Abstract
Galectins, which were first characterized in the mid-1970s, were assigned a role in the recognition of endogenous (‘self) carbohydrate ligands in embryogenesis, development and immune regulation. Recently, however, galectins have been shown to bind glycans on the surface of potentially pathogenic microorganisms, and function as recognition and effector factors in innate immunity. Some parasites subvert the recognition roles of the vector or host galectins to ensure successful attachment or invasion. This Review discusses the role of galectins in microbial infection, with particular emphasis on adaptations of pathogens to evasion or subversion of host galectin-mediated immune responses.
Multicellular organisms and their commensal, symbiotic, or pathogenic microorganisms have co-evolved over millions of years. The evolutionary success of individual consortium members has depended either on the maintenance of a homeostatic balance for mutual benefit (symbiosis or commensalism) or on a colonization of the host that is beneficial to the microorganism but reduces fitness in the host (pathogenic)1. These cross-kingdom interactions take place through the sensing of soluble signals, such as hormone-like factors that are produced by both the host and the microorganism2, and direct cell–cell contacts3. Because virtually all bacterial and eukaryotic cells, as well as many viruses, display surface carbohydrates, it is thought that these molecules have a crucial role in the establishment of cell-to-cell contacts3. Indeed, many host–microorganism interactions have recently been shown to involve protein–carbohydrate recognition. Rapid modifications of exposed carbohydrate moieties by either microbial or host glycosyltransferases and glycosidases, and the equally dynamic patterns of expression of their protein receptors, further add to the complexity of host–microorganism interactions4,5. Similarly, within multicellular organisms, protein–carbohydrate recognition is crucial to intracellular processes, such as protein folding and transport, as well as interactions between cells, or cells and the extracellular matrix in functions ranging from cell differentiation, adhesion and migration, to embryogenesis, development and immune responses3–5. Therefore, it is recognized that cell surface glycans, including glycoproteins, glycolipids and polysaccharides, encode complex information that is decoded by specific binding to cell surface-associated or soluble carbohydrate–protein receptors, which are mainly known as lectins6.
Lectin–glycan interactions are ubiquitous and essential to biological systems, not simply as the ‘glue’ between cells, but as the initiators of a functional crosstalk that modulates their physiology and homeostatic balance7. Several microbial lectins are involved in host colonization, including viral haemagglutinins, bacterial adhesins and parasite lectins8,9. Likewise, some animal lectins can function as pattern-recognition receptors (PRRs)10, a group of diverse, soluble and membrane-associated molecules that include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NODs) and NK cell receptors (FIG. 1).
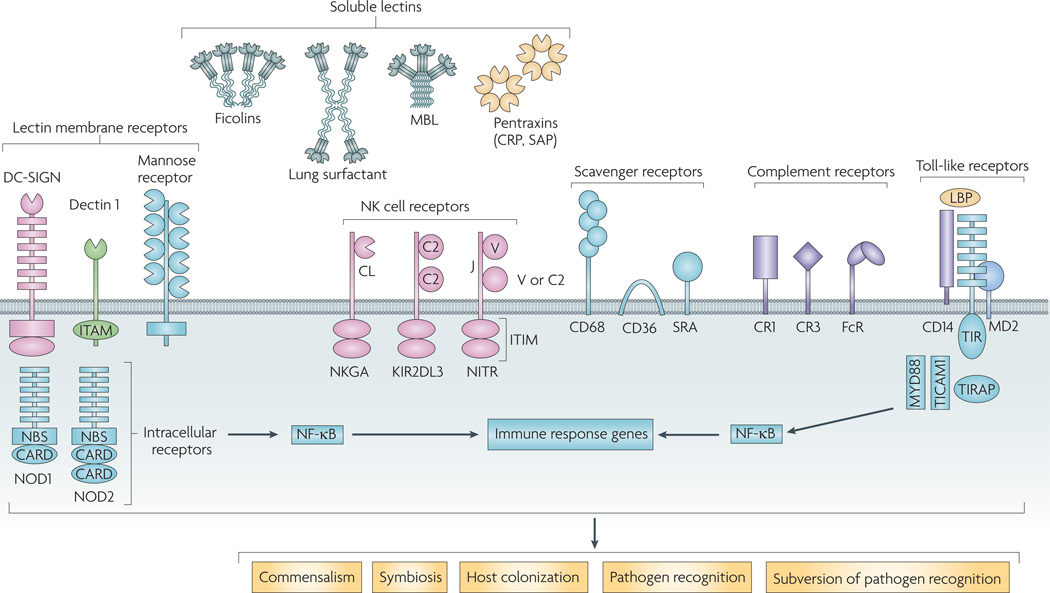
Figure 1. Pattern-recognition receptors and their participation in microbial-metazoan interactions.
Extracellular pattern-recognition receptors (PRRs) include the soluble lectins, such as collectins (for example, mannose-binding lectins and lung surfactants), ficolins and pentraxins (for example, C-reactive proteins and serum amyloid-P), and integral membrane C-type lectins, including dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN), dectin 1 and the macrophage mannose receptor. Other extracellular receptors shown are the natural killer (NK) cell receptors, scavenger receptors and complement receptors. The extracellular Toll-like receptors and the intracellular nucleotide-binding oligomerization domain (NOD) receptors, both of which are rich in leucine rich repeats, activate immune genes through nuclear factor- κB (NF- κB). Soluble and membrane-associated lectins mediate interactions with microorganisms that may lead to mutualistic interactions (commensalism or symbiosis), host colonization, immune recognition by the host or ‘subversion’ of the non-self recognition functions of the receptors by the microorganisms for attachment to the vector or host invasion. CARD, caspase recruitment domain; CRP, C-reactive protein; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibition motif; KIR2DL3, killer cell immunoglobulin-like receptor 2DL3; LBP, lipopolysaccharide-binding protein; MBL, mannan-binding lectin; NBS, nucleotide-binding site; NKGA, natural killer glycoprotein C-lectin receptor; NITR, novel immune-type receptor; SAP serum amyloid protein; TICAM1, TIR domain-containing adapter molecule 1; TIRAP, Toll/interleukin-1 receptor domain-containing adapter protein. Figure adapted, with permission, from REF. 147 ©(2003) Elsevier Science.
Galectins (formerly known as S-type lectins) were initially thought to bind only endogenous ‘self ‘ glycans and mediate developmental processes, including cell differentiation, tissue organization11 and, more recently, regulation of immune homeostasis12,13. In the past few years, however, it has become clear that galectins also bind glycans on the surface of potentially pathogenic microorganisms and parasitic worms, and mediate recognition and effector functions in innate immunity14.
In this Review, the roles of galectins in microbial infection are discussed, with a particular emphasis on adaptations to evade or subvert the host galectin-mediated immune responses.
Lectin–sugar interactions in consortia
The term lectin commonly refers to a wide range of carbohydrate-binding proteins and glycoproteins from viruses, bacteria (including adhesins and soluble lectins), fungi, protista, plants and animals6–9,15 (see Further information for a link to A Genomics Resource for Animal Lectins and the Functional Glycomics Gateway). Most lectins are organized as homooligomers or heterooligomers of non-covalently bound polypeptide subunits displaying carbohydrate-recognition domains (CRDs) that bind to the sugar ligand, usually a non-reducing terminal monosaccharide or oligosaccharide. Lectins have a diverse array of roles in symbioses, host colonization by microbial pathogens and immune responses of the host7 (FIG. 1).
Lectin–carbohydrate recognition is not only crucial to the establishment of specific mutualistic associations of symbiotic microorganisms with plants, corals and squid, but can also modulate the physiology of either the host or the symbiont16–18. This carbohydrate-mediated crosstalk is particularly intriguing in host gut–microbial symbioses19 in which reciprocal, tightly regulated interactions modulate the composition and function of the microbiota20. One mechanism that contributes to the tolerance of the resident microbiota is molecular mimicry, whereby bacteria display surface molecules that resemble those of the host’s surface to render them immunologically inert, while enzymatically modifying the host mucosal glycome for a mutually beneficial microbial colonization of the gut21.
During the host colonization process, pathogens ranging from viruses to protists bind to host cell surface glycans through surface lectins8,9. Influenza A haemagglutinins bind to sialic acids on the surface glycans of bird and mammalian cells22. Similarly, specific adhesins in pili and fimbriae mediate interactions between bacteria and cell surface carbohydrate ligands on specific host tissues23. Host colonization by protozoan parasites, such as Entamoeba hystolytica24, Plasmodium falciparum25, Cryptosporidium species26 and Toxoplasma gondii27, is also mediated by lectins. Reciprocally, soluble and membrane-associated lectins of the host cell are crucial recognition molecules that can facilitate the establishment of favourable mutualistic interactions with the colonizing microorganisms, or initiate innate and adaptive immune responses against pathogens. In addition to pathogen recognition, lectins also mediate downstream effector functions, such as agglutination, immobilization, opsonization and phagocytosis of potential pathogens, as well as leukocyte migration and complement activation7. The increasing availability of amino acid sequences and crystal structures of C- and S-type characteristic sequence motifs in animal lectins28 has allowed us to classify them into several families (TABLE 1). In the C- and F-type lectin families, CRDs can be found in combination with a range of unrelated domains to form multifunctional chimerical molecules29–31. Although interactions between animal lectins and their legends on the microbial surface are weak compared with other immune recognition molecules, such as antibodies, high avidity for the target is achieved when multiple CRDs interact with the ligand simultaneously, as observed in collectins and other oligomeric lectins32. A less frequent organizational plan is the presence of tandemly arrayed CRDs within a polypeptide, such as that observed in the macrophage mannose receptor33, immulectins34, F-type lectins30,31 and tandem repeat-type galectins11. C-type lectins, ficolins, pentraxins, F-type lectins and galectins are implicated in immune functions7.
Table 1.
Classification of animal lectins
| lectin family | ca2+ use | Specificity | Defining features | localization |
|---|---|---|---|---|
| C type | Yes | Variable | C-type sequence motif | Cell membrane; extracellular |
| Calnexin and calreticulin | Yes | Glc1Man3–9 | Calnexin sequence motif | ER |
| Chilectins | No | Chitooligosaccharides | TIMβ or TIMα barrel fold | Extracellular |
| F box | No | GlcNAc2 | F-box sequence motif | Cytoplasm |
| F type | Variable | l-fucose | F-type sequence motif and F-type fold | Extracellular |
| Ficolin | Yes | GlcNAc and GalNAc | Ficolin sequence motif | Cell membrane; extracellular |
| Galectins | No | β-galactosides | S-type sequence motif | Cytoplasm and nucleus; extracellular |
| Heparin-binding type | No | Heparin or Heparan-SO42− | Basic amino acid clusters | Cytoplasm and nucleus; extracellular |
| Siglecs (I type) | No | Variable | Immunoglobulin-like domains | Cell membrane |
| L type | Yes | Variable | L-type sequence motif | ER, ERGICs and Golgi |
| M type | Yes | Man8 | M-type sequence motif | ER |
| P type (M-6-P R) | Variable | Man-6-P | P-type sequence motif | Cell membrane |
| Pentraxins | Most | Phosphocholine and galactosides | Multimeric binding motif | Extracellular |
| R type | No | Variable | R-type sequence motif | Golgi and cell membrane |
| Intelectins (X type) | Yes | Gal, galactofuranose and pentoses | Intelectin sequence motif | Cell membrane; extracellular |
ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment; Gal, galactose; GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Man, mannose.
Galectins: structure–function relationships
Galectins constitute a lectin family with a characteristic domain organization, affinity for β-galactosides (BOX 1), a characteristic CRD sequence motif and wide taxonomic distribution35. Based on structural features, mammalian galectins have been classified as proto, chimera or tandem repeat types36 (FIG. 2a). Proto-type galectins contain one CRD per subunit, and are usually homodimers of non-covalently linked subunits. Chimera-type galectins have a carboxy-terminal CRD that is similar to the proto type, joined to an amino-terminal peptide that is rich in glycine, tyrosine and proline. In the tandem repeat galectins, two CRDs are joined by a functional linker peptide. The proto and tandem repeat types comprise several distinct subtypes, which have been numbered according to the order of their discovery, and, so far, 15 have been described in mammals37. Galectins 1, 2, 5, 7, 10, 11, 13, 14 and 15 are of the proto type, galectin 3 is the only galectin of the chimera type and galectins 4, 6, 8, 9 and 12 are of the tandem repeat type. By contrast, nonmammalian galectins are named based on the organism in which they were discovered35,37.
