1.11). Values are mean ± standard error of mean
Each group consisted of 10 mice, RUT and QRT was administered 5 consecutive days. One hour after the last administration, on the 5th day, animals were exposed to various doses of gamma radiation. Values are mean ± SEM from 10 mice per group.
DISCUSSION
Total body exposure to ionizing radiation suppresses hematopoiesis, which results in decreased production of blood cells.[28,29] The hematopoietic, or BM syndrome is induced in the range of 3-8 Gy and requires several weeks to express physical, visual symptoms, as radiation energy kills the immune progenitor cells.[30] Subsequently, many compounds have been tested for their ability to modify the effect of radiation on hematological parameters.[11,28,29,31,32] The polyphenol compounds are considered as potential radioprotectors because of their adaptogenic ability and abundance in the diet. Present study demonstrates the potential of RUT and QRT, a dietary polyphenol in ameliorating radiation induced toxicity under in vivo condition.
In the assessment of optimum protective dose of RUT and QRT against gamma radiation, mice exposed to 10 Gy gamma rays developed typical radiation symptoms such as a reduction in the food and water intake, irritability, watering of eyes, epilation, weight loss, emaciation, lethargy, diarrhea, facial edema etc., with an anticipated mortality. The survival of animals even after this sub-lethal dose may be attributed to the multi-system efforts of animals in combating infection, maintaining vital functions, and thereby the wholesome integrity of body during the period of repair at the cellular level. Administration of RUT (10 mg/kg. b. wt. for 5 consecutive days) and QRT (20 mg/kg. b. wt. for 5 consecutive days) prior to irradiation enhanced survival of mice with an optimum survival rate of 69.7% and 69.2% respectively, further increment in the RUT and QRT dose did not result in an increased survival. This observation is consistent with several other antioxidant radioprotectors in vivo such as 2-mercapto-propionylglycine, dimethyl sulphoxide DMSO, Orientin and Vicenin, and Zingerone.[13,33,34,35]
In animal studies, irradiating mice with and without administered agent at a range of radiation doses and then comparing the endpoint of interest typically determine DRF or dose modifying factor. The DRF clearly gives an indication of the quantitative capacity of the radioprotective agent to enhance the tolerance of tissues and its ability to palliate radiation-induced sickness and mortality. In the present investigation, mice exposed to radiation the LD50/30 dose was found to be 9.1 Gy, whereas the combination of RUT and Irradiation increased to 10.59 Gy giving a dose DRF value of 1.15. In QRT treated group QRT administered mice exposed to radiation has shown LD50/30 10.43 giving a dose DRF value 1.11. Further, the combination treatment (drug + irradiation) was also delayed the appearance of the radiation sickness such as weight loss, irritability, lethargy, ruffling of hair, emaciation, and epilation.[36] The DRF of RUT and QRT is 1.15 and 1.11 respectively, this clearly demonstrate the radioprotecive potential of RUT and QRT in Swiss mice.
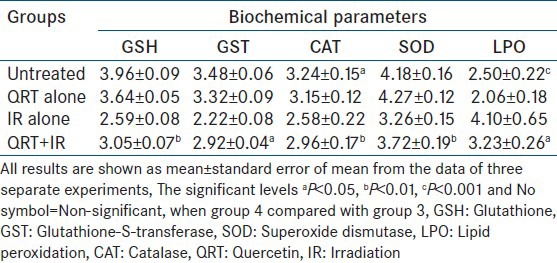
Irradiation of mice with different doses of gamma radiation resulted in a dose dependent decline in GSH, GST, glutathione peroxidase GPx, SOD, and CAT activities in the mice liver [Tables 1 and 2]. While the LPO, which results from a cascade of events induced by ionizing radiation increased. SOD dismutases the superoxide into hydrogen peroxide thus minimizing the deleterious effects. CAT is another intracellular antioxidant, which takes care of hydrogen peroxide by converting it into the water and minimizes the radiation toxicity by preventing the formation of OH. In this study, it is observed that decrease in the levels of antioxidants such as SOD, CAT, GST, and GSH in liver of irradiated mice; this decrease in the intracellular antioxidants was normalized to some extent with RUT and QRT [Tables 1 and 2]. This may be attributed to the scavenging of free radicals formed during irradiation resulting in decreased oxidative stress in animals.
Table 1.
