Abstract
Background
Leukocyte depletion at the time of transplantation with alemtuzumab (Campath-1H) has been demonstrated to be a potential strategy for reducing long term exposure to immunosuppressive drugs. While the impact of alemtuzumab treatment on the immune system has been explored, the effects of long-term immunosuppressive therapy in alemtuzumab treated patients still need to be elucidated.
Methods
T regulatory cells and Th1/Th17 responses were assessed by flow cytometry and real-time PCR > 3 years after transplantation in 10 kidney recipients treated with alemtuzumab induction. 7 patients were converted to sirolimus monotherapy at 12 months post-transplant while the remaining 3 patients with history of graft rejection were treated with sirolimus and mycophenolate mofetil (MMF). Additionally, we sorted and expanded IL17A-producing CCR6+CD4+ T cells and assessed their susceptibility to suppression by regulatory T (Treg) cells in vitro suppression tests.
Results
3 years of mTOR inhibitor monotherapy correlates with an increase in the number of IL-17A producing cells, compared to patients treated with sirolimus and MMF. In these patients, IL-17A expression was compensated for by an increase in Treg cell frequency and number. Additionally, we demonstrated that both proliferation and cytokine production by Th17 cells can be effectively regulated by Treg cells.
Conclusions
Our results demonstrate that history of rejection and long-term maintenance immunosuppression has an impact on the number of circulating Treg and Th17 cells. But more importantly, we have shown that Treg can effectively regulate Th17cells both in vitro and in vivo.
Keywords: CAMPATH-1H, IL-17, T regulatory cells, kidney transplant, Sirolimus
Introduction
Long-term success in solid organ transplantation is limited by the immune-mediated process of allograft rejection. Although advances in immunosuppressive drug regimens over the last decades have led to significant improvement in 1 year transplant outcome (1) managing subclinical destructive processes leading to graft dysfunction and chronic rejection remains a major clinical challenge. Importantly, long-term immunosuppressive therapy has serious side effects leading to nephrotoxicity, chronic rejection and vascular disease. Leukocyte depletion at the time of transplantation with alemtuzumab (Campath-1H) has been demonstrated to be one of the ways of reducing immunosuppressive drug load without increasing the rate of acute rejection (2-4). We have previously demonstrated that alemtuzumab induction modulates the response of repopulating leukocytes to donor antigens leaving them unresponsive to graft antigens, even though maintenance immunosuppression has been significantly reduced (5). In this protocol steroids are avoided and patients treated with tacrolimus and mycophenolate mofetil (MMF), followed by conversion from tacrolimus to sirolimus (SRL) at 6 months and withdrawal of MMF at 12 months. During the first 15 months after transplantation we demonstrated that the CD8+ T cell compartment was repopulated predominantly by immunosenescent CD28−CD8+ cells with suppressive capacity, while classical Treg cells (CD25hiCD4+) remained low until the switch from tacrolimus to sirolimus at 6 months (6). An increase in Treg cells after alemtuzumab induction has been demonstrated to be most pronounced in calcineurin inhibitor (CNI) sparing protocols with early introduction of sirolimus (7, 8), however the effect of long-term maintenance therapy on regulatory and effector populations remains to be elucidated.
Th17 cells are a novel subset of CD4+ T cells characterised by the ability to produce IL-17A, IL-17F, IL-21, IL-22 and expression of the transcription factor retinoid-related orphan receptor γt (RORγt). Th17 cells have been demonstrated to be protective against extracellular pathogens but detrimental in autoimmune conditions (9). Although the role of Th17 cells in transplantation immunity is not fully elucidated, the involvement of IL-17 and Th17 cells in the rejection of allografts has been suggested (10, 11), especially in proinflammatory conditions (12). Human circulating Th17 cells are found within CCR6+ population (13, 14) and an increase in this population has been observed in autoimmune diseases (14).
In this study, we investigated the effect of long-term reduced immunosuppressive therapy on Treg and T effector cell populations. The initial group of 10 patients were treated with alemtuzumab induction followed by low dose tacrolimus and MMF for the first six months after transplantation. At 6 months post-transplant, patients were converted from tacrolimus to sirolimus and at 12 months MMF was withdrawn. During the first year post-transplant 3 patients experienced rejection episodes (one acute rejection at day 70 and two chronic rejection at about 1 year) that were treated successfully by reintroducing MMF into the drug regimen. Analysis of non-rejecting patients following 3 years of sirolimus monotherapy demonstrated an increase in IL-17A producing cells with IL-17A expression compensated for by increase in Treg cell frequency and number. Additionally, we demonstrated that such Th17 cells could be effectively regulated by Treg cells in vitro.
