Abstract
As the second synapse in the central gustatory pathway of the rodent, the parabrachial nucleus of the pons (PbN) receives information about taste stimuli directly from the nucleus of the solitary tract (NTS). Data show that NTS cells amplify taste responses before transmitting taste-related signals to the PbN. NTS cells of varied response profiles send converging input to PbN cells, though input from NTS cells with similar profiles is more effective at driving PbN responses. PbN cells follow NTS input for the first 3 s of taste stimulation for NaCl, HCl, and quinine, but are driven in cyclic bursts throughout the response interval for sucrose. Analyses of the temporal characteristics of NTS and PbN responses show that both structures use temporal coding with equal effectiveness to identify taste quality. Thus, the NTS input to the PbN is comprehensive, in that PbN cells receive NTS input that could support broad sensitivity, systematic, in that the time course of PbN firing patterns depend reliably on the tastant, and efficient, in that information from the NTS is preserved as it is communicated across structures. Comparisons of NTS and PbN taste responses in rats form the basis for our speculation that in primates, where the central gustatory pathway does not synapse in the PbN, the function of the PbN in taste processing may have been incorporated into that of the NTS.
Keywords: taste, parabrachial pons, temporal coding
The parabrachial nucleus of the pons (PbN) is an obligatory synapse in the central gustatory pathway from the nucleus of the solitary tract (NTS) to the thalamus in rodents,1 but not in many primates,2 including humans. To understand why evolution has chosen to delete this synapse in primates, it may be useful to understand how the PbN in rodents modifies or transforms gustatory information that it receives from the NTS. Here we study this issue using large data sets of taste responses recorded from NTS and PbN neurons and data from simultaneous recordings of pairs of taste-responsive neurons, one in the NTS and the other in the PbN. We find that the transfer of information from the NTS to the PbN is comprehensive, in that PbN cells receive NTS input that could support broad sensitivity, systematic, in that the time course of PbN firing patterns depend reliably on the tastant, and efficient, in that information from the NTS is preserved as it is communicated across structures.
Transfer of Information to the Parabrachial Nucleus of the Pons: General Characteristics
Comparisons of taste-evoked response rates across neural structures have yielded conflicting results. Earlier studies comparing responses in the chorda tympani (CT) nerve, NTS, and PbN suggested that taste responses in the NTS were larger than those in both the CT and the PbN.3 However, when we compared response rates evoked by taste stimuli in the NTS and PbN collapsed across several studies,4–11 we found that taste responses in the PbN were larger, though only by ~10%, than those in the NTS (see Fig. 1). In studies of NTS cells identified electrophysiologically as NTS-PbN relay cells, we and others found that these cells showed larger taste responses than nonrelay NTS cells,12–14 suggesting that the amplification of taste responses that is seen in the PbN actually originates in NTS cells that provide their input.
Figure 1.
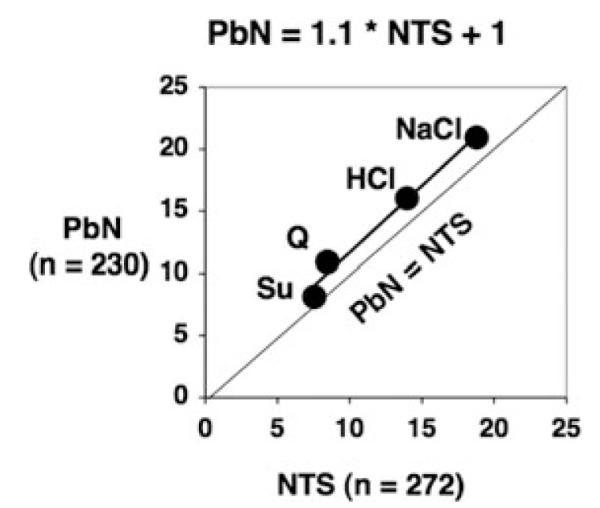
Relationship of taste response magnitude in the nucleus of the solitary tract (NTS) versus the parabrachial nucleus of the pons (PbN). Shown is a regression line fitted to the data; R2 = 0.99. Abbreviations are: Q, quinine; Su, sucrose.
