Abstract
Nematode sterol-binding protein 1 (NSBP-1) is a homolog of nucleosome assembly protein 1 in mammals that is expressed widely in Caenorhabditis elegans. NSBP-1 mutants are biologically lethal, demonstrating the significance of the gene in growth and development. We investigated how cholesterol influences the insulin signaling pathway through this novel sterol-binding protein in C. elegans. Here we report that NSBP-1 influences many biological processes mediated by insulin signaling, such as longevity, dauer formation, fat storage, and resistance to oxidative stress. We found that NSBP-1 is phosphorylated by AKT-1 downstream of insulin signaling. In the absence of insulin signaling, NSBP-1 is translocated to the nucleus and binds to DAF-16, a FOXO transcription factor, in a cholesterol-dependent manner. Moreover, NSBP-1 and DAF-16 regulate a common set of genes that can directly modulate fat storage, longevity, and resistance to stress. Together, our results present a new steroid-binding molecule that can connect sterol signaling to insulin signaling through direct interaction with FOXO.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1221-0) contains supplementary material, which is available to authorized users.
Keywords: Cholesterol, Cholesterol-binding protein, C. elegans, D2096.8, Insulin/IGF-1 signaling, DAF-16
Introduction
Cholesterol serves as a plasma membrane component to control membrane fluidity and permeability. It is also a precursor for biologically active molecules, including vitamin D, oxysterols, steroid hormones (such as ecdysone or dafachronic acid), and bile acids. Sterols usually need sterol-binding proteins (SBPs) to regulate sterol homeostasis, trafficking, and cell metabolism. For instance, SREBP and LXR are transcription factors that regulate the expression of genes involved in cholesterol homeostasis [1, 2], lipoproteins and NPCs are transporters of cholesterol, and caveolin located in the membrane regulates both cholesterol transport and signal transduction [3–5]. As in mammals, cholesterol in Caenorhabditis elegans has diverse functions [6–9] including an interaction with the insulin signaling pathway [10, 11]. A key difference between worms and mammals, however, is that worms require only a very small amount of cholesterol, suggesting that cholesterol primarily functions in signaling. For example, it is a precursor of dafachronic acid, a dauer molting hormone.
Insulin is involved in many cellular processes including cell growth and differentiation, carbohydrate metabolism, fat mobilization, lipogenesis, and aging [12–14]. Insulin resistance contributes to the pathogenesis of numerous metabolic diseases, such as type 2 diabetes, hypertension, lipid disorders, atherosclerosis, and reproductive abnormalities [13, 14]. The insulin pathway has been shown to regulate metabolism, development, stress resistance, and life span is conserved between C. elegans and mammals [15, 16]. In worms, the insulin-like receptor DAF-2 [17] activates a kinase signaling cascade from PI 3-kinase (AGE-1/AAP-1) [18] to downstream serine/threonine kinases such as PDK-1, AKT-1, AKT-2, and SGK-1 [19–21]. Once activated, these kinases negatively regulate the Forkhead (FOXO) transcription factor, DAF-16 [22, 23], which regulates genes involved in longevity, dauer formation, fat accumulation, and stress resistance [22–24]. Recently, FOXO was found to interact with several histone remodeling factors such as the histone deacetylase SIRT1 [25]. This suggests that DAF-16 may differentiate its regulation by inducing different subsets of targets depending on its binding partner.
In this study, we identified nematode sterol-binding protein 1 (NSBP-1), a homolog of nucleosome assembly protein 1 (NAP1) in mammals, as a new cholesterol-binding protein. NSBP-1 interacts with the insulin signaling pathway and is translocated to the nucleus in the absence of the insulin signaling. In the nucleus, it binds to DAF-16 to control metabolic processes such as fat accumulation in a cholesterol concentration-dependent manner. These results suggest that NSBP-1 provides a novel molecular mechanism for DAF-16 to regulate insulin response in conjunction with cholesterol signaling.
Materials and methods
General methods for culturing worms and strains
All strains were cultured using standard C. elegans handling and culture methods [26] with the OP50 Escherichia coli strain as a food source. The wild-type strain was C. elegans variant Bristol, strain N2. Other strains used were CB1370 daf-2(e1370), CF1038 daf-16(mu86), RB759 akt-1(ok525), RT130 pwIs23[vit-2::GFP], CF1407 daf-16(mu86) I; muIs71[pKL99 (daf-16a::GFP/bKO) + pRF4(rol-6)]. The daf-2(e1370); muIs84[Psod-3::gfp] strain was generated by crossing daf-2(e1370) males to Psod-3::gfp(muIs84) hermaphrodites.
Expression vector constructs
To produce GST-D2096.8 protein, a D2096.8 cDNA fragment was amplified by PCR and cloned into the EcoRI and NotI sites of pGEX-4T-3. This expression vector was transformed into E. coli Rosetta (DE3), overexpressed, and purified using glutathione-Sepharose 4B. To produce a GFP expression construct of NSBP-1, a PCR fragment containing the nsbp-1 coding region with 1.4 kb of the upstream promoter region was amplified and cloned into the SphI and BamHI sites of the Fire lab vector pPD 95.75 to generate the NSBP-1::GFP construct. Approximately 50 ng/μl co-injection marker pRF4 (rol-6(sd)) was mixed with the NSBP-1::GFP construct (at a final concentration of 100 ng/μl). The T203A variant (NSBP-1T203A::GFP) was generated by site-directed mutagenesis of NSBP-1::GFP according to the manufacturer’s recommendations (Stratagene, La Jolla, CA, USA). The mutation was verified by nucleotide sequencing. The primers used for PCR analysis are listed in Table S3.
Cholesterol feeding conditions
Worms were grown in different cholesterol-containing nematode growth medium (NGM) or RNAi plates. Low cholesterol medium (LCM) is the same as NGM, except that no cholesterol was added. High cholesterol medium (HCM) is the same as NGM, except that instead of 5 µg/ml added cholesterol, it contains 20 µg/ml. Other conditions were the same as previously described [9].
RNAi assay
To prepare the bacterial RNAi expression construct, an nsbp-1 cDNA fragment was amplified by PCR from C. elegans and cloned into the XbaI and HindIII sites of the Fire lab vector pPD126.36 vector. RNAi plates were prepared by supplementing NGM with 100 μg/ml ampicillin and 1 mM IPTG. RNAi bacteria were grown overnight at 37 °C in LB media supplemented with 100 μg/ml ampicillin. After 1 day, the cultures were diluted (1:50) into LB broth containing 100 μg/ml ampicillin and grown at 37 °C to an OD600 of 0.5, and then 1 mM IPTG was added. After 3 h, approximately 200 μl of the bacterial suspension was seeded onto each RNAi plate.
Dauer assay
For the dauer assays, approximately five adult worms were transferred onto the RNAi bacteria and maintained at 15 °C for approximately 3 days. F1 adult worms were then picked, transferred to a fresh RNAi plate, and allowed to lay eggs. Approximately 120 eggs were picked from these plates, transferred onto three fresh plates containing the RNAi bacteria, and then incubated at the indicated temperatures. The plates were scored for the presence of dauers or non-dauers after 3.5–4 days, with some exceptions.
Analysis of resistance to oxidative stress
Following NSBP-1 and DAF-16 knockdown, wild-type N2, daf-2, or daf-16 worms were fed E. coli HT115 (DE3) cells expressing the indicated double-stranded RNA. Young adult worms were transferred to an M9 solution containing 100 mM paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride, Sigma-Aldrich, St. Louis, MO, USA) and incubated at 20 °C. The number of dead worms was counted by the continuous absence of swimming movements and pharyngeal pumping every 5 h for up to 20 h. Thirty worms from each strain were treated with 100 mM paraquat. This experiment was performed three times.