Results
Impaired renal function in patients with a history of rejection following alemtuzumab induction
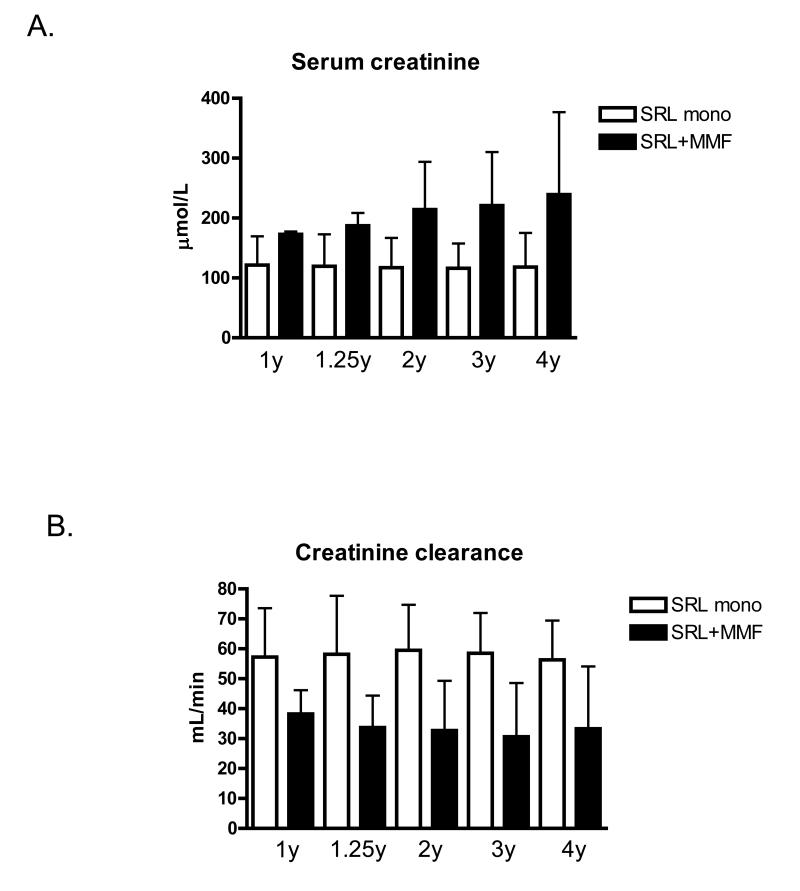
Alemtuzumab induced patients participating in this study (n=10) were divided according to their history of rejection and current immunosuppressive drug regimen. 7 patients remained rejection free and at 1 year after transplantation were converted to sirolimus monotherapy. The remaining 3 patients experienced biopsy proven rejection (one acute rejection at day 70 and two chronic rejection at about 1 year) and therefore remained on sirolimus and MMF therapy. As demonstrated on Fig 1, patients with a history of rejection had impaired renal function during the whole period of stable maintenance immunosuppressive therapy (between 1 and 4 years after transplantation) compared to the sirolimus monotherapy group.
Figure 1.
Serum creatinine and calculated creatinine clearance at different time points post-transplant in renal transplant recipients divided according to maintenance immunosuppression regimen.
Higher number of IL-17A-producing CCR6+CD4+ T cells in renal transplant patients treated with sirolimus monotherapy
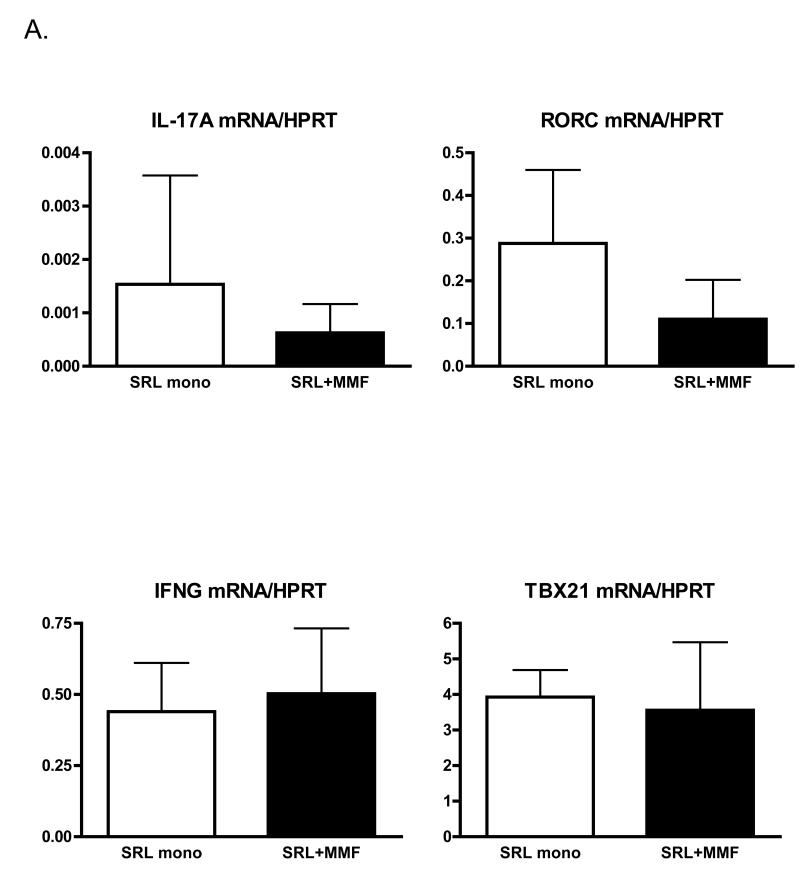
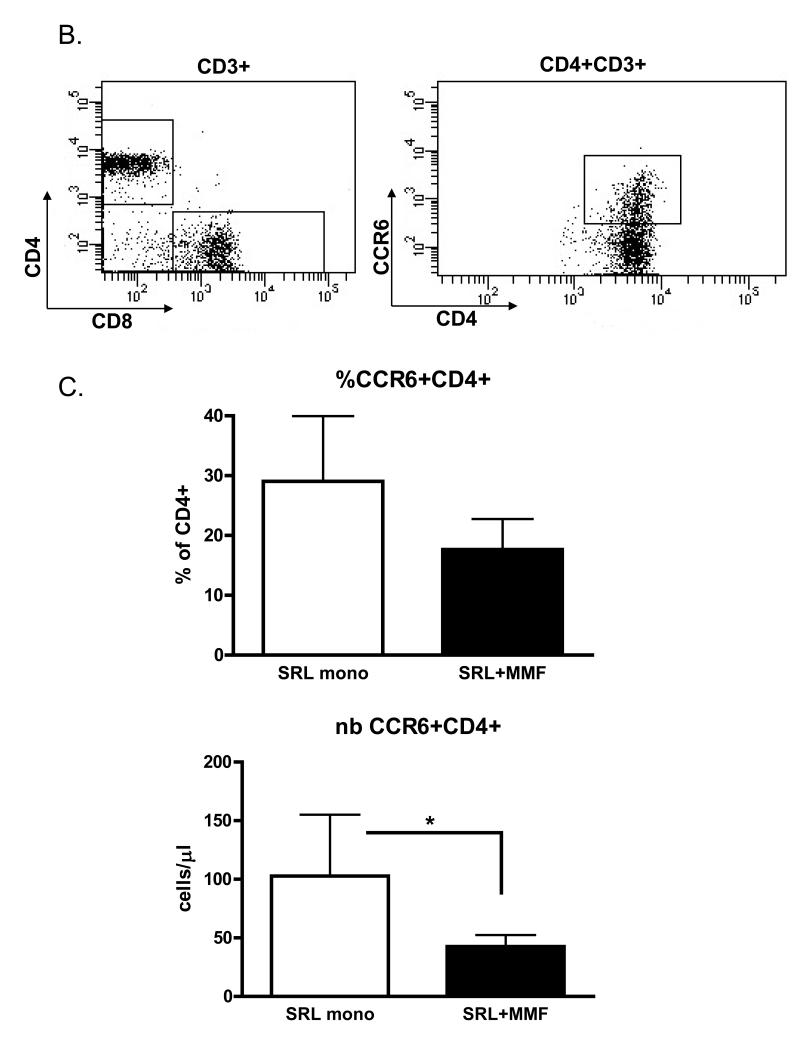
To determine the effect of immunosuppressive drug regimen on T cell cytokine and transcription factor signatures in the longer term after transplantation (>4 years) we analysed peripheral blood from renal transplant recipients treated with the same immunosuppressive drug regimen for a minimum of 3 years. Expression of IFNγ (Th1 signature cytokine), TBX21 (gene encoding T-bet – Th1 transcription factor) as well as IL-17A (Th17 signature cytokine) and RORC (gene encoding RORγt – Th-17 transcription factor) mRNA levels were quantified. Expression levels of