In a study aimed at examining the nature of NTS input to PbN cells,11 we have shown that input from a heterogeneous assortment of NTS cell types, identified by the taste stimulus that evokes the largest response, that is, the best stimulus, converges onto single PbN cells. In that study, taste responses in 37 pairs of NTS and PbN cells were recorded simultaneously. Of these, 13 NTS-PbN pairs showed a significant peak in the cross-correlation function, implying that they were functionally connected. Seven pairs of connected cells had matching best stimuli (six pairs were both NaCl best and one pair were both HCl best), but in six pairs of NTS-PbN cells, the best stimulus of the NTS cell differed from that of the PbN cell (three NaCl-HCl, two HCl-NaCl, and one sucrose-NaCl). We then calculated the proportion of PbN response-related spikes that followed an NTS spike within 3 msec as an indication of the potential efficacy of the NTS input to a functionally connected PbN cell. Results showed that input from NTS cells that shared the same best stimulus with the PbN target was more effective in driving the response to the best stimulus than to other “side band” stimuli. In contrast, NTS input from cells that did not share the same best stimulus with the PbN target was nonstimulus selective. The observation that taste-responsive PbN cells receive input from all types of best stimulus types of NTS cells supports the contention that PbN cells may be latently responsive to a broad range of tastants. That is, the observation that PbN cells receive information about stimuli to which they show no response implies that there may be circumstances under which they would respond. A good example of this can be seen in the effects of taste adaptation. Specifically, following adaptation of the tongue to some tastants, some cells will respond to tastants to which they normally do not respond.15
Transfer of Information to the Parabrachial Nucleus of the Pons: Temporal Characteristics
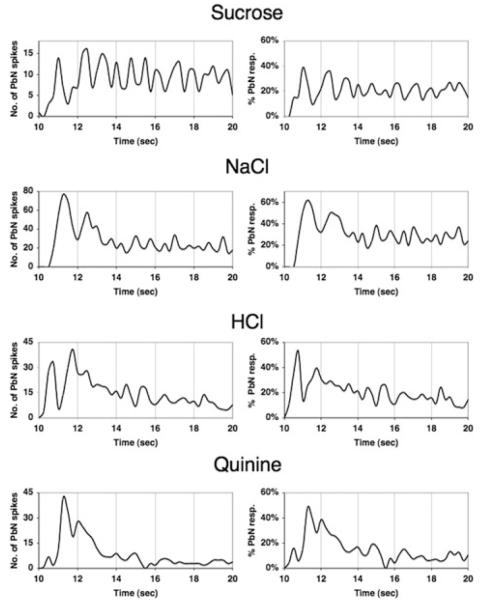
Because of our interest in the temporal coding of taste responses, we revisited the data collected from pairs of simultaneously recorded NTS-PbN cells to examine the time course of transfer of information.11 As above, we examined the timing of PbN spikes that occurred within 3 msec of an NTS spike in “connected” pairs, but we also noted the time of occurrence of these “NTS-driven” PbN spikes. The averaged time course of NTS input across functionally connected NTS-PbN cells is shown in Figure 2. In Figure 2 (left panels), it can be seen that input from the NTS is most effective during the initial ~ 3 s of the response for NaCl, HCl, and quinine. The time courses of this input could be roughly described as a damped oscillation. In contrast, the oscillatory NTS input for sucrose was sustained throughout the response interval. Figure 2 (right panels) shows the proportion of the total number of PbN spikes that were purportedly driven by NTS input over the time course of the (4 s) response. These plots indicate that, on average, NTS input contributes equally to all taste stimuli tested but there are dramatic differences in the time course of the efficacy of the NTS in driving PbN spikes.
Figure 2.