Lifespan measurement
Synchronized populations were obtained by allowing adults to lay eggs at 20 °C for 5–6 h on RNAi plates. After larval development, the worms were transferred onto RNAi plates containing 0.05 mg/ml FUdR. The number of living animals was scored every 1–2 days until death, which was defined as the failure to respond to gentle prodding on the head and tail with a platinum wire.
Coupling of cholesterol 3-hemisuccinate to EAH Sepharose 4B for cholesterol affinity chromatography
Cholesterol-substituted EAH-Sepharose 4B was prepared according to Cuatrecasas et al. [27–29] with a few modifications. EAH-Sepharose 4B (1 ml, GE Healthcare, Piscataway, NJ, USA) was swollen in 20 ml of 0.5 M NaCl and washed with 5 ml of distilled water (adjusted to pH 4.5 with 0.1 M HCl). The swollen EAH-Sepharose 4B beads were placed in 1 ml of diethylene dioxide (1,4-dioxane) containing 0.0486 g cholesterol-hemisuccinate (10 mmol) and 1 ml of water (pH 4.5). Cholesterol-3 hemisuccinate was coupled to 1,6-diaminohexyl (EAH)-substituted Sepharose 4B using the carbodiimide-promoted condensation reaction described previously [30, 31]. Briefly, 0.055 g of 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide (0.13 M) was added to 0.2 ml of water (pH 4.5), manually inverted for 60 min, and then stirred for 24 h at 4 °C. The resulting cholesterol-substituted EAH-Sepharose 4B was washed successively with 20 ml of 1,4-dioxane, 100 ml of 80 % (v/v) ethyl alcohol, 10 ml of acetone, 20 ml of distilled water, and 20 ml of 10 mM potassium phosphate buffer, pH 7.5 containing 20 % (v/v) glycerol.
Proteomic analysis of NSBP-1 using nano-liquid chromatography–tandem mass spectrometry (LC–MS/MS)
For the identification of purified NSBP-1, nano-LC–MS/MS analysis was performed using an Agilent Nano HPLC 1100 system coupled with a linear trap quadrupole mass spectrometer (Thermo Electron, San Jose, CA) as described [32]. Preparation of capillary columns, mobile phase solution, gradient conditions, and flow rates were the same as described previously [32]. Xcalibur 2.1 (Thermo Electron) was used for peak list generation. Acquisition of mass spectra, scanning of MS/MS, temperature, and collision energy was performed as described [32].
[3H]-labeled cholesterol-binding assay
We performed the binding assay as described previously [33]. Each assay tube contained 100 nM [3H]-labeled cholesterol in buffer A (50 mM Tris–HCl at pH 7.5, 150 mM NaCl, 1 mM dithiothreitol, 0.1 % Fos-Choline 13, and 0.005 % sodium azide) in a final volume of 100 μl, and was incubated at 25 °C in the presence of GST-fused proteins for the indicated times. After incubation, the mixture was washed with 2 ml of buffer A, and the bound protein was eluted with 100 μl elution buffer (10 mM reduced glutathione in 50 mM Tris–HCl, pH 8.0). Eluted fractions were measured for radioactivity using a liquid scintillation counter.
RNA isolation and qRT-PCR
Total RNA was isolated using the RNAspin Mini isolation kit (GE Healthcare), and reverse transcribed using the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics Corp., Indianapolis, IN, USA). The cDNA was quantified using Nanodrop (ND-1000) and used in qRT-PCR reactions. These reactions were performed on an MJ Research Chromo4 Detector using the QuantiTect SYBR Green PCR kit according to the manufacturer’s protocol (QIAGEN, Valencia, CA, USA) using 500 ng of cDNA per sample in a total volume of 50 μl. Target genes (i.e., sod-3, mtl-1) were amplified using specific primers. Amplification and expression analysis were performed in triplicate. mRNA levels in the tested strains were normalized to those in the vector control RNAi group and compared to NSBP-1- or DAF-16-specific RNAi-treated samples. Serially diluted vector RNAi cDNA (500–0.05 ng) was used to construct a standard curve for qRT-PCR using actin as an internal control. All of the primers used are listed in Table S3. Unless otherwise specified, the data presented throughout this paper represent the mean (±SEM) from three independent experiments performed in triplicate.
Immunoprecipitation and Western blotting
Wild-type, daf-16::gfp, daf-2(e1370); daf-16::gfp, or Psod-3::gfp mixed-staged worms were collected by washing with water, pelleted by centrifugation at 3,000 × g for 30 s, and then frozen in liquid nitrogen in approximately 100-μl aliquots. Depending on the size of the frozen worm pellet, an approximately 4× volume of lysis buffer (50 mM Tris–HCl at pH 7.5, 150 mM NaCl, 1 mM dithiothreitol, 0.1 % Fos-Choline 13, 0.005 % sodium azide, and protease inhibitor cocktail) was added. The pellets underwent three freeze/thaws and were then homogenized. For immunoprecipitation, the lysate was incubated with antibody (10 μl antibody per 1 mg total protein) for 2 h at 4 °C. The anti-NSBP-1 antibody was generated against a peptide from the C-terminus of NSBP-1 (FEPPKSKDERNEDED) as reported previously [34]. A 1/10 volume of protein A slurry was then added and the mixture incubated for 4 h at 4 °C. The protein A beads were then washed three times with lysis buffer, and bound proteins were eluted by boiling in 4X sample buffer for 5 min prior to Western blotting. The primary antibodies used were anti-DAF-16 (rabbit, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GFP (mouse, Santa Cruz Biotechnology), and anti-NSBP-1 (rabbit, Abfrontier, Seoul). Anti-mouse and anti-rabbit secondary antibodies were obtained from Santa Cruz Biotechnology.
Fat assessment
Sudan black staining of stored fat was performed as described previously [17]. Briefly, wild-type and daf-2(e1370) adult worms were transferred onto fresh RNAi plates and grown to the L3 stage. The worms were then washed off the plates and incubated in M9 buffer for 30 min on a shaker at RT. After three washes with M9 buffer, the worms were fixed in 1 % paraformaldehyde, and then sequentially dehydrated with washes in 25, 50, and 70 % ethanol. Saturated Sudan Black (Sigma-Aldrich) solution was prepared fresh in 70 % ethanol. The fixed worms were incubated for 1–4 h in 200 μl of Sudan Black solution on a shaker at 4 °C.
Oil-Red O staining was conducted by as previously reported [35]. Briefly, worms collected after RNAi treatment and washed with PBS (pH 7.4) and were then resuspended in 120 μl of PBS to which an equal volume of 2× MRWB buffer containing 2 % paraformaldehyde was added. Samples were gently stirred for 1 h at room temperature and worms were washed with 1× PBS. Worms were dehydrated with 60 % isopropanol. Oil-Red-O solution was prepared as follows: a 0.5 g/100 ml isopropanol stock solution equilibrated for several days was freshly diluted to 60 % with water and gently stirred for at least 1 h and filtrated. The worms were incubated for 1 h in 1 ml of 60 % Oil-Red O solution at room temperature.