both Th1 signature genes were similar in both groups of patients (Fig 2A). Unexpectedly, in patients treated with sirolimus monotherapy we observed tendency towards higher expression of both IL-17A and RORC genes, suggesting activation of the Th17 lineage (Fig 2A). Both IL-17A and RORγt have been demonstrated to be expressed by a variety of cell types in addition to CD4+ T cells, including NKT cells, gamma,delta T cells and CD8+ T cells (15-17). To determine if the observed upregulation in IL-17A and RORC expression in the peripheral blood was indeed correlated with polarisation towards the Th17 pathway we analysed the frequency and absolute number of CCR6+CD4+ T cells. The chemokine receptor, CCR6 has previously been described as a phenotypic marker of Th17 cells (13, 14). In agreement with the gene expression data (Fig 2A), we observed a higher percentage and absolute number of CCR6+CD4+ T cells in the peripheral blood of patients treated with sirolimus monotherapy (Fig 2B and C), however only the difference in the number of cells was statistically significant. As expected for a differentiated effector population, CCR6+CD4+ T lymphocytes mainly possessed a memory phenotype (data not shown).
Figure 2.
An increased number of Th17 cells in patients treated with sirolimus monotherapy. (A) Quantitative PCR measurement of IL-17A, RORC, IFNG and TBX21 mRNA in peripheral blood of kidney transplant recipients after 3 years of the indicated maintenance immunosuppressive treatment. Data expressed in arbitrary units calculated using the 2−ΔΔCt method normalized to HPRT expression. (B) Example CCR6+CD4+ T cells gating. (C) Frequency and absolute number of CCR6+CD4+ T cells in the peripheral blood of renal transplant recipients after 3 years of the indicated maintenance immunosuppressive treatment.