Time course of nucleus of the solitary tract (NTS) input to the parabrachial nucleus of the pons (PbN). (left panels) Plot of average number of PbN spikes that were preceded within 3 msec by an NTS spike in pairs of NTS and PbN neurons that were recorded simultaneously. For each stimulus, n = 10. (right panels) Plot of the proportion of the total number of PbN spikes that were preceded within 3 msec by an NTS spike over time in pairs of NTS and PbN neurons that were recorded simultaneously.
We next asked whether taste-responsive cells in the PbN exploit the temporal characteristics of their responses to aid in the identification of taste quality. Based on reports that spike timing in taste-evoked spike trains can contribute significantly to information conveyed by single cells in the NTS,16–18 we have applied similar analyses to taste-responsive cells in the PbN. Specifically, we isolated single taste-responsive cells in the PbN of urethaneanesthetized (1.5 gm/kg) rats and presented repeated trials of 0.1 M NaCl, 0.5 M sucrose, 0.01 M quinine HCl, and 0.01 M HCl, both preceded and followed by distilled water rinse. Spike trains during the first 2 s of response were analyzed with metric space analyses19,20 to determine the relative contribution of spike count (rate coding) and spike timing to the information conveyed by each cell about taste quality.
In brief, metric space analyses provide an index of the similarity of two spike trains in terms of both the number of spikes, called Dcount, and the timing of spikes within a response, called Dspike. With this method, the “cost” of transforming one spike train into another is calculated by first adding or deleting spikes (each event incurring a cost of “1”) and then by moving spikes in time. The cost of the latter procedure depends on the level of temporal precision, called “q,” applied to the measurement. The cost of moving a spike in time then is set at 1/q where q is in units of 1/s. The information conveyed by spike timing is then calculated at various values of q to determine the level of temporal precision that is maximally informative.19,20 At q = 0, the information is termed Hcount and is an index of how much information is conveyed by spike count alone. Hmax indicates the maximum amount of information conveyed by spike timing for that cell. In control analyses, H is recalculated for data sets where the spike trains are randomly assigned to taste stimuli, called Hshuffle, and when the timing of spikes in each response is randomly rearranged but the rate envelope remains intact, called Hexchange.
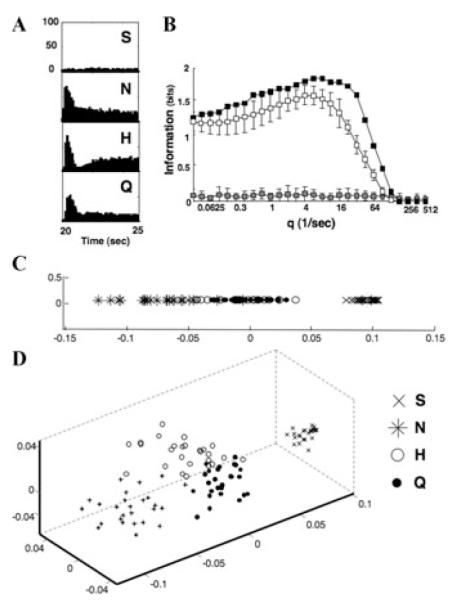
The results from one PbN cell are shown in Figure 3. In this broadly tuned cell (Fig. 3A), the information conveyed by spike count alone is 1.26 (out of a maximum of 2 bits to discriminate among four stimuli). However, consideration of spike timing in addition to spike count boosts the information to 1.84 bits (Fig. 3B). Multidimensional scaling (MDS) analyses of individual responses based on their spike count alone as a measure of similarity, shown in Figure 3C, shows that responses to NaCl, HCl and quinine evoke overlapping response magnitudes across trials. Sucrose is separated from the other three tastants because it does not evoke a response. In Figure 3D, results of MDS analyses in three dimensions using Dspike at qmax as a measure of similarity shows that responses to each taste stimulus are grouped in separate “clouds.” This implies that the temporal characteristics of their responses can be used to distinguish among them.
Figure 3.