To quantitate total triglyceride content, wild-type and daf-2(e1370) adult worms were picked and transferred onto fresh RNAi plates and grown until the L4 stage. The worms were then frozen in liquid nitrogen and stored at −80 °C until needed. Approximately 30 mg of nematodes were weighed and ground in a nitrogen-chilled mortar with 250 μl of frozen phosphate buffer. Extracts were centrifuged for 7 min at 12,000 × g to clear the lysate. Fat content was determined using a commercially available TG determination kit (Sigma-Aldrich) as previously described [36] and normalized to the total amount of protein in each homogenate using the Bradford assay (Bio-Rad, Hercules, CA). For each treatment condition and time point indicated, at least three independent biological samples were generated, with duplicate preparations of lysates from each sample and two measurements for every preparation.
Results
NSBP-1 binds to cholesterol
In C. elegans, cholesterol is required for reproductive growth and gonad development [7, 8]. Cholesterol functions in part through its binding proteins. However, so far vitellogenin (a functional homolog of mammalian low-density lipoprotein, LDL) is the only known cholesterol-binding protein in worms [37]. To identify new cholesterol-binding protein(s), we used affinity chromatography to screen for cholesterol (sterol)-binding proteins in cell lysates from wild-type worms. We isolated D2096.8 and identified it as a homolog of human NAP1 (with 42 % identity) with proteomic analysis using nano-LC–MS/MS analysis coupled with a linear trap quadrupole mass spectrometer (Thermo Electron, San Jose, CA, USA) as described in the “Materials and methods” section among others including vitellogenin (Online Resource Fig. S1 and Table S1) [37]. We focused on D2096.8 because it was also isolated from our previous screen for daf-2 downstream target genes, yet whether it functions in cholesterol signaling, especially in conjunction with the insulin signaling pathway, had not been addressed [34, 38].
To verify that D2096.8 binds to cholesterol, we made a recombinant GST-tagged fusion protein and performed an in vitro cholesterol-binding assay as described previously (Online Resource Fig. S1B) [33, 39]. Recombinant GST-D2096.8 bound to [3H]-labeled cholesterol in a concentration-dependent manner with a K d of 87.5 ± 27.7 nM (Fig. 1a). The binding was saturated after 30 min of incubation (Fig. 1b). In competition assays with unlabeled cholesterol, we measured an IC50 of 40 ± 5.6 nM (Fig. 1c). Competition assays using sterols other than cholesterol (e.g., 7-dehydrocholesterol, desmosterol, β-sitosterol, 25-hydroxycholesterol, and lanosterol) showed that while 25-hydroxycholesterol could compete with [3H]-labeled cholesterol at a higher concentration (IC50 ≈ 0.63 μM), no other sterol tested could (Fig. 1c), confirming that D2096.8 binding is mostly specific to cholesterol. We named it nematode sterol-binding protein-1 (NSBP-1).
Fig. 1.
D2096.8 (NSBP-1) binds cholesterol. a GST-D2096.8 binds to cholesterol in a concentration-dependent manner: D2096.8-GST (100 pmol) was incubated with increasing concentrations of [3H]-labeled cholesterol and binding activity measured as described (see “Materials and methods”). b The binding was saturated after 30 min of incubation: D2096.8-GST was incubated with [3H]-labeled cholesterol (100 nM) for the indicated time. c D2096.8-GST was incubated with 100 nM [3H]-labeled cholesterol in the presence of increasing concentrations of unlabeled sterols as indicated. a–c Each data point represents the mean ± SEM (n = 3) from two independent experiments
NSBP-1 function and expression
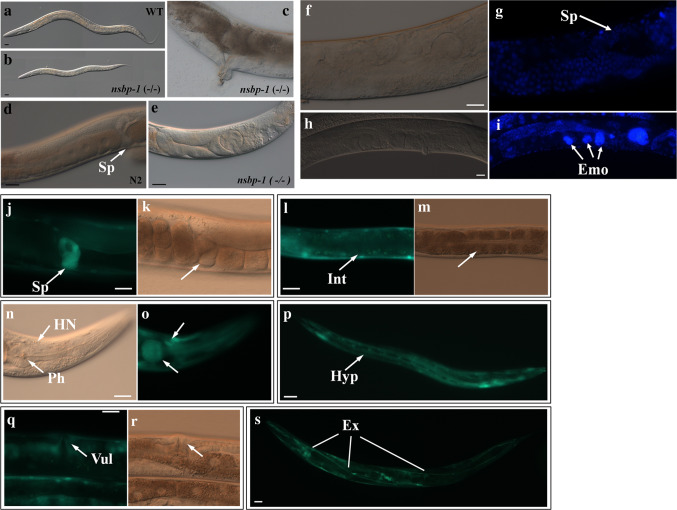
To investigate the physiological function of NSBP-1, we examined the phenotype of the nsbp-1(tm1278) deletion mutant and worms treated with RNAi against nsbp-1 (Online Resource Fig S3). nsbp-1 mutants were either arrested as larvae or sterile as adults. Homozygous progeny from a heterozygous mother were scrawny and thin, grew slowly, and showed severe germline defects compared to wild-type siblings (Fig. 2a–e). Even nsbp-1 heterozygotes grew noticeably slowly and had reduced brood sizes without any visible defects in either body size or the germline, suggesting haploinsufficiency of this gene in development and reproduction (Online Resource Fig. S2). RNAi knockdown of nsbp-1 beginning at the egg stage resulted in viable adults with a normal rate of development. However, a few nsbp-1(RNAi) adults showed abnormal migration of the distal gonad tip, a spermatheca developmental defect, endomitotic oocytes, or abnormal vulval development (Fig. 2f–i and data not shown). In contrast, most worms treated with nsbp-1 RNAi for two generations showed an abnormal gonad phenotype and became sterile, mimicking the phenotype of nsbp-1 mutants. This confirmed that nsbp-1 is an essential gene involved in development and growth. These phenotypes mimic the main defects caused by cholesterol deficiency, supporting an NSBP-1 role in cholesterol signaling [7]. To examine where NSBP-1 functions, we generated transgenic worms carrying the nsbp-1 promoter and gene fused with GFP to visualize expression. NSBP-1::GFP was widely expressed in various tissues, including the pharynx, vulva, spermatheca, intestine, excretory cells, and hypodermis (Fig. 2j–s), consistent with the pleiotropic phenotypes and broad expression patterns. In the cell, NSBP-1::GFP was expressed diffusely throughout nucleus and cytoplasm.
Fig. 2.