Increased frequency and number of Treg cells in patients treated with sirolimus monotherapy
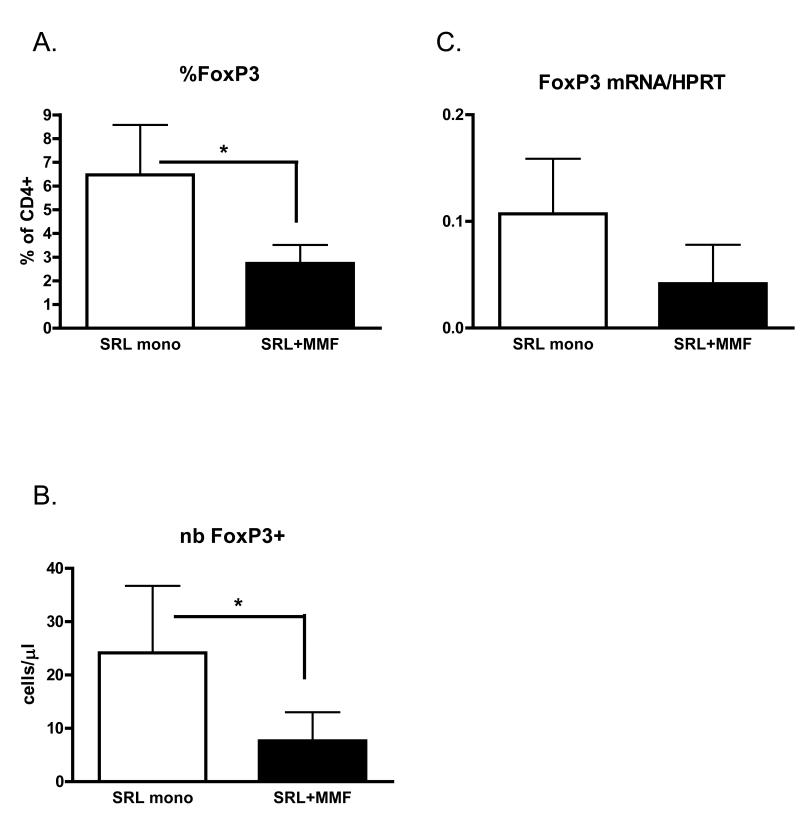
Interestingly, despite the increase in the number of IL-17A-producing cells, patients treated with sirolimus monotherapy had better renal function (Fig 1). The alloresponse depends not simply on the presence of effector populations, but rather on the fine balance between effector and regulatory mechanisms. We therefore hypothesised that Th17 cells present in the peripheral blood of these patients may be controlled by Treg cells. Indeed, we observed a higher frequency and absolute number of Treg cells in the peripheral blood of these patients as compared to patients treated with sirolimus and MMF (Fig 3A and B). These results were confirmed by detection of higher FoxP3 mRNA levels in peripheral blood in the sirolimus monotherapy group (Fig 3C).
Figure 3.
Increased frequency and number of Treg cells in patients treated with sirolimus monotherapy. (A) Frequency and (B) number of FoxP3+CD4+ T cells in patients divided according to maintenance immunosuppression regimen. Cells were gated as FoxP3+CD4+CD3+7-AAD−. (C) Quantitative PCR measurement of FoxP3 mRNA in peripheral blood. Data expressed in arbitrary units calculated using the 2−ΔΔCt method normalized to HPRT expression.
Regulation of Th17 cells by T regulatory cells
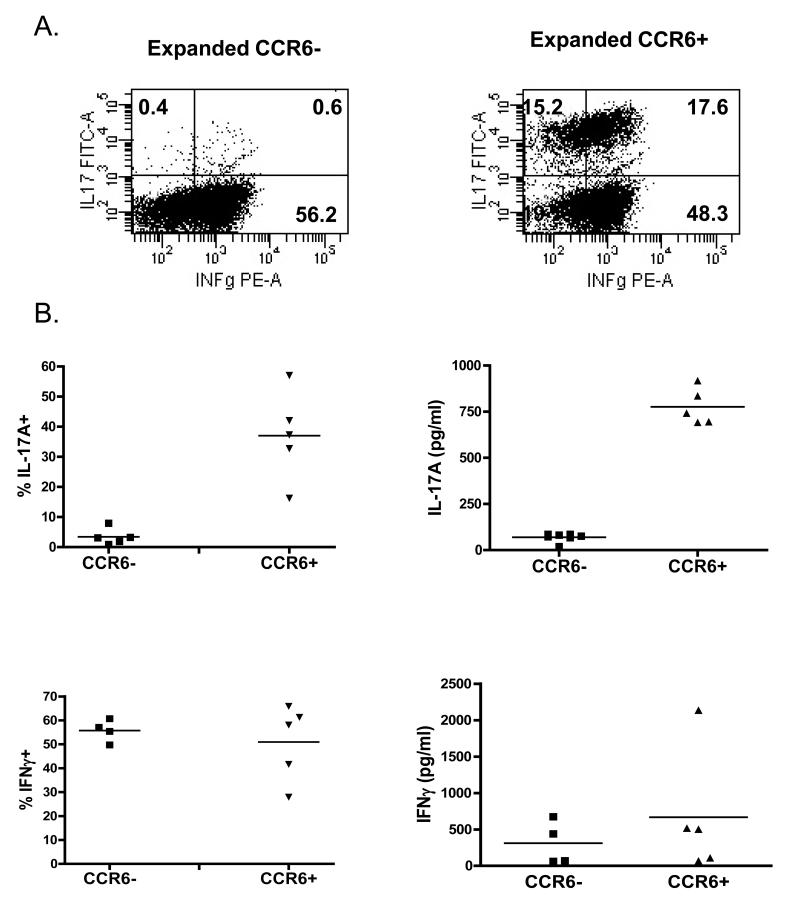
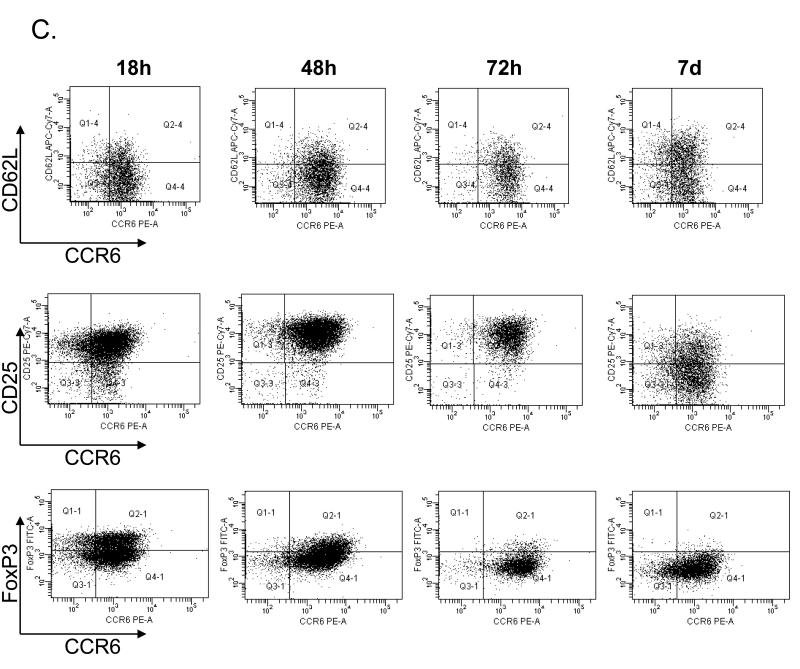
In order to study the functional properties of memory Th17 cells, we developed a strategy to purify this cell population and expand them in vitro. CCR6+CD25−CD4+ and CCR6−CD25−CD4+ T cells were sorted by flow cytometry. The CD25 marker was included into the sorting strategy to exclude a small population of CCR6+CD25+FoxP3+CD4+ Treg cells (data not shown). Sorted cells were expanded in the presence of anti-CD3/anti-CD28 beads and IL-2. As expected, CCR6+ cultures included cells producing either IL-17A or IFNγ only or both cytokines, whereas in the CCR6− cultures only IFNγ-producing cells were found (Fig 4A). As shown in Fig 4B differences in intracellular IL-17A or IFNγ production were also reflected by cytokines levels present in the culture supernatants. We detected significantly more IL-17A in culture supernatants of CCR6+ than CCR6− cells, whereas IFNγ production was similar in both cell populations studied (Fig 4B). As expected, activation of CCR6+CD4+ T cells results in temporal upregulation of CD25 and FoxP3 which decreased with time, whereas CCR6 expression remained high during and after expansion (Fig 4C). Transient upregulation of FoxP3 in non-Treg cells upon activation is well described in literature (18), and as we demonstrate here, Th17 cells are no different from other CD4+ T cells in this aspect.
