Abstract
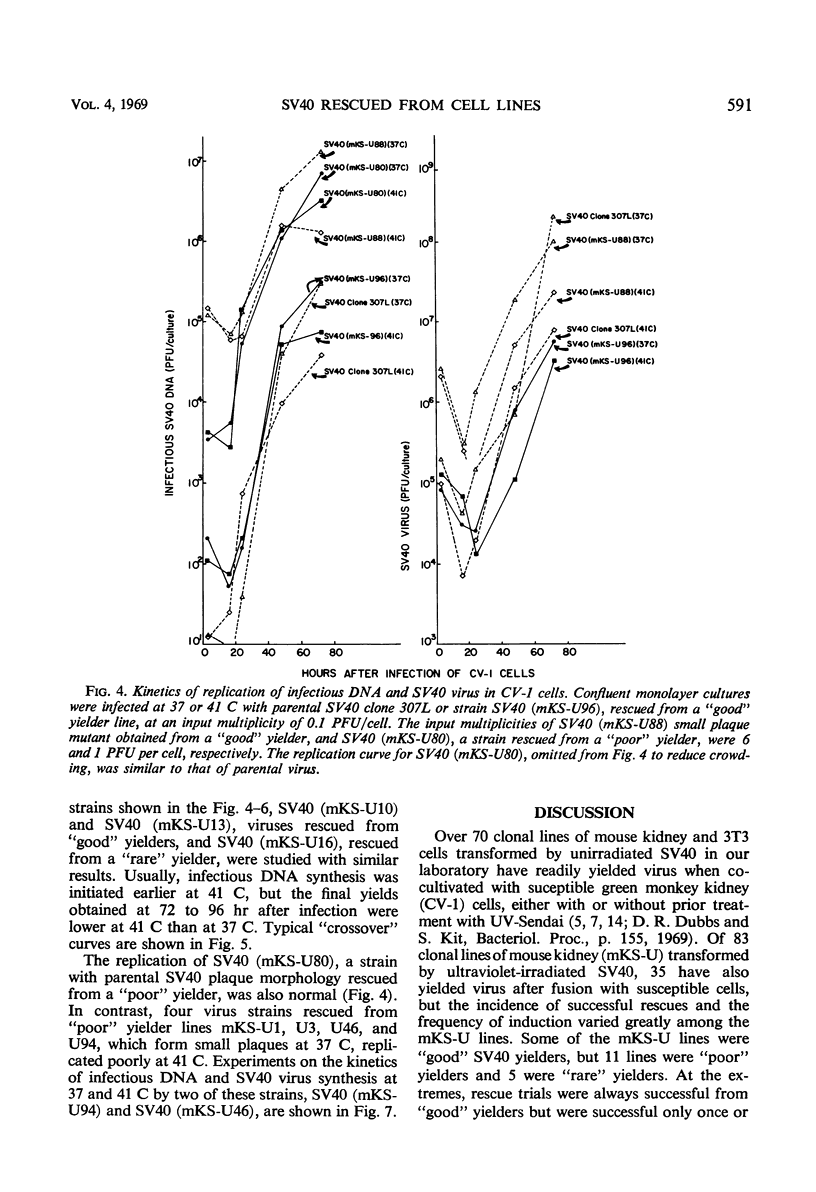
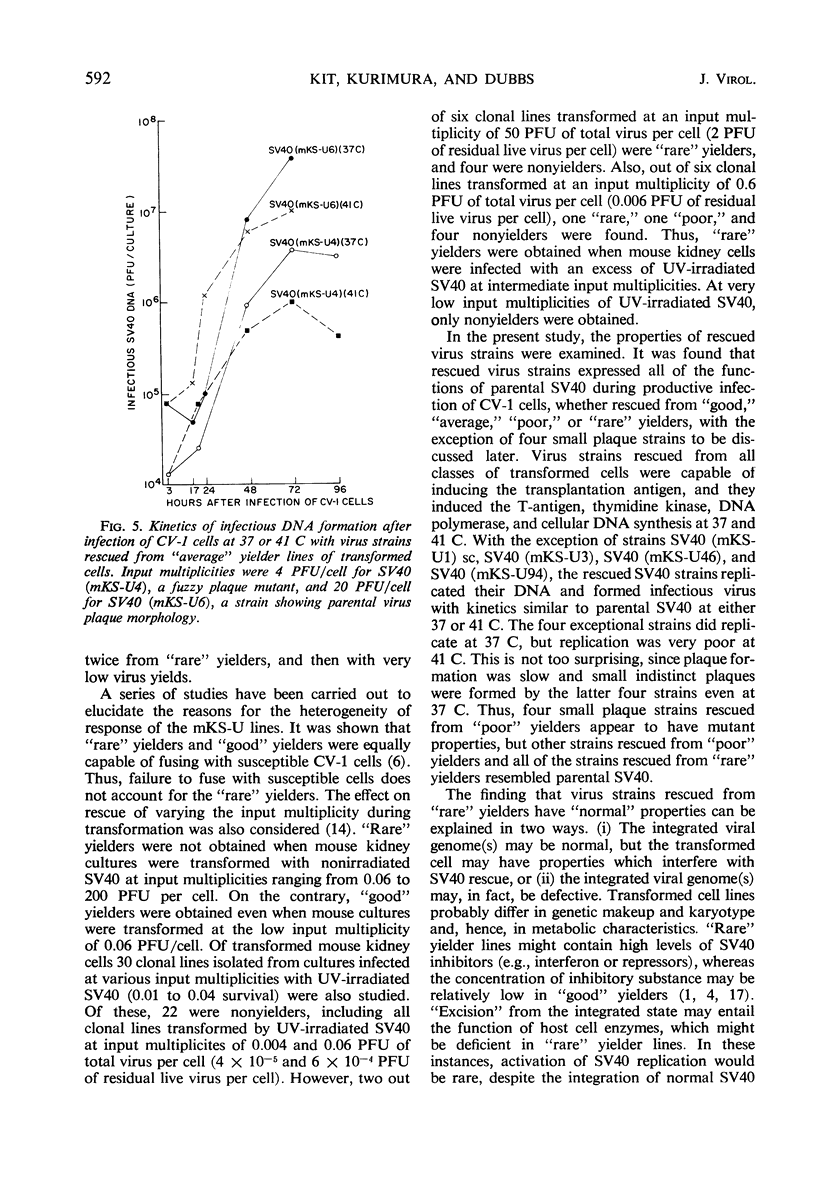
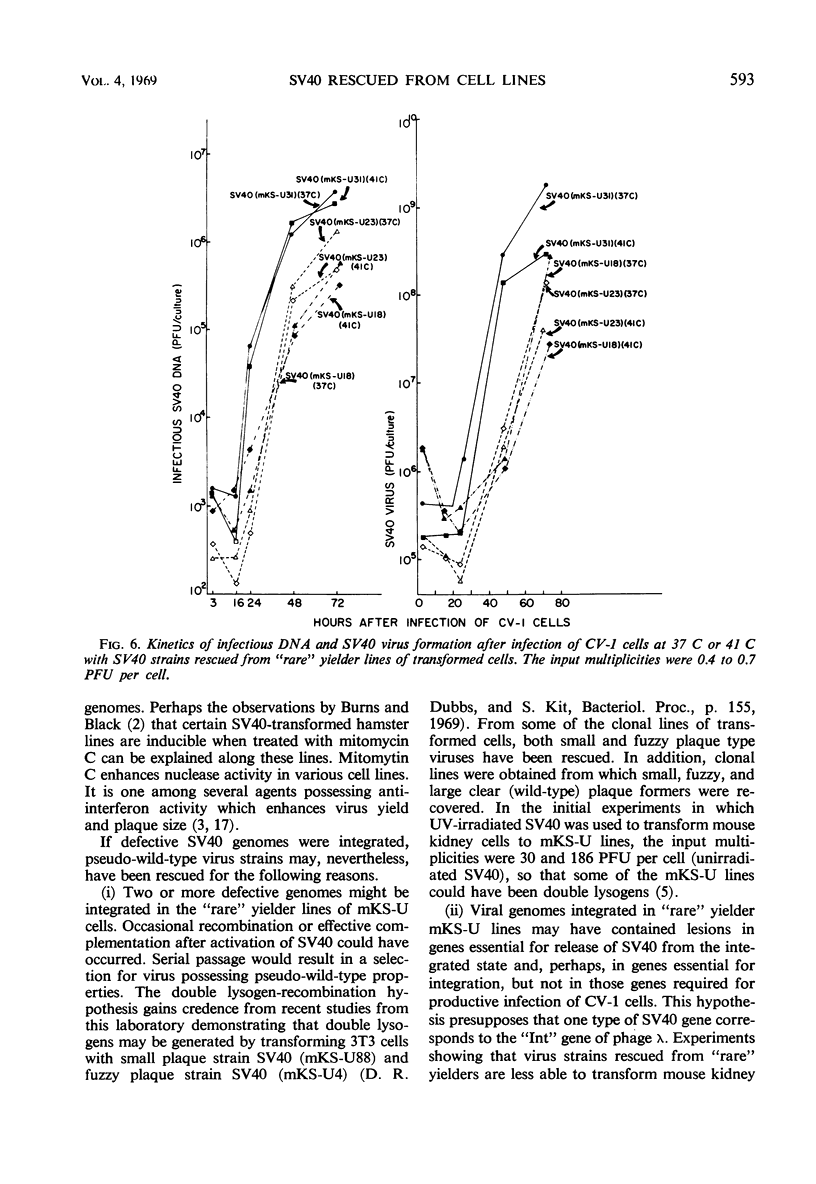
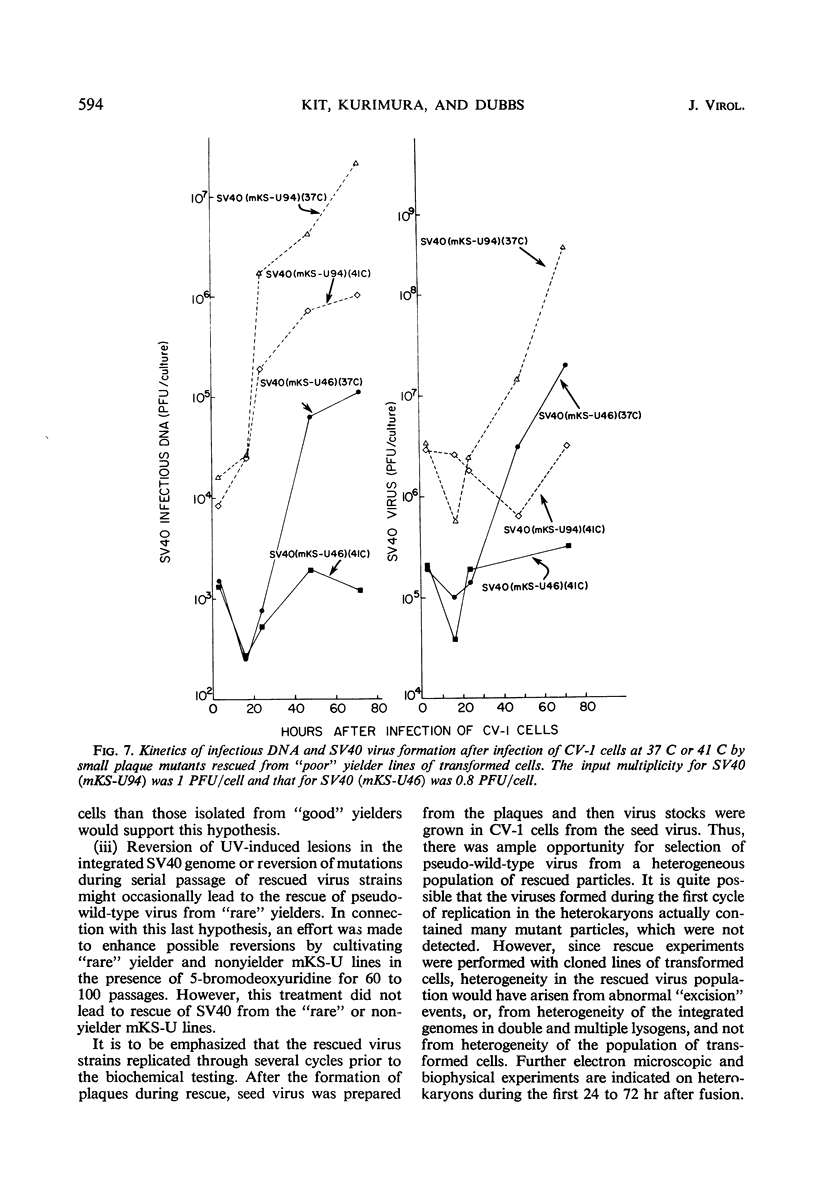
Simian virus 40 (SV40) strains have been rescued from various clonal lines of mouse kidney cells that had been transformed by ultraviolet (UV)-irradiated SV40. To learn whether some of the rescued SV40 strains were mutants, monkey kidney (CV-1) cells were infected with the rescued virus strains at 37 C and at 41 C. The SV40 strains studied included strains rescued from transformed cell lines classified as “good,” “average,” “poor,” and “rare” yielders on the basis of total virus yield, frequency of induction, and incidence of successful rescue trials. Four small plaque mutants isolated from “poor” yielder lines and fuzzy and small plaque strains isolated from an “average” and a “good” yielder line, respectively, were among the SV40 strains tested. Virus strains rescued from all classes of transformed cells were capable of inducing the transplantation antigen, and they induced the intranuclear SV40-T-antigen, thymidine kinase, deoxyribonucleic acid (DNA) polymerase, and cellular DNA synthesis at 37 C