Temporal coding analyses in one parabrachial nucleus of the pons (PbN) neuron, cell 1. (A) Peristimulus time histograms for all taste stimuli tested. Twenty-six trials were recorded for each taste stimulus. Abbreviations are: S, sucrose; N, NaCl; H, HCl; Q, quinine. (B) Results of metric space analyses for cell 1. The information conveyed by spike timing in the response peaks at Hmax = 1.84 at a value of q = 5.657. This value is greater than the value of Hexchange ± 2 SD at that q, indicating that spike timing conveys information above and beyond that conveyed by the rate envelope of the response. (C) Multidimensional scaling plot in one dimension of individual responses using Dspike at q = 0, that is, indicating spike count alone, as an index of similarity. Responses to different stimuli evoke similar response magnitudes on many trials. (D) Multidimensional scaling plot in three dimensions of individual responses using Dspike at q = 5.657 as an index of similarity. Response to all four stimuli form separate clouds in the space, indicating that their temporal patterns are distinct from each other.
Preliminary conclusions are based on recordings from 10 PbN cells. Thus far, results show that PbN cells are similar to NTS cells in that they use spike timing to convey information about taste quality. Importantly, the average contribution of spike count and spike timing in both structures are nearly identical: in both the PbN and the NTS16 the average Hcount is nearly identical (for the PbN, 0.96 ± 0.15 SEM; for the NTS, 0.96 ± 0.06 SEM); the average proportional contribution of spike timing in the PbN is 41% ± 9.4% SEM (excluding one outlier) and in the NTS is 45% ± 9% SEM.16 These results indicate that the information conveyed through temporal coding is preserved as it travels from the NTS to the PbN.
Speculation about Why the Parabrachial Nucleus of the Pons in Primates Does Not Seem to Be Part of the Gustatory Pathway
Since the pioneering study of Norgren and Pfaffmann in 197521 describing taste-responsive cells in the PbN, dozens of studies have focused on what the PbN might add to the process of gustation. In spite of this intense effort, few differences between taste-responsive cells in the PbN and its main source of taste-related input, the NTS, have been revealed. To illustrate this point, we compared taste processing in the NTS and PbN by constructing “taste spaces” for NTS and PbN responses in two dimensions using MDS analyses, with Pearson correlations as measures of interstimulus response similarities. Results, shown in Figure 4, confirm that taste responses to basic tastes are arranged identically in both structures.
Figure 4.
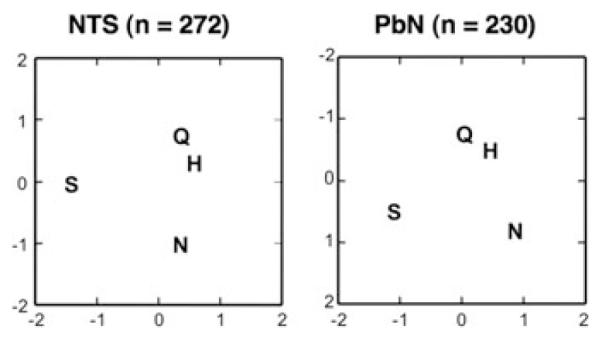
Results of multidimensional scaling analyses of taste responses in nucleus of the solitary tract (NTS) (left) and parabrachial nucleus of the pons (PbN) (right) cells. Responses are based on the average firing rate (spikes per s) over the first 5 s of response minus the baseline firing rate (no tastant presented). Abbreviations are: N, NaCl; H, HCl; Q, quinine; S, sucrose.
