Abstract
Aims:
To evaluate the effects of melatonin premedication on anxiety and pain scores of patients, operating conditions, and intraocular pressure during cataract surgery under topical anesthesia.
Materials and Methods:
Sixty patients were randomly assigned to receive either sublingual melatonin 3 mg or placebo 60 min before surgery. Verbal anxiety scores and verbal pain scores, heart rate, systolic and diastolic blood pressure, intraocular pressure, and quality of operating conditions were recorded.
Results:
Melatonin significantly reduced the anxiety scores (median, interquartile range) from 5 and 5–3 to 3 and 2–4 after premedication and to 3 and 2–3 during surgery and to 0 and 0–1 postoperatively before discharge from the recovery room. There were significant differences between two groups in anxiety scores after premedication (95% CI 3–3.5; P = 0.023), intraoperatively (95% CI 2.5–3.5; P = 0.007), and postoperatively (95% CI 0.5–1; P = 0.007). The surgeon reported better quality of operating conditions in the melatonin group (P = 0.001). No significant difference in intraoperative and postoperative pain scores, intraocular pressure, heart rate, and systolic and diastolic blood pressure between two groups was recorded.
Conclusion:
Sublingual melatonin premedication for patients undergoing cataract surgery under topical anesthesia reduced the anxiety scores in patients and provided excellent operating conditions.
Keywords: Anxiety, cataract, intraocular pressure, melatonin, pain, topical anesthesia
Most cataract surgeries in recent years are performed by phacoemulsification under topical anesthesia. Topical anesthesia protects patients from the possibility of globe perforations, optic nerve injury, and risk of respiratory arrest.[1]
Topical anesthesia has additional advantages such as lack of interference with visual function, immediate visual improvement, absence of injection-related pain, and unlimited ocular motility without an increase in orbital volume.[2]
In a study by Fung et al. it was reported that although most patients had negligible preoperative anxiety, some patients (14%) were found to have high levels of anxiety and thus would like to take more sedation if they were to have surgery sometime in the future.[3] In such cases, systemic analgesia and sedation might be necessary to relieve patient distress and enhance the patient's satisfaction of surgery. The combined effects of premedication, the surgical draping, and simultaneous respiratory or cardiovascular diseases may increase morbidity in elderly patients.
Several drugs such as propofol, benzodiazepines, opioids, and dexmedetomidine have been used for sedation during this procedure.[4,5] Propofol may cause oversedation, disorientation, and respiratory depression; benzodiazepines may result in confusion; opioids are associated with increased risk of respiratory depression and oxygen desaturation; and dexmedetomidine is associated with serious complications such as hypotension, bradycardia, and cardiovascular depression.[4,5] Additionally, the reduction of cognitive functioning following administration of these drugs is a well-known and important aspect of hypnotics use which is considered as an undesirable outcome of such drugs during cataract surgery. Obviously, minimizing perioperative pain may require more sedation but at the same time the presence of unchanged vigilance during cataract surgery is of prime importance. Alternatively, each of these drugs has its own limitations, leading to impairment of patient's cooperation during surgery and thereby these medications work less than ideal agents for management of conscious sedation. Therefore, the potential clinical advantages of new drugs in this setting remain to be evaluated.
Melatonin or N-acetyl-methoxytryptamine, is mainly a neurohormone produced by the pineal gland.[6] In addition to the pineal gland as the principal location for production of melatonin, the eyes are also considered as another organ to produce this substance.[6] Melatonin, to great extent, has been investigated in the retina, where it can modulate visual physiology.[7]
The presence of melatonin receptors in nonpigmented ciliary epithelial cells suggests that it modifies physiological processes such as the production of aqueous humor and intraocular pressure (IOP).[8,9] Furthermore, the IOP is higher during the daytime than at night and it has been suggested that the melatonergic mechanisms in the eyes could be responsible for the diurnal rhythm in IOP.[9]
Although several studies have reported increased IOP following melatonin administration,[10] nevertheless, there are other reports emphasizing on a decrease in IOP.[8,9]
Other actions mediated by this hormone include its role in reducing blood pressure and decreasing catecholamine levels.[11] Moreover, melatonin is reported to be associated with analgesic effects in patients with major tissue injuries.[12]
Several studies have reported that melatonin given as preoperative premedication is associated with sedation and preoperative anxiolysis without cognitive dysfunction including the memory recall and driving performances.[13,14] Hence, these favorable effects may be valuable when melatonin is used as a premedicant for cataract surgery.