Figure 4.
CCR6+CD4+ T cells may be expanded in vitro and retain IL-17A production. (A) Example of intracellular cytokine staining for IL-17A and IFNγ of expanded CCR6− CD4+ (left) and CCR6+CD4+ T cells (right panel). (B) Percentage of cytokine producing cells measured by intracellular cytokine staining of expanded CCR6−CD4+ and CCR6+CD4+ T cells (left panels). Supernatant levels of IL-17A and IFNγ from expanded CCR6−CD4+ and CCR6+CD4+ T cells (right panels). (C) Expression of CCR6, CD25, CD62L and FoxP3 on CCR6+CD4+ T cells during in vitro expansion.
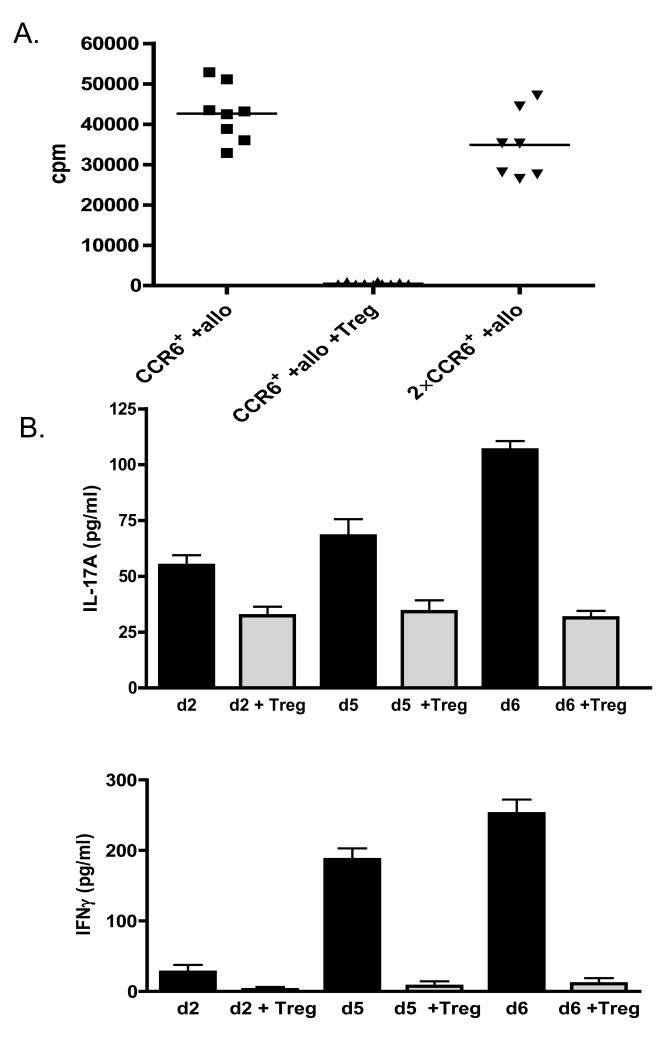
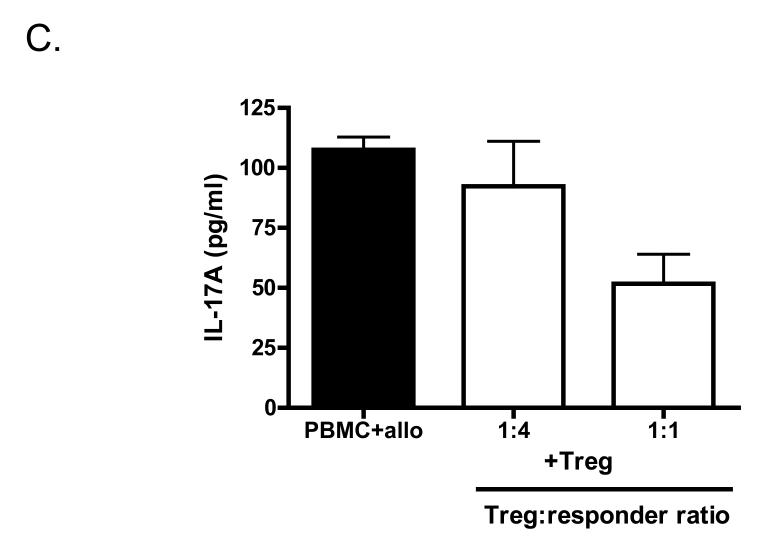
Finally, we asked whether CCR6+ T cells can be regulated by Treg cells. We have previously demonstrated that ex vivo expanded CD127loCD25+CD4+ Treg cells are suppressive both in vitro and in vivo in a humanized mouse model of transplant arteriosclerosis (19). Importantly, we demonstrated that Treg cells efficiently suppress IFNγ production in vitro and in vivo in the allograft itself. Here, we hypothesise that Treg cells may be effective suppressors of Th17 cells. Expanded CCR6+ T cells were therefore stimulated with allogeneic PBMC and co-cultured with expanded CD127loCD25+CD4+ Treg cells at a 1:1 ratio (Fig 5A). In the presence of Treg cells proliferation of CCR6+ T cells was completely inhibited. Additionally, production of both IL-17A and IFNγ was inhibited by addition of Treg cells (Fig 5B). Interestingly, Treg-mediated suppression of IFNγ production was much more effective than inhibition of IL-17A production (Fig 5B). Finally, we asked if Treg cells are able to suppress IL-17A production by allo-stimulated unmanipulated PBMC. As demonstrated in Fig 5C, the amount of IL-17A secreted into the culture medium is reduced by co-culture with Treg cells in a dose dependent manner. These results suggest that Treg cells are able to suppress not only ex vivo expanded Th17 cells but also unmanipulated cells.
Figure 5.
CCR6+CD4+ T cell proliferation and cytokine production is controlled by Treg cells. (A) Expanded CCR6+CD4+ T cells were stimulated with irradiated allogeneic PBMC and incubated with or without expanded Treg cells at a 1:1 ratio. Double the number of allo-stimulated CCR6+CD4+ T cells (2×CCR6+) served as a control. Cells were incubated for 7 days, 3H-Thymidine was added for the last 16-18h of the culture. Results are depicted as counts per minute (cpm). (B) Measurement of supernatant levels of IL-17A and IFNγ taken on day 2, 5 and 6 of allo-stimulated expanded CCR6+CD4+ T cells cultured in absence or presence of expanded Treg cells at a 1:1 ratio. (C) IL-17A levels in supernatants from PBMC stimulated with irradiated allogeneic PBMC cultured in absence or presence of expanded Treg cells in 1:1 and 1:4 Treg:responder ratio.