Phenotypes of the nsbp-1(tm1278) deletion mutant. a Wild type. Homozygous nsbp-1 mutants showed b small body size, c abnormal vulva development, d spermatheca (Sp) of wild type, and e abnormal migration of the distal gonad tip and spermatheca development defects (indicated by arrow). f–i nsbp-1 RNAi in the wild type also caused gonad development defects, such as defects in spermatheca development (f, g) and endomitotic oocytes (h, i). g and i were stained by DAPI and (f) and (h) are corresponding DIC images (Emo endomitotic oocyte, Sp spermatheca). j–s Transgenic worms carrying NSBP-1::GFP in a wild-type background showed expression of the protein in the spermatheca (Sp, j, k), the intestine (Int, l, m), head neurons and pharynx (HN and Ph, n, o), the hypodermis (Hyp, p), the vulva (Vul, q, r) and the excretory cell (Ex, s). A representative image from one of three independent experiments is shown (n = 30). j–s Fluorescence images of transgenic worms while k, m, o, r show the DIC images corresponding to j, l, n, q. All scale bars represent 20 μm
NSBP-1 influences multiple biological processes affected by the insulin pathway
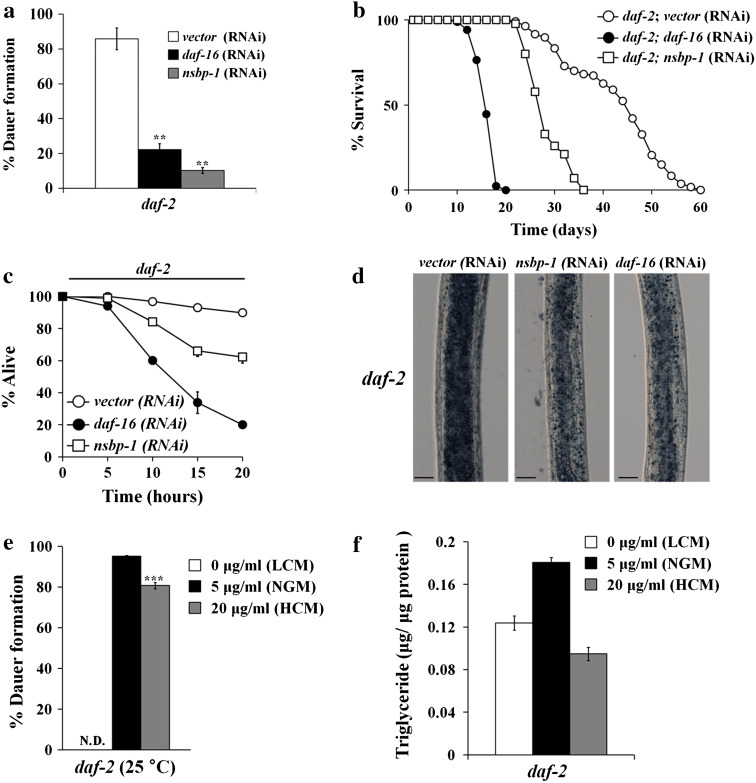
In the absence of cholesterol, worms arrest as dauer larvae, a dormant form to survive harsh environments [6, 11]. Dauer formation is regulated by insulin, TGFβ, and cGMP pathways, all of which converge on synthesis of cholesterol-derived ligands of a nuclear hormone receptor, DAF-12 [40]. Because NSBP-1 binds to cholesterol, which regulates the dauer formation pathway, and because NSBP-1 is located at the downstream of DAF-2 signaling [34], we tested if NSBP-1 regulates dauer formation in daf-2 mutants. Since nsbp-1 is an essential gene, we used RNAi to partially reduce gene activity. When fed control RNAi, daf-2 mutants became dauers at 25 °C. However, knockdown of nsbp-1 in daf-2 mutants reduced dauer formation (Fig. 3a), suggesting NSBP-1 interacts with the insulin signaling pathway.
Fig. 3.
NSBP-1 influences multiple biological processes affected by the insulin pathway. a nsbp-1 knockdown decreased dauer formation in daf-2 mutants at 25 °C. **p ≤ 0.001. Unless described otherwise, all values represent the average of three independent experiments (n ≥ 100) and error bars indicate the SEM. p values were calculated using Student’s t test. b nsbp-1 knockdown reduced the lifespan of daf-2 mutants. Mean lifespan [days ± SEM (sample number)] of control RNAi = 42.7 ± 0.5 days (n = 100), nsbp-1 RNAi = 28.4 ± 0.4 days (n = 90), p < 0.0001; daf-16 RNAi = 16.3 ± 0.2 days (n = 85), p < 0.0001. c nsbp-1 knockdown reduced resistance to oxidative stress in daf-2 mutants. Percent living animals at 20 h were 90 % (vector RNAi), 20 % (daf-16 RNAi, p < 0.001), and 62 % (nsbp-1 RNAi, p < 0.001). Worms (n = 30 for each strain) were treated with paraquat and then survival was assessed. p < 0.001 at the 20-h time point. d nsbp-1 knockdown reduced fat accumulation in daf-2 mutants similarly to daf-16 knockdown. A representative image from one of three independent experiments (n = 20) is shown. e Cholesterol imbalance decreased dauer formation in daf-2 mutants 25 °C (n ≥ 100). ND not detected. ***p ≤ 0.001 (LCM low cholesterol medium; NGM normal cholesterol medium; HCM high cholesterol medium; see key in panel f). f Cholesterol imbalance reduced fat storage in daf-2 worms. Total triglyceride content was altered in daf-2 worms in response to changes in cholesterol concentration
We further examined life span, fat storage, and resistance to oxidative stress, all of which are known to be regulated by the insulin signaling pathway [41]. RNAi of nsbp-1 reduced the lifespan of daf-2 mutant by approximately 33 % compared to control RNAi (Fig. 3b). To examine resistance to oxidative stress, as a measure of increased sod-3 expression, we measured the survival of worms treated with 100 mM paraquat. After 20 h of paraquat treatment, 90 % of control RNAi-treated daf-2 mutants survived, whereas only 62 % of nsbp-1 RNAi-treated daf-2 mutants did (Fig. 3c). Lastly, RNAi of nsbp-1 reduced fat storage in the daf-2 mutant as measured by Sudan black staining [17] (Fig. 3d). These data show that nsbp-1 may have a partial role in mediating dauer formation, longevity, oxidative stress resistance, and fat accumulation in daf-2 mutants, suggesting that nsbp-1 influences multiple biological processes regulated by the insulin signaling pathway in C. elegans.
Because NSBP-1 binds to cholesterol and because knockdown of nsbp-1 by RNAi alleviates several daf-2 mutant phenotypes, we reasoned that if we manipulated cholesterol concentrations to disrupt nsbp-1 function, daf-2 might show phenotypes similar to those caused by treatment with nsbp-1 RNAi. When we treated daf-2 mutants with three different cholesterol concentrations, 0 μg/ml (low cholesterol medium, LCM), 5 μg/ml (NGM) and 20 μg/ml (high cholesterol medium, HCM), we found that not only low cholesterol but also high cholesterol treatment reduced dauer formation and fat storage, mimicking the nsbp-1 RNAi effect on daf-2 mutants (Fig. 3e, f). That cholesterol imbalance induced a similar phenotype as treating daf-2 mutants with nsbp-1 RNAi suggests that cholesterol is necessary for daf-2 mutants to show their phenotypes and the cholesterol function is mediated by nsbp-1. However, it is not clear why increased and decreased cholesterol reduces dauer formation and fat storage in daf-2 animals. We speculate that there may be another factor that can sense the level of cholesterol (e.g., SCAP in mammals) and mediate concentration-dependent modulation of cholesterol effect on such daf-2 mutant phenotypes.
NSBP-1 is translocated into the nucleus in the absence of insulin signaling
To understand the molecular mechanisms by which nsbp-1 regulates the insulin signaling pathway, we first compared the localization of NSBP-1 in the wild type to that in daf-2 mutants. In wild-type intestinal cells NSBP-1 protein was diffusely localized throughout the cell. However, in daf-2 mutants, NSBP-1 protein was mainly in the nucleus, showing that in the absence of insulin signaling, NSBP-1 proteins translocated into the nucleus (Fig. 4a–d, i–k). Because DAF-16, the main downstream target of the insulin signaling pathway, also translocates into the nucleus in the absence of insulin signaling [42, 43] and because it is known that NAP-1 family proteins, homologs of NSBP-1, also translocate into the nucleus to function [44], we hypothesized that NSBP-1 might change its intracellular localization through phosphorylation by kinases in the insulin pathway.
Fig. 4.