The aim of this study was to evaluate the effects of melatonin premedication on anxiety and pain scores of patients, operating conditions, and IOP during elective phacoemulsification cataract surgery under topical anesthesia.
Materials and Methods
The present study was a placebo-controlled, randomized, double-blind clinical trial in which the patients, investigators, anesthesiologist, and the surgeon were blinded to the given treatment. Patients were fully informed about the study protocol and provided written informed consent. The study was approved by the institutional ethics committee and performed during September 2009 to June 2010. Inclusion criteria were patients aged 25–80 years, scheduled for elective cataract surgery with intraocular lens implantation using phacoemulsification under topical anesthesia for the first time according to the American Society of Anesthesiologists (ASA) physical status classification[15] I–III (the ASA classification method is a system to assess the risk associated with anesthesia in patients undergoing surgery and is divided into six categories). Exclusion criteria were patients with sleep disorders, autoimmune disease, diabetes, depressive disorder or expected compliance problems (known psychiatric disease), epilepsy, leukemia, insufficient pupillary dilation, nystagmus, deafness, allergy to the study drugs, ongoing treatment with hypnotics or psychotropic drugs (including opioids) within a week before admission, daily analgesic treatment, ß blockers, Coumadin derivates, and those who could not tolerate Shioetz tonometer during IOP measurement. Using a computer-generated randomization schedule, patients were given either melatonin 3 mg tablet (Melatonin, Nature Made/Pharmavite LLC USA) (M group; n = 30) or a placebo tablet (P group; n = 30) sublingually 60 min before surgery. Patients were given the study drugs by a nurse who was unaware of the study. Other sedative or analgesic agents were not used. At the preoperative visit, the verbal pain scores (VPS) ranging from 0 to 10 (0 = no pain and 10 = worst pain imaginable) and also the verbal anxiety score (VAS) ranging between 0 and 10 (0 = completely calm, 10 = the worst possible anxiety)[16] were explained to patients. Later, a 20 gauge cannula was inserted into one of the two hands. Patients were monitored with electrocardiogram, noninvasive measurement of blood pressure, and pulseoximetry (SPO2). Anxiety score and pain score were recorded for each patient before premedication (T1), 60 min after premedication, on arrival at the operating room (T2), during the operation period (T3) and also postoperatively before discharge from the recovery room (T4). At the end of surgery, the patients were asked about the average level of their anxiety and pain during the operation period according to the VAS and VPS criteria explained before premedication. The ophthalmologist, who was blinded to the group, measured the IOP using a Shioetz tonometer under topical analgesia in the nonoperated eye. The IOP was recorded before the premedication and at the end of surgery.
Moreover, the ophthalmologist, who was unaware of patient assignment, applied the topical anesthesia by instillation of two drop of tetracaine 0.5% in the lower fornix 5 min before surgery. The lids and periocular areas were painted with povidone iodine 5% solution twice and the patient was draped. While fully draped, the eye speculum was inserted and soon after 0.1 ml of the solution (2.5 ml lidocaine 2% and 0.5 ml epinephrine 1/10000 and 2 ml balanced salt solution) prepared by a nurse, was generously poured on the exposed ocular surface. The entry into the anterior chamber was followed by an intra-cameral injection of 0.1 ml of the solution mentioned above. Supplemental oxygen at a rate of 4L/min was administered to all patients through nasal cannula. Surgery was performed early in the morning. Systolic blood pressure (SBP) and diastolic blood pressure (DBP), heart rate (HR), and peripheral oxygen saturation (SPO2) values were recorded before premedication (T1), 60 min after premedication, on arrival at the OR (T2), 5 min after the start of surgery (T3), and postoperatively before discharge from the recovery room (T4). Fentanyl 0.5 μg/kg IV was administered during the surgery if the patient complained of pain while having a VPS more than 4. The number of patients who needed to be administered with fentanyl was also recorded.
