Abstract
The developmental transcription factor NeuroD1 is anomalously expressed in a subset of aggressive neuroendocrine tumors. Previously, we demonstrated that TrkB and neural cell adhesion molecule (NCAM) are downstream targets of NeuroD1 that contribute to the actions of neurogenic differentiation 1 (NeuroD1) in neuroendocrine lung. We found that several malignant melanoma and prostate cell lines express NeuroD1 and TrkB. Inhibition of TrkB activity decreased invasion in several neuroendocrine pigmented melanoma but not in prostate cell lines. We also found that loss of the tumor suppressor p53 increased NeuroD1 expression in normal human bronchial epithelial cells and cancer cells with neuroendocrine features. Although we found that a major mechanism of action of NeuroD1 is by the regulation of TrkB, effective targeting of TrkB to inhibit invasion may depend on the cell of origin. These findings suggest that NeuroD1 is a lineage-dependent oncogene acting through its downstream target, TrkB, across multiple cancer types, which may provide new insights into the pathogenesis of neuroendocrine cancers.
Keywords: NeuroD1, neuroendocrine, TrkB, migration
Introduction
Aberrant expression of basic helix loop helix transcription factors such as neurogenic differentiation 1 (NeuroD1) and achaete-scute homolog1 has been observed in aggressive small cell lung cancer (SCLC), neural and neuroendocrine lung carcinomas.1, 2, 3, 4 Although the developmental roles of these basic helix loop helix proteins are well established, their possible causative roles in the pathogenesis of neuroendocrine carcinomas are less understood. Neuroendocrine tumors can initiate from almost any organ system. Although described based on organ of origin, this feature is not limiting, as many of these tumors share pathological characteristics such as expression of the neuroendocrine markers synaptophysin and chromogranin A, and the neural cell adhesion molecule (NCAM, also known as CD56).5, 6, 7 Neuroendocrine tumors are thought to originate from neuroendocrine cells, or to undergo an epithelial to neuroendocrine differentiation that leads to more aggressive carcinomas, as observed in melanoma and cancers of the gastrointestinal tract and prostate.2, 8, 9, 10, 11, 12, 13, 14 To investigate the role of NeuroD1 in tumorigenesis, we examined several tissue types. We report that several pigmented melanoma cell lines express high amounts of NeuroD1 and confirmed previous findings in prostate cell lines.2 We find that regulation of TrkB is conserved across multiple tissue types. Additionally, downregulation of both NeuroD1 and TrkB prevented viability and migration of several carcinomas; however, inhibition of TrkB activity only had an effect in cell lines with the neuroendocrine feature as defined by the presence of the neuroendocrine markers synaptophysin and NCAM. We also determined that loss of p53 is permissive for increased expression of NeuroD1, possibly in a lineage-dependent manner.
Results
NeuroD1 is highly expressed in aggressive neuroendocrine cancers
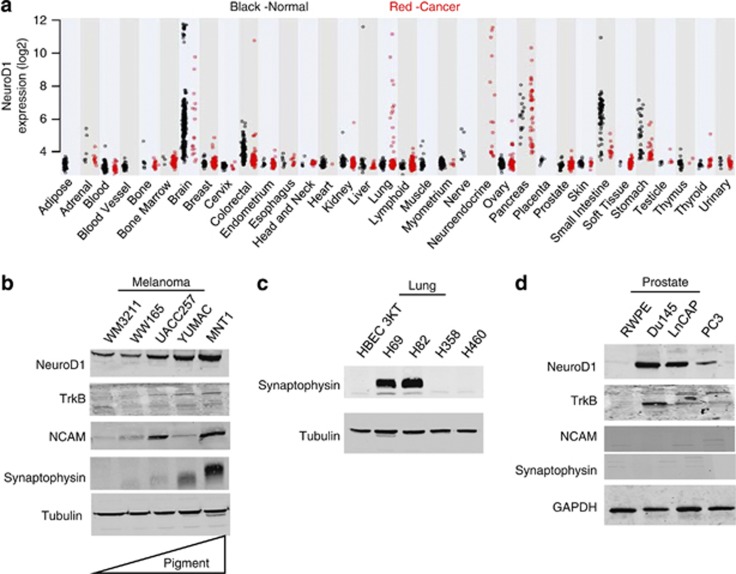
To investigate the clinical significance, expression of NeuroD1 was examined in a data set including more than 5400 patient samples taken from tumor and normal tissues. Elevated NeuroD1 expression was observed in several malignant tumors, including those from the neuroendocrine tissues, pancreas, brain, and lung, all of which were SCLC (Figure 1a, Supplementary Tables S1 and S2). Previously, we have demonstrated that NeuroD1 promotes tumor cell survival and metastasis in aggressive neuroendocrine lung tumors through regulation of the tyrosine kinase receptor, TrkB.15 To complement studies in the lung, we examined the role of NeuroD1 in cell lines from non-neural or non-neuroendocrine cells that undergo neuroendocrine differentiation.2, 16 The consequences of NeuroD1 expression have not been investigated in malignant melanoma, even though it has been suggested to induce neuroendocrine differentiation in conjunction with oncogenic B-RAFV600E under certain circumstances.17, 18 NeuroD1 was observed in prostate cancer; however, its expression in several of the commonly used prostate cancer cell lines was only noted upon in vitro differentiation with cAMP.2 We observed that NeuroD1, TrkB, and NCAM expression was greater in melanoma cell lines that were reported to have higher pigmentation and metastatic potential19, 20 (Figure 1b and Supplementary Figure 1a). The tendency of increasing expression of the three factors with increasing pigmentation appeared to be independent of the mutational status of B-RAF, as all cell lines with the exception of WM3211 have V600 mutations.21, 22, 23 As in SCLC, increased expression of the neuroendocrine marker synaptophysin was also detected in melanomas with high NeuroD1 (Figures 1b and c). NeuroD1 was also expressed in undifferentiated malignant prostate cell lines; however, neither of the neuroendocrine markers, synaptophysin or NCAM, were detected (Figure 1d).