Estimation of antioxidant and lipid peroxidation level in liver sample of swiss mice exposed to 4.5 Gy of gamma radiation with or without RUT given orally for 5 consecutive days
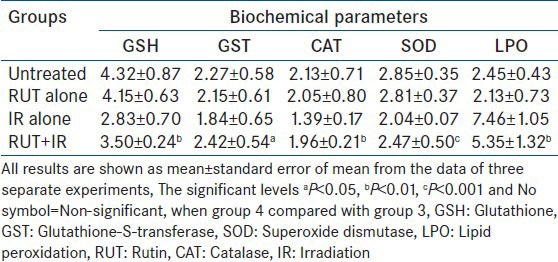
Table 2.
Estimation of antioxidant and lipid peroxidation level in liver sample of swiss mice exposed to 4.5 Gy of gamma radiation with or without QRT given orally for 5 consecutive days
Results obtained on gamma irradiated mice pre-treated for 5 consecutive days with RUT and QRT showed radioprotective potential and increase in the survivability of the animals. The observed effect may be attributed to the possible mechanisms such as normalization of intracellular antioxidant levels, anti-lipid peroxidative effect as well as free radical scavenging activity. Further, investigations are essential for the promotion of RUT and QRT as a radiation antagonistic agent for its detailed mechanistic study.
ACKNOWLEDGMENTS
We acknowledge the financial support by Board of Research in Nuclear Sciences (BRNS), Mumbai through Center for Application of Radioisotopes and Radiation Technology (CARRT), Mangalore University, Manglaore. We thank Prof. Satish Rao, Head, Radiolobiology Division, Manipal Lifes Sciences Center, Prof. Vidhya Sagar, Head, Radiotherapy, and Prof. J.G.R. Solomon, Head, Medical Physics, Shirdi Saibaba Cancer Hospital, Manipal, for the use of the irradiation facility and for radiation dosimetry respectively.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hall EJ. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. Acute effects of total-body irradiation. [Google Scholar]
- 2.Halliwell B, Gutteridge JM. Vol. 3. Free Radic Biol Med Oxford: Clarendon Press; 1989. Lipid peroxidation: A radical chain reaction; pp. 188–276. [Google Scholar]
- 3.Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 4.Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: Current status and new directions. Oncology. 2002;63(Suppl 2):2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 6.Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, et al. Radioprotection by plant products: Present status and future prospects. Phytother Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 7.Uma Devi P. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi) Indian J Exp Biol. 2001;39:185–90. [PubMed] [Google Scholar]
- 8.Devasagayam TP, Sainis KB. Immune system and antioxidants, especially those derived from Indian medicinal plants. Indian J Exp Biol. 2002;40:639–55. [PubMed] [Google Scholar]
- 9.Prasanna PG, Uma Devi P. Modification of WR-2721 radiation protection from gastrointestinal injury and death in mice by 2-mercaptopropionylglycine. Radiat Res. 1993;133:111–5. [PubMed] [Google Scholar]
- 10.Prabhakar KR, Veerapur VP, Parihar KV, Priyadarsini KI, Rao BS, Unnikrishnan MK. Evaluation and optimization of radioprotective activity of Coronopus didymus Linn. in gamma-irradiated mice. Int J Radiat Biol. 2006;82:525–36. doi: 10.1080/09553000600876686. [DOI] [PubMed] [Google Scholar]
- 11.Vijayalaxmi KK, Venu R. In vivo anticlastogenic effects of L-ascorbic acid in mice. Mutat Res. 1999;438:47–51. doi: 10.1016/s1383-5718(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 12.Rao BN, Rao BS. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis. 2010;25:577–87. doi: 10.1093/mutage/geq043. [DOI] [PubMed] [Google Scholar]
- 13.Rao BS, Rao N, Archana PR, Gayathri P, Shetty P. Antioxidant and radiation antagonistic effect of Saraca indica. J Environ Pathol Toxicol Oncol. 2010;29:69–79. doi: 10.1615/jenvironpatholtoxicoloncol.v29.i1.90. [DOI] [PubMed] [Google Scholar]
- 14.Rao BS, Shanbhoge R, Upadhya D, Jagetia GC, Adiga SK, Kumar P, et al. Antioxidant, anticlastogenic and radioprotective effect of coleus aromaticus on Chinese hamster fibroblast cells (V79) exposed to gamma radiation. Mutagenesis. 2006;21:237–42. doi: 10.1093/mutage/gel023. [DOI] [PubMed] [Google Scholar]