The striking similarity between taste spaces in the NTS and PbN points to the possibility that any differences in their roles may be a consequence of their outputs. For example, it is not unreasonable to suggest that the main function of the NTS may orchestrate orofacial reflexes while the PbN may parse information for sensory and hedonic evaluation related to ingestion. The respective anatomical relationships of each structure support this contention.1 To study this issue, we used the taste responses from the 37 pairs of simultaneously recorded NTS and PbN cells11 to construct taste spaces using MDS analyses as above. In these analyses we separated the early (first 0.5 s) from the later (3.5 to 4.0 s) portions of the response, given our data suggesting that after ~3 s taste processing in NTS and PbN are relatively independent. For each structure, a single MDS was conducted that included both the early and later portions of the response. Figure 5 shows the results of these analyses. During the earliest parts of the response, both the NTS and PbN can easily differentiate all four taste stimuli in the taste space. However, by the later portion of the response, the NTS mainly differentiates HCl and quinine versus NaCl and sucrose, that is, hedonically negative versus hedonically positive tastants. In contrast, the PbN singles out sucrose from the other tastants, suggesting a focus on nutritive value as well as hedonics.
Figure 5.
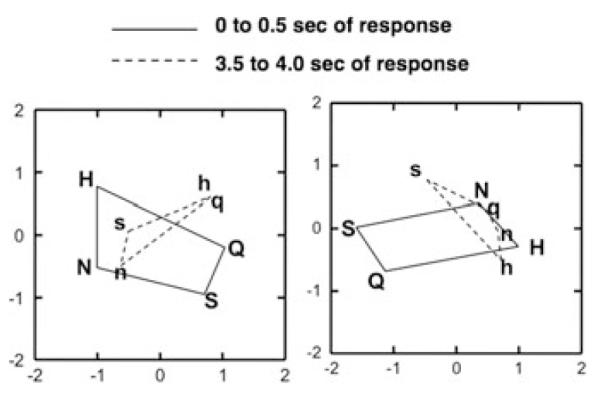
Results of multidimensional scaling analyses of taste responses in nucleus of the solitary tract (NTS) (left) and parabrachial nucleus of the pons (PbN) (right) cells that were recorded simultaneously (n = 37). Firing rates (in spikes per s) for each stimulus were based on the first 0.5 s or the period between 3.5 and 4.0 s of the response. Spontaneous rate was included. Abbreviations are: N and n, NaCl; H and h, HCl; Q and q, quinine; S and s, sucrose.
The fact that a synapse in the PbN along the central gustatory is missing in many primates2 begs the question of what, if any, neural structures in primates do what the PbN does in rodents. Here, we would like to speculate that the PbN became a vestigial structure for gustatory processing as evolution progressed and that its function with regard to taste was incorporated into more complex circuitry in the NTS in primates. Other functions, such as those associated with eating, for example, might still be the purview of the PbN in higher animals.
Summary and Conclusions
In conclusion, we have presented evidence concerning several aspects of the communication between the NTS and PbN. First, although PbN responses are larger than those in the NTS, the amplification takes place in NTS cells and is then conveyed directly to the PbN. Second, there is evidence that both NTS and PbN cells have the capability to respond more broadly across taste qualities than they normally express. This is supported by evidence that PbN cells receive input from a heterogeneous array of best stimulus cell types in the NTS, though input from NTS cells with similar profiles is more effective at driving PbN responses. Third, the efficacy of NTS input to the PbN is most prominent in the initial 3 s of response for NaCl, HCl, and quinine, but is cyclic throughout the entire response interval for sucrose. This suggests that the information channel for sweet is subject to less feedback inhibition than channels associated with other tastants. Fourth, analyses of temporal coding in PbN cells show that the temporal characteristics of NTS response are transmitted to the PbN with a high degree of fidelity.
Finally, we speculated on why the PbN is not part of the gustatory pathway in primates. We argued that in rodents, the NTS may orchestrate orofacial reflexes related to hedonic evaluation of taste stimuli while the PbN may parse taste information related to sensory, hedonic, and nutritive value. We further hypothesize that the PbN became vestigial with respect to sensory processing of taste, so that in primates the NTS has incorporated what the PbN does in rodents into its own circuitry.
Acknowledgments
Supported by NIDCD Grants RO1DC006914 to P. Di Lorenzo and RO1-MH-68012 to D. Gardner.