Figure 1.
NeuroD1 is expressed in aggressive neuroendocrine lung cancers. (a) Expression of NeuroD1 mRNA compared across diverse normal (N=3879; black dots) and malignant tissues (N=1605; red dots) using the Affymetrix HGU133 Plus v2 GeneChip. (b, d) Melanoma and prostate cancer cell lines were lysed, and 25 μg total protein was loaded and then immunoblotted for NeuroD1, TrkB, NCAM, synaptophysin and tubulin or GAPDH (as loading controls). Melanoma cell lines were loaded by increasing pigmentation. (c) Lung cancer cell lines were lysed and 25 μg of total protein was immunoblotted for synaptophysin and tubulin (as loading controls).
Loss of p53 increased NeuroD1 expression
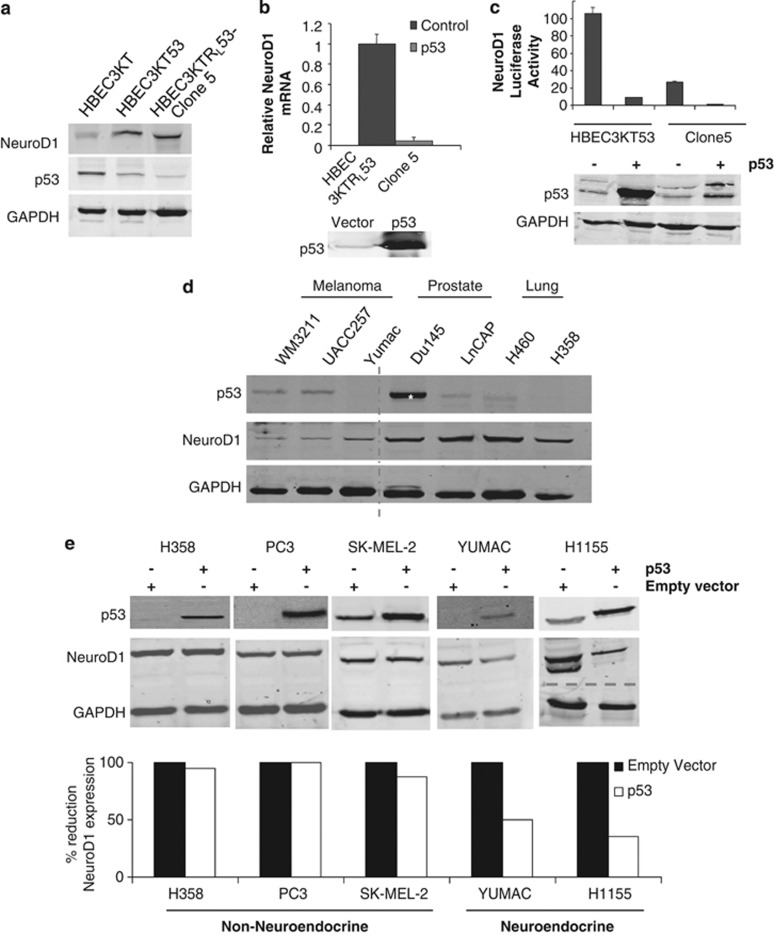
We next sought to investigate specific onco-genotypes possibly responsible for expression of NeuroD1. To do this we utilized human bronchial epithelial cell (HBEC) lines that were assigned a number to distinguish lines from different individuals and immortalized them by overexpression of cyclin-dependent kinase 4 and human telomerase reverse transcriptase (for example, HBEC3KT).24 The immortalized HBEC3KT cell line was sequentially transformed by knockdown of the tumor suppressor p53 and expression of K-RasV12 (HBEC3KTRL53)25 (also, Sato et al26). NeuroD1 expression was increased in HBEC3KT53 cells, a non-tumorigenic derivative with stable knockdown of p53 (Figure 2a). Additionally, isogenic derivatives of HBEC3KT that were transformed from normal to tumorigenic cells followed by clonal selection (HBEC3KTRL53-Clone 5, hereafter called Clone 5) exhibited spontaneous expression of NeuroD1 (Figure 2a). Sustained inactivation of p53 is suggested to enhance tumorigenesis at multiple stages, including initiation and progression.13, 24, 27, 28, 29, 30 To test a possible relationship between p53 and NeuroD1, we re-expressed p53 in the tumorigenic cell line Clone 5 and found a substantial decrease in NeuroD1 mRNA (Figure 2b). Next, we utilized a luciferase construct driven by the mouse Neurod1 proximal promoter to determine if p53 expression affected promoter activity. A 100-fold increase in Neurod1 promoter activity was observed in immortalized HBEC3KT53 compared with the parental HBEC3KT (Supplementary Figure 1A). Furthermore, re-expression of p53 in HBEC3KT53 and Clone 5 led to a dramatic reduction in Neurod1 promoter reporter activity (Figure 2c). From this HBEC model we concluded that loss of p53 induced NeuroD1 expression, suggesting that p53 may regulate NeuroD1 early in the pathogenesis of neuroendocrine lung cancer.