- 15.Rao BS, Upadhya D, Adiga SK. Protection of ionizing radiation-induced cytogenetic damage by hydroalcoholic extract of Cynodon dactylon in Chinese hamster lung fibroblast cells and human peripheral blood lymphocytes. J Environ Pathol Toxicol Oncol. 2008;27:101–12. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.30. [DOI] [PubMed] [Google Scholar]
- 16.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 17.Kostyuk VA, Potapovich AI. Antiradical and chelating effects in flavonoid protection against silica-induced cell injury. Arch Biochem Biophys. 1998;355:43–8. doi: 10.1006/abbi.1998.0708. [DOI] [PubMed] [Google Scholar]
- 18.Deschner EE, Ruperto JF, Wong GY, Newmark HL. The effect of dietary quercetin and rutin on AOM-induced acute colonic epithelial abnormalities in mice fed a high-fat diet. Nutr Cancer. 1993;20:199–204. doi: 10.1080/01635589309514287. [DOI] [PubMed] [Google Scholar]
- 19.Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4’- glucoside do not differ in humans. J Nutr. 2000;130:1200–3. doi: 10.1093/jn/130.5.1200. [DOI] [PubMed] [Google Scholar]
- 20.Park JB, Levine M. Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and Jurkat cells. J Nutr. 2000;130:1297–302. doi: 10.1093/jn/130.5.1297. [DOI] [PubMed] [Google Scholar]
- 21.Matthews D, Farewell V. Using and Understanding Medical Statistics. Basel: Karger; 1985. Kaplan-Meier or actuarial survival curves; pp. 67–78. [Google Scholar]
- 22.Miller NJ, Miller LC, Tainter ML. Estimation of the ED50 and its error by means of logarithmic-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261–4. [Google Scholar]
- 23.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–63. [PubMed] [Google Scholar]
- 27.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 28.Macková NO, Fedorocko P. Effect of liposomal muramyl tripeptide phosphatidylethanolamine and indomethacin on hematopoietic recovery in irradiated mice. Physiol Res. 2002;51:511–21. [PubMed] [Google Scholar]
- 29.Mouthon MA, Van der Meeren A, Gaugler MH, Visser TP, Squiban C, Gourmelon P, et al. Thrombopoietin promotes hematopoietic recovery and survival after high-dose whole body irradiation. Int J Radiat Oncol Biol Phys. 1999;43:867–75. doi: 10.1016/s0360-3016(98)00477-5. [DOI] [PubMed] [Google Scholar]
- 30.Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic Biol Med. 2003;34:689–95. doi: 10.1016/s0891-5849(02)01372-2. [DOI] [PubMed] [Google Scholar]
- 31.Nothdurft W, Kreja L, Selig C. Acceleration of hemopoietic recovery in dogs after extended-field partial-body irradiation by treatment with colony-stimulating factors: RhG-CSF and rhGM-CSF. Int J Radiat Oncol Biol Phys. 1997;37:1145–54. doi: 10.1016/s0360-3016(97)00112-0. [DOI] [PubMed] [Google Scholar]
- 32.Momm F, Bechtold C, Rudat V, Strnad V, Tsekos A, Fischer K, et al. Alteration of radiation-induced hematotoxicity by amifostine. Int J Radiat Oncol Biol Phys. 2001;51:947–51. doi: 10.1016/s0360-3016(01)01710-2. [DOI] [PubMed] [Google Scholar]
- 33.Nagata H, Sugahara T, Tanaka T. Radiation protection by 2-mercaptopropionylglycine in mice. J Radiat Res. 1972;13:163–6. doi: 10.1269/jrr.13.163. [DOI] [PubMed] [Google Scholar]
- 34.Littlefield LG, Joiner EE, Colyer SP, Sayer AM, Frome EL. Modulation of radiation-induced chromosome aberrations by DMSO, an OH radical scavenger. 1: Dose-response studies in human lymphocytes exposed to 220 kV X-rays. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:875–90. doi: 10.1080/09553008814551241. [DOI] [PubMed] [Google Scholar]
- 35.Vrinda B, Uma Devi P. Radiation protection of human lymphocyte chromosomes in vitro by orientin and vicenin. Mutat Res. 2001;498:39–46. doi: 10.1016/s1383-5718(01)00263-7. [DOI] [PubMed] [Google Scholar]
- 36.Uma Devi P. Normal tissue protection in cancer therapy: Progress and prospects. Acta Oncol. 1998;37:247–52. doi: 10.1080/028418698429531. [DOI] [PubMed] [Google Scholar]


