Abstract
Three isolates of PARA (particle aiding replication of adenovirus)-adenovirus 7 out of a total of 112 clonal progeny derived by two successive plaque purifications in green monkey kidney cells (GMK) were found to induce the synthesis of simian papovavirus40 (SV 40) tumor (T) antigen in the cytoplasm of infected cells. The variant viruses induced plaque formation in human embryonic kidney cells which followed one-hit kinetics. In GMK cells, plaque formation followed two-hit kinetics which converted to first-order kinetics in the presence of additional helper adenovirus type 7. Analysis of plaque progeny from human cells showed that the progeny could replicate only in human cells, whereas progeny from monkey cells could multiply in both human and monkey cells. Heterologous human adenoviruses were able to enhance plaque formation by the variant viruses in monkey kidney cells. Neutralization tests indicated that both components of the populations had a type 7 adenovirus capsid. All three viruses were capable of inducing SV40 transplantation immunity in weanling hamsters. These results indicate the three variants are PARA-adenovirus 7 populations. Response of the induction of the synthesis of the cytoplasmic antigen to metabolic inhibitors was the same as for the synthesis of the nuclear SV40 T antigen. Different pools of sera which reacted with the intranuclear SV40 T antigen also detected the cytoplasmic antigen induced by the variant viruses. An adsorption experiment with cells containing either nuclear or cytoplasmic T antigen to remove tumor antibody from hamster sera also indicated that it is probably SV40 T antigen which is responsible for the cytoplasmic reaction. The species of the host cell—human, simian, or rabbit—appeared to play no role in the altered localization of this antigen. It is postulated that these PARA variants are further defective in some virus-mediated transport mechanism which shifts the T antigen from the cytoplasm to the nucleus.
Full text
PDF

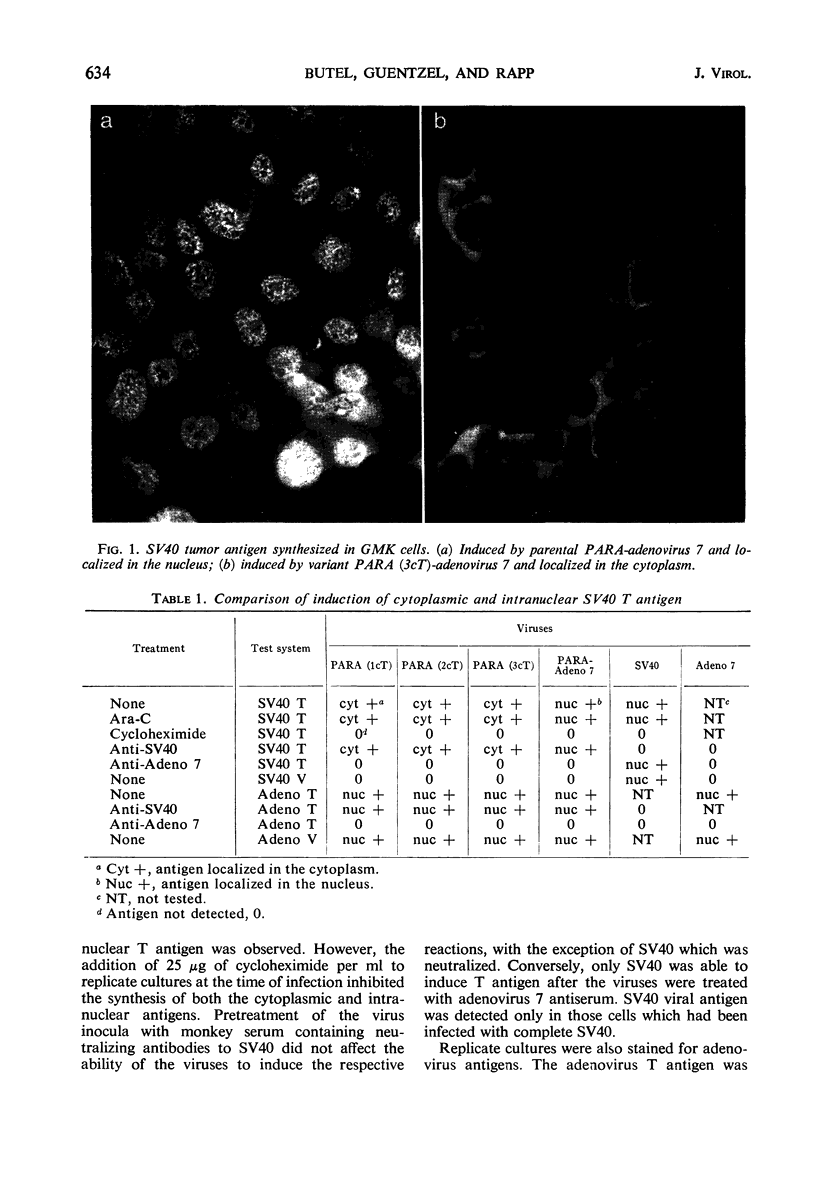
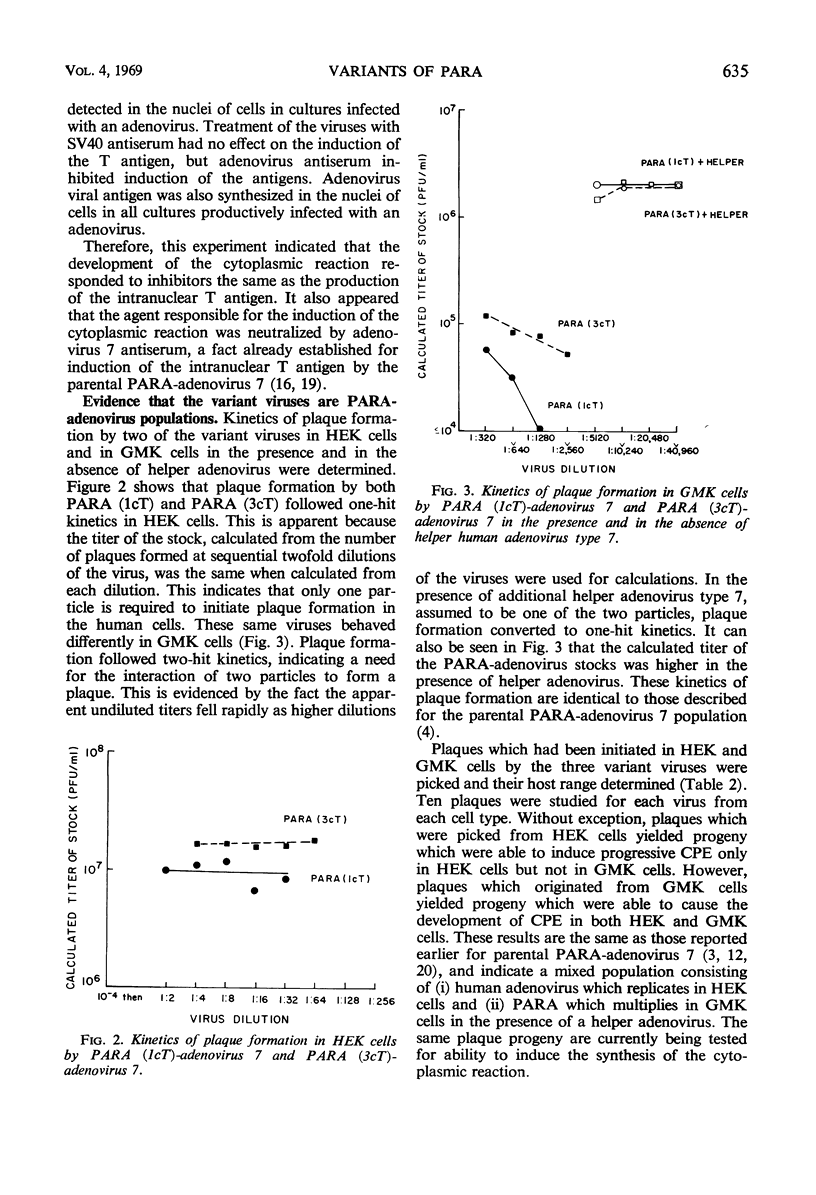


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHKENAZI A., MELNICK J. L. Tumorigencity of simian papovavirus SV40 and of virus-transformed cells. J Natl Cancer Inst. 1963 Jun;30:1227–1265. [PubMed] [Google Scholar]