Figure 2.
Loss of p53 is permissive for expression of NeuroD1. (a) NeuroD1, p53 and GAPDH (loading control) were immunoblotted in lysates of HBEC3KT, HBEC3KT53 and Clone 5. (b) qRT–PCR analysis of NeuroD1 in Clone 5 cells transfected as indicated. A representative p53 immunoblot is shown from one of three independent experiments. (c) HBEC3KT53 and Clone 5 were transfected with pGL3-NeuroD1-luciferase with and without p53. p53 was immunoblotted and luciferase activity was measured; one of six experiments shown. (d) Melanoma, prostate and lung cancer cell lines were lysed; 50 μg total protein was immunoblotted for p53, NeuroD1 and GAPDH (as loading control). The dashed line indicates discontinuity in gel. The asterisk represents a loss-of-function mutation in p53.43 (e) Cell lines with loss of or mutation in p53 were transfected with control vector or vector encoding p53. Cells were lysed and immunoblotted for p53. Overexpression was quantified using Odyssey software.
To evaluate p53 as a determinant of NeuroD1 expression in neuroendocrine cancers, we analyzed its expression in lung, prostate and melanoma cells with loss of (H358, PC3, and YUMAC) or mutation in (H1155, M14, SK-MEL-2 and SK-MEL28) p5331, 32 (Figure 2d). Overexpression of p53 only suppressed NeuroD1 in cells that also had neuroendocrine features, not in the three non-neuroendocrine cell lines (Figure 2e). Together, these results suggest a role for loss of p53 being permissive for NeuroD1 expression, not only in neuroendocrine lung cancers but also, as recently suggested, in melanoma pathogenesis.33
NeuroD1 and TrkB regulates viability and migration of prostate and melanoma cell lines
Previously, we have demonstrated that knockdown of NeuroD1 and its downstream target TrkB led to a decrease in survival and migration of neuroendocrine lung cancers.15 We observed that loss of NeuroD1 resulted in loss of TrkB in all lines tested, indicating a conserved connection between NeuroD1 and TrkB across multiple cancer types (Supplementary Figure 2a). NeuroD1 has the ability to regulate the promoter of TrkB in neural and neuroendocrine lung cancers.15, 34 We sought to investigate if NeuroD1 bound the promoter of TrkB in the melanoma and the prostate cell lines using chromatin immunoprecipitation. Endogenous NeuroD1 was bound to the TrkB promoter in all cell lines; the enrichment of NeuroD1 on the TrkB promoter was greater than 100-fold in both YUMAC and Mnt1 cell lines at the more distal site and closer to 10-fold or less in the other cell lines (Supplementary Figure 3).
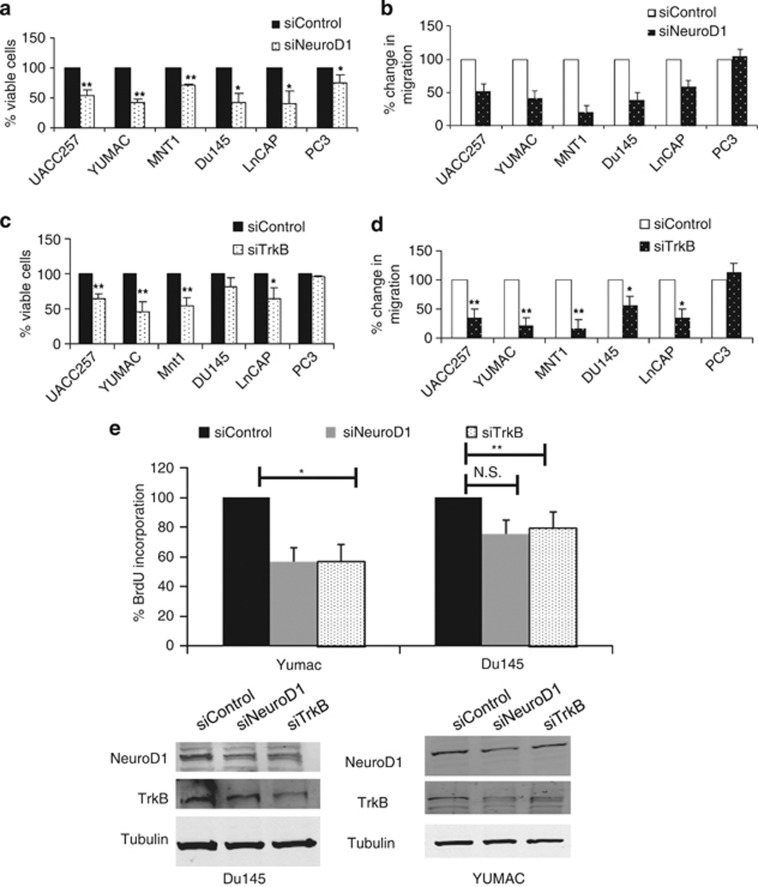
Next we tested the possible functions of NeuroD1 and TrkB in the prostate and melanoma cell lines. Loss of NeuroD1 in prostate and melanoma cell lines significantly reduced viability and migration (Figures 3a and b). Depletion of TrkB significantly decreased the viability and migration of all the melanoma cell lines (Figures 3c and d and Supplementary Figure 4), but prostate cancer cell lines differed, as loss of TrkB decreased migration but not viability (Figures 3c and d and Supplementary Figure 4). Additionally, knockdown of either NeuroD1 or TrkB led to a significant decrease in proliferation as measured by BrdU incorporation in Mnt1 and YUMAC, melanoma cells, and the LnCAP and Du145, prostate cells (Figure 3e and Supplementary Figure 2b). Taken together, these results indicate that NeuroD1 and TrkB regulate proliferation, viability and migration in several melanoma and prostate cancer cell lines.
