Summary
A common principle of tissue regeneration is the reactivation of previously employed developmental programs [1–3]. During zebrafish heart regeneration, cardiomyocytes in the cortical layer of the ventricle induce the transcription factor gene gata4 and proliferate to restore lost muscle [4–6]. A dynamic cellular mechanism initially creates this cortical muscle in juvenile zebrafish, where a small number of internal cardiomyocytes breach the ventricular wall and expand upon its surface [7]. Here, we find that emergent juvenile cortical cardiomyocytes induce expression of gata4 similarly as during regeneration. Clonal analysis indicates that these cardiomyocytes make biased contributions to build the ventricular wall, whereas gata4+ cardiomyocytes have little or no proliferation hierarchy during regeneration. Experimental microinjuries or conditions of rapid organismal growth stimulate production of ectopic gata4+ cortical muscle, implicating biomechanical stress in morphogenesis of this tissue and revealing clonal plasticity. Induced transgenic inhibition defined an essential role for Gata4 activity in morphogenesis of the cortical layer and the preservation of normal cardiac function in growing juveniles, and again in adults during heart regeneration. Our experiments uncover an injury-responsive program that prevents heart failure in juveniles by fortifying the ventricular wall, one that is reiterated in adults to promote regeneration after cardiac damage.
Results and Discussion
gata4 Expression is Activated in Emergent Cortical Cardiomyocytes of the Growing Juvenile Zebrafish Ventricle
As juvenile zebrafish mature into adults, a layer of cortical muscle encapsulates the ventricle. Multicolor clonal analysis demonstrated that this cortical muscle is created by a small number (~8) of proliferative cardiomyocytes (Figure 1A–1C; Figures S1A and S1B). These cells emerge from inner trabecular muscle through the overlying primordial layer in rare breaching events, before proliferating upon the ventricular surface (Figure 1A) [7]. To characterize these emergent cardiomyocytes, we examined whether they express the transcription factor gata4, a marker induced in a subpopulation of cardiomyocytes that regenerates lost muscle after resection of the adult ventricular apex [5]. The functions of Gata4 have been extensively studied in murine cardiomyocytes during embryonic cardiac patterning, pathological remodeling, and reprogramming [8–13].
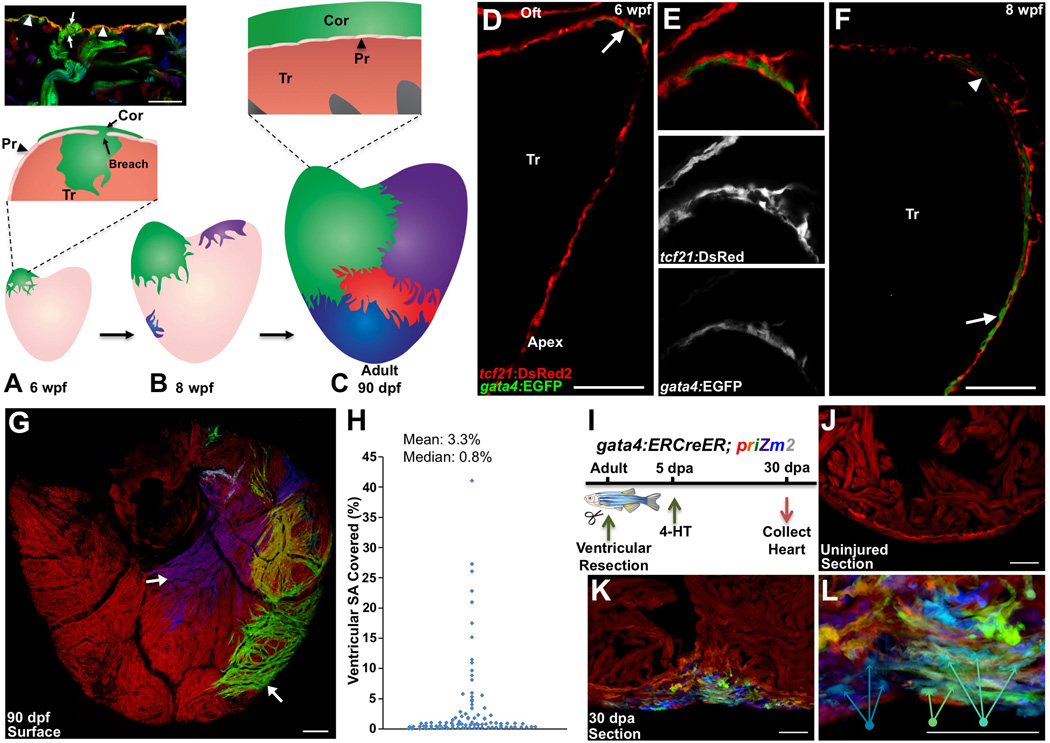
Figure 1. gata4 Marks and Traces Emerging Juvenile Cortical Cardiomyocytes and Regenerating Cardiomyocytes.