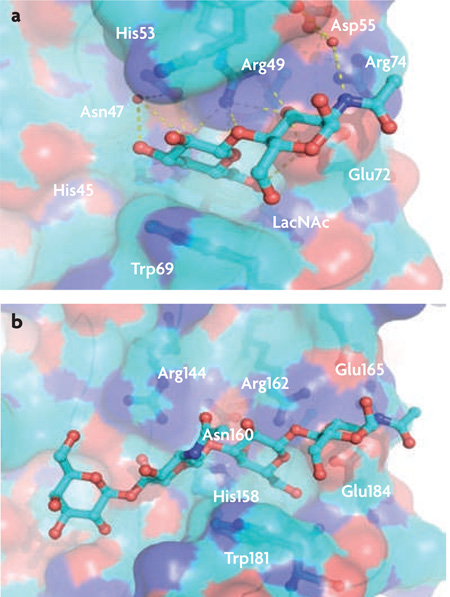
Galectin–oligosaccharide complexes.
Galectins are β -galactoside-binding lectins. Their preferred ligands are N-acetyll actosamine (LacNAc; Galβ 1,4GlcNAc) and related disaccharides, and they have dissociation constants in the order of 10−5 M31. Glycans that contain polylactosamine chains ((Galβ 1,4GlcNAc)n), such as laminin, fibronectin, lysosome-associated membrane proteins and mucins, are the preferred endogenous ligands for galectins from vertebrates48–51. Determination of the crystal structure of bovine galectin 1 complexed with LacNAc (see the figure, part a) revealed the galectin structural fold, and enabled the identification of the amino acids in the galectin polypeptide and the hydroxyl groups of the sugar ligands that participate in protein-carbohydrate interactions44–47. Unlike galectin 1, galectin 3 (see the figure, part b) has an extended carbohydrate-binding site formed by a cleft open at both ends, which leads to increased affinity for glycans with multiple lactosamine units and substitution of the non-reducing terminal galactose moiety with ABH blood group oligosaccharides (Fucα 1,2, GalNAcα 1,3 (Fucα 1,2) and Galα 1,3(Fucα 1,2))47,48.
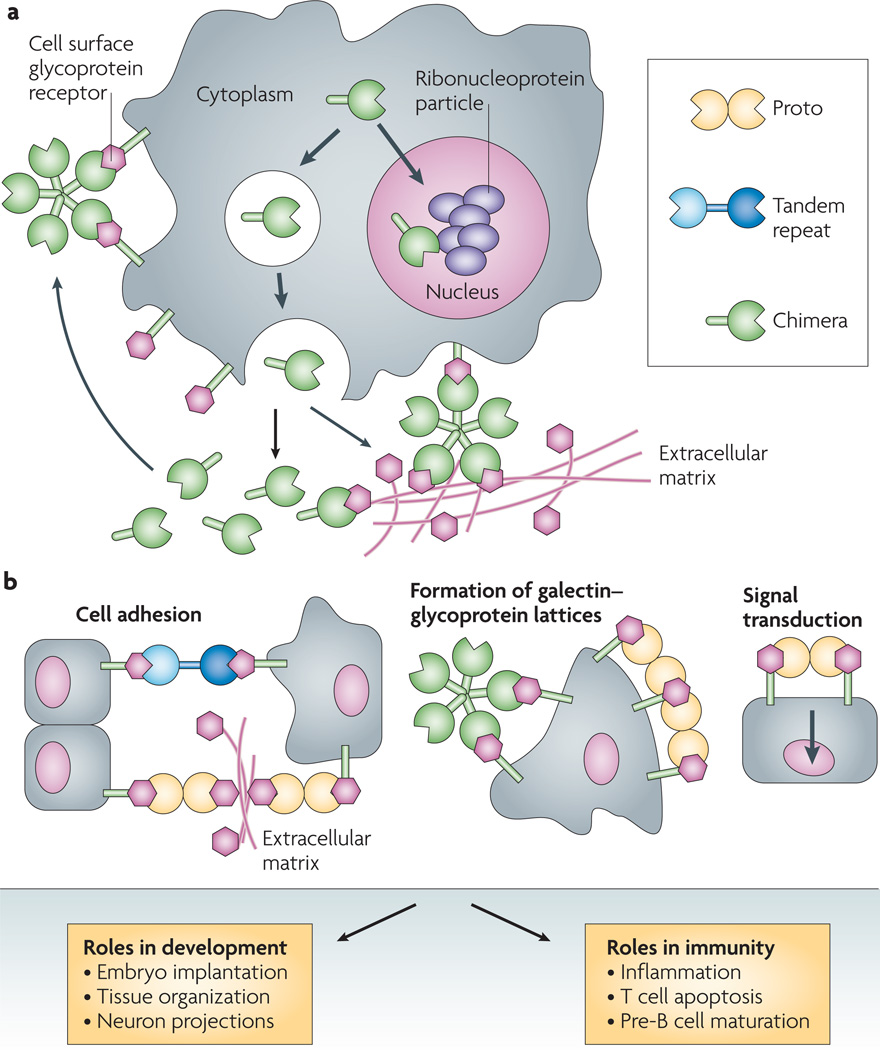
Figure 2. galectin types, binding activity and biological roles at the cell surface.
a | Subcellular localization and secretion. Based on structural features, galectins have been classified into three types: proto, chimera and tandem repeat. Proto-type galectins contain one carbohydrate-recognition domain (CRD) per subunit and are usually homodimers of non-covalently linked subunits (such as mammalian galectins 1, 2, 5, 7, 10, 11, 13, 14 and 15). By contrast, chimera-type galectins are monomeric with a carboxy-terminal CRD that is similar to the proto type, joined to anamino-terminal peptide that contains a collagen-like sequence rich in proline and glycine (such as mammalian galectin 3). Tandem-repeat galectins, in which two CRDs are joined by a linker peptide, are also monomeric (such as mammalian galectins 4, 6, 8, 9 and 12). From the cytosol, galectins can be translocated into the nucleus or into vesicles, or can accumulate at sub-cytosolic sites. Although galectins lack a typical secretion signal peptide, they can be targeted for secretion by non-classical mechanisms by direct vesicle translocation across the plasma membrane. Once in the extracellular space, they can bind to cell surface glycans and crosslink them with carbohydrate moieties in the extracellular matrix. b | Binding activity at the cell surface and biological roles. Secreted galectins can bind to the cell surface in cis or in trans, and exert a range of functions. The binding of galectins to cell surface glycans can crosslink glycans on neighbouring cells, leading to cell adhesion. Galectins can also form homogeneous lattices at the cell surface that can activate signalling pathways of functional relevance in the control of receptor endocytosis, host-pathogen interactions, and activation and homeostasis of immune cells. Part a adapted, with permission, from REF. 148 ©(2003) Oxford Univ. Press.
Most galectins are non-glycosylated soluble proteins, although galectins with transmembrane domains have been reported38,39. The presence of a galectin fold in the protistan parasite T. gondii 40, and lactose-binding galectin-like proteins in the fungus Coprinopsis cinerea41 and in the sponge Geodia cydonium42 reveal the early emergence and structural conservation of galectins in eukaryotic evolution. By contrast, galectin-like proteins, such as the lens crystallin protein galectin-related inter-fibre protein (GRIFIN)43 and galectin-related protein (GRP; previously known as HSPC159)44, lack carbohydrate-binding activity and are considered to be products of evolutionary co-option43.
Glycans that contain polylactosamine chains ((Galβ1,4GlcNAc)n), such as laminin, fibronectin, lysosome-associated membrane proteins and mucins, are the preferred endogenous galectin ligands45–52 (BOX 1). Most metazoans are endowed with a complex galectin repertoire, with members exhibiting multiple isoforms53 and subtle variations in carbohydrate specificity. This, together with the plasticity observed in sugar binding47, suggests that there is substantial diversity in their glycan-recognition properties.
Although galectins lack a typical secretion signal peptide, they are present not only in the cytosol and the nucleus, but also in the extracellular space37,54,55. From the cytosol, galectins can be targeted for secretion by non-classical mechanisms, possibly by direct translocation across the plasma membrane55 (FIG. 2a). The binding properties and biological functions of galectins in the oxidative extracellular environment, however, may depend on their immediate binding to ligand, which prevents the oxidation of free cysteine residues, as well as galectin susceptibility to proteolysis45,46,56. In solution, galectins can form multivalent species in a concentration-dependent equilibrium57, and their binding to β-galactoside-containing glycolipids and glycoproteins exposed on the cell surface (FIG.2b) can lead to the formation of lattices that cluster these ligands into lipid raft microdomains required for optimal transmission of signals relevant to cell function58–60 (BOX 2).
Galectin–ligand lattices at the cell surface lead to immunoregulatory activity.
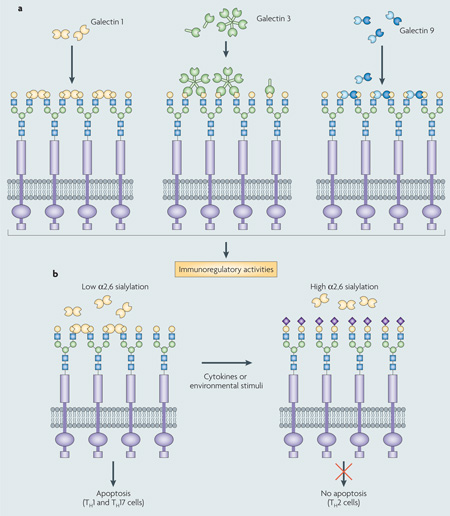
Proto-type galectins (for example, galectin 1) associate as non-covalently bound dimers through a hydroph obic interphase, whereas chimera galectins (galectin 3) associate through their amino-terminal domain to form oligomers that, in the presence of multivalent oligosaccharides, display binding cooperativity31 (see the figure, part a). Bivalent tandem repeat-type galectins (for example, galectin 9) can recognize ligands with a single polypeptide, although they can also form higher-order aggregates that enhance their avidity31,56 Galectin-mediated lipid raft assembly can modulate turnover of endocytic receptors, signal transduction pathways that lead to T cell activation and cytokine secretion or apoptosis, B cell maturation, activation and tolerance, and neutrophil activation that leads to phagocytosis, oxidative burst, and protease and cytokine release57. Galectin-glycoprotein lattices at the cell surface have therefore been proposed to function as an ‘on-off switch’ that regulates cell proliferation, differentiation and survival, including immune cell responsiveness and tolerance31,57.
Galectins form cell surface lattices and polarize the T helper response (TH1, TH2 or TH17) through galectin 1-induced apoptosis (see the figure, part b). Differential sialylation of cell surface glycoproteins selectively influences the formation of galectin-carbohydrate lattices in distinct TH cells, thereby regulating their susceptibility to galectin 1-induced cell death. T 1 and TH17 effector cells express cell surface glycans that enable the formation of galectin-glycoprotein lattices. By contrast, TH2 cells are protected from galectin 1 by differential α2,6-sialylation of surface glycoproteins. Figure adapted, with permission, from REF. 58 © (2007) Elsevier Science.
Roles of galectins in immune homeostasis
Galectins are ubiquitously expressed and distributed in mammalian tissues, including most cells of the innate (dendritic cells, macrophages, mast cells, natural killer cells, gamma and delta T cells, and B1 cells) and adaptive (activated B and T cells) immune systems61,62. The roles of galectins in the development and regulation of innate and adaptive immunity homeostasis, as well as responses to infectious and allergic challenge and cancer, have been recently reviewed12,13.
During the infection process, galectins have diverse effects on cells involved in innate immune responses, including macrophages, dendritic cells, neutrophils, eosinophils and mast cells (Supplementary information S1 (table)). Galectin 1 displays anti-inflammatory activities by blocking or attenuating signalling events that lead to leukocyte infiltration, migration and recruitment61,62. By contrast, galectin 3 shows pro-inflammatory activity, enhances macrophage survival, and positively modulates macrophage recruitment and antimicrobial activity63. Galectin 9 induces chemotaxis, activation, oxidative activity and degranulation of eosinophils. Similarly to galectin 1, it also induces monocyte-derived dendritic cell maturation64, although through different mechanisms and with different outcomes65,66.