NSBP-1 translocated into the nucleus in the absence of insulin signaling. a–d NSBP-1::GFP accumulated in intestinal nuclei when DAF-2 activity was reduced. a, b NSBP-1::GFP in the wild type. c, d NSBP-1::GFP in daf-2 mutants. e–h Alanine substitution of a predicted AKT phosphorylation site within NSBP-1 (T203A) resulted in nuclear accumulation of NSBP-1::GFP. E, NSBP-1::GFP in the wild type (f is its DIC image), g NSBP-1T203A::GFP in the wild type (h is its DIC image). White arrows indicate the intestinal nuclei. a–h A representative image from one of three independent experiments is shown (n = 30). i–k Quantitation of NSBP-1::GFP and NSBP-1T203A::GFP in intestinal nuclei; i indicates high, j indicates medium, and k indicates low level of NSBP-1::GFP in intestinal nuclei
AGE-1, a worm homolog of PI3 kinase, and AKT-1 are two kinases downstream of daf-2. Mutants for these genes show phenotypes identical to those of daf-2 mutants; they become dauers because the mutants cannot phosphorylate DAF-16 to keep it in the cytoplasm. As a result, DAF-16 gets dephosphorylated and then translocates into the nucleus. In age-1 and akt-1 mutants, NSBP-1 was localized in the nuclei (Online Resource Fig. S4, S5), showing that the cytoplasmic localization of NSBP-1 was dependent on insulin signaling pathway kinases. Interestingly, however, NSBP-1 was still localized in the nucleus in daf-16; daf-2 double mutants (Online Resource Fig. S4). This result was verified by treating daf-16 and wild-type worms with daf-2 RNAi; daf-2 RNAi translocates NSBP-1 from cytosol to nucleus in both wild-type and daf-16 worms (Online Resource Fig. S5, S6). These results indicated that NSBP-1 translocates into the nucleus when insulin signaling is absent. However, the translocation does not seem to require DAF-16.
To determine whether phosphorylation plays a role in NSBP-1 localization in the absence of insulin signaling, we searched for potential AGE-1 or AKT-1 phosphorylation sites using the SCANSITE program (http://scansite.mit.edu/). This analysis identified threonine 203 (Thr203) and serine 239 (Ser239) of NSBP-1 as potential AKT-1 phosphorylation sites. To test if either of these was the phosphorylation site to regulate NSBP-1′s localization in the absence of insulin signaling, we generated three different transgenic worms that carried NSBP-1 fused with GFP, [36] a construct in which Thr203 of NSBP-1::GFP was substituted with alanine, or [34] a construct in which Ser239 of NSBP-1::GFP was substituted with glycine [45]. NSBP-1 T203A::GFP was constitutively localized in nuclei even in the wild type, whereas NSBP-1 S239G::GFP and NSBP-1::GFP remained in the cytoplasm (Fig. 4e–h, i–k and Online Resource Fig. S7). These results strongly suggest that phosphorylation of NSBP-1 on threonine 203, most likely by AKT-1, keeps NSBP-1 proteins in cytoplasm in the presence of insulin signaling, and dephosphorylation of the site in the absence of insulin signaling translocates the protein into the nucleus.
NSBP-1 directly binds to DAF-16
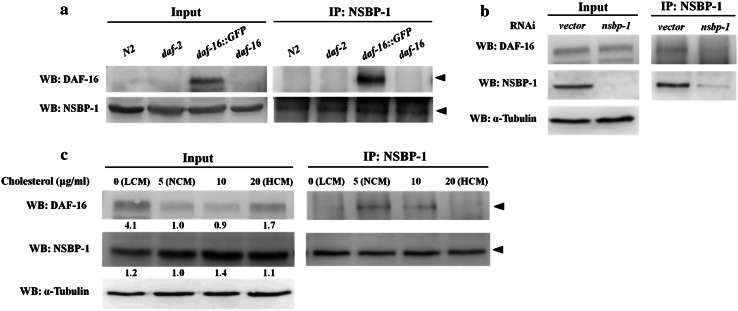
Our results show that NSBP-1 can be located in nuclei, and that translocation was dependent on the insulin signaling pathway. Based on the similarity between NSBP-1 and DAF-16, which also translocates into nucleus in the absence of insulin signaling, we examined if NSBP-1 directly interacts with DAF-16 using co-immunoprecipitation (Fig. 5). We used daf-16; muIs71 worms, which express GFP fused to DAF-16 [42], because it is difficult to detect endogenous DAF-16 in wild-type worms. DAF-16::GFP was detected in daf-16; muIs71 but not in the wild type or daf-2 (Fig. 5a). To confirm that the interaction was specific to DAF-16 but not to GFP, we immunoprecipitated NSBP-1 from a lysate muIs84[Psod-3::GFP] worms, which express an unrelated GFP fusion protein. There was no detectable interaction (Online Resource Fig. S8), confirming that the interaction is specific for DAF-16 but not for GFP. To further confirm the specificity, we performed RNAi of nsbp-1 to reduce amounts of NSBP-1 protein. No DAF-16 could be immunoprecipitated with NSBP-1 antibody when nsbp-1 was knocked down by RNAi, confirming the specificity (Fig. 5b).
Fig. 5.
NSBP-1 directly binds to DAF-16. a Lysates from the indicated genotypes were co-immunoprecipitated (IP) with antibodies against NSBP-1, then analyzed by Western blot (WB) with anti-DAF-16 and anti-NSBP-1 antibodies. b Transgenic worms carrying daf-16::GFP were grown on RNAi plates containing L4440 vector or nsbp-1 RNAi bacteria. Lysates were co-immunoprecipitated using anti-NSBP-1 antibody and analyzed by Western blot with anti-DAF-16 and anti-NSBP-1 antibodies. c NSBP-1 and DAF-16 interaction was affected by cholesterol concentration. Transgenic worms carrying daf-16::GFP were grown at various cholesterol concentrations. Lysates were co-immunoprecipitated using anti-NSBP-1 antibody and analyzed by Western blot with anti-DAF-16 and anti-NSBP-1 antibodies. Quantification of fold change in protein normalized with α-tubulin is shown below each lane
Because NSBP-1 also binds to cholesterol, we examined whether binding between NSBP-1 and DAF-16 is regulated by cholesterol. When we examined their interaction using cell lysates from wild-type worms grown in various cholesterol concentrations, both extracts obtained from worms grown in low and high (but not normal cholesterol) medium show reduced in binding between NSBP-1 and DAF-16 (Fig. 5c). This suggests the binding prefers an optimum concentration of ~5 μg/ml cholesterol. This also supports our previous observation (Fig. 3) of a cholesterol imbalance effect on daf-2 mutants; both high and low cholesterol treatment alleviated some of daf-2 mutant phenotypes (fat accumulation and dauer phenotype). This suggests that when there was too much or too little cholesterol, NSBP-1 could not bind to DAF-16 and therefore DAF-16 activity was reduced, which in turn alleviated several daf-2 phenotypes in life span, fat storage, and oxidative stress.