- BENYESH-MELNICK M., ROSENBERG H. S. THE ISOLATION OF ADENOVIRUS TYPE 7 FROM A FATAL CASE OF PNEUMONIA AND DISSEMINATED DISEASE. J Pediatr. 1964 Jan;64:83–87. doi: 10.1016/s0022-3476(64)80321-8. [DOI] [PubMed] [Google Scholar]
- BOEYE A., MELNICK J. L., RAPP F. ADENOVIRUS-SV40 "HYBRIDS": PLAQUE PURIFICATION INTO LINES IN WHICH THE DETERMINANT FOR THE SV40 TUMOR ANTIGEN IS LOST OR RETAINED. Virology. 1965 Jul;26:511–512. doi: 10.1016/0042-6822(65)90016-4. [DOI] [PubMed] [Google Scholar]
- Boeyé A., Melnick J. L., Rapp F. SV40-adenovirus "hybrids": presence of two genotypes and the requirement of their complementation for viral replication. Virology. 1966 Jan;28(1):56–70. doi: 10.1016/0042-6822(66)90306-0. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Melnick J. L., Rapp F. Detection of biologically active adenovirions unable to plaque in human cells. J Bacteriol. 1966 Aug;92(2):433–438. doi: 10.1128/jb.92.2.433-438.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Rapp F. Inactivation and density studies with PARA-adenovirus 7 (SV40-adenovirus "hybrid" population). J Immunol. 1966 Oct;97(4):546–553. [PubMed] [Google Scholar]
- Butel J. S., Rapp F. Replication in simian cells of defective viruses in an SV40-adenovirus "hybrid" population. J Bacteriol. 1966 Jan;91(1):278–284. doi: 10.1128/jb.91.1.278-284.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROWE W. P., BLACK P. H., HUEBNER R. J. PRODUCTION OF "TUMOR-SPECIFIC" ANTIGENS BY ONCOGENIC VIRUSES DURING ACUTE CYTOLYTIC INFECTIONS. Proc Natl Acad Sci U S A. 1965 Jan;53:12–19. doi: 10.1073/pnas.53.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., CHANOCK R. M., RUBIN B. A., CASEY M. J. INDUCTION BY ADENOVIRUS TYPE 7 OF TUMORS IN HAMSTERS HAVING THE ANTIGENIC CHARACTERISTICS OF SV40 VIRUS. Proc Natl Acad Sci U S A. 1964 Dec;52:1333–1340. doi: 10.1073/pnas.52.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELNICK J. L., KHERA K. S., RAPP F. PAPOVAVIRUS SV40: FAILURE TO ISOLATE INFECTIOUS VIRUS FROM TRANSFORMED HAMSTER CELLS SYNTHESIZING SV40-INDUCED ANTIGENS. Virology. 1964 Jul;23:430–432. doi: 10.1016/0042-6822(64)90267-3. [DOI] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., MELNICK J. L. VIRUS-INDUCED INTRANUCLEAR ANTIGEN IN CELLS TRANSFORMED BY PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1131–1135. doi: 10.3181/00379727-116-29472. [DOI] [PubMed] [Google Scholar]