Postoperatively, the operating surgeon, who was unaware of patient assignment, was asked to assess the adequacy of intraoperative conditions according to the following scale: excellent (complete calmness and cooperating with the surgeon), good (slight undesirable movements of eye, and poor (sever undesirable movements of the eye and un-cooperating). The occurrence of adverse effects such as agitation, airway obstruction, respiratory depression, nausea and vomiting, excessive dizziness, unresponsiveness, and insomnia or headache was recorded. The anesthetist was blinded to the patient's group assignment, and the study data were recorded by a blinded observer. Based upon previously published data,[17,18,19,20] we assumed the placebo could have an effect in reducing the anxiety scores in 5% of patients, whereas in case of melatonin, a decreasing effect on anxiety scores would be obvious in at least 40% of patients and this provided an 80% power with an error equal to 0.05, therefore, a sample size of 22 patients per group seemed to be sufficient. To compensate for dropout cases and shifting from normality in data distribution, a total of 30 cases were studied in each group.
Parametric data were expressed as the mean ± SD. Normality of the data was tested by one sample Kolmogorov–Smirnov test. The t-test analysis was used for continuous parametric variables such as, weight, height, age, duration of surgery, HR, SBP and DBP, SPO2, and IOP. Nonparametric data were expressed as the median interquartile range. The Mann–Whitney rank-sum test was employed for comparison of VAS and VPS values between the groups. The Wilcoxon's test was performed to compare the variables at different times. The chi square and Fisher's exact tests were used for comparison of surgery field conditions between the groups. A P value < 0.05 was considered as significant. Statistical analysis was carried out using SPSS version 16 for Windows (SPSS, Chicago, IL).
Results
Among 65 patients initially enrolled in this study, 5 cases had to be excluded because of logistical reasons or other violations of the study protocol. Sixty patients were included and randomly assigned to their treatment groups [Fig. 1].
Figure 1.
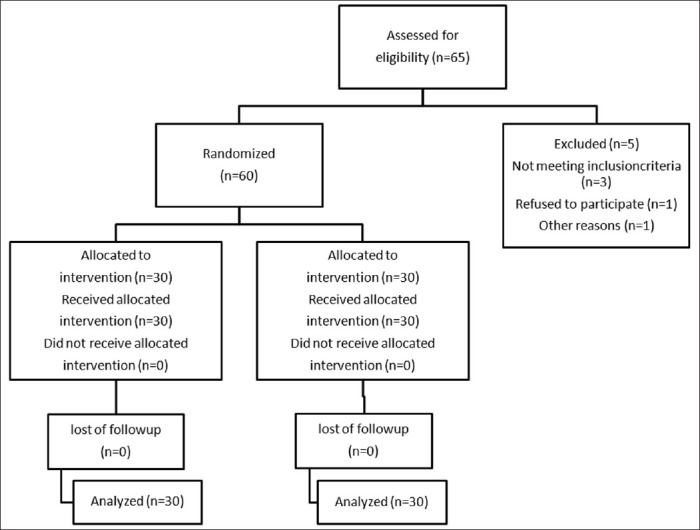
Consort flow diagram of the trial
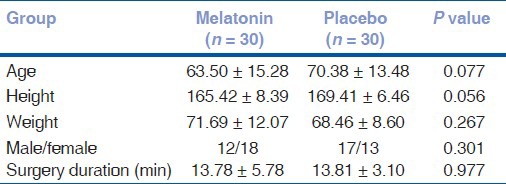
There were no significant differences among two groups regarding the demographic features including the age, gender, body weight, height, and duration of surgery [Table 1].
Table 1.
Patients characteristics
Baseline characteristics such as HR, SBP, DBP, and IOP were similar in both study groups [Table 2].
Table 2.