Figure 3.
NeuroD1 and TrkB regulates viability and migration of prostate and melanoma. (a) NeuroD1 was transiently knocked down in melanoma or prostate in 96-well formats. Cells were assayed for viability using Cell-Titer Blue. Graph represents fold mean±s.d. of three independent experiments in triplicate (**P<0.005, *P<0.05; one-way ANOVA). (b) NeuroD1 was transiently knocked down in prostate and melanoma cell lines and cells were subjected to transwell migration assay. (c) TrkB was transiently knocked down in melanoma or prostate in 96-well formats. Cells were assayed for viability using Cell-Titer Blue. Graph represents fold mean±s.d. of three independent experiments in duplicate (**P<0.005, *P<0.05; one-way ANOVA). (d) TrkB was transiently knocked down in melanoma or prostate and subjected to transwell migration assay. Graph represents fold mean±s.d. of three independent experiments in triplicate (**P<0.005, *P<0.05; one-way ANOVA). (e) NeuroD1 and TrkB were transiently knocked down in melanoma or prostate cells in 96-well format for 3 days. Cells were then assayed for BrdU incorporation.
TrkB regulates cell survival and migration in melanoma but not prostate cancer cell lines
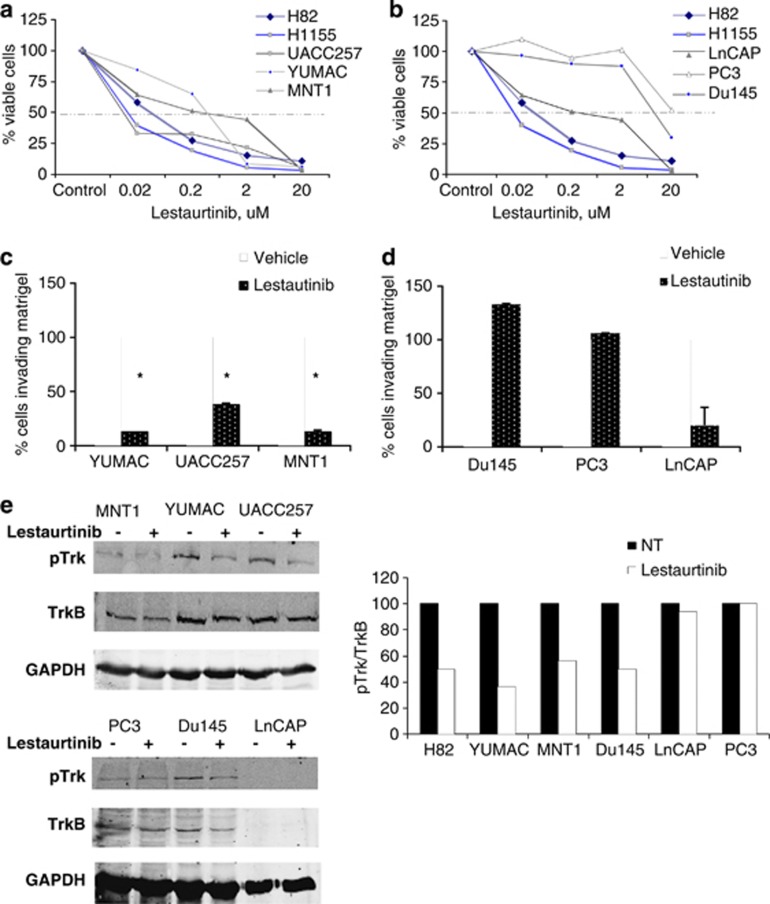
We next investigated if chemical inhibition of TrkB would phenocopy the loss of NeuroD1 in melanoma and prostate cancer cells. We then treated the melanoma and prostate cell lines with the pan-Trk inhibitor lestaurtinib to ascertain if chemical inhibition would mimic loss of TrkB. The melanoma cell lines exhibited a phenotype similar to the neuroendocrine lung cancer cell lines in that treatment with nanomolar concentrations of lestaurtinib reduced cell viability (Figure 4a). The prostate cell lines, however, were resistant to the inhibitor below micromolar concentrations, suggesting a divergence in the actions of TrkB in non-neuroendocrine cells (Figure 4b). The capacity of melanoma cells to invade through matrigel was inhibited by lestaurtinib (Figure 4c), whereas the prostate cell lines were resistant (Figure 4d). Treatment with lestaurtinib decreased active TrkB in the melanoma cell line similar to its effect on the SCLC cell line, H82 (Figure 4e). Lestaurtinib had no effect on phosphorylated Trk in the LnCAP or PC3 prostate cell lines, apparently explaining the lack of an effect on migration or viability (Figure 4e). Lestaurtinib did decrease viability and migration of LnCAP; however, these effects were not through a decrease in phosphorylated TrkB and may be due to another target of the drug (Figures 4b and d). We hypothesized that even if NeuroD1 was highly expressed, its mechanisms of action through TrkB may be dependent on the cell of origin. Neuroendocrine differentiation, defined by the presence of synaptophysin, was observed in the melanoma and neuroendocrine lung cancer cell lines, but not in the prostate cell lines (Figures 1b and d). We suggest that NeuroD1 and TrkB regulate migration in neuroendocrine and non-neuroendocrine cancer cells, but the efficacy of targeting TrkB may depend on the cell of origin.