With regards to adaptive immune responses, galectins are regulators of immune cell development and homeos-tasis4,12,61–65. Interactions between stromal cells from the bone marrow and thymic compartments and lymphocyte precursors are crucial to their development, selection and further progression to the periphery. Galectins can modulate B cell maturation and differentiation both at the central and peripheral immune compartments67. Galectin 1 can have pro- or anti-apoptotic effects on T cells depending on the developmental stage and activation status of the cell, and the microenvironment in which the exposure takes place68. The effects of galectin 3 on T cell survival, however, are dependent on whether the protein acts endogenously (anti-apoptotic) or by exogenous exposure (pro-apoptotic)63. Galectins also exert regulatory functions in T cell homeostasis, and signalling cascades triggered by their binding and lattice formation at the T cell surface have implications in a range of downstream events that modulate their differentiation, functional activation, and production of pro- and anti-inflammatory cytokines12,58 (BOX 2). Finally, given the regulatory roles of galectins on cells that mediate both innate and adaptive immune responses, their effects can be beneficial or detrimental for pathological conditions that are based on exacerbated or depressed immune function, such as inflammatory, allergic and autoimmune disorders, and cancer12,63,69.
Galectins as PRRs
Recently, galectins have been discovered to bind glycans on the surface of viruses, bacteria, protista and fungi14,70–72. Thus, the potential role of galectins as PRRs has become an area of increased attention.
Viruses
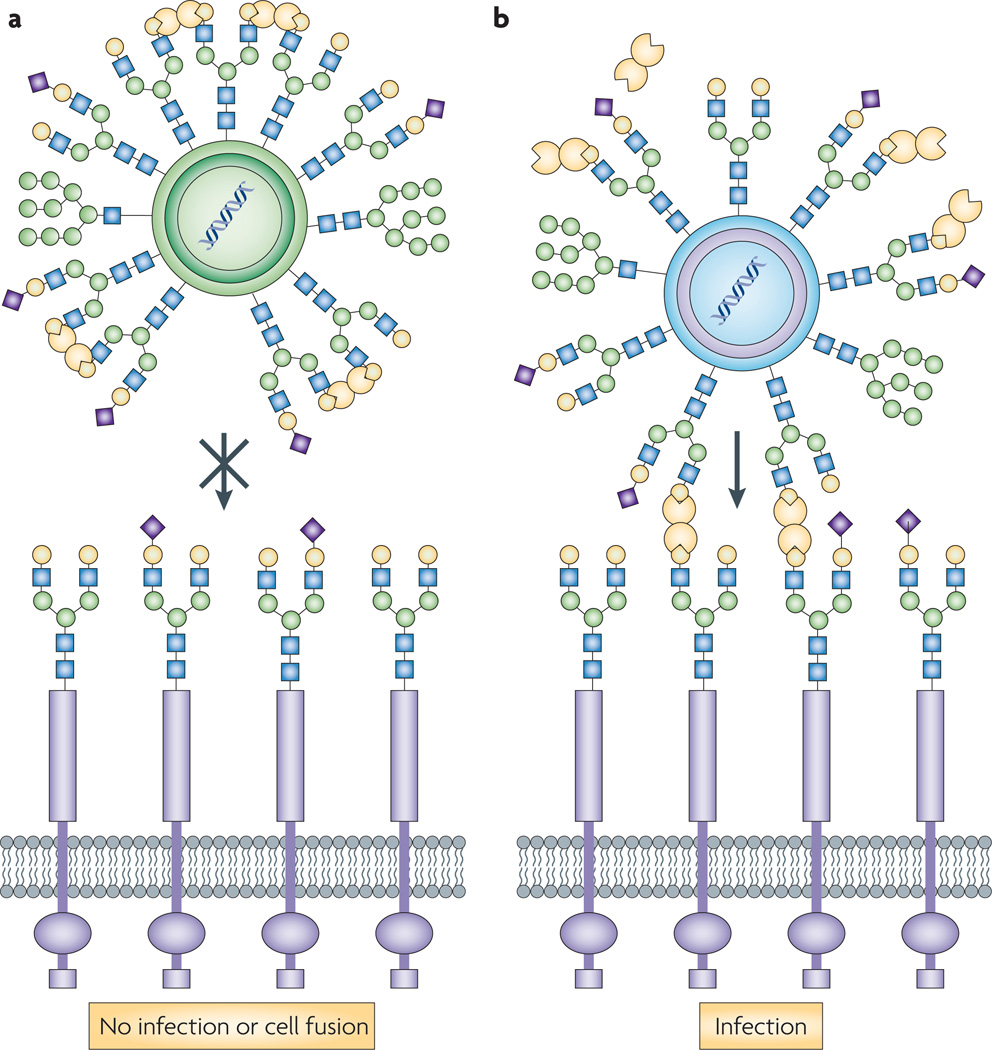
Galectin 1 can inhibit virus attachment and host cell fusion by binding N-linked oligosaccharides from the virion envelope or capsid glycoproteins, and promoting their crosslinking and oligomerization. Infection by the Nipah virus, a paramyxovirus that causes severe encephalitis, takes place by fusion and attachment of its envelope glycoproteins to the host endothelial cells through their ephrinB2, and alternatively ephrinB3 receptors, leading to cell–cell fusion and formation of syncytia72. In both Nipah virus and Hendra virus (another paramyxovirus that affects humans and animals), the dimeric galectin 1 specifically crosslinks the N glycans displayed in the envelope glycoproteins, causing aberrant oligomerization and blocking cell–cell fusion73 (FIG. 3a). experimental removal of the Nipah virus N glycans resulted in higher fusogenicity, but also increased sensitivity to neutralization by specific antisera, suggesting that although the viral glycans can hinder viral infectivity to some extent, they serve a protective function against the neutralizing host antibodies74. Modulatory effects of viral infection (for example, by herpesvirus 1, Newcastle disease and epstein–Barr virus)75–77 on galectin expression and function suggest that galectins participate at various levels of antiviral defence, from the initial recognition and blocking of envelope and fusion glycoproteins, to the activation and amplification of the innate and adaptive immune responses.
Figure 3. galectin 1 functions as a pattern-recognition receptor for paramixoviruses and HIV-1.
a | In both Nipah virus and Hendra virus (Paramyxoviridae family), galectin 1 specifically crosslinks the N glycans displayed in the envelope glycoproteins, causing aberrant oligomerization and blocking cell–cell fusion73. b | Galectin 1 promotes infection by HIV-1 by mediating viral attachment to host cell surface glycans113. Galectin 1 enhances HIV-1 adsorption kinetics on monocyte-derived host macrophages, which facilitates HIV-1 infectivity by shortening the time required to establish an infection114.
Bacteria
Galectins can interact directly with bacterial surface glycans. Furthermore, bacterial infection can modulate galectin expression, which in turn regulates leukocyte function and inflammatory responses. Both Gram-positive bacteria, such as Streptococcus pneumoniae, and Gram-negative bacteria, such as Klebsiella pneumoniae, Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, and Pseudomonas aeruginosa, display surface carbohydrate galectin ligands70,78–81. Galectin 3 binds to bacterial lipopolysaccharides (LPSs) in a dual manner: the C terminus CRD binds to lactosyl moieties of K. pneumoniae LPS, whereas the non-carbohydrate-binding N-terminal domain of galectin 3 binds to the lipid A moiety of Salmonella enterica subsp. enterica serovar Minnesota LPS70. Galectin 3 also binds mycobacterial phosphatidylinositol mannosides, glycolipids that accumulate on the membrane of Mycobacterium-containing phagosomes during infection82. Recent studies have shown that galectin 3 recognizes the O-antigen side chains on Helicobacter pylori, a species that can cause gastritis, in some cases leading to peptic ulcer, gastric adenocarcinoma or gastric lymphoma. Adhesion of H. pylori to the gastric lining upregulates expression and secretion of galectin 3 by gastric epithelial cells83, suggesting that galectin 3 might play a part in the host response to infection by promoting recruitment of phagocytic cells to the site of infection and induction of an inflammatory response, and by disrupting the interaction of the pathogen with the host cells. Furthermore, transcript profiling of H. pylori-infected gastric tissue in rhesus macaques revealed increased expression of galectin 8, and marginally, of galectins 3 and 4, whereas in in vitro infected gastric epithelial AGS cells, galectins 1 and 3 were upregulated84,85. Galectin 3 also has a role in neutrophil extravasation and recruitment during S. pneumoniae infection86,87. expression of galectin 9 in periodontal ligament cells is elevated after both in vitro and in vivo exposure to LPS from Porphyromonas gingivalis88. The galectin BbtGal-L from the cephalochordate amphioxus (Branchiostoma belcheri) specifically recognizes Vibrio vulnificus but not Vibrio parahaemolyticus or Staphylococcus aureus, and its expression is upregulated by bacterial challenge89. Taken together, these observations indicate that host galectins can bind directly to glycoconjugates on the surface of bacteria, either facilitating or inhibiting pathogen entry, followed by positive and negative regulation of host innate and adaptive immunity. Interestingly, a recent report suggests that galectin 3 protects the host from Salmonella LPS-induced endotoxin shock, but, paradoxically, favours bacterial survival90.
Fungi
Galectins can recognize surface glycans on the surface of saprophytic or pathogenic fungi. The binding of a galectin 3 homologue from the J774 mouse cell line to polysaccharides and glycolipids that display β1,2-linked oligomannosides on the cell wall of Candida albicans suggested that galectin 3 could function as a macrophage receptor for this opportunistic fungal pathogen91, either leading to a successful immune response or enabling intracellular parasitism. The differential recognition of C. albicans and Saccharomyces cerevisiae by macrophages takes place through a cooperative mechanism that involves TLR2 and galectin 3, which probably binds to carbohydrate ligands that are specific to the C. albicans cell wall92. In a mouse model of experimental colitis, pre-existing inflammatory conditions strongly promoted C. albicans colonization, which in turn augmented the inflammatory process with upregulation of TLR2 expression93. This supports the roles of galectin 3 in both inflammation and the regulation of host immune responses to C. albicans93. Interestingly, the selective binding of galectin 3 to the C. albicans cell wall glycans is fungicidal94, an observation that underscores how galectins have direct effector functions in innate immunity.
Protistan parasites
It has been recently recognized that host galectins can also directly bind carbohydrate moieties displayed on the parasite surface and function as PRRs. Lipophosphoglycan (LPG) on the surface of Leishmania parasites is recognized by both galectin 3 and 9, although with different outcomes95,96. Leishmania species are intracellular protozoan parasites that are transmitted by phlebotomine sandflies to vertebrate hosts, including humans, where they invade macrophages, and eventually reside within their fusogenic phagolysosomes and block macrophage activation97. Depending on the Leishmania species, the disease can take cutaneous (for example, Leishmania major, Leishmania pifanoi and Leishmania mexicana) or visceral (for example, Leishmania donovani and Leishmania infantum) clinical forms. Leishmania promastigotes, the parasite’s infective stage, have a complex glycocalyx composed of proteophosphoglycans, glycosylinositolphospholipids, the protease gp63 and abundant LPG. LPG consists of a phosphoglycan domain linked through a hexasaccharide glycan core to a 1-O-alkyl-2-lyso-phosphatidylinositol lipid anchor97. The LPG phosphoglycan moieties share a common backbone of PO4-6Gal(β1,4)Manα1 repeating units, in which the C3 position of the Gal residue can be either unsubstituted (for example, L. donovani), partially substituted with glucose side chains (for example, L. donovani, L. mexicana and Leishmania chagasi) or completely substituted with side chain sugars that terminate primarily in glucose and arabinose (for example, Leishmania tropica) or galactose (for example, L. major)71,98. In L. major, the poly-β-galactosyl moieties (Galβ1,3)n are recognized with similar affinity by galectin 3 and 9 (REFS 95,96). The significantly lower affinity of galectin 1 for (Galβ1,3)n can be attributed to the lack of an extended CRD that in galectin 3 would accommodate oligosaccharide repeats (BOX 1). Interestingly, Trichomonas vaginalis LPG is strongly recognized by galectin 1, which facilitates attachment of the parasite to the cervical epithelial cells99, suggesting that there are substantial differences in the structure of LPG from both parasite species. Furthermore, the recently elucidated structure of T. vaginalis LPG revealed the presence of polylactosamine100, which could be a good target for galectin 3 recognition.