Parametric variables measured in the study
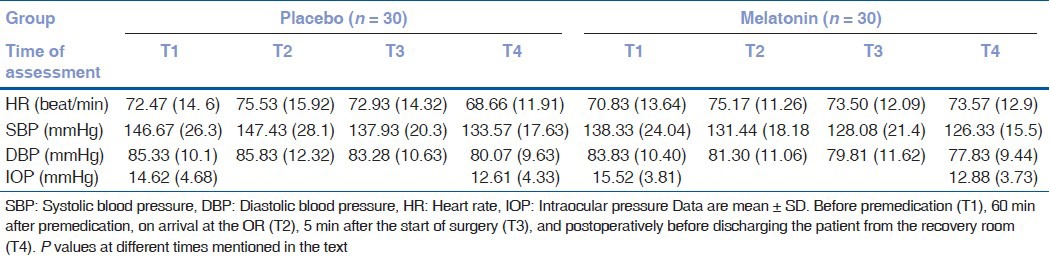
Table 3 indicates that the anxiety scores significantly decreased in the melatonin group after premedication (95% CI 3–4; P = 0.000). On the contrary and as shown in Table 3, the anxiety scores increased in the placebo group following premedication (95% CI 3–3.5; P = 0.011). There were significant differences between two groups in anxiety scores after premedication (95% CI 3–3.5; P = 0.023), intraoperatively (95% CI 2.5–3.5; P = 0.007), and postoperatively before discharging the patient from the recovery room (95% CI 0.5–1; P = 0.007).
Table 3.
Nonparametric variables measured in the study
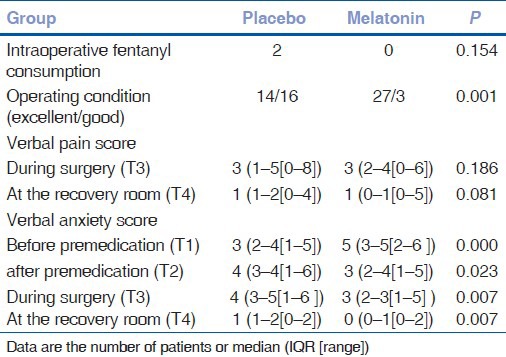
Neither the intraoperative pain scores (P = 0.186) nor the postoperative pain scores (P = 0.081) were different between the groups [Table 3]. Two patients in the placebo group needed fentanyl boluses but none in the melatonin group; there was no significant difference (P = 0.154) between two groups [Table 3]. In group M, the quality of operating conditions was excellent in 27 patients and good in 3 others whereas in group P, 14 patients had excellent conditions and the rest (16 cases) with good operating conditions. There was a significant difference (P = 0.001) between two groups [Table 3].
No significant difference in HR, SBP, DBP, and IOP between two groups was recorded at different stages [Table 2]. As shown in Table 2, after premedication and at the end of surgery, the IOP decreased in both M (2.388 ± 5.220) and P (1.813 ± 3.986) groups, however, the difference between two groups was found to be insignificance (P = 0.644) [Table 2]. Furthermore, following premedication and on arrival at the OR, the SBP decreased (5.96 ± 18.62) in group M, whereas there was an increase (0.766 ± 21.712) in SBP in group P nevertheless, the difference (P = 0.217) was not significant [Table 2]. Moreover, the DBP in group M decreased (3.14 ± 9.21) after premedication (T2) while in group P there was an increase (0.5 ± 11.013) although the difference (P = 0.183) was not statistically significant [Table 2]. Finally, there was no significant difference in HR between two groups recorded at different stages [Table 2]. No patient developed hypoxia, hypotension, or bradycardia. Only one patient in the melatonin group complained of mild headache.
Discussion
We have demonstrated that the patients who received premedication with 3mg sublingual melatonin had a significant decrease in anxiety levels [Table 3]. Although the study failed to show a melatonin-associated decrease in pain score, IOP, and BP although, there was a decrease in SBP, DBP, and IOP after premedication (T2) in group M but the difference between two groups turned out to be insignificant.