Figure 4.
TrkB activity regulates cell survival and migration in melanoma but not prostate cancer cell lines. (a, b) Viability of neuroendocrine lung, prostate and melanoma cells was measured after a treatment with 0.02, 0.2, 2, 20 μM lestaurtinib for 96 h, using Cell-Titer Blue. (c, d) Invasion capabilities were measured after being embedded in matrigel along treatment with 2 μM lestaurtinib for 48 h. (e) SCLC, melanoma and prostate cells were treated with 10 μM lestaurtinib for 8 h. Cells were lysed and immunoblotted with TrkB antibodies and GAPDH. Blots were quantified using Odyssey software.
Discussion
Neurogenic basic helix loop helix transcription factors, including NeuroD1, are found to have increased expression in neural and neuroendocrine tumors. Whether their expression was causative or solely a consequence of disease had not been determined.1, 4, 9, 14, 35 Recently, NeuroD1 was implicated in the tumorigenesis of neuroblastoma.36 Our data reveal a novel function for NeuroD1 in the induction and coordination of signal transduction pathways that regulate survival and migration of non-neural/neuroendocrine cancers. We now demonstrate that NeuroD1 promotes survival and migration in neuroendocrine lung and other carcinomas at least in part through TrkB.
HBEC models provided a useful system to explore NeuroD1 function. Our studies suggest that p53 negatively regulates NeuroD1 expression not only in HBEC but also in carcinomas with neuroendocrine features. Loss of p53 did not unilaterally result in an increase in NeuroD1 expression, as observed in the non-neuroendocrine lung and prostate cell lines. Unlike cells of the prostate, melanocytes derive from neural crest cells migrating from the dorsal neural tube to the dermis, making them neuroectoderm in origin.37, 38 Furthermore, p53 is not only a potent tumor suppressor, but also suppresses self-renewal of adult neural stem cells.39 NeuroD1 has also been shown to enhance proliferation of committed neuronal progenitor cells.40 Perhaps p53-mediated inhibition of NeuroD1 in neuroendocrine cells present in non-neural tissues may in some respects parallel its effect on determination of neuronal cell fate.
Surprisingly, inhibition of TrkB kinase activity is apparently not equally significant in the three cancer types examined: lung, melanoma, and prostate. TrkB activity is important for neither migration nor viability in prostate cancer cell lines. We speculate this may be because of the following reasons: (1) prostate cancer cells do not express the neuroendocrine marker synaptophysin in contrast to melanoma and SCLC, which possibly links them in a neuroendocrine pathology; (2) prostate, unlike melanoma or SCLC, expresses smaller forms of TrkB, possibly TrkB splice variants thought to be inhibitory due to lack of the kinase domain;41 (3) the effects of NeuroD1 on prostate viability and migration may be mediated by pathways independent of TrkB. Ultimately, additional characteristics must be identified to distinguish between TrkB inhibitor-sensitive and insensitive tumor types.