- RAPP F., KITAHARA T., BUTEL J. S., MELNICK J. L. SYNTHESIS OF SV40 TUMOR ANTIGEN DURING REPLICATION OF SIMIAN PAPOVAVIRUS (SV40). Proc Natl Acad Sci U S A. 1964 Nov;52:1138–1142. doi: 10.1073/pnas.52.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., BUTEL J. S., KITAHARA T. THE INCORPORATION OF SV40 MATERIAL INTO ADENOVIRUS 7 AS MEASURED BY INTRANUCLEAR SYNTHESIS OF SV40 TUMOR ANTIGEN. Proc Natl Acad Sci U S A. 1964 Dec;52:1348–1352. doi: 10.1073/pnas.52.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Butel J. S., Melnick J. L. SV40-adenovirus "hybrid" populations: transfer of SV40 determinants from one type of adenovirus to another. Proc Natl Acad Sci U S A. 1965 Sep;54(3):717–724. doi: 10.1073/pnas.54.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Butel J. S., Tevethia S. S., Melnick J. L. Comparison of ability of defective foreign genomes (PARA and MAC) carried by human adenoviruses to induce SV40 transplantation immunity. J Immunol. 1967 Aug;99(2):386–391. [PubMed] [Google Scholar]
- Rapp F., Jerkofsky M. Replication of PARA (defective SV40)-adenoviruses in simian cells. J Gen Virol. 1967 Jul;1(3):311–321. doi: 10.1099/0022-1317-1-3-311. [DOI] [PubMed] [Google Scholar]
- Rapp F., Pauluzzi S., Butel J. S. Variation in properties of plaque progeny of PARA (defective simian papovavirus 40)-adenovirus 7. J Virol. 1969 Nov;4(5):626–631. doi: 10.1128/jvi.4.5.626-631.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Tevethia S. S., Melnick J. L. Papovavirus SV40 transplantation immunity conferred by an adenovirus-SV40 hybrid. J Natl Cancer Inst. 1966 Apr;36(4):703–708. doi: 10.1093/jnci/36.4.703. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Bau S. G. Studies of adenovirus SV40 hybrid viruses. II. Defectiveness of the hybrid particles. J Exp Med. 1965 Nov 1;122(5):955–966. doi: 10.1084/jem.122.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABIN A. B., KOCH M. A. SOURCE OF GENETIC INFORMATION FOR SPECIFIC COMPLEMENT-FIXING ANTIGENS IN SV40 VIRUS-INDUCED TUMORS. Proc Natl Acad Sci U S A. 1964 Nov;52:1131–1138. doi: 10.1073/pnas.52.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEVETHIA S. S., KATZ M., RAPP F. NEW SURFACE ANTIGEN IN CELLS TRANSFORMED BY SIMIAN PAPOVAVIRUS SV40. Proc Soc Exp Biol Med. 1965 Jul;119:896–901. doi: 10.3181/00379727-119-30330. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]