We chose to administer 3 mg of melatonin sublingually 60 min before surgery in our study because the onset of melatonin-induced sedation is reported to begin approximately 20–30 min after sublingual administration, and also the fact that melatonin concentrations remain constant for approximately 1.5 h at its peak concentration.[21,22,23] In addition, it is also reported that melatonin at doses between 0.3 and 10 mg has similar hypnotic effects.[24]
Our finding regarding the anxiolytic effect of melatonin is consistent with several previous studies.[17,18,20,22] However, Capuzzo et al.[25] and Isik et al.[26] reported that melatonin premedication failed to reduce anxiety at a level higher than placebo in elderly and anxious children undergoing surgery. The difference between these results could be explained by differences in populations (age, gender, and types of surgery) or methodologies. In current study, all patients with diseases that could interfere with the results of our study regarding the anxiety and pain or those with possibility of exacerbation of their diseases following the use of melatonin were excluded. Therefore, by the exclusion criteria used in the present study, the results of this research are more likely to be reliable. The anxiolytic effects of melatonin may be related to GABAergic system activation.[27] Although a number of previous studies[17,18,20] have reported a significantly lower pain scores in melatonin group compared with control group nevertheless, we could not demonstrate that melatonin has a decreasing effect on pain scores. The dissimilarity between the results of these studies may be due to several factors such as lower pain scores of our patients, the type of surgery, kind of topical anesthesia used by the surgeon, and the shorter duration of surgery. In a report by Caumo et al., it was declared that the coadministration of melatonin and clonidine as a preoperative premedication decreased the postoperative morphine use by more than 30% in patients undergoing abdominal hysterectomy with moderate and severe anxiety, whereas in mildly anxious patients, it was not associated with an analgesic effect.[20] According to this study, the level of anxiety score in most of our patients was mild and therefore it seems that melatonin had no effect on pain. In addition, in the current study, the surgeon used tetracaine 0.5% plus an intracameral injection of 0.1 ml of a mixed anesthetic solution (2.5 ml lidocaine 2%, 0.5 ml epinephrine 1/10000, and 2 ml balanced salt solution), while in Ismail's study,[17] a sponge soaked with lidocaine 2% was inserted into the upper fornix and removed before draping and that the surgeon only used oxybuprocaine 0.4%. Gupta et al.,[28] Ezra et al.,[29] and Ho et al.[30] also reported of a decreased intraoperative pain by intracameral lidocaine during cataract surgery under topical anesthetic. The mean duration of surgery in the present study was almost 13 min while in Ismail's study[17] it was 30 min. In our study although the reduction of IOP following premedication in group M was greater than that of group P but the difference was not significant. However, some studies have reported of an increased IOP following melatonin administration[10] while others failed to show such an effect.[8,9,17] These apparently controversial results may be due to either the difference in methodology used for measurements such as application of different tonometers and calibration methods or population variation and dose of melatonin. We chose a dose of 3 mg sublingual melatonin whereas in the Ismail's study[17] a higher dose (10 mg) of oral melatonin was used. In our study, although the SBP and DBP in group M decreased after melatonin premedication, these two parameters slightly increased in group P but the difference was found to be insignificant. This finding is in contrast with the result obtained in the Ismail's study in which a significant decrease in MAP, after melatonin premedication, was reported. Nevertheless, he also emphasized that this difference, at some points, was insignificant among the study groups. It is likely that if a larger dose of melatonin was used, the results could have been more obvious. As mentioned earlier, the selected dose of sublingual melatonin (3 mg) used was based on previous studies.[18,22] In several studies by Naguib et al., it was declared that premedication with sublingual melatonin 5 mg,[22] 0.05, or 0.01 mg/kg[18] was associated with preoperative anxiolysis and sedation. However, a melatonin dose with analgesic potentials is still unclear. Moreover, minimizing the perioperative anxiety during cataract surgery under topical anesthesia requires more sedation but at the same time the maintenance of vigilance during cataract surgery is of prime importance. Otmani declared that the prolonged release melatonin (PR-M) alone had no effect on any cognitive measures including the memory recall and driving performances but the cognitive functions were impaired by zolpidem within 5 h postadministration.[13] Naguib also reported that melatonin premedication like midazolam is associated with sedation and preoperative anxiolysis, however, unlike midazolam these effects are not associated with impaired psychomotor skills or the quality of recovery.[14] In the current study, none of our patients in melatonin group were found to develop impaired vigilance and cooperation or respiratory and cardiovascular depression. Therefore, it seems that melatonin to be a valuable premedicant for cataract surgery under topical anesthesia. Further studies are required to determine either the analgesic and anxiolysis efficacy of combined melatonin with other analgesic agents or good counseling as premedication during cataract surgery. In conclusion, our data indicate that melatonin is a valuable premedicant for patients undergoing cataract surgery under topical anesthesia as it produces anxiolytic effect and improves the operating conditions during cataract surgery under topical anesthesia.