Taken together these data demonstrate that the increase in cells with Th17 characteristics observed in the peripheral blood of renal transplant recipients converted to sirolimus monotherapy may be compensated for by an increase in Treg cells. Additionally, we have shown that Treg cells are able to control proliferation and cytokine production of these Th17 cells.
Discussion
Th17 cells are a recently described subpopulation of CD4+ T cells with a well demonstrated role in induction and pathogenesis of various autoimmune diseases. Their possible role in allograft rejection and alloantigen-driven immune responses remain unsolved. Expression of IL-17 in transplant recipients was demonstrated long before the discovery of Th17 cells. For example, IL-17 mRNA and protein expression was found in human borderline rejection renal allograft biopsy tissue (20) and shown to be upregulated in bronchoalveolar lavage during acute rejection after lung transplantation (21). Interestingly, higher IL-17A serum levels have been shown in patients awaiting a second renal transplant following graft dysfunction when compared to patients with end-stage renal disease (22). Finally, in chronically rejected renal allografts IL-17 expression correlated with faster progression of rejection (23). Although the presence of Th17 cells has been associated with the rejection process (24), relatively little is known about the impact of immunosuppressive treatment on these cells.
Although limited by number of patients available for analysis in this pilot study, our data demonstrate an interesting effect of immunosuppressive drug regimen on the balance betweenTh17/Treg cells in the long term (> 4 years) after kidney transplantation. Sirolimus and MMF maintenance therapy used to treat patients with a history of rejection was associated with lower IL-17 gene expression in the blood and lower number of circulating, IL-17 producing T cells compared to patients treated with sirolimus monotherapy. Interestingly, one of the major side effects of MMF treatment is its myelosuppressive effect (25). In a mouse model, MMF was found to induce neutropenia by inhibiting IL-17 production by T cells and subsequent G-CSF induction (26). In an elegant study, von Vietinghoff and colleagues demonstrated that administration of MMF resulted in a dose-dependent decrease in blood neutrophils and this effect could be overcome by injection of IL-17 during drug treatment. Moreover, mycophenolic acid suppressed IL-17 production by T cells both in vitro and in vivo (26). Therefore, we postulate that the relative decrease in IL-17A expression and Th17 cells levels we observed in patients treated with sirolimus and MMF maybe a result of MMF activity.