NSBP-1 and DAF-16 co-regulate transcription response
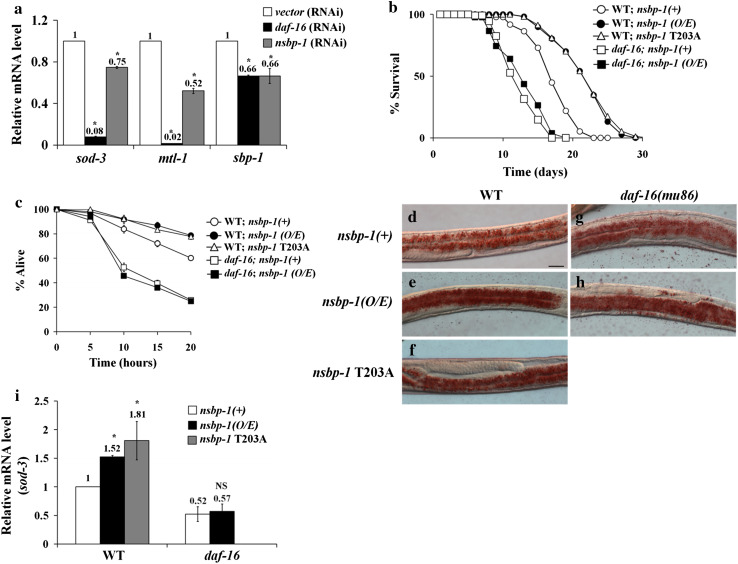
We have shown that both NSBP-1 and DAF-16 are regulated by the insulin signaling pathway and both translocate into the nucleus in the absence of insulin signaling. Furthermore, we showed that NSBP-1 and DAF-16 bind to each other. To understand the physiological consequences of this binding, we measured gene expression of known daf-16 targets, sod-3 and mtl-1, using quantitative RT-PCR (qRT-PCR). The expression of both genes decreased by nsbp-1 RNAi (Fig. 6a), indicating that NSBP-1 regulates DAF-16 transcriptional activity positively.
Fig. 6.
NSBP-1 regulates DAF-16-dependent transcription. a Decreased sod-3, mtl-1, and sbp-1 mRNA in daf-2 and wild-type worms treated with nsbp-1-RNAi. qRT-PCR was performed to assess gene expression. *p ≤ 0.05 versus control, NS not significant. b Overexpression (O/E) of nsbp-1 extended lifespan. Mean lifespan of the wild type 17.2 ± 0.1 (n = 96); nsbp-1(O/E) 21.6 ± 0.5 (n = 107), p = 0.0025; nsbp-1 T203A 22.0 ± 0.5 (n = 164), p < 0.0001; mean lifespan of daf-16 13.3 ± 0.2 (n = 108); daf-16; nsbp-1(O/E) 13.0 ± 0.5 (n = 133), p = 0.63. c Overexpression (O/E) of nsbp-1 increased resistance to oxidative stress. Worms (n = 30 for each strain) were treated with paraquat, and then survival was assessed. In the wild-type background, survival rate was nsbp-1(+) 61 %; nsbp-1(O/E) 78 %, p < 0.001 at 20 h. In the daf-16 background, nsbp-1 overexpression did not improve survival rate. p ≤ 0.001 versus nsbp-1(+), NS not significant. d–g Fat storage measured by Oil-Red O staining. Fat storage in both the wild type (d–f) and daf-16 (g–h) increased when nsbp-1 was overexpressed. A representative image from one of three independent experiments (n = 20) is shown. i nsbp-1(O/E) upregulated sod-3, and this upregulation depended on DAF-16 activity. sod-3 expression determined by qRT-PCR. *p ≤ 0.05 versus each control, NS not significant
Fatty acid and cholesterol metabolism are related in many aspects of lipid metabolism [46]. Of all the insulin-mediated physiological processes that NSBP-1 influenced, fat storage was most affected (Fig. 3). To understand how nsbp-1 regulates fat accumulation in interaction with DAF-16, we examined four genes that are known to function most significantly in fat accumulation and storage, fat-7, sbp-1, lipl-4, and C46C11.1. Among them we found that sbp-1, a homolog of SREBP, whose expression is known to be regulated by FOXO in mammals [47], is regulated by both NSBP-1 and DAF-16; when we measured sbp-1 mRNA level in worms treated with nsbp-1- or daf-16-specific RNAi, sbp-1 expression was decreased (Fig. 6a). These results indicated that NSBP-1 and DAF-16 act together to regulate both oxidative stress and fat storage.
Increased expression of NSBP-1 enhanced DAF-16 activity
To investigate if nsbp-1 overexpression (nsbp-1 O/E) influences insulin signaling pathway and daf-16 activity, we examined the effects of nsbp-1 O/E on life span, stress resistance, fat storage, and sod-3 expression. Lifespan increased about 25 % in both nsbp-1(O/E) and nsbp-1(T203A O/E) worms (Fig. 6b, Online Resource Table S3). In addition, nsbp-1(O/E) worms are resistant to oxidative stress when changes in survival rate in parquet treatment and in sod-3 expression were measured as an index of oxidative stress; after 20 h of paraquat treatment, nsbp-1(O/E) worms showed 78 % survival, whereas control worms exhibited 60 % viability (Fig. 6c). Fat storage and sod-3 expression were also increased in both nsbp-1(O/E) and nsbp-1(T203A O/E). However, increase in fat storage by overexpression of wild-type or constitutively active NSBP-1 was not abolished by the daf-16 mutation, suggesting that NSBP-1 may have another way to regulate fat storage independent of DAF-16 (Fig. 6d–h). The increases in resistance to oxidative stress, in sod-3 expression and in lifespan were all abolished in daf-16; nsbp-1(O/E) (Fig. 6i). That the phenotypes caused by overexpression and constitutively active gene function show phenotypes exactly opposite to those caused by knockdown of the gene shows that all phenotypes are indeed mediated by nsbp-1. Moreover, that the phenotypes caused by overexpression mimic daf-2 mutant phenotypes mediated by DAF-16 and that most of those phenotypes were abolished by DAF-16 mutation strongly supports the hypothesis that NSBP-1 interacts with the insulin pathway by regulating DAF-16 activity.
Discussion
Although C. elegans lack de novo cholesterol biosynthesis, they require cholesterol for germ cell development, growth, stress resistance, dauer formation, fat storage, cuticle formation, and modulation of other lipids [7, 9, 10, 48–50]. Changes in exogenous or endogenous cholesterol profoundly affect C. elegans cellular function and cholesterol cannot be replaced by other lipid molecules. For example, disturbances in cholesterol uptake are known to cause defects in germ cell development, growth, cuticle development, and motility [48, 51]. In this regard, C. elegans represents a nice model for studying cholesterol-mediated signaling [9]. Thus, we believe that many of the physiological changes caused by various cholesterol conditions (e.g., disturbance in uptake or hypercholesterolemia) are relevant to worm physiology [9].
It will be intriguing in the future to determine whether the link between cholesterol and insulin signaling via NSBP-1 that we discovered in C. elegans can also exist in a similar manner in mammals, including humans. NSBP-1 is a homolog of human NAP1, which is involved in such biological functions as histone chaperoning, nucleocytoplasmic shuttling, chromatin remodeling, enzyme inhibition, replication, transcription, silencing, and apoptosis [52]. This protein is essential in mammals since deletion leads to embryonic lethality or poor viability in adults [53, 54], similar to homozygous deletion of nsbp-1 in C. elegans.
NSBP-1 has cholesterol-binding activity. It possesses binding affinity for cholesterol (K d = 87 nM; Fig. 1) comparable to that of other known sterol-binding proteins (e.g., PBR, K d = 10 nM; NPC2, K d = 30–50 nM; NPC1, K d = 100 nM) [55–57]. Interestingly, like NPC2, NSBP-1 lacks any known sterol-sensing domain. Because NPC2 binds sterols via a hydrophobic region within its β-sheet [57], we speculate that NSBP-1 may bind to cholesterol via a highly conserved hydrophobic region within the β-sheet [52].