Footnotes
Conflicts of Interest The authors declare no conflicts of interest.
References
- 1.Lundy RF, Jr., Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego, CA: 2004. pp. 891–921. [Google Scholar]
- 2.Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J. Comp. Neurol. 1980;190:259–282. doi: 10.1002/cne.901900205. [DOI] [PubMed] [Google Scholar]
- 3.Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J. Neurophysiol. 2003;89:265–275. doi: 10.1152/jn.00870.2001. [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo PM, Lemon CH. The neural code for taste in the nucleus of the solitary tract of the rat: effects of adaptation. Brain Res. 2000;852:383–397. doi: 10.1016/s0006-8993(99)02187-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen J-Y, Di Lorenzo PM. Responses to binary taste mixtures in the nucleus of the solitarytract: neural coding with firing rate. J. Neurophysiol. 2008;99:2144–2157. doi: 10.1152/jn.01020.2007. [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo PM. Corticofugal influence on taste responses in the parabrachial pons of the rat. Brain Res. 1990;530:73–84. doi: 10.1016/0006-8993(90)90658-x. [DOI] [PubMed] [Google Scholar]
- 7.Di Lorenzo PM, Lemon CH, Reich CG. Dynamic coding of taste stimuli in the brain stem: effects of brief pulses of taste stimuli on subsequent taste responses. J. Neurosci. 2003;23:8893–8902. doi: 10.1523/JNEUROSCI.23-26-08893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo PM, Monroe S. Taste responses in the parabrachial pons of male, female and pregnant rats. Brain Res. Bull. 1989;23:219–227. doi: 10.1016/0361-9230(89)90151-2. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo PM, Monroe S. Corticofugal input to taste-responsive units in the parabrachial pons. Brain Res. Bull. 1992;29:925–930. doi: 10.1016/0361-9230(92)90167-v. [DOI] [PubMed] [Google Scholar]
- 10.Di Lorenzo PM, Monroe S. Corticofugal influence on taste responses in the nucleus of the solitary tract in the rat. J. Neurophysiol. 1995;74:258–272. doi: 10.1152/jn.1995.74.1.258. [DOI] [PubMed] [Google Scholar]
- 11.Di Lorenzo PM, Monroe S. Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Res. 1997;763:167–181. doi: 10.1016/s0006-8993(97)00217-5. [DOI] [PubMed] [Google Scholar]
- 12.Cho YK, Li CS, Smith DV. Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem. Senses. 2002;27:81–90. doi: 10.1093/chemse/27.1.81. [DOI] [PubMed] [Google Scholar]
- 13.Monroe S, Di Lorenzo PM. Taste responses in neurons in the nucleus of the solitary tract that do and do not project to the parabrachial pons. J. Neurophysiol. 1995;74:249–257. doi: 10.1152/jn.1995.74.1.249. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa H, Imoto T, Hayama T. Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Exp. Brain Res. 1984;54:349–358. doi: 10.1007/BF00236236. [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo PM. The neural code for taste in the brain stem: response profiles. Physiol. Behav. 2000;69:87–96. doi: 10.1016/s0031-9384(00)00191-8. [DOI] [PubMed] [Google Scholar]
- 16.Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J. Neurophysiol. 2003;90:1418–1431. doi: 10.1152/jn.00177.2003. [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo PM, Victor JD. Neural coding mechanisms for flow rate in taste-responsive cells in the nucleus of the solitary tract of the rat. J. Neurophysiol. 2007;97:1857–1861. doi: 10.1152/jn.00910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roussin AT, Victor JD, Chen J-Y, et al. Variability in responses and temporal coding of tastants of similar quality in the nucleus of the solitary tract of the rat. J. Neurophysiol. 2008;99:644–655. doi: 10.1152/jn.00920.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J. Neurophysiol. 1996;76:1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- 20.Victor JD, Purpura KP. Metric-space analysis of spike trains: theory, algorithms and application. Network. 1997;8:127–164. [Google Scholar]
- 21.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]