and at 41 C. With the exception of four small plaque strains rescued from “poor” yielders, the rescued SV40 strains replicated their DNA and formed infectious virus with kinetics similar to parental SV40 at either 37 or 41 C. The four exceptional strains did replicate at 37 C, but replication was very poor at 41 C. Thus, only a few of the rescued virus strains exhibited defective SV40 functions in CV-1 cells. All of the virus strains rescued from the “rare” yielder lines were similar to parental SV40. Several hypotheses consistent with the properties of the rescued virus strains are discussed, which may account for the significant variations in virus yield and frequency of induction of the transformed cell lines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brailovsky C. A., Berman L. D., Chany C. Decreased interferon sensitivity and production in cells transformed by SV40 and other oncogenic agents. Int J Cancer. 1969 Mar 15;4(2):194–203. doi: 10.1002/ijc.2910040209. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. V. Variability of virus recovery from cell clones inducible with mitomycin C and cell fusion. J Virol. 1968 Jun;2(6):606–609. doi: 10.1128/jvi.2.6.606-609.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassingena R., Tournier P. Mise en évidence d'un "répresseur" spéfique dans des cellules d'espèces différentes transformées par le virus SV 40. C R Acad Sci Hebd Seances Acad Sci D. 1968 Dec 16;267(25):2251–2254. [PubMed] [Google Scholar]
- Dubbs D. R., Kit S., De Torres R. A., Anken M. Virogenic properties of bromodeoxyuridine-sensitive and bromodeoxyuridine-resistant simian virus 40-transformed mouse kidney cells. J Virol. 1967 Oct;1(5):968–979. doi: 10.1128/jvi.1.5.968-979.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Heterokaryon formation of simian virus 40-transformed cells in the presence of ultraviolet-irradiated Sendai virus. J Virol. 1969 May;3(5):536–538. doi: 10.1128/jvi.3.5.536-538.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of defective lysogens from Simian virus 40-transformed mouse kidney cultures. J Virol. 1968 Nov;2(11):1272–1282. doi: 10.1128/jvi.2.11.1272-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., HSU T. C. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). III. Radioautographic and biochemical studies of thymidine-H3 uptake into DNA of L-M cells and rabbit cells in primary culture. Virology. 1963 Jan;19:13–22. doi: 10.1016/0042-6822(63)90019-9. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Brown M. Rescue of Simian Virus 40 from Cell Lines Transformed at High and at Low Input Multiplicities by Unirradiated or Ultraviolet-irradiated Virus. J Virol. 1969 Sep;4(3):226–230. doi: 10.1128/jvi.4.3.226-230.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Salvi M. L., Dubbs D. R. Activation of infectious SV40 synthesis in transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1239–1246. doi: 10.1073/pnas.60.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Piekarski L. J., Dubbs D. R. DNA polymerase induced by Simian virus 40. J Gen Virol. 1967 Apr;1(2):163–173. doi: 10.1099/0022-1317-1-2-163. [DOI] [PubMed] [Google Scholar]
- Rapp F., Vanderslice D. Enhancement of the replication of measles virus by mitomycin C. J Immunol. 1965 Oct;95(4):753–758. [PubMed] [Google Scholar]
- Takemoto K. K., Todaro G. J., Habel K. Recovery of SV40 virus with genetic markers of original inducing virus from SV40-transformed mouse cells. Virology. 1968 May;35(1):1–8. doi: 10.1016/0042-6822(68)90299-7. [DOI] [PubMed] [Google Scholar]