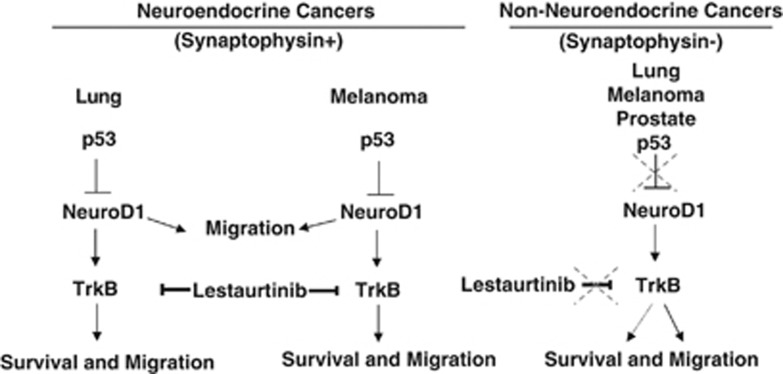
The findings here suggest that NeuroD1 acts as a lineage-specific regulator of survival and migration in cells with neuroendocrine features mainly through TrkB, which may be potentiated by loss of p53 (Figure 5). The discovery of downstream targets of NeuroD1 in non-neuroendocrine/non-neural tumors is ongoing. The actions of the NeuroD1/TrkB axis may differ outside of cancers with neuroendocrine features. The development of drugs that act as inhibitors of transcription factors has proven extremely difficult. Cell surface proteins offer greater opportunities for therapeutic intervention. In particular, TrkB, a receptor and enzyme, has gained attention as a potential target of drug development for neuronal and non-neuronal metastatic carcinomas. NeuroD1-expressing neuroendocrine carcinomas should now also be considered for sensitivity to TrkB inhibitors.
Figure 5.
Working model. Model of NeuroD1 induction and mechanism of action in neuroendocrine and non-neuroendocrine cancers.
Materials and methods
Reagents, antibodies, immunoblotting
Immunoblot analyses were done as previously described using equal amounts of protein from each sample.42 The following antibodies were used for blotting, immunoprecipitation and chromatin immunoprecipitation: goat NeuroD1 (N-19), rabbit pan-phospho-Trk (E-6), synaptophysin (H-8), p53 (DO-1), GAPDH (FL-335) (Santa Cruz, Dallas, TX, USA); rabbit TrkB (Chemicon/Millipore Billerica, MA, USA), mouse CD56/NCAM (Cell Signaling, Danvers, MA, USA), α-tubulin hybridoma was purchased from the Hybridoma Bank Studies at the University of Iowa. Lestaurtinib was purchased from LC Labs, Woburn, MA, USA, and BDNF from R&D Systems (Minneapolis, MN, USA). Band intensities were quantified using LICOR Odyssey Infrared Imaging System (Linclon, NE, USA).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as previously described.42 Twenty-five nanograms of total DNA was used for the quantitative RT-PCR reactions. TrkB primers were as described previously.34
Quantitative real-time PCR
Total RNA from the xenograft tumors and cell lines was isolated with TRI Reagent (Sigma-Aldrich, St Louis, MO, USA). RNA from tumor samples was from MD Anderson Cancer Center (Houston, TX, USA). complementary DNA was synthesized using iSCRIPT cDNA Synthesis Kit (Bio-Rad, Laboratories, Hercules, CA, USA). RNAs for mouse and human NeuroD1, TrkB, NCAM and 18s ribosomal RNA were quantified by RT-PCR with iTaq (Bio-Rad) master mix using TaqMan probes (Applied Biosystems, Life Technologies/Invitrogen, Grand Island, NY, USA) on an ABI 7500 thermocycler. Relative transcript levels were normalized to 18s rRNA. Transcript amounts in knockdown cells were plotted as fold change relative to control. Data were analyzed using ABI 7500 system software (Life Technologies/Invitrogen).
Cell viability and proliferation assay
Cells were plated at a density of 105/well and reverse transfected with small interfering RNA for 3 days. Viability after knockdown or drug was assayed using Cell-Titer Blue Reagent according to the manufacturer's protocol by measuring fluorescence as readout. Proliferation was measured by incorporation of BrdU in cells for 24 h following reverse transfection with indicated small interfering RNA for 3 days. Incorporation was measured using the Cell Signaling Assay Kit #6813S.
Cell culture
SCLC and non-small-cell lung carcinoma lines were obtained from the Hamon Cancer Center Collection (UT Southwestern). SCLC, non-small cell lung cancers with neuroendocrine differentiation, HBEC3KTRL53-Clone 5 (Sato et al., submitted) and prostate cell lines were cultured in RPMI 1640 medium with 10% fetal bovine serum. Melanoma cell lines were cultured in DMEM with 10% fetal bovine serum. Immortalized HBECs and RWPE (normal immortalized prostate cells) (except HBEC3KTRL53-Clone 5)25 were cultured in KSFM (Life Technologies/Invitrogen) with 5 ng/ml epidermal growth factor and 50 μg/ml bovine pituitary extract. The lung cancer cell lines were DNA fingerprinted using the PowerPlex 1.2 kit (Promega, Madison, WI, USA) and confirmed to be the same as the DNA fingerprint library maintained either by ATCC or by the Hamon Cancer Center. The lines were also tested to be free of mycoplasma by e-Myco kit (Boca Scientific, Boca Raton, FL, USA).
Migration assays
For migration assays, cells were seeded 48 h following knockdown of NeuroD1 or TrkB. Transwell migration was assayed in Transwell permeable supports (Corning #3422, Corning, NY, USA). Cells were seeded in the top chamber in RPMI with 1% fetal bovine serum and allowed to migrate along a concentration gradient through a polycarbonate membrane with 8 μm pores to the bottom chamber containing medium with 10% fetal bovine serum. After 24 h cells were fixed, stained (with hematoxylin and eosin stain), and counted. For invasion assays 1.5 × 105 cells were embedded in growth factor reduced matrigel in the presence or absence of 100 nM lestaurtinib in transwell permeable supports. Cells were allowed to migrate for 48 h across membranes with a gradient of 10% serum in the bottom chamber.