(A) The 30 dpf ventricle is comprised of an outer primordial layer of single cardiomyocyte thickness. By ~6 weeks post fertilization (wpf), inner trabecular cardiomyocytes (Tr) (green) breach the primordial layer (Pr) to establish a cortical cardiomyocyte clone (Cor) at the base of the ventricle. (Top) Histologic section from multicolor clonal analysis, indicating breaching (arrows) of the primordial layer (arrowheads) by a trabecular myofiber (green).
(B) During maturation, the initial basal clone (green) expands while other clones emerge on the ventricular surface.
(C) (Bottom) Cortical layer development is completed by adulthood, with a small number of clones contributing to the entire cortical layer. (Top) The adult ventricle retains an architecture with 3 muscle types.
(D–F) Tissue sections of 6 (D and E), and 8 (F) weeks post-fertilization (wpf) ventricular portions, assessed for tcf21:DsRed2+ epicardial cells (red) and gata4:EGFP+ cardiomyocytes (green). At 6 wpf, a cluster of gata4:EGFP+ surface myocytes is visible at the base of the heart in an area of dense epicardial tissue (arrow in (D)). By 8 wpf, gata4:EGFP+ cortical myocytes are detected at the ventricular midpoint with cortical gata4:EGFP expression strongest in cardiomyocytes closer to the apex (arrow), and weaker in basal myocytes (arrowhead) (n = 8–12). Tr, trabeculae. Oft, Outflow tract.
(G) gata4:ERCreER; priZm2 animals were pulsed with 4-HT at 6 wpf, and ventricles are analyzed at 90 dpf. Image shows surface muscle from a ventricle in these experiments with arrows indicating large clones.
(H) Percentage surface area (SA) occupied by each clone from experiments in (G), (96 total counted clones from 9 ventricular halves).
(I) Cartoon of lineage-tracing experiments to determine clonal contributions in regenerating gata4+ cardiomyocytes.
(J) Section through a ventricle from an uninjured animal 10 days after 4-HT treatment, indicating no recombination in the absence of injury.
(K) Section through a ventricular apex at 30 dpa, visualizing multiple colored cardiomyocyte clones in regenerated muscle. Over 15 colors can be distinguished in this 10-micron section of the regenerate. A maximum projection image from confocal slices is shown (n = 6).
(L) Higher magnification view of regenerate shown in (K). Arrows indicate 3 differently colored multicellular clones.
Scale bars = 100 µm (D–G); 50 µm (J–L).
We did not detect fluorescence in primordial or trabecular cardiomyocytes of 5 weeks post-fertilization (wpf) juvenile gata4:EGFP ventricles (Figures S1C and S1D). By contrast, at 6 wpf, the onset of cortical layer formation, gata4:EGFP was evident in a small population of emergent cortical cardiomyocytes positioned between epicardial cell layers at the base of the ventricle near the outflow tract (Figures 1D and 1E; Movie S1). By 8 wpf, gata4:EGFP was present in cortical cardiomyocytes that spanned to the chamber midpoint. Myocytes closer to the apex displayed a gradually stronger EGFP signal, indicating higher levels of gata4 expression (Figure 1F). Thus, gata4 regulatory sequences are activated in emergent cortical muscle, resembling their activation during adult heart regeneration.
Clonal Analysis of gata4+ Cardiomyocytes during Cortical Morphogenesis and Regeneration
In previous clonal analysis experiments, we consistently observed a large cortical clone at the base of the adult ventricle that could cover 30–60% of the ventricular surface [7]. To directly test whether juvenile gata4+ cardiomyocytes at the ventricular base are the source of the adult cortical layer, we performed a clonal lineage trace of these cells. gata4:ERCreER transgenic zebrafish [5] were crossed to different priZm multicolor lineage-tracing lines. One line was identified that enabled recombination only in the presence of 4-HT, Tg(β-act2:Brainbow1.0L)pd50 (referred to hereafter as priZm2). To label gata4+ cardiomyocytes at the ventricular base, we pulsed 6 wpf gata4:ERCreER; priZm2 animals with 4-HT. Ventricles were analyzed in 90 dpf adults, and 9 of 32 ventricular surfaces contained labeled (non-red) clones of cortical muscle, often spanning into the apical portion (Figure 1G). No labeling of trabecular muscle was detected. These fate-mapping results indicate that gata4+ cardiomyocytes at the base of the 6 wpf ventricle proliferate to create the adult cortical layer.
Upon quantification, we identified a clear proliferation bias, with several large clones apparent among many smaller clones (Figure 1H). Analysis of these data indicated that clone sizes did not follow a normal distribution. Rather, they were more consistent with a model of two distinct cell populations with low and high proliferative capacities (Figures S1E and S1F). These populations appeared to be regionally separable, as clones positioned toward the apex were on average over 10 times larger than clones located at the base (9.8% surface area vs. 0.8%; Figure 1H; Figure S1G). Thus, emergent cardiomyocytes that induce gata4 show proliferative heterogeneity, with cardiomyocytes that generate apical muscle making larger contributions. From our fatemapping experiments (and Figure 4 experiments), we expect that gata4+ myocytes make the primary contribution to cortical layer formation, but we cannot exclude minor contributions from other source cardiomyocytes.