The effects of immunosuppressive drugs on Treg cells have been studied more extensively. The detrimental effect of calcineurin inhibitors (CNI) on Treg cells has been well documented in vitro and in vivo in animal models (27). Additionally, results from clinical studies demonstrate that conversion from CNI-based protocols to sirolimus monotherapy resulted in an increase in percentage of circulating Treg cells (28). In contrast, the mTOR inhibitor sirolimus was shown to be beneficial for Treg generation and function in vivo in mouse models (27) and in cultures of human Treg cells (29). Alemtuzumab treated patients maintained on sirolimus monotherapy were found to have significantly higher levels of Treg cells than patients treated with anti-CD25 induction therapy followed by conventional long-term immunosuppression using cyclosporine A, steroids and MMF (7). Our own results presented here also suggest that sirolimus monotherapy is more beneficial for Treg cells than combined therapy with sirolimus and MMF. While the effect of MMF on Treg cells has not been studied so intensively, Zeiser et al. demonstrated that, in contrast to cyclosporine A, both rapamycin and MMF preserved mouse Treg both in vitro and in vivo in GVHD model. However, the MMF effect was inferior to rapamycin, especially in vivo (30).
Our results demonstrate that patients with a history of rejection, treated with sirolimus and MMF maintenance therapy, were characterised by decreased percentage and number of Treg when compared to nonrejecting patients treated with sirolimus monotherapy. However, we can not elucidate whether the observed differences were the effect of the rejection process itself or rather were influenced by MMF treatment. But more importantly, we have shown that Treg cells induced in sirolimus treated patients can inhibit Th1/Th17cell proliferation and cytokine production. The ability of Treg cells to suppress Th17 responses remains controversial. First reports indicated that Th17 cells are not regulated by Tregs, but subsequent studies demonstrated the ability of Treg cells to suppress Th17 cells and Th17 responses, especially in vivo (31, 32). Our data demonstrate ability of human Treg cells to suppress Th17 cell proliferation and cytokine production in vitro and suggest that the same may be true in vivo. It needs to be emphasised, however, that unlike IFNγ, IL-17A production was only partially suppressed, even though a high, 1:1 ratio of Treg to responder cells was used, what suggests the limited capacity of Treg in regulation of Th17 responses. However, observations made in this study due to limited number of patients need to be treated with caution and would require further in-depth analysis in a larger cohort of patients.
Material and Methods
Patients
Ten kidney transplant recipients were treated at the time of transplantation with alemtuzumab and maintained on steroid free reduced maintenance immunosuppression. Patients gave informed consent when enrolling in this study. The study was approved by Oxfordshire Research Ethics Committee B under the reference number C02.225. During the first 6 months patients were treated with tacrolimus (to the level 5-8ng/ml) and MMF (500mg, twice daily), after this period tacrolimus was switched to low dose of sirolimus (adjusted to 5-8mg/ml). After 12 months MMF was withdrawn and patients remained on sirolimus monotherapy. During the follow-up period 3 patients experienced rejection episode. Patients with rejection (one acute rejection at day 70 and two chronic rejections at about 1 year) were successfully treated; their maintenance immunosuppression in a period 1-4 years after transplantation was sirolimus and MMF (250 or 500mg, twice daily). Graft function was monitored as serum creatinine levels and calculated creatinine clearance.
Specimen preparation
Venous blood samples were collected from patients at 4 years after transplantation into TEMPUS™ tubes (for RNA isolation) and EDTA tubes (for PBMC isolation). PBMC from patients and healthy blood donors (National Blood Service, UK) were obtained by centrifugation over Ficoll-Hypaque gradient (GE Healthcare).
Flow cytometry
The following monoclonal antibodies were used in flow cytometry analyses: CD25 PE-Cy7, CD127 PE, CD8 APC Cy7, CD62L APC Cy7, IFN-γ PE (all BD); CCR6 PE, IL-17A FITC, CD3 Pacific Blue and FoxP3 FITC and PE (eBioscience); CD4 ECD (Beckman Coulter). FoxP3 staining buffers were supplied by eBioscience. Analysis of patients’ leukocyte phenotype was performed using eight-colour flow cytometry (Aria, BD) and standard staining procedures. Analysis of intracellular cytokine production was performed after 5h restimulation with PMA (phorbol 12-myristate 1-acetate; 100ng/ml, Sigma) and ionomycin (1μg/ml, Sigma) in the presence of Golgi Stop (1 μl/ml, BD). FoxP3 staining buffers were used as fixation/permeabilisation and permeabilisation buffers.
Sorting and cell expansion
Th17 cells were sorted as CCR6+CD25−CD4+ T cells using Aria sorter and ex vivo expanded in the presence of antiCD3/antiCD28 beads (Invitrogen) and 100U/ml recombinant human IL-2 (Chiron) over two rounds of expansion 7 days each. Treg cells were sorted as CD127loCD25+CD4+ T cells and expanded in the presence of antiCD3/antiCD28 beads (Invitrogen) and 1000U/ml recombinant human IL-2 (Chiron) over two rounds of expansion 7 days each. In order to bring cells to the resting state, expansion was followed by two days of culture with no beads and in a reduced amount of IL-2 (20U/ml for CCR6+CD4+ cells and 200U/ml for Tregs) (19).