The binding of oligomeric galectin 3 to L. major during the early stages of infection leads to its cleavage by the gp63 protease in the parasite glycocalyx96. Pelletier and Sato have proposed that this cleavage might lead to lattice disruption and initiation of signalling pathways that skew the immune response balance towards a T helper 1 (TH1)-type profile (BOX 2), thereby inducing a strong local inflammation that limits the infection to the dermal tissues and results in cutaneous lesions96. Leishmania species, such as L. donovani, that fail to cleave galectin 3 and only promote a limited inflammatory response at the primary site of infection would migrate further, leading to the visceral form of the disease96. Therefore, it is tempting to speculate that by its sensitivity to proteolytic attack, galectin 3 could also function as a ‘sensor’ for microbial infection. By contrast, the binding of galectin 9 would actually promote interactions between L. major and the host macrophages, although the underlying mechanisms that control this binding have not been characterized in detail95.
In the early stages of infection by the protozoan parasite Trypanosoma cruzi, the causative agent of Chagas disease, recognition of the parasite glycosylphosphatidylinositol anchors and DNA is carried out by TLR2 and TLR9, respectively, on macrophages and dendritic cells. This leads to the activation of both myeloid differentiation primary-response gene 88 (MYD88)- and TIR domain-containing adapter molecule 1 (TICAM1; also known as TRIF)-dependent pathways101. However, T. cruzi trypomastigotes are also specifically recognized by galectin 3 through its C terminus CRD, which mediates adhesion of the parasite to laminin and possibly facilitates entry into human coronary artery smooth muscle cells102. Reciprocally, T. cruzi infection upregulates expression of galectin 3 and its ligands on dendritic cells, which affects dendritic adhesion and migration103. This is concurrent with intense thymus atrophy followed by depletion of CD4+CD8+ immature cortical thymocytes through galectin 3-mediated migration and premature escape to the peripheral compartment, and increased apoptosis104. It also leads to interleukin-4 (IL-4)-induced survival of activated B cells, favouring their differentiation towards a plasma cell pathway, and increasing immunoglobulin (Ig) production and parasite clearance105. unlike galectin 3, galectin 1 does not recognize T. cruzi surface glycans, although its upregulation during T. cruzi infection can induce macrophage apoptosis and inhibit parasite replication106.
Infection by the apicomplexan parasite T. gondii is characterized by a TH1-polarized immune response in which galectin 3 has important roles both as a proinflammatory factor and in the regulation of dendritic cell function107.
Helminths and nematodes
Glycans are also present on the surface of parasitic worms, such as trematode helminths, commonly known as blood (Schistosoma spp.) or liver (Fasciola spp.) flukes, and nematodes, and are recognized by soluble and cell surface-associated C-type lectin receptors that modulate macrophage cytokine production and dendritic cell maturation, and initiate innate immune responses108. During Schistosoma mansoni infection, galectin 3 functions as a PRR that mediates LacdiNAc (LDN; composed of GalNAcβ1,4GlcNAc) recognition and phagocytosis by macrophages109. Galectin 1 does not recognize LDN, possibly because galectin 3 can accommodate the O-2 N-acetyl moiety of the GalNAc residue, whereas in galectin 1 the histidine residue at position 52 may prevent GalNAc binding48. A comparative study on acute and chronic phases of S. mansoni infection in wild-type and galectin 3–/– mice suggests that galectin 3 has an essential role in the modulation of monocyte– macrophage and B cell–plasma cell differentiation110, although this is under debate111.
Intestinal parasitic nematodes can upregulate galectin expression. In sheep, larval nematode infection leads to upregulation of a galectin-like protein (galectin 11) that is secreted from epithelial cells into the gut lumen in association with an eosinophil-rich inflammatory response that is typical for larval infections112. As a functional analogue of galectin 1, galectin 11 might crosslink mucus glycans and form an effective physical barrier as part of the inflammatory response to the parasite. The higher rate of expression during a secondary challenge suggests that galectin 11 might be involved in both the innate and adaptive immune response to gastrointestinal parasite infection112.
In summary, the experimental evidence for galectin binding to the surface of virus, bacteria, protista and helminth pathogens and parasites suggests that galectins can function as effective PRRs. Furthermore, the considerable diversity of the galectin repertoire in each organism and the substantial or subtle variations in the specificity of each galectin towards the target glycans, which are determined by oligosaccharide repeats, branchings or substitutions, suggest that there is extensive diversity and plasticity in the capacity of galectins for non-self recognition. The presence of canonical and extended CRDs45–48, and the carbohydrate-independent binding properties of the N terminus region of galectin 3 (REF. 63), further suggests that galectins have a high recognition capacity. Moreover, because galectins from all three types (proto, chimera or tandem repeat) can form oligomers, their multivalent binding properties, including increased avidity32, clearly enable galectins to participate effectively both in direct recognition of pathogens and parasites, and downstream processes that lead to activation of innate and adaptive immune responses. whether galectin-mediated recognition is an effective defence mechanism with a clear benefit for the host is not entirely clear, except for a few examples. It is noteworthy that a particular glycan on the surface of a microorganism or parasite can be recognized by multiple galectins, and that the outcome of the interaction differs substantially depending on the galectin type involved and the concentration of the galectin in a particular cell surface or extracellular microenvironment. This, in turn, determines the level of oligomerization and cooperative binding to ligand, and the potentially antagonistic or synergistic activation of pathogen signalling pathways (for example, modulation of immune cell activation, or cytokine production and secretion).
Subversion of galectin PRR function
Several pathogens and parasites can subvert the roles of host or vector galectins as PRRs to attach to or gain entry into their cells.
HIV-1 infection
In contrast to the inhibitory role of galectin 1 in paramyxovirus infection, elegant work by Sato and colleagues has shown that galectin 1, which is abundant in organs that represent major reservoirs for HIV-1, such as the thymus and lymph nodes, promotes infection by HIV-1 by facilitating virus attachment to the host cell surface glycans113 (FIG. 3b). Furthermore, galectin 1 enhances HIV adsorption kinetics on monocyte-derived host macrophages, facilitating HIV-1 infectivity by shortening the time required to establish an infection114. Galectin 1 could also function as a soluble scavenger receptor and enhance the uptake of the virus by macrophages, thereby facilitating sexual transmission of HIV-1 (REF. 114). Galectin 3 has no effect on HIV-1 adsorption, entry or infection113,114, although its expression is upregulated by the HIV Tat protein in several human cell lines115, and in cells infected with other retroviruses116,117, suggesting that it may participate in regulation of antiviral immunity. This underscores the relevance of the subtle differences in galectin specificity and affinity that may determine different recognition and effector outcomes.
Attachment of Leishmania to the insect vector
After a sandfly feeds on blood from a host infected with a Leishmania species, the ingested Leishmania amastigotes mature into promastigotes, which attach to the insect midgut epithelium to prevent their excretion along with the digested blood meal (FIG. 4A). These promastigotes then undergo numerous divisions before differentiating into free-swimming infective metacyclics71. The Phlebotomus papatasi sandfly midgut receptor for the procyclic L. major LPG was identified as a 35.4 kDa tandem repeat galectin (PpGalec) that is only expressed by epithelial midgut cells and is upregulated in blood-feeding females98. Binding of PpGalec is restricted to Leishmania promastigotes that express poly-Gal(β1–3) side chains on their LPG, such as those of L. major, and is responsible for specific attachment of the parasite to the vector’s midgut linings. These polygalactose epitopes are masked with arabinose during L. major metacyclogenesis, and therefore the free-swimming, infective, metacyclic promastigotes are unable to bind to PpGalec and are released from the midgut for transmission from the sandfly to the mammalian host98. Thus, the sandfly midgut galectin is not only crucial for parasite survival during the promastigote stage, but also determines the species specificity of the vector– parasite interaction. expression of PpGalec is restricted to P. papatasi and Phlebotomus duboscqi, closely related sandfly species that naturally transmit L. major. unlike P. papatasi, the sandfly Lutzomyia longipalpis is broadly permissive to the development of different Leishmania species118,119 and expresses four putative galectin-like proteins, of which LulongGale A is a tandem-repeat galectin with high similarity to PpGalec120. However, L. infantum (also known as L. chagasi), a species transmitted by L. longipalpis, does not display galactose residues on its surface LPG120, and lectin-like molecules on the Leishmania surface bind to the sandfly midgut glycoproteins118,119, a reverse interaction that might contribute to the adaptation of Leishmania species to new vectors and expand their geographical distribution119. Furthermore, the Leishmania–midgut interactions might not be limited simply to parasite anchoring to avoid expulsion, as a comparative analysis of the Lutzomyia longipalpis midgut transcriptome revealed that, during blood meal digestion, the parasite alters the expression profile of midgut factors that could be crucial to its survival in the sandfly121.
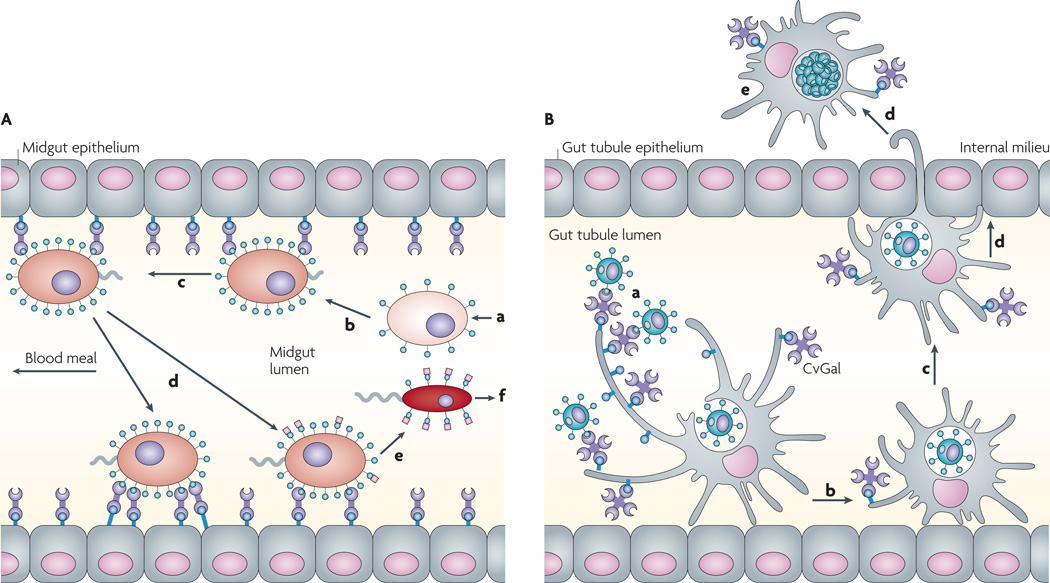
Figure 4. galectins function as protein-recognition receptors for parasites.
A | Recognition of Leishmania major lipophosphoglycan (LPG) by the sandfly galectin PpGalec facilitates attachment of the parasite to the midgut. The L. major amastigotes (a) ingested by the Phlebotomus papatasi sandfly from an infected host attach to the vector midgut epithelium (b) to prevent their excretion along with the digested blood meal, mature into promastigotes (c) and undergo numerous divisions (d) before differentiating into infective metacyclics (e,f)71. The parasite binds through PpGalec, a tandem repeat galectin expressed by the sandfly midgut that recognizes poly-Gal(β1–3) side chains (green circles) on the parasite LPG. During metacyclogenesis, PpGalec can be downregulated or capped with arabinose (pink squares), and is no longer recognized by PpGalec. This allows the free-swimming infective metacyclic promastigotes to detach from the midgut and migrate forward for transmission from the sandfly to the mammalian host98. B | Recognition of Perkinsus marinus trophozoites by the oyster (Crassostrea virginica) galectin CvGal facilitates infection127: CvGal displays four canonical galectin carbohydrate-recogniton domains (CRDs), a domain organization that is unlike any of the known galectin types. CvGal is translocated to the periphery and secreted by attached haemocytes, and binds to the cell surface. P. marinus trophozoites (a) ingested by filter-feeding are recognized by CvGal on the surface of haemocytes that coat the gut tubules, phagocytosed (b) and transported through the gut epithelium (c,d) into the internal milieu. The parasite inhibits intracellular killing by the host haemocytes and proliferates (e), thereby causing systemic infection and eventually death of the host.