Microarray analysis
Five micrograms of total RNA was labeled and hybridized to Affymetrix GeneChips HG-U133A and B according to the manufacturer's protocol (http://www.affymetrix.com) while 0.5 micrograms of total RNA was used for Illumina BeadChip HumanWG-6 V3 (http://www.illumina.com). These data are available in GEO (accession no. GSE4824 and GSE32036). Array data were pre-processed with MAS5 (Affymetrix algorithm for probe summarization) or MBCB (Illumina algorithm for background subtraction (Ding et al, NAR 36 (10), 2008)), quantile-normalized and log-transformed
Microarray expression data of NeuroD1 mRNA were also compared across diverse benign (N=3879; black dots) and malignant tissues (N=1605; red dots) using the Affymetrix HGU133 Plus v2 GeneChip. These data were obtained from Gene Logic, Inc. (Gaithersburg, MD, USA). The analysis shown is for probe set ID 206282_at. The microarray data were normalized using the RMA method.
Plasmids, primers, and luciferase assays
Oligonucleotides used were the following: NeuroD1-1 sense 5′-CGAAUUUGGUGUGGCUGUA-3′, antisense 5′-UACAGCCACACCAAAUUCG-3′ (QIAGEN, Valencia, CA, USA); NeuroD1-AB sense 5′-GGAUCAAUCUUCUCAGGCA-3′, antisense 5′-UGCCUGAGAAGAUUGAUCC-3′ (AMBION, Life Technologies/Invitrogen); NTRK2–5 sense 5′-GACGAGUUUGUCUAGGAAA-3′, antisense 5′-UUUCCUAGACAAACUCGUC-3′ (QIAGEN). For p53 experiments, cells were transfected with pCMV5, SV40- internal control, pcDNA.1-p53 or pGL3-NeuroD1 constructs using Fugene HD. Luciferase assays used the Promega dual luciferase kit according to the manufacturer's protocol.
Statistical analyses
Student's t test, one-way analysis of variance (ANOVA), Pearson's test and linear regression were used to determine statistical significance. Statistical significance for all tests was assessed by calculating the P values and was defined as <0.05.
Acknowledgments
We thank A-Young Lee, Elma Zaganjor, Andres Lorente-Rodriguez, Rolf Brekken, Joseph Albanesi and Banu Eskiocak for comments on the manuscript and Dionne Ware for administrative assistance. We thank Jenny Hsieh for NeuroD1-luciferase. We thank Banu Eskiocak, Elisabeth Martinez and JT Hsieh for melanoma and prostate cell lines. This work was supported by grants from the National Institutes of Health (R01DK55310 to MHC and P50CA70907 to JDM), grants from the Cancer Prevention and Research Institute of Texas (CPRIT) to MHC and JDM, and DOD PROSPECT and Longenbaugh Foundation to JDM. JKO is supported by NIGMS pharmacological sciences training grant 5-T32 GM007062. JEL is supported by NHMRC Biomedical Fellowship (494511)TSANZ/Allen & Hanburys Respiratory Research Fellowship.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis)
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
References
- Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- Cindolo L, Franco R, Cantile M, Schiavo G, Liguori G, Chiodini P, et al. NeuroD1 expression in human prostate cancer: can it contribute to neuroendocrine differentiation comprehension. Eur Urol. 2007;52:1365–1373. doi: 10.1016/j.eururo.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Hiroshima K, Iyoda A, Shida T, Shibuya K, Iizasa T, Kishi H, et al. Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis. Mod Pathol. 2006;19:1358–1368. doi: 10.1038/modpathol.3800659. [DOI] [PubMed] [Google Scholar]
- Rostomily RC, Bermingham-McDonogh O, Berger MS, Tapscott SJ, Reh TA, Olson JM. Expression of neurogenic basic helix-loop-helix genes in primitive neuroectodermal tumors. Cancer Res. 1997;57:3526–3531. [PubMed] [Google Scholar]
- Kaufmann O, Georgi T, Dietel M. Utility of 123C3 monoclonal antibody against CD56 (NCAM) for the diagnosis of small cell carcinomas on paraffin sections. Hum Pathol. 1997;28:1373–1378. doi: 10.1016/s0046-8177(97)90226-4. [DOI] [PubMed] [Google Scholar]
- Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gonen M, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34:300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Cantile M, Kisslinger A, Cindolo L, Schiavo G, D'Anto V, Franco R, et al. cAMP induced modifications of HOX D gene expression in prostate cells allow the identification of a chromosomal area involved in vivo with neuroendocrine differentiation of human advanced prostate cancers. J Cell Physiol. 2005;205:202–210. doi: 10.1002/jcp.20384. [DOI] [PubMed] [Google Scholar]
- Eyden B, Moss J, Shore I, Banerjee SS. Metastatic small cell malignant melanoma: a case requiring immunoelectronmicroscopy for the demonstration of lattice-deficient melanosomes. Ultrastruct Pathol. 2005;29:71–78. doi: 10.1080/01913120590910612. [DOI] [PubMed] [Google Scholar]
- Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402–409. doi: 10.1111/j.1365-2559.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- Pilozzi E, Cacchi C, Di Napoli A, Pini B, Duranti E, D'Andrilli A, et al. Primary malignant tumour of the lung with neuroendocrine and melanoma differentiation. Virchows Arch. 2011;459:239–243. doi: 10.1007/s00428-011-1109-7. [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, et al. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci USA. 2004;101:4471–4476. doi: 10.1073/pnas.0307983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JK, Larsen JE, Shields MD, Gonzales JX, Shames DS, Sato M, et al. NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM. Proc Natl Acad Sci USA. 2013;110:6524–6529. doi: 10.1073/pnas.1303932110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119–129. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- Bhat KM, Maddodi N, Shashikant C, Setaluri V. Transcriptional regulation of human MAP2 gene in melanoma: role of neuronal bHLH factors and Notch1 signaling. Nucleic Acids Res. 2006;34:3819–3832. doi: 10.1093/nar/gkl476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddodi N, Bhat KM, Devi S, Zhang SC, Setaluri V. Oncogenic BRAFV600E induces expression of neuronal differentiation marker MAP2 in melanoma cells by promoter demethylation and down-regulation of transcription repressor HES1. J Biol Chem. 2010;285:242–254. doi: 10.1074/jbc.M109.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo M, Nicotra MR, Apollonj C, Fraioli R, Giacomini P, Natali PG. Production and characterization of the murine monoclonal antibody 2G10 to a human T4-tyrosinase epitope. J Invest Dermatol. 1991;96:446–451. doi: 10.1111/1523-1747.ep12470092. [DOI] [PubMed] [Google Scholar]
- Kluger HM, McCarthy MM, Alvero AB, Sznol M, Ariyan S, Camp RL, et al. The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by Phenoxodiol is associated with Carboplatin sensitization. J Transl Med. 2007;5:6. doi: 10.1186/1479-5876-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Krauthammer M, Pelizzola M, Cheng E, Kovacs D, Sznol M, et al. Integrative analysis of epigenetic modulation in melanoma cell response to decitabine: clinical implications. PLoS One. 2009;4:e4563. doi: 10.1371/journal.pone.0004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8:2079–2085. doi: 10.1158/1535-7163.MCT-09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, et al. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- Sato M, Larsen JE, Lee W, Sun H, Shames DS, Dalvi MP, et al. Human Lung Epithelial Cells Progressed to Malignancy through Specific Oncogenic Manipulations. Mol Cancer Res. 2013;11:638–650. doi: 10.1158/1541-7786.MCR-12-0634-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468:572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Donehower LA, Bradley A, Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993;74:813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- Yang G, Rajadurai A, Tsao H. Recurrent patterns of dual RB and p53 pathway inactivation in melanoma. J Invest Dermatol. 2005;125:1242–1251. doi: 10.1111/j.0022-202X.2005.23931.x. [DOI] [PubMed] [Google Scholar]
- Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39 (Database issue:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapajarvi T, Pitkanen K, Laiho M. Human melanoma cell line UV responses show independency of p53 function. Cell Growth Differ. 1999;10:163–171. [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Encinas M, Comella JX, Aldea M, Gallego C. Basic helix-loop-helix proteins bind to TrkB and p21(Cip1) promoters linking differentiation and cell cycle arrest in neuroblastoma cells. Mol Cell Biol. 2004;24:2662–2672. doi: 10.1128/MCB.24.7.2662-2672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Browne C, Kim S, Case T, Paul M, et al. Neuroendocrine differentiation in the 12T-10 transgenic prostate mouse model mimics endocrine differentiation of pancreatic beta cells. Prostate. 2008;68:50–60. doi: 10.1002/pros.20650. [DOI] [PubMed] [Google Scholar]
- Huang P, Kishida S, Cao D, Murakami-Tonami Y, Mu P, Nakaguro M, et al. The neuronal differentiation factor NeuroD1 downregulates the neuronal repellent factor Slit2 expression and promotes cell motility and tumor formation of neuroblastoma. Cancer Res. 2011;71:2938–2948. doi: 10.1158/0008-5472.CAN-10-3524. [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- Pavan WJ, Raible DW. Specification of neural crest into sensory neuron and melanocyte lineages. Dev Biol. 2012;366:55–63. doi: 10.1016/j.ydbio.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkina N, Grashchuck M, Buchman VL, Davies AM. TrkB variants with deletions in the leucine-rich motifs of the extracellular domain. J Biol Chem. 1997;272:13019–13025. doi: 10.1074/jbc.272.20.13019. [DOI] [PubMed] [Google Scholar]
- Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- Gurova KV, Rokhlin OW, Budanov AV, Burdelya LG, Chumakov PM, Cohen MB, et al. Cooperation of two mutant p53 alleles contributes to Fas resistance of prostate carcinoma cells. Cancer Res. 2003;63:2905–2912. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.