Acknowledgment
Supported in part by Endocrine Research center of Booali Hospital. We thank the effort of the OR team. We also acknowledge Zahra Mohamadi for her help in the preparation of this manuscript.
Footnotes
Source of Support: Supported in part by Endocrine Research center of Booali Hospital
Conflict of Interest: Nil.
References
- 1.Eke T, Thompson JR. Serious complications of local anaesthesia for cataract surgery: A 1 year national survey in the United Kingdom. Br J Ophthalmol. 2007;91:470–5. doi: 10.1136/bjo.2006.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen PJ. Immediate visual capability after cataract surgery: Topical versus retrobulbar anesthesia. J Cataract Refract Surg. 1995;21:302–4. doi: 10.1016/s0886-3350(13)80137-x. [DOI] [PubMed] [Google Scholar]
- 3.Fung D, Cohen M, Stewart S, Davies A. What determines patient satisfaction with cataract care under topical local anesthesia and monitored sedation in a community hospital setting? Anesth Analg. 2005;100:1644–50. doi: 10.1213/01.ANE.0000154206.81132.B9. [DOI] [PubMed] [Google Scholar]
- 4.Janzen PR, Christys A, Vucevic M. Patient- controlled sedation using propofol in elderly patients in day-case cataract surgery. Br J Anaesth. 1999;82:635–6. doi: 10.1093/bja/82.4.635. [DOI] [PubMed] [Google Scholar]
- 5.Alhashemi JA. Dexmedetomidine vs midazolam for monitored anesthesia care during cataract surgery. Br J Anaesth. 2006;96:722–6. doi: 10.1093/bja/ael080. [DOI] [PubMed] [Google Scholar]
- 6.Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: Location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–22. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Zawilska JB, Lorenc A, Berezinska M, VivienRoels B, Pevet P, Skene D. Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina. Gen Comp Endocrinol. 2006;145:162–8. doi: 10.1016/j.ygcen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Wiechmann AF, Wirsig-Wiechmann CR. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp Eye Res. 2001;73:617–23. doi: 10.1006/exer.2001.1073. [DOI] [PubMed] [Google Scholar]
- 9.Pintor J, Pelaez T, Hoyle CH, Peral A. Ocular hypotensive effects of melatonin receptor agonists in the rabbit: Further evidence for an MT3 receptor. Br J Pharmacol. 2003;138:831–6. doi: 10.1038/sj.bjp.0705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohde BH, McLaughlin MA, Chiou LY. Existence and role of endogenous ocular melatonin. J Ocul Pharmacol. 1985;1:235–43. doi: 10.1089/jop.1985.1.235. [DOI] [PubMed] [Google Scholar]
- 11.Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, et al. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol. 1999;83:1417–9. doi: 10.1016/s0002-9149(99)00112-5. [DOI] [PubMed] [Google Scholar]
- 12.Ebadi M, Govitrapong P, Phansuwan-Pujito P, Nelson F, Reiter RJ. Pineal opioid receptors and analgesic action of melatonin. J Pineal Res. 1998;24:193–200. doi: 10.1111/j.1600-079x.1998.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 13.Otmani S, Demazières A, Staner C, Jacob N, Nir T, Zisapel N, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- 14.Naguib M, Gottumukkala V, Goldstein P. Melatonin and anesthesia: A clinical perspective. J Pineal Res. 2007;42:12–21. doi: 10.1111/j.1600-079X.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 15.Saklad M. Grading of patients for surgical procedures. Anesthesiol. 1941;2:281–4. [Google Scholar]