Another important function of NSBP-1 is to regulate DAF-16 activity. Although NAP1 has been reported to bind other transcription factors [58, 59], we show differential binding of NSBP-1 to a FOXO factor in C. elegans. Unlike other nuclear factors (e.g., SMK-1, HCF-1, and BAR-1), NSBP-1 not only binds differentially to DAF-16 to control some of insulin signaling in a cholesterol-dependent manner, but also translocates to the nucleus depending on AKT. DAF-16 plays a major role in mediating changes in resource allocation in response to environmental changes such as heat stress and starvation [60]. DAF-16 translocates to the nucleus upon heat stress [60], whereas NSBP-1 does not seem to respond to this stress: there was no change in location of DAF-16 or NSBP-1 in response to changes in cholesterol level (data not shown). So we suggested that nsbp-1 does not exactly overlap with daf-16 function while cholesterol imbalance is different from stresses (e.g., heat stress).
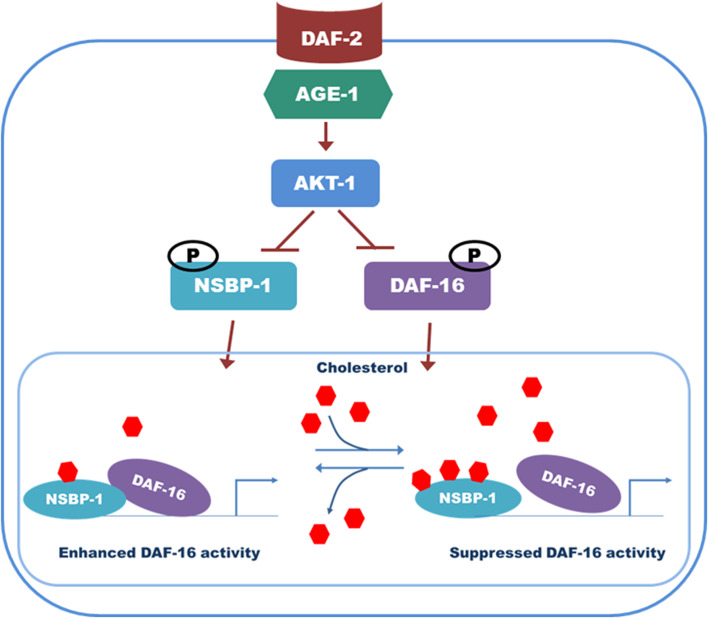
Based on our results, we propose a model of how NSBP-1 mediates various cellular functions (Fig. 7). In the presence of active insulin signal, NSBP-1 and DAF-16 remain in the cytosol. In the absence of insulin signal, however, NSBP-1 translocates to the nucleus and interacts with DAF-16 to enhance its activity. This interaction increases dauer formation, oxidative stress resistance, and fat storage. However, under low- or high-cholesterol conditions, NSBP-1 can bind only weakly to DAF-16 and therefore DAF-16 activity is reduced (Figs. 5, 7). We interpret these data that the interaction between NSBP-1 and DAF-16 is dependent on cholesterol concentration and that interaction with DAF-16 is critical for NSBP-1-mediated regulation of the insulin signaling pathway. Studies showed that hyperactivation of FOXO transcription factors causes insulin-resistant diabetes in mammals [61, 62]. To prevent FOXO hyperactivation, a feedback control mechanism modulates the insulin signaling pathway [63]. Our finding that NSBP-1 as a cholesterol-binding protein regulates DAF-16 activity suggests that to prevent FOXO hyperactivation in worms, high cholesterol may suppress DAF-16 activity by controlling its binding to NSBP-1. In fact, DAF-16 hyperactivation could also be harmful to worms [64]. Thus, it is reasonable to propose that NSBP-1 acts as a cholesterol-dependent modulator of DAF-16 function, which can lead to fine-tuning of aging, dauer formation, fat storage, and stress resistance in C. elegans. In mammals, binding of CBP/p300 to FOXO factors is essential for FOXO-mediated transcription [65], and NAP1-p300 complexes promote transcriptional activation [66]. Our results suggest that NAP1-FoxO complexes could also play an important role in mammals, as NSBP-1-DAF-16 does in C. elegans.
Fig. 7.
Proposed model for the potential role of NSBP-1 in the insulin pathway. DAF-2 signals through the AGE-1 signaling cascade activate AKT-1 by phosphorylation. These kinases in turn regulate DAF-16 and NSBP-1 negatively. In the absence of insulin signaling, NSBP-1 binds to DAF-16 in the nucleus to regulate insulin signaling-mediated dauer formation, fat storage, and oxidative stress
In conclusion, we report the discovery of NSBP-1 as a novel cholesterol-binding protein that mediates many cellular processes by differentially interacting with DAF-16 in response to cholesterol concentration. Insulin signaling-dependent nucleocytoplasmic translocation of NSBP-1 was found to be modulated in a cholesterol concentration-dependent manner. This finding expands our understanding of insulin resistance, as well as other metabolic disease mechanisms in mammals, suggesting screens for new therapeutic drugs for type II diabetes or treatment strategies for other metabolic syndromes in humans [67].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by National Research Foundation of Korea grant 2011-0028112 (to Y.-K. P.), World Class University Program grant R31-2008-000-10086-0 funded by the Korean government (Ministry of Education, Science and Technology), and National Project for Personalized Genomic Medicine grant A111218-11-CP01 (to Y.-K. P.). We also thank the National BioResource Project (NBRP) of Japan for providing the nsbp-1(tm1278) strain.
Abbreviations
- FOXO
Forkhead transcription factor
- HCM
High cholesterol medium
- LCM
Low cholesterol medium
- MLS
Mean lifespan
- NAP
Nucleosome assembly protein
- NSBP
Nematode sterol-binding protein
References
- 1.Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev. 1998;8(5):571–575. doi: 10.1016/S0959-437X(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281(42):31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 4.Borresen AL, Berg K. Serum reserve cholesterol binding capacity (SRCBC): the relative importance of different lipoprotein classes. Artery. 1981;9(2):96–119. [PubMed] [Google Scholar]
- 5.Scheel J, Srinivasan J, Honnert U, Henske A, Kurzchalia TV. Involvement of caveolin-1 in meiotic cell-cycle progression in Caenorhabditis elegans . Nat Cell Biol. 1999;1(2):127–129. doi: 10.1038/10100. [DOI] [PubMed] [Google Scholar]
- 6.Matyash V, Entchev EV, Mende F, Wilsch-Brauninger M, Thiele C, Schmidt AW, Knolker HJ, Ward S, Kurzchalia TV. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol. 2004;2(10):e280. doi: 10.1371/journal.pbio.0020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim YH, Chun JH, Lee EY, Paik YK. Role of cholesterol in germ-line development of Caenorhabditis elegans . Mol Reprod Dev. 2002;61(3):358–366. doi: 10.1002/mrd.10099. [DOI] [PubMed] [Google Scholar]
- 8.Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126(3):597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 9.Paik YK, Jeong SK, Lee EY, Jeong PY, Shim YH. C. elegans: an invaluable model organism for the proteomics studies of the cholesterol-mediated signaling pathway. Expert Rev Proteomics. 2006;3(4):439–453. doi: 10.1586/14789450.3.4.439. [DOI] [PubMed] [Google Scholar]
- 10.Cheong MC, Na K, Kim H, Jeong SK, Joo HJ, Chitwood DJ, Paik YK. A potential biochemical mechanism underlying the influence of sterol deprivation stress on Caenorhabditis elegans longevity. J Biol Chem. 2011;286(9):7248–7256. doi: 10.1074/jbc.M110.189183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Devel. Cell. 2001;1(6):841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 12.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 13.Accili D. Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes. 2004;53(7):1633–1642. doi: 10.2337/diabetes.53.7.1633. [DOI] [PubMed] [Google Scholar]
- 14.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9(10):759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antebi A. Genetics of aging in Caenorhabditis elegans . PLoS Genet. 2007;3(9):1565–1571. doi: 10.1371/journal.pgen.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans . Science. 1997;277(5328):942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 18.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans . Nature. 1996;382(6591):536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 19.Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell. 2004;6(4):577–588. doi: 10.1016/s1534-5807(04)00095-4. [DOI] [PubMed] [Google Scholar]
- 20.Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans . Genes Dev. 1999;13(11):1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12(16):2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans . Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 23.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans . Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 24.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans . Genetics. 1998;150(1):129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 26.Brenner S. The genetics of Caenorhabditis elegans . Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuatrecasas P, Wilchek M, Anfinsen CB. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci USA. 1968;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970;245(12):3059–3065. [PubMed] [Google Scholar]