Mixed lymphocyte reaction
The suppressive effect of Treg on expanded CCR6+ T cells was tested by measuring proliferation after 7 days of culture of 1×105 CCR6+ T cells stimulated with 1×105 of irradiated allogeneic PBMC in the presence of 1×105 expanded Treg cells. Proliferation was assessed by adding 3H-thymidine (Perkin Elmer) for the last 16-18h of culture.
ELISA
IFN-γ and IL-17A ELISA was supplied by eBioscience and performed according to manufacturer’s instructions.
Real-time RT-PCR
Total RNA was isolated from blood samples with TEMPUS™ Spin RNA Isolation Kit (Applied Biosysytems), followed by cDNA sythesis using the High Capacity RNA-to-cDNA kit (Applied Biosysytems). Real-time PCR was performed with Strategene Mx3000P (Agilent Technologies) Sybr Green technology for HPRT, FoxP3, IFNG and IL-17A (primer sequences available on request) and TaqMan Gene Expression Assays (Applied Biosystems) for TBX21 and RORC. Quantification of mRNA expression was analyzed by the ΔCt method (33) and results were expressed in relation to the expression of housekeeping gene, HPRT.
Statistical methods
Statistical analysis was performed using Graphpad Prism software. Due to the low number of samples in the groups and inability to analyze normal distribution, the analysis was performed by the nonparametric Mann-Whitney test, p <0.05 was considered statistically significant.
Acknowledgements
The authors thank all patients taking part in the study and Sally Ruse and staff of the Oxford Transplant Centre for help with sample acquisition.
Abbreviations
- CNI
calcineurine inhibitor
- IFN
interferon
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- MMF
mycophenolate mofetil
- mTOR
mammalian target of rapamycin
- PBMC
peripheral blood mononuclear cells
- SRL
sirolimus
- Treg cell
regulatory T cell
Footnotes
Authorship: J.H., P.F. and K.J.W. participated in study design, J.H., N.M. performed the experiments, J.H. analyzed experimental data, S.S. analyzed clinical data, M.C-G. design primers and participated in some experiments, J.H. and K.J.W. wrote the article
Support: This work was supported by grants from The Wellcome Trust (KJW), Medical Research Council UK (KJW) and European Union – RISET framework 6 Integrated Project (JH and KJW)
Authors declare no conflict of interest.
References
- 1.Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993-2004. Transplantation. 2007;84(5):611. doi: 10.1097/01.tp.0000280553.23898.ef. [DOI] [PubMed] [Google Scholar]
- 2.Calne R, Moffatt SD, Friend PJ, et al. Campath IH allows low-dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation. 1999;68(10):1613. doi: 10.1097/00007890-199911270-00032. [DOI] [PubMed] [Google Scholar]
- 3.Knechtle SJ, Pirsch JD, Fechner H, J J, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3(6):722. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 4.Watson CJ, Bradley JA, Friend PJ, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation--efficacy and safety at five years. Am J Transplant. 2005;5(6):1347. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 5.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82(10):1342. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 6.Trzonkowski P, Zilvetti M, Chapman S, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8(2):338. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 7.Bloom DD, Chang Z, Fechner JH, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8(4):793. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- 8.Knechtle SJ, Pascual J, Bloom DD, et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. Am J Transplant. 2009;9(5):1087. doi: 10.1111/j.1600-6143.2009.02581.x. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 10.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001;72(2):348. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Ahmed E, Wang T, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182(10):6217. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachitskaya AV, Hansen AM, Horai R, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180(8):5167. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182(4):1794. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 18.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 19.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197(3):322. doi: 10.1002/path.1117. [DOI] [PubMed] [Google Scholar]
- 21.Vanaudenaerde BM, Dupont LJ, Wuyts WA, et al. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J. 2006;27(4):779. doi: 10.1183/09031936.06.00019405. [DOI] [PubMed] [Google Scholar]
- 22.San Segundo D, Lopez-Hoyos M, Fernandez-Fresnedo G, et al. T(H)17 versus Treg cells in renal transplant candidates: effect of a previous transplant. Transplant Proc. 2008;40(9):2885. doi: 10.1016/j.transproceed.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184(9):5344. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 24.Heidt S, San D, Chadha R, Wood KJ. The impact of Th17 cells on transplant rejection and the induction of tolerance. Curr Opin Organ Transplant. 2010;15(4):456. doi: 10.1097/MOT.0b013e32833b9bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zafrani L, Truffaut L, Kreis H, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009;9(8):1816. doi: 10.1111/j.1600-6143.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- 26.von Vietinghoff S, Ouyang H, Ley K. Mycophenolic acid suppresses granulopoiesis by inhibition of interleukin-17 production. Kidney Int. 2010;78(1):79. doi: 10.1038/ki.2010.84. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7(7):1722. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrikx TK, Velthuis JH, Klepper M, et al. Monotherapy rapamycin allows an increase of CD4 CD25 FoxP3 T cells in renal recipients. Transpl Int. 2009;22(9):884. doi: 10.1111/j.1432-2277.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 30.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffar Z, Ferrini ME, Girtsman TA, Roberts K. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B-cell recruitment and polymeric IgA and IgM levels in the airways. Eur J Immunol. 2009;39(12):3307. doi: 10.1002/eji.200939498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]