Galectins have also been identified in other insect species122–125, some of which are parasite vectors or intermediate hosts. A galectin identified in Anopheles spp. is upregulated in response to Plasmodium infection123, and galactose-specific lectins mediate attachment of Trypanosoma rangeli to the salivary glands of its reduvid vector, Rhodnius prolixus124. A galectin has been recently characterized from the argasid tick Ornithodoros moubata, a vector of various viral and borrelian diseases125. whether these parasites similarly misuse recognition by the insect vector’s galectins remains to be determined.
Perkinsus marinus entry into phagocytic cells
The protozoan parasite P. marinus is a facultative intracellular parasite that causes Dermo disease in the eastern oyster Crassostrea virginica, and is responsible for catastrophic damage to shellfisheries and the estuarine environment in North America126. Oyster haemocytes (which are phagocytic cells) recognize P marinus through a galectin (CvGal) that has four canonical CRDs, a domain organization that is unlike any of the known galectin types127 (FIG. 4B). CvGal is present in the cytoplasm of circulating haemocytes, and following recognition of a foreign surface, CvGal is translocated to the cell periphery and secreted. Some CvGal binds to the cell surface, whereas the remaining galectin is released to the extracellular environment. Interestingly, the soluble CvGal binds in a carbohydrate-specific manner to bacteria, phytoplankton components and, preferentially, to Perkinsus spp. trophozoites, suggesting that CvGal has a direct role in recognition and opsonization of potential microbial pathogens and algal food. Inhibition of phagocytosis of P.marinus trophozoites by pre-treatment of haemocytes with anti-CvGal antibodies confirmed that the haemocyte surface-associated CvGal is a phagocytosis receptor for P.marinus. P.marinus might therefore have evolved in such a way that its glycocalyx is strongly recognized by CvGal, thereby subverting the innate immune and feeding recognition mechanism of the oyster to gain entry into host cells127. A two-CRD galectin (BgGal) was subsequently identified in the embryonic cell line and circulating haemocytes of the snail Biomphalaria glabrata, the intermediate host of S. mansoni, and was proposed to mediate snail–parasite interactions128.
T. vaginalis attachment to host epithelia
A recent study identified galectin 1 as the receptor for the protozoan parasite T. vaginalis99, the causative agent of the most prevalent non-viral sexually transmitted human infection in both women and men. As an obligate extracellular parasite, establishment and persistence of T. vaginalis infection requires adherence to the host epithelial cell surface129. T. vaginalis displays a surface LPG rich in galactose and N-acetyl glucosamine. This LPG is recognized in a carbohydrate-dependent manner by galectin 1, which is expressed by the epithelial cells in the cervical linings, as well as in other tissue colonized by the parasite, such as placenta, prostate, endometrial and decidual tissue99,129. Galectin 7 neither binds to nor mediates attachment of T. vaginalis to host cells, suggesting that the interaction of galectin 1 and T. vaginalis has some specificity, although it does not exclude the possibility that other galectins may also contribute to this interaction99.
The examples discussed above reveal that some pathogens and parasites subvert the roles of galectins as PRRs to either attach to suitable epithelia in their insect vector or final host, or to enter the host cells to proliferate and disseminate systemically. These interactions not only confirm the role of galectins as non-self recognition receptors, but also reveal the co-evolutionary adaptations of the glycocalyx in these highly specialized microorganisms.
Parasite galectins that promote infection
Galectin-like proteins have been also identified in parasitic worms and protozoa. Initial studies on parasitic nematodes and helminths identified galectins in Onchocerca volvulus130, Teladorsagia circumcincta131, Haemonchus contortus132 and Trichostrongylus colubriformis133. The flatworms Taenia serialis and Fasciola hepatica also express galectin-like cross-reactive molecules133.
The galectins from the free-living nematode Caenorhabditis elegans134 have been characterized in most detail and are thought to mediate developmental processes. By contrast, galectins from parasitic nematodes and helminths might contribute to parasitic invasion or downregulation of the host immune response135, suggesting that galectins from free-living worms and protista might have been co-opted during evolution to parasitism to ensure their entry and survival in the host environment. Products secreted or excreted by parasitic nematodes and helminths significantly modulate the host immune response135,136. Proteomic analysis of these products in T. circumcincta, Brugia malayi and Ancylostoma caninum has identified galectin-like proteins, in addition to a range of proteolytic, antioxidative and glycolytic enzymes137–139. The eosinophil-specific chemokinetic activity of the H. contortus-infective larvae (L3 stage) is mediated by the nematode galectins in a carbohydrate-dependent manner, and may mimic the activity of the host’s galectin 9 (a known eosinophil chemokine) to the parasite’s advantage140. Perhaps the strongest evidence for a crucial role of parasite galectins in infection is the observation that in sheep resistant to the gastrointestinal nematode T. colubriformis, antibodies specific for the galectins expressed by the infective larvae are dominant141. A recent vaccination trial in goats that used the recombinant galectins rHco-gal-m and rHco-gal-f from H. contortus as immunogens significantly reduced faecal egg output and worm burdens, and increased IgG levels in the vaccinated groups relative to the controls142. Taken together, these findings suggest that worm galectins actively participate in the infection process.
The protistan parasite T. gondii has lactose-binding lectin activity that is associated with micronemal protein 1 (MIC1)143. Although further studies identified a galectin-like domain in the MIC4–MIC1–MIC6 protein complex of the microneme, this domain was devoid of carbohydrate-binding activity40. The MIC1 tandem MAR domains resemble thrombospondin type 1 repeats27. These repeats recognize negatively charged sulphated glycosaminoglycans, which, together with sialic acid, have been implicated as cell surface glycan receptors for T. gondii attachment144. The current evidence therefore suggests that the T. gondii galectin domain only participates indirectly in host invasion, by facilitating micronemal protein assembly.
Conclusions and perspectives
A growing body of evidence from recent studies indicates that host galectins can function as PRRs that target glycans on the surfaces of viruses, bacteria and helminths. The specific carbohydrate moieties galectins recognize in these surface glycans can be identified tentatively in a limited number of species, based on the available information about galectin binding specificity and affinity45–52,91,94–96,99,109,113,114,145 (FIG. 5). In most cases, however, these carbohydrate ligands remain largely unknown and rigorous studies for each galectin–microorganism (or galectin–parasite) interaction will be required to experimentally isolate, identify and characterize them.
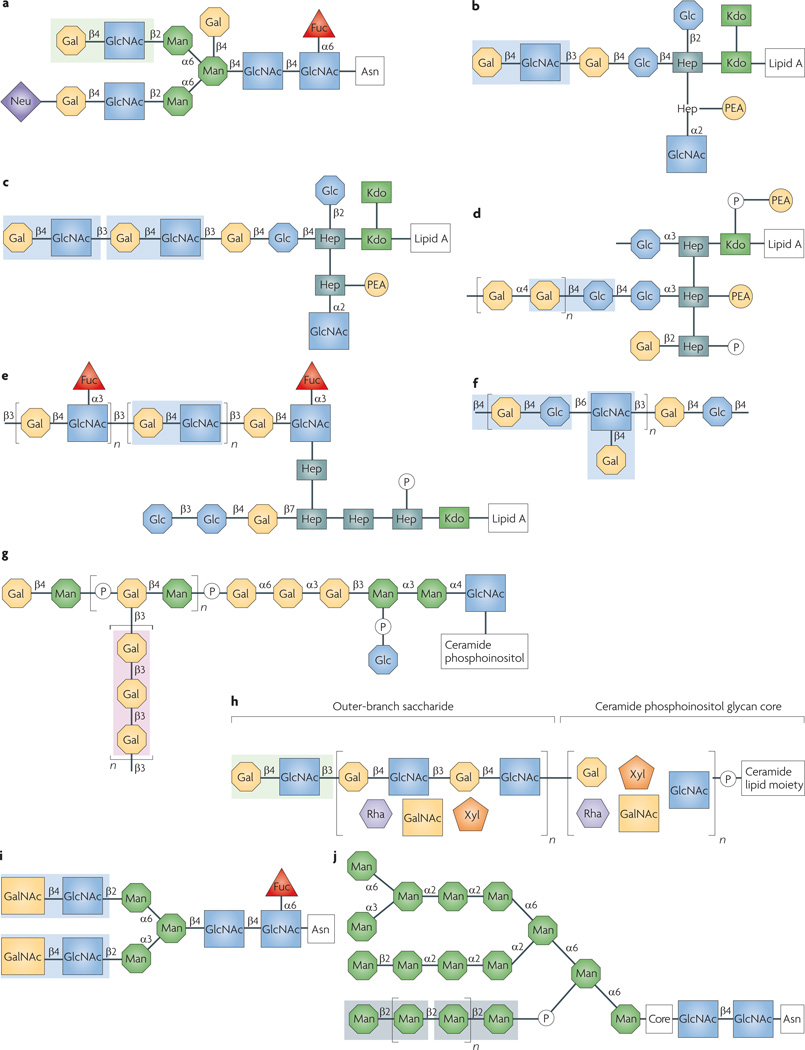
Figure 5. glycans on the surface of selected microorganisms and protistan or metazoan parasites potentially recognized by galectins 1, 3 and 9.
Complex type N-linked oligosaccharide from the HIV-1 gp120 envelope glycoprotein149 (a); meningococcal (Neisseria meningitidis) lipopolysaccharide (LPS)150,151 (b); gonococcal (Neisseria gonorrhoeae) lipooligosaccaride151 (c); Haemophilus influenzae LPS152 (d); Helicobacter pylori LPS O-antigen side chain153 (e); Streptococcus pneumoniae polysaccharide type XIV154 (f); Leishmania major lipophosphoglycan (LPG)71,96,155 (g); Trichomonas vaginalis LPG100 (h); Schistosoma mansoni LacdiNAc (LDN)156 (i); Candida albicans oligomannan94,148,157 (j). Carbohydrate moieties that could be recognized by galectins are highlighted: galectin 1, light green; galectin 3, light blue; galectins 3 and 9, pink. Because there is glycan microheterogeneity and variability within and among microbial and parasite strains and species, either only the most abundant or representative glycan is shown. In the T. vaginalis LPG sequence (only partially elucidated), the presence of polylactosamine has been confirmed100 (h). Although these carbohydrate moieties are probably strongly recognized by galectin 3, because only recognition of the T. vaginalis LPG by galectin 1 has been reported to date99, only the terminal LacNAc disaccharide is highlighted. For the C. albicans oligomannan (j), the boxed oligosaccharide indicates its requirement for recognition by galectin 3, without implying direct binding91–94. Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Hep, heptose; KDO, 2-keto-3-deoxyoctonate; Man, mannose; Rha, rhamnose; Neu, N-acetylneuraminic acid; PEA, phosphoethanolamine; Xyl, xylose.
Nevertheless, a perplexing paradox arises, as the host galectins also recognize endogenous lactosamine-containing glycans on the host cell surface, which are important for certain developmental processes and regulation of immune homeostasis. Based on the janeway and Medzhitov model10 for non-self recognition, PRRs recognize pathogens through highly conserved and widely distributed microbial surface molecules, such as LPS or peptidoglycan, which are absent from the host. Thus, this premise does not seem to apply to galectins, which bind similar self or non-self molecular patterns. This paradox underscores, first, the oversimplification in the use of PRR terminology, and second, the gaps in our knowledge of recognition properties of the host galectin repertoire and the dynamic and mechanistic aspects of the subcellular compartmentalization and secretion of its members, as well as the detailed structural and biophysical aspects of their interactions with microbial carbohydrate moieties. All these aspects warrant further investigation.