- 16.Kindler CH, Harms C, Amsler F, Ihde-Scholl T, Scheidegger D. The visual analog scale allows effective measurement of preoperative anxiety and detection of patients’ anesthetic concerns. Anesth Analg. 2000;90:706–12. doi: 10.1097/00000539-200003000-00036. [DOI] [PubMed] [Google Scholar]
- 17.Ismail SA, Mowafi HA. Melatonin provides anxiolysis, enhances analgesia, decreases intraocular pressure, and promotes better operating conditions during cataract surgery under topical anesthesia. Anesth Analg. 2009;108:1146–51. doi: 10.1213/ane.0b013e3181907ebe. [DOI] [PubMed] [Google Scholar]
- 18.Naguib M, Samarkandi AH. Premedication with melatonin: A double-blind, placebo controlled comparison with midazolam. Br J Anaesth. 1999;82:875–80. doi: 10.1093/bja/82.6.875. [DOI] [PubMed] [Google Scholar]
- 19.Mowafi HA, Ismail SA. Melatonin improves tourniquet tolerance and enhances postoperative analgesia in patients receiving intravenous regional anesthesia. Anesth Analg. 2008;107:1422–6. doi: 10.1213/ane.0b013e318181f689. [DOI] [PubMed] [Google Scholar]
- 20.Caumo W, Levandovski R, Hidalgo M. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: A double-blind, randomized, placebo-controlled study. J Pain. 2009;10:100–8. doi: 10.1016/j.jpain.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–13. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 22.Naguib M, Samarkandi AH. The comparative dose-response effect of melatonin and midazolam for premedication of adult's patients: A double-blinded placebo-controlled study. Anesth Analg. 2000;91:473–9. doi: 10.1097/00000539-200008000-00046. [DOI] [PubMed] [Google Scholar]
- 23.Markantonis SL, Tsakalozou E, Paraskeva A, Staikou C, Fassoulaki A. Melatonin pharmacokinetics in premenopausal and postmenopausal healthy female volunteers. J Clin Pharmacol. 2008;48:240–5. doi: 10.1177/0091270007311112. [DOI] [PubMed] [Google Scholar]
- 24.Arendt J. Melatonin and human rhythms. Chronobiol Int. 2006;23:21–37. doi: 10.1080/07420520500464361. [DOI] [PubMed] [Google Scholar]
- 25.Capuzzo M, Zanardi B, Schiffino E, Buccoliero C, Gragnaniello D, Bianchi S, et al. Melatonin does not reduce anxiety more than placebo in the elderly undergoing surgery. Anesth Analg. 2006;103:121–3. doi: 10.1213/01.ane.0000222476.62547.ed. [DOI] [PubMed] [Google Scholar]
- 26.Isik B, Baygin O, Bodur H. Premedication with melatonin vs midazolam in anxious children. Paediatr Anaesth. 2008;18:635–41. doi: 10.1111/j.1460-9592.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 27.Wan Q, Man HY, Liu F, Braunton J, Niznik HB, Pang SF, et al. Differential modulation of GABAA receptor function by Mel 1a and Mel 1b receptors. Nat Neurosci. 1999;2:401–3. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SK, Kumar A, Kumar D, Agrawal S. Manual small incision cataract surgery under topical anesthesia with intracameral lignocaine: Study on pain evaluation and surgical outcome. Indian J Ophthalmol. 2009;57:3–7. doi: 10.4103/0301-4738.44488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezra DG, Nambiar A, Allan BD. Supplementary intracameral lidocaine for phacoemulsification under topical anesthesia: A meta-analysis of randomized controlled trials. Ophtalmology. 2008;115:455–87. doi: 10.1016/j.ophtha.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Ho AL, Zakrzewski PA, Braga-Mele R. The effect of combined topical-intracameral anaesthesia on neuroleptic requirements during cataract surgery. Can J Ophthalmol. 2010;45:52–7. doi: 10.3129/i09-204. [DOI] [PubMed] [Google Scholar]