- 29.Shaltiel S, Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci USA. 1973;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhara K, Gomi T, Sato H, Itagaki E, Takemori S, Katagiri M. Purification and immunochemical characterization of the two adrenal cortex mitochondrial cytochrome P-450-proteins. Arch Biochem Biophys. 1978;190(1):290–299. doi: 10.1016/0003-9861(78)90278-3. [DOI] [PubMed] [Google Scholar]
- 31.Gibson GG, Schenkman JB. Purification and properties of cytochrome P-450 obtained from liver microsomes of untreated rats by lauric acid affinity chromatography. J Biol Chem. 1978;253(17):5957–5963. [PubMed] [Google Scholar]
- 32.Lee HJ, Kwon MS, Lee EY, Cho SY, Paik YK. Establishment of a PF2D-MS/MS platform for rapid profiling and semiquantitative analysis of membrane protein biomarkers. Proteomics. 2008;8(11):2168–2177. doi: 10.1002/pmic.200701022. [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan A, Sun LP, Kwon HJ, Brown MS, Goldstein JL. Direct binding of cholesterol to the purified membrane region of SCAP: mechanism for a sterol-sensing domain. Mol Cell. 2004;15(2):259–268. doi: 10.1016/j.molcel.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 3rd;317(5838):660–663. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- 35.Rourke EJ, Soukas AA, Carr CE, Ruvkun G. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metabol. 2009;10(5):430–435. doi: 10.1016/j.cmet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ristow M, Pfister MF, Yee AJ, Schubert M, Michael L, Zhang CY, Ueki K, Michael MD, 2nd, Lowell BB, Kahn CR. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc Natl Acad Sci USA. 2000;97(22):12239–12243. doi: 10.1073/pnas.220403797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, Grant B, Maxfield FR, Kurzchalia TV. Distribution and transport of cholesterol in Caenorhabditis elegans . Mol Biol Cell. 2001;12(6):1725–1736. doi: 10.1091/mbc.12.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park YJ, Luger K. Structure and function of nucleosome assembly proteins. Biochem Cell Biol. 2006;84(4):549–558. doi: 10.1139/o06-088. [DOI] [PubMed] [Google Scholar]
- 39.Wyles JP, Perry RJ, Ridgway ND. Characterization of the sterol-binding domain of oxysterol-binding protein (OSBP)-related protein 4 reveals a novel role in vimentin organization. Exp Cell Res. 2007;313(7):1426–1437. doi: 10.1016/j.yexcr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22(16):2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123(7):765–771. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 42.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 43.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvert ME, Keck KM, Ptak C, Shabanowitz J, Hunt DF, Pemberton LF. Phosphorylation by casein kinase 2 regulates Nap1 localization and function. Mol Cell Biol. 2008;28(4):1313–1325. doi: 10.1128/MCB.01035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips JE, Gersbach CA, Wojtowicz AM, Garcia AJ. Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J Cell Sci. 2006;119(Pt 3):581–591. doi: 10.1242/jcs.02758. [DOI] [PubMed] [Google Scholar]
- 46.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/S0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 47.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118(6):2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi BK, Chitwood DJ, Paik YK. Proteomic changes during disturbance of cholesterol metabolism by azacoprostane treatment in Caenorhabditis elegans . Mol Cell Proteomics. 2003;2(10):1086–1095. doi: 10.1074/mcp.M300036-MCP200. [DOI] [PubMed] [Google Scholar]
- 49.Merris M, Kraeft J, Tint GS, Lenard J. Long-term effects of sterol depletion in C. elegans: sterol content of synchronized wild-type and mutant populations. J Lipid Res. 2004;45(11):2044–2051. doi: 10.1194/jlr.M400100-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Lee EY, Shim YH, Chitwood DJ, Hwang SB, Lee J, Paik YK. Cholesterol-producing transgenic Caenorhabditis elegans lives longer due to newly acquired enhanced stress resistance. Biochem Biophys Res Commun. 2005;328(4):929–936. doi: 10.1016/j.bbrc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Brown G, Ailion M, Lee S, Thomas JH. NCR-1 and NCR-2, the C. elegans homologs of the human Niemann-Pick type C1 disease protein, function upstream of DAF-9 in the dauer formation pathways. Development. 2004;131(22):5741–5752. doi: 10.1242/dev.01408. [DOI] [PubMed] [Google Scholar]
- 52.Park YJ, Luger K. The structure of nucleosome assembly protein 1. Proc Nat Acad Sci USA. 2006;103(5):1248–1253. doi: 10.1073/pnas.0508002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lankenau S, Barnickel T, Marhold J, Lyko F, Mechler BM, Lankenau DH. Knockout targeting of the Drosophila nap1 gene and examination of DNA repair tracts in the recombination products. Genetics. 2003;163(2):611–623. doi: 10.1093/genetics/163.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogner UC, Spyropoulos DD, Le Novere N, Changeux JP, Avner P. Control of neurulation by the nucleosome assembly protein-1-like 2. Nat Genet. 2000;25(4):431–435. doi: 10.1038/78124. [DOI] [PubMed] [Google Scholar]
- 55.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283(2):1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 56.Jamin N, Neumann JM, Ostuni MA, Vu TK, Yao ZX, Murail S, Robert JC, Giatzakis C, Papadopoulos V, Lacapere JJ. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol Endocrinol. 2005;19(3):588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- 57.Ko DC, Binkley J, Sidow A, Scott MP. The integrity of a cholesterol-binding pocket in Niemann-Pick C2 protein is necessary to control lysosome cholesterol levels. Proc Natl Acad Sci USA. 2003;100(5):2518–2525. doi: 10.1073/pnas.0530027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eckey M, Hong W, Papaioannou M, Baniahmad A. The nucleosome assembly activity of NAP1 is enhanced by Alien. Mol Cell Biol. 2007;27(10):3557–3568. doi: 10.1128/MCB.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shikama N, Chan HM, Krstic-Demonacos M, Smith L, Lee CW, Cairns W, La Thangue NB. Functional interaction between nucleosome assembly proteins and p300/CREB-binding protein family coactivators. Mol Cell Biol. 2000;20(23):8933–8943. doi: 10.1128/MCB.20.23.8933-8943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11(24):1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 61.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14(7):778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116(9):2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19(20):2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115(4):489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 65.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends Biochem Sci. 2005;30(2):81–86. doi: 10.1016/j.tibs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Asahara H, Tartare-Deckert S, Nakagawa T, Ikehara T, Hirose F, Hunter T, Ito T, Montminy M. Dual roles of p300 in chromatin assembly and transcriptional activation in cooperation with nucleosome assembly protein 1 in vitro. Mol Cell Biol. 2002;22(9):2974–2983. doi: 10.1128/MCB.22.9.2974-2983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5(3):150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.