From an evolutionary standpoint, it has been proposed that the microbial and host glycomes and their receptors continuously evolve to escape mutual recognition, in a process known as the Red Queen effect146, by which the microorganisms avoid recognition by the host PRRs, while the host avoids colonization through microbial factors (agglutinins, adhesins and lectins). Given the key parts played by galectins in host development and immunoregulation through the recognition of self lactosamine moieties, strong functional constraints should prevent galectins from undergoing dramatic evolutionary changes in carbohydrate specificity, which is to some extent supported by the apparent structural conservation within this lectin family. Furthermore, as pathogens and parasites seem to subvert host galectins to attach or gain entrance into host cells, it seems plausible that instead of avoiding recognition by the host, they have evolved their glycomes to mimic their hosts’ in a ‘Trojan horse’ model127 and rely on the host’s self-recognition molecules, such as galectins, for attachment to the vector or host invasion. It is noteworthy that most (if not all) of these pathogens and parasites are endowed with diverse and powerful mechanisms to evade intracellular killing by the host and/or downregulate downstream immune responses. The complex strategies developed by microbial pathogens to successfully colonize, enter, proliferate and disseminate within and among their vectors or hosts are the products of strong selective pressures. Such pressures have led to adaptations that ensure the survival of microorganisms in the most hostile environment of all, and therefore represent a substantial challenge for the development of novel strategies for intervention in human disease. The novel insights provided by the realization that galectins are directly involved in pathogen recognition has opened new avenues of research aimed at disrupting their roles in parasite–vector interactions or host invasion.
Online-only summary
Virtually all bacterial and eukaryotic cells, as well as many viruses, display surface carbohydrates, which have a crucial role in the establishment of host-microorganism complex interactions through their recognition by protein receptors, mainly known as lectins.
Lectin-glycan interactions are ubiquitous and essential to biological systems, not simply as the ‘glue’ between cells, but as the initiators of a functional crosstalk that modulates their physiology and homeostatic balance. Microbial lectins, including viral haemagglutinins, bacterial adhesins and parasite lectins, are involved in host colonization, whereas some animal lectins can function as pattern recognition receptors in immune responses against microbial pathogens and parasites.
Among the various lectin families, the galectins are proteins that are characterized by a unique binding-site sequence motif, affinity for β-galactosides and wide taxonomic distribution. Most metazoans are endowed with a complex galectin repertoire, with members exhibiting multiple isoforms and subtle variations in carbohydrate specificity, which together with a certain level of plasticity in sugar binding suggests they have substantial diversity in recognition properties.
Galectins were initially thought to only bind endogenous ‘self glycans and mediate developmental processes, including cell differentiation and tissue organization, and more recently, regulation of immune homeostasis. In the past few years, however, it has become clear that galectins also bind non-self glycans on the surface of potentially pathogenic microorganisms (viruses, bacteria, protista and fungi) and parasitic worms, and mediate recognition and effector functions in innate immunity.
Some pathogens and parasites subvert the roles of galectins as PRRs to either attach to suitable epithelia in their insect vector or final host, or to enter the host cells to proliferate and disseminate systemically. Furthermore, galectins from parasites might contribute directly or indirectly to host invasion, or downregulation of the host immune response.
In summary, the recent evidence discussed in this Review indicates that host galectins can function as recognition receptors that target non-self glycans on the surfaces of viruses, bacteria, protista and helminth pathogens and parasites, and either prevent or facilitate infection. Because galectins also bind self glycans on the host cell surface as the first step in immunoregulation and developmental processes, galectins do not fit current models of innate immune self or non-self recognition or defence.
Gaps in our knowledge about the diversity of the host galectins, their subcellular compartmentalization and secretion, and structural and biophysical aspects of their interactions with the microbial carbohydrate moieties warrant further investigation. The novel insights provided by the realization that galection are directly involved in pathogen recognition has opened new avenues of research aimed at disrupting their roles in parasite-vector interactions or host invasion.
Supplementary Material
Acknowledgements
The author thanks M. Bianchet, Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, for providing the galectin structural models in BOX 1. He also thanks R.D. Cummings, Department of Biochemistry, Emory University School of Medicine, S. Sato, Research Center for Infectious Diseases, Laval University, and L.G. Baum, Department of Pathology and Laboratory Medicine, UCLA School of Medicine, for useful comments on FIG. 5. Research in the authors laboratory was supported by grant R01 GM070589-01 from the National Institutes of Health, grants IOB-0618409 and IOS-0822257 from the National Science Foundation and grant NA05NMF4571243 from the National Oceanic and Atmospheric Administration. The author apologizes to the numerous investigators whose articles could not be cited owing to space constraints.
Glossary
- Pattern-recognition receptor
Soluble or membrane-associated receptor displayed by the metazoan host that can recognize complex molecular patterns on the surface of microorganisms.
- Innate immunity
Constitutive immunity that in contrast to acquired or adaptive immunity, is not inducible to specific antigens and does not require prior sensitization through an antigen from, for example, an infection or vaccination.
- Glycome
The entire carbohydrate repertoire, whether free or present in more complex molecules, of an organism.
- Adaptive immune response
A response to a stimulating agent, such as protein, carbohydrate, or a pathogen, that improves recognition of the agent and, after neutralization of the agent, allows the system to retain immunological memory.
- Collectin
A C-type lectin characterized by a collagen-like domain. A coiled-coil neck domain connects the carbohydrate recognition domains with the collagen-like domain, and a cysteine-rich domain is present at the amino terminus. Collectin subunits are usually organized as trimers, and trimeric units are assembled in numbers that differ among members of the collectin family.
- Apoptosis
Programmed cell death in multicellular organisms that is characterized by cell shrinkage, cytoskeleton break-up, chromatin condensation and chromosomal fragmentation. In contrast to necrosis, elimination of apoptotic cells or their fragments has no detrimental effects on the organism.
- Metacyclogenesis
The process by which non-infective promastigotes mature into the infective parasite life stage. In Leishmania spp., this process takes place in the midgut of the insect vector, and the changes in metabolism (such as protein kinase A activity) and expression of surface molecules that take place during the process are major determinants of parasite virulence.
- Microneme
Small, osmiophilic, elongated secretory organelle that forms part of the apical complex located within the extreme apical region of the apicomplexan parasite, under the inner membrane complex. Micronemes discharge their contents (micronemal proteins) during initial contact of the parasite’s apical pole with the host cell surface.
Biography
Author biography
Gerardo R. Vasta earned his Ph.D. degree in biochemistry from the university of La Plata, Argentina, in 1978, and his Ph.D. degree in zoology in 1980. He conducted postdoctoral studies at the Roswell Park Cancer Institute, New York, uSA, and at the Medical university of South Carolina, uSA. He is currently Professor at the university of Maryland Biotechnology Institute, Maryland, uSA. His primary scientific interests include structural, functional and evolutionary aspects of protein-carbohydrate interactions in innate immunity, host-parasite associations and development.
Footnotes
DATABASES
Entrez Genome Project: http://www.ncbi.nlm.nih.gov/ entrez/query.fcgi?db=genomeprj
Ancylostoma caninum | Biomphalaria glabrata | Brugia malayi | Candida albicans | Caenorhabditis elegans | Coprinopsis cinerea | Crassostrea virginica | Entoamoeba hystolytica | Fasciola hepatica | Haemophilus influenzae | Helicobacter pylori | Klebsiella pneumoniae | Leishmania infantum | Leishmania major | Lutzomyia longipalpis | Neisseria gonorrhoeae | Neisseria meningitidis | Perkinsus marinus | Phlebotomus papatasi | Plasmodium falciparum | Porphyromonas gingivalis | Pseudomonas aeruginosa | Rhodnius prolixus | Saccharomyces cerevisiae | Staphylococcus aureus | Streptococcus pneumoniae | Toxoplasma gondii | Trichomonas vaginalis | Trypanosoma cruzi | Trypanosoma rangeli | Vibrio parahaemolyticus | Vibrio vulnificus
UniProtKB: http://www.uniprot.org
FURTHER INFORMATION
Gerardo R. Vasta’s homepage: http://www.umbi.umd.edu/comb/faculty-directory/vasta/index.php
A Genomics Resource for Animal Lectins:http://www.imperial.ac.uk/research/animallectins/
Functional Glycomics Gateway: http://www.functionalglycomics.org/static/index.shtml
Roles of galectins in infection
Gerardo R. Vasta
Galectins are important for recognition of carbohydrate ligands during embryogenesis, development and immune regulation. In addition, recent work has shown that galectins also function as receptors for glycans expressed on the surface of potentially pathogenic microorganisms. In this Review, Gerardo Vasta discusses the roles of galectins in host immunity and how pathogens have evolved to evade or subvert galectin-mediated immune responses.
References
- 1.Casadevall A, Pirofski LA. Host–pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 2000;68:6511–6518. doi: 10.1128/iai.68.12.6511-6518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nature Rev. Microbiol. 2008;6:111–120. doi: 10.1038/nrmicro1836. A review that describes the quorum sensing that takes place between the metazoan host and its microbiota
- 3.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 4.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nature Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 5. Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nature Rev. Immunol. 2008;8:874–887. doi: 10.1038/nri2417. References 4 and 5 provide excellent, comprehensive overviews of the roles of cell surface glycans in microbial infection and host immune responses
- 6.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007;282:2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 7.Vasta GR, Ahmed H, editors. Animal Lectins: A Functional View. Boca Raton: CRC; 2008. [Google Scholar]
- 8.Doyle RJ, Slifkin M. Lectin– Microorganism Interactions. United States: Routledge; 1994. [Google Scholar]
- 9.Mirelman D. Microbial Lectins and Agglutinins: Properties and Biological Activity. New York: John Wiley & Sons; 1986. [Google Scholar]
- 10. Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. A detailed analysis of the self and non-self recognition mechanisms and effector pathways in immune responses
- 11.Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj. J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- 12.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovich GA, Toscano MA. Turning sweet on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nature Rev. Immunol. doi: 10.1038/nri2536. (in the press) [DOI] [PubMed] [Google Scholar]
- 14. Sato S, Nieminen J. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 2004;19:583–591. doi: 10.1023/B:GLYC.0000014089.17121.cc. This article was the first to propose that intracellular galectins function as ‘danger signals’
- 15.Vasta GR, Ahmed H, Odom EO. Structural and functional diversity of lectin repertoires in invertebrates, protochordates and ectothermic vertebrates. Curr. Opin. Struct. Biol. 2004;14:617–630. doi: 10.1016/j.sbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Mathis R, et al. Lipopolysaccharides as a communication signal for progression of legume endosymbiosis. Proc. Natl Acad. Sci. USA. 2005;102:2655–2660. doi: 10.1073/pnas.0409816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 2006;8:1985–1993. doi: 10.1111/j.1462-5822.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- 18. Nyholm SV, McFall-Ngai MJ. The winnowing: establishing the squid-Vibrio symbiosis. Nature Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. References 17 and 18 show that protein-carbohydrate interactions are crucial to the establishment of invertebrate-microorganism and invertebrate-algae symbioses
- 19.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nature Rev. Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bishop JR, Gagneux P. Evolution of carbohydrate antigens — microbial forces shaping host glycomes? Glycobiology. 2007;17:23R–34R. doi: 10.1093/glycob/cwm005. References 20 and 21 describe in detail mechanistic aspects of how the mammalian gut symbiotic microbiota regulates the host-microorganism homeostatic balance
- 22.Stevens J, et al. Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. J. Mol. Biol. 2006;355:1143–1155. doi: 10.1016/j.jmb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Mandlik A, Swierczynski A, Das A, Ton-That H. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 2008;16:33–40. doi: 10.1016/j.tim.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frederick JR, Petri WA., Jr. Roles for the galactose-/N-acetylgalactosamine-binding lectin of Entamoeba in parasite virulence and differentiation. Glycobiology. 2005;15:53R–59R. doi: 10.1093/glycob/cwj007. [DOI] [PubMed] [Google Scholar]
- 25. von Itzstein M, Plebanski M, Cooke BM, Coppel RL. Hot, sweet and sticky: the glycobiology of Plasmodium falciparum . Trends Parasitol. 2008;24:210–218. doi: 10.1016/j.pt.2008.02.007. Comprehensive report that reveals the various carbohydrate-mediated mechanisms for P. falciparum recognition and invasion of the host
- 26.Bhat N, Joe A, Pereira Perrin M, Ward HD. Cryptosporidium p30, a galactose/ N-acetylgalactosamine-specific lectin, mediates infection in vitro . J. Biol. Chem. 2007;282:34877–34887. doi: 10.1074/jbc.M706950200. [DOI] [PubMed] [Google Scholar]
- 27. Hager KM, Carruthers VB. MARveling at parasite invasion. Trends Parasitol. 2008;24:51–54. doi: 10.1016/j.pt.2007.10.008. First rigorous description of a Toxoplasma micronemal protein for host cell colonization
- 28. Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 1988;263:9557–9560. First identification of sequence motifs that define the animal C-type and S-type lectin families
- 29.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 30.Odom EW, Vasta GR. Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis) J. Biol. Chem. 2006;281:1698–1713. doi: 10.1074/jbc.M507652200. [DOI] [PubMed] [Google Scholar]
- 31.Bianchet MA, Odom EW, Vasta GR, Amzel LM. A novel fucose recognition fold involved in innate immunity. Nature Struct. Biol. 2002;9:628–634. doi: 10.1038/nsb817. [DOI] [PubMed] [Google Scholar]
- 32. Dam TK, Brewer CF. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry. 2008;47:8470–8476. doi: 10.1021/bi801208b. A rigorous analysis of cooperative effects in multivalent lectin-ligand interactions
- 33.van Vliet SJ, Saeland E, van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 2008;29:83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 34. Yu XQ, Zhu YF, Ma C, Fabrick JA, Kanost MR. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem. Mol. Biol. 2002;32:1287–1293. doi: 10.1016/s0965-1748(02)00091-7. First description of C-type lectins (immulectins) that activate melanization pathways
- 35.Houzelstein D, et al. Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 2004;21:1177–1187. doi: 10.1093/molbev/msh082. [DOI] [PubMed] [Google Scholar]
- 36.Hirabayashi J, Kasai K. The family of metazoan metal-independent β-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 37.Cooper DN. Galectinomics: finding themes in complexity. Biochim. Biophys. Acta. 2002;1572:209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 38.Lipkowitz MS, Leal-Pinto E, Cohen BE, Abramson RG. Galectin 9 is the sugar-regulated urate transporter/channel UAT. Glycoconj. J. 2004;19:491–498. doi: 10.1023/B:GLYC.0000014078.65610.2f. [DOI] [PubMed] [Google Scholar]
- 39.Gorski JP, Liu FT, Artigues A, Castagna LF, Osdoby P. New alternatively spliced form of galectin-3, a member of the β-galactoside-binding animal lectin family, contains a predicted transmembrane-spanning domain and a leucine zipper motif. J. Biol. Chem. 2002;277:18840–18848. doi: 10.1074/jbc.M109578200. [DOI] [PubMed] [Google Scholar]
- 40.Saouros S, et al. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J. Biol. Chem. 2005;280:38583–38591. doi: 10.1074/jbc.C500365200. [DOI] [PubMed] [Google Scholar]
- 41.Walser PJ, Kües U, Aebi M, Künzler M. Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet. Biol. 2005;42:293–305. doi: 10.1016/j.fgb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Stalz H. The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology. 2006;16:402–414. doi: 10.1093/glycob/cwj086. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed H, Vasta GR. Unlike mammalian GRIFIN, the zebrafish homologue (DrGRIFIN) represents a functional carbohydrate-binding galectin. Biochem. Biophys. Res. Commun. 2008;371:350–355. doi: 10.1016/j.bbrc.2008.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou D, et al. Crystal structure of the C-terminal conserved domain of human GRP, a galectin-related protein, reveals a function mode different from those of galectins. Proteins. 2008;71:1582–1588. doi: 10.1002/prot.22003. [DOI] [PubMed] [Google Scholar]
- 45.Lobsanov YD, Gitt MA, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-Å resolution. J. Biol. Chem. 1993;268:27034–27038. doi: 10.2210/pdb1hlc/pdb. [DOI] [PubMed] [Google Scholar]
- 46.Liao DI, Kapadia G, Ahmed H, Vasta GR, Herzberg O. Structure of S-lectin, a developmentally regulated vertebrate β-galactoside-binding protein. Proc. Natl Acad. Sci. USA. 1994;91:1428–1432. doi: 10.1073/pnas.91.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchet MA, Ahmed H, Vasta GR, Amzel LM. Soluble β-galactosyl-binding lectin (galectin) from toad ovary: crystallographic studies of two protein-sugar complexes. Proteins. 2000;40:378–388. doi: 10.1002/1097-0134(20000815)40:3<378::aid-prot40>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48. Seetharaman J, et al. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-Å resolution. J. Biol. Chem. 1998;273:13047–13052. doi: 10.1074/jbc.273.21.13047. References 45 –48 provided the first structures of galectins 1 and 3, which enabled us to understand their distinct binding properties
- 49.Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992;267:6983–6990. [PubMed] [Google Scholar]
- 50.Zhou Q, Cummings RD. The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch. Biochem. Biophys. 1990;281:27–35. doi: 10.1016/0003-9861(90)90408-q. [DOI] [PubMed] [Google Scholar]
- 51.Fang R, Mantle M, Ceri H. Characterization of quail intestinal mucin as a ligand for endogenous quail lectin. Biochem. J. 1993;293:867–872. doi: 10.1042/bj2930867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozeki Y, et al. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology. 1995;5:255–261. doi: 10.1093/glycob/5.2.255. [DOI] [PubMed] [Google Scholar]
- 53.Shoji H, Nishi N, Hirashima M, Nakamura T. Characterization of the Xenopus galectin family. Three structurally different types as in mammals and regulated expression during embryogenesis. J. Biol. Chem. 2003;278:12285–12293. doi: 10.1074/jbc.M209008200. [DOI] [PubMed] [Google Scholar]
- 54.Patterson RJ, Dagher SF, Vyakarnam A, Wang JL. Nuclear galectins: functionally redundant components in processing of pre-mRNA. Trends Glycosci. Glycotechnol. 1997;9:77–85. [Google Scholar]
- 55.Cleves AE, Cooper DNW, Barondes SH, Kelly RB. A new pathway for protein export in Saccharomyces cerevisiae . J. Cell Biol. 1996;133:1017–1026. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guévremont M, et al. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann. Rheum. Dis. 2004;63:636–643. doi: 10.1136/ard.2003.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris S, et al. Quaternary solution structures of galectins-1, −3, and −7. Glycobiology. 2004;14:293–300. doi: 10.1093/glycob/cwh029. [DOI] [PubMed] [Google Scholar]
- 58.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin- glycoprotein lattices. Curr. Opin. Struct. Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Partridge EA, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 60. Ohtsubo K, et al. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. References 59 and 60 established that galectins function in modulating the turnover and function of cell surface transporters and receptors
- 61.Stowell SR, et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 62.Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 2007;66:143–158. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 63. Liu FT, Hsu DK. The role of galectin-3 in promotion of the inflammatory response. Drug News Perspect. 2007;20:455–460. doi: 10.1358/dnp.2007.20.7.1149628. Excellent review of the various roles of galectin 3 in inflammation
- 64.Hirashima M, et al. Galectin-9 in physiological and pathological conditions. Glycoconj. J. 2004;19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f. [DOI] [PubMed] [Google Scholar]
- 65.Perone MJ, et al. Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses in naive and activated T cells. J. Immunol. 2006;176:7207–7220. doi: 10.4049/jimmunol.176.12.7207. [DOI] [PubMed] [Google Scholar]
- 66.Fulcher JA, et al. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J. Immunol. 2006;177:216–226. doi: 10.4049/jimmunol.177.1.216. [DOI] [PubMed] [Google Scholar]
- 67.Rossi B, Espeli M, Schiff C, Gauthier L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J. Immunol. 2006;177:796–803. doi: 10.4049/jimmunol.177.2.796. [DOI] [PubMed] [Google Scholar]
- 68. Toscano MA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nature Immunol. 2007;8:825–834. doi: 10.1038/ni1482. First description of the galectin-mediated mechanism that leads to the polarization of TH1, TH2 and TH17 effector cells
- 69.Salatino M, et al. Galectin-1 as a potential therapeutic target in autoimmune disorders and cancer. Expert Opin. Biol. Ther. 2008;8:45–57. doi: 10.1517/14712598.8.1.45. [DOI] [PubMed] [Google Scholar]
- 70. Mey A, Leffler H, Hmama Z, Normier G, Revillard JP. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J. Immunol. 1996;156:1572. First report of the mechanism of bacterial LPS recognition by galectin 3
- 71.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Lee B. Envelope? receptor interactions in Nipah virus pathobiology. Ann. NY Acad. Sci. 2007;1102:51–65. doi: 10.1196/annals.1408.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Levroney EL, et al. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 2005;175:413–420. doi: 10.4049/jimmunol.175.1.413. First description of galectin 1 as a recognition (or effector) receptor for viral envelope glycans
- 74.Aguilar HC, et al. N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 2006;80:4878–4889. doi: 10.1128/JVI.80.10.4878-4889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez MI, et al. Regulated expression of galectin-1 after in vitro productive infection with herpes simplex virus type 1: implications for T cell apoptosis. Int. J. Immunopathol. Pharmacol. 2005;18:615–623. doi: 10.1177/039463200501800402. [DOI] [PubMed] [Google Scholar]
- 76.Almkvist J, Dahlgren C, Leffler H, Karlsson A. Newcastle disease virus neuraminidase primes neutrophils for stimulation by galectin-3 and formyl-Met-Leu-Phe. Exp. Cell Res. 2004;298:74–82. doi: 10.1016/j.yexcr.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Pioche-Durieu C, et al. In nasopharyngeal carcinoma cells, Epstein-Barr virus LMP1 interacts with galectin 9 in membrane raft elements resistant to simvastatin. J. Virol. 2005;79:13326–13337. doi: 10.1128/JVI.79.21.13326-13337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vinogradov E, Perry MB. Structural analysis of the core region of the lipopolysaccharides from eight serotypes of Klebsiella pneumoniae . Carbohydr. Res. 2001;335:291–296. doi: 10.1016/s0008-6215(01)00216-6. [DOI] [PubMed] [Google Scholar]
- 79.Mandrell RE, Apicella MA, Lindstedt R, Leffler H. Possible interaction between animal lectins and bacterial carbohydrates. Methods Enzymol. 1994;236:231–254. doi: 10.1016/0076-6879(94)36019-7. [DOI] [PubMed] [Google Scholar]
- 80.John CM, et al. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell. Microbiol. 2002;4:649–662. doi: 10.1046/j.1462-5822.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- 81. Gupta SK, Masinick S, Garrett M, Hazlett L. D., Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 1997;65:2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997.This interesting report proposed thatP. aeruginosa binds to host galectin 3 to establish corneal infection
- 82.Barboni E, Coade S, Fiori A. The binding of mycolic acids to galectin-3: a novel interaction between a host soluble lectin and trafficking mycobacterial lipids? FEBS Lett. 2005;579:6749–6755. doi: 10.1016/j.febslet.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 83. Fowler M, Thomas RJ, Atherton J, Roberts IS, High NJ. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell. Microbiol. 2006;8:44–54. doi: 10.1111/j.1462-5822.2005.00599.x. First comprehensive analysis of the effects of H. pylori infection on galectin 3 expression and secretion
- 84.Huff JL, Hansen LM, Solnick JV. Gastric transcription profile of Helicobacter pylori infection in the rhesus macaque. Infect. Immun. 2004;72:5216–5226. doi: 10.1128/IAI.72.9.5216-5226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int. J. Biochem. Cell Biol. 2003;35:1284–1296. doi: 10.1016/s1357-2725(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 86.Farnworth SL, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am. J. Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae . J. Immunol. 2008;180:2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- 88.Kasamatsu A, et al. Elevation of galectin-9 as an inflammatory response in the periodontal ligament cells exposed to Porphylomonas gingivalis lipopolysaccharide in vitro and in vivo . Int. J. Biochem. Cell Biol. 2005;37:397–408. doi: 10.1016/j.biocel.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y, et al. Molecular and biochemical characterization of galectin from amphioxus: primitive galectin of chordates participated in the infection processes. Glycobiology. 2007;17:774–783. doi: 10.1093/glycob/cwm044. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, et al. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 91. Fradin C, Poulain D, Jouault T. β-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. First description of the galectin-mediated recognition of surface glycans in a pathogenic fungus
- 92.Jouault T, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 93.Jawhara S, et al. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008;197:972–980. doi: 10.1086/528990. [DOI] [PubMed] [Google Scholar]
- 94. Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific β-1,2-linked mannans. J. Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. First description of the fungicidal activity of a host galectin
- 95.Pelletier I, et al. Specific recognition of Leishmania major poly-β-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 2003;278:22223–22230. doi: 10.1074/jbc.M302693200. [DOI] [PubMed] [Google Scholar]
- 96. Pelletier I, Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. The authors proposed, for the first time, a mechanism for how the susceptibility of galectin 3 to proteolytic attack from L. major determines the specific cutaneous or visceral clinical manifestations of the infection
- 97.Späth GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl Acad. Sci. USA. 2003;100:9536–9541. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kamhawi S, et al. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. First report that a galectin expressed in the midgut of the insect vector mediates attachment of the parasite for maturation to the next stage of its life cycle
- 99.Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis . Cell. Microbiol. 2008;1:2078–2090. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh BN, et al. Structural details and composition of Trichomonas vaginalis lipophosphoglycan in relevance to the epithelial immune function. Glycoconj. J. 2009;26:3–17. doi: 10.1007/s10719-008-9157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarleton RL. Immune system recognition of Trypanosoma cruzi . Curr. Opin. Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Kleschenko YY, et al. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect. Immun. 2003;72:6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vray B, et al. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology. 2004;14:647–657. doi: 10.1093/glycob/cwh068. [DOI] [PubMed] [Google Scholar]
- 104. Silva-Monteiro E, et al. Altered expression of galectin-3 induces cortical thymocyte depletion and premature exit of immature thymocytes during Trypanosoma cruzi infection. Am. J. Pathol. 2007;170:546–556. doi: 10.2353/ajpath.2007.060389. This study revealed the dramatic galectin 3-mediated effects of T. cruzi infection on T cells in both the central and peripheral compartments
- 105.Acosta-Rodríguez EV, et al. Galectin-3 mediates IL-4-induced survival and differentiation of B cells: functional cross-talk and implications during Trypanosoma cruzi infection. J. Immunol. 2004;172:493–502. doi: 10.4049/jimmunol.172.1.493. [DOI] [PubMed] [Google Scholar]
- 106.Zúñiga E, Gruppi A, Hirabayashi J, Kasai KI, Rabinovich GA. Regulated expression and effect of galectin-1 on Trypanosoma cruzi-infected macrophages: modulation of microbicidal activity and survival. Infect. Immun. 2001;69:6804–6812. doi: 10.1128/IAI.69.11.6804-6812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bernardes ES, et al. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am. J. Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Liempt E, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 2007;44:2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 109. van den Berg TK, et al. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 2004;173:1902–1907. doi: 10.4049/jimmunol.173.3.1902. A direct demonstration of galectin 3 as a PRR for S. mansoni glycans
- 110.Oliveira FL, et al. Kinetics of mobilization and differentiation of lymphohematopoietic cells during experimental murine schistosomiasis in galectin-3−/− mice. J. Leukoc. Biol. 2007;82:300–310. doi: 10.1189/jlb.1206747. [DOI] [PubMed] [Google Scholar]
- 111.Bickle Q, Helmby H. Lack of galectin-3 involvement in murine intestinal nematode and schistosome infection. Parasite Immunol. 2007;29:93–100. doi: 10.1111/j.1365-3024.2006.00923.x. [DOI] [PubMed] [Google Scholar]
- 112.Dunphy JL, et al. Isolation and characterization of a novel inducible mammalian galectin. J. Biol. Chem. 2000;275:32106–32113. doi: 10.1074/jbc.M003739200. [DOI] [PubMed] [Google Scholar]
- 113. Ouellet M, et al. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 2005;174:4120–4126. doi: 10.4049/jimmunol.174.7.4120. An elegant study that shows how, in contrast to the paramixovirus, galectins promote HIV-1 infection
- 114.Mercier S, et al. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. 2008;371:121–129. doi: 10.1016/j.virol.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 115.Fogel S, Guittaut M, Legrand A, Monsigny M, Hébert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383–387. doi: 10.1093/glycob/9.4.383. [DOI] [PubMed] [Google Scholar]
- 116.Schröder HC, et al. Expression of nuclear lectin carbohydrate-binding protein 35 in human immunodeficiency virus type 1-infected Molt-3 cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995;9:340–348. [PubMed] [Google Scholar]
- 117.Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the β-galactoside-binding lectin, galectin-3. Am J. Pathol. 1996;148:1661–1670. [PMC free article] [PubMed] [Google Scholar]
- 118.Volf Pt, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2006;22:439–445. doi: 10.1016/j.pt.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myskova J, Svobodova M, Beverley SM, Volf P. P. A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect. 2007;9:317–324. doi: 10.1016/j.micinf.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dillon RJ, et al. Analysis of ESTs from Lutzomyia longipalpis sand flies and their contribution toward understanding the insect-parasite relationship. Genomics. 2006;88:831–840. doi: 10.1016/j.ygeno.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jochim RC, et al. The midgut transcriptome of Lutzomyia longipalpis: comparative analysis of cDNA libraries from sugar-fed, blood-fed, post-digested and Leishmania infantum chagasi-infected sand flies. BMC Genomics. 2008;9:15. doi: 10.1186/1471-2164-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pace KE, Baum LG. Insect galectins: roles in immunity and development. Glycoconj. J. 2004;19:607–614. doi: 10.1023/B:GLYC.0000014092.86763.2f. [DOI] [PubMed] [Google Scholar]
- 123.Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Ribeiro JM. Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito. Insect Biochem. Mol. Biol. 2003;33:717–732. doi: 10.1016/s0965-1748(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 124.Basseri HR, Tew IF, Ratcliffe NA. Identification and distribution of carbohydrate moieties on the salivary glands of Rhodnius prolixus and their possible involvement in attachment/ invasion by Trypanosoma rangeli . Exp. Parasitol. 2002;100:226–234. doi: 10.1016/s0014-4894(02)00026-7. [DOI] [PubMed] [Google Scholar]
- 125.Huang X, et al. Molecular characterization and oligosaccharide-binding properties of a galectin from the argasid tick Ornithodoros moubata . Glycobiology. 2007;17:313–323. doi: 10.1093/glycob/cwl070. [DOI] [PubMed] [Google Scholar]
- 126.Harvell CD, et al. Emerging marine diseases — climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 127.Tasumi S, Vasta GR. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus . J. Immunol. 2007;179:3086–3098. doi: 10.4049/jimmunol.179.5.3086. [DOI] [PubMed] [Google Scholar]
- 128.Yoshino TP, Dinguirard N, Kunert J, Hokke CH. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni . Gene. 2008;411:46–58. doi: 10.1016/j.gene.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bastida-Corcuera FD, Okumura CY, Colocoussi A, Johnson PJ. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell. 2005;4:1951–1958. doi: 10.1128/EC.4.11.1951-1958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Klion AD, Donelson JE. OvGalBP, a filarial antigen with homology to vertebrate galactoside-binding proteins. Mol. Biochem. Parasitol. 1994;65:305–315. doi: 10.1016/0166-6851(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 131.Greenhalgh CJ, Loukas A, Newton SE. The organization of a galectin gene from Teladorsagia circumcincta . Mol. Biochem. Parasitol. 1999;101:199–206. doi: 10.1016/s0166-6851(99)00075-4. [DOI] [PubMed] [Google Scholar]
- 132.Newlands GF, Skuce PJ, Knox DP, Smith SK, Smith WD. Cloning and characterization of a β-galactoside-binding protein (galectin) from the gut of the gastrointestinal nematode parasite Haemonchus contortus . Parasitology. 1999;119:483–490. doi: 10.1017/s003118209900503x. [DOI] [PubMed] [Google Scholar]
- 133.Greenhalgh CJ, Beckham SA, Newton SE. Galectins from sheep gastrointestinal nematode parasites are highly conserved. Mol. Biochem. Parasitol. 1999;98:285–289. doi: 10.1016/s0166-6851(98)00167-4. [DOI] [PubMed] [Google Scholar]
- 134.Nemoto-Sasaki Y, et al. Caenorhabditis elegans galectins LEC-1-LEC-11: structural features and sugar-binding properties. Biochim. Biophys. Acta. 2008;1780:1131–1142. doi: 10.1016/j.bbagen.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 135.Rabinovich GA, Gruppi A. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol. 2005;27:103–114. doi: 10.1111/j.1365-3024.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 136.Young AR, Meeusen EN. Galectins in parasite infection and allergic inflammation. Glycoconj. J. 2004;19:601–606. doi: 10.1023/B:GLYC.0000014091.00844.0a. [DOI] [PubMed] [Google Scholar]
- 137.Craig H, Wastling JM, Knox DP. A preliminary proteomic survey of the in vitro excretory/secretory products of fourth-stage larval and adult Teladorsagia circumcincta . Parasitology. 2006;132:535–543. doi: 10.1017/S0031182005009510. [DOI] [PubMed] [Google Scholar]
- 138.Hewitson JP, et al. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol. Biochem. Parasitol. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 139.Mulvenna J, et al. Proteomic analysis of the excretory/secretory component of the blood-feeding stage of the hookworm, Ancylostoma caninum . Mol. Cell Proteomics. 2009;8:109–121. doi: 10.1074/mcp.M800206-MCP200. [DOI] [PubMed] [Google Scholar]
- 140.Turner DG, Wildblood LA, Inglis NF, Jones DG. Characterization of a galectin-like activity from the parasitic nematode, Haemonchus contortus, which modulates ovine eosinophil migration in vitro . Vet. Immunol. Immunopathol. 2008;122:138–145. doi: 10.1016/j.vetimm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 141.Kiel M, et al. Identification of immuno-reactive proteins from a sheep gastrointestinal nematode, Trichostrongylus colubriformis, using two-dimensional electrophoresis and mass spectrometry. Int. J. Parasitol. 2007;37:1419–1429. doi: 10.1016/j.ijpara.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 142. Yanming S, et al. Vaccination of goats with recombinant galectin antigen induces partial protection against Haemonchus contortus infection. Parasite Immunol. 2007;29:319–326. doi: 10.1111/j.1365-3024.2007.00949.x. The first direct demonstration of the protective effects of immunization against a parasite worm galectin
- 143.Lourenço EV, et al. Toxoplasma gondii micronemal protein MIC1 is a lactose-binding lectin. Glycobiology. 2001;11:541–547. doi: 10.1093/glycob/11.7.541. [DOI] [PubMed] [Google Scholar]
- 144.Ortega-Barria E, Boothroyd JC. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 1999;274:1267–1276. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 145.Hirabayashi J, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 146. Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. An excellent article that analyses the evolutionary aspects of protein-carbohydrate interactions in microbial infection and host defence
- 147.McGuinness DH, Dehal PK, Pleass RJ. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 2003;19:312–319. doi: 10.1016/s1471-4922(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 148.Taylor MA, Drickamer K. Introduction to Glycobiology. New York: Oxford Univ. Press; 2003. [Google Scholar]
- 149.Mizuochi T, et al. Diversity of oligosaccharide structures on the envelope glycoprotein gp 120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J. Biol. Chem. 1990;265:8519–8524. [PubMed] [Google Scholar]
- 150.Cox AD, et al. Identification of a novel inner-core oligosaccharide structure in Neisseria meningitidis lipopolysaccharide. Eur. J. Biochem. 2003;270:1759–1766. doi: 10.1046/j.1432-1033.2003.03535.x. [DOI] [PubMed] [Google Scholar]
- 151.Mandrell RE, Griffiss JM, Macher BA. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J. Exp. Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masoud H, Martin A, Thibault P, Moxon ER, Richards JC. Structure of extended lipopolysaccharide glycoforms containing two globotriose units in Haemophilus influenzae serotype b strain RM7004. Biochemistry. 2003;42:4463–4475. doi: 10.1021/bi026632a. [DOI] [PubMed] [Google Scholar]
- 153.Aspinall GO, Monteiro MA, Pang H, Walsh EJ, Moran AP. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 1996;35:2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- 154.Kolkman MA, Morrison DA, Van Der Zeijst BA, Nuijten PJ. The capsule polysaccharide synthesis locus of Streptococcus pneumoniae serotype 14: identification of the glycosyl transferase gene cps14E . J. Bacteriol. 1996;178:3736–3741. doi: 10.1128/jb.178.13.3736-3741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moody SF, Handman E, McConville MJ, Bacic A. The structure of Leishmania major amastigote lipophosphoglycan. J. Biol. Chem. 1993;268:18457–18466. [PubMed] [Google Scholar]
- 156.Cummings RD, Nyame AK. Schistosome glysoconjugates. Biochim. Biophys. Acta. 1999;1455:363–374. doi: 10.1016/s0925-4439(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 157.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nature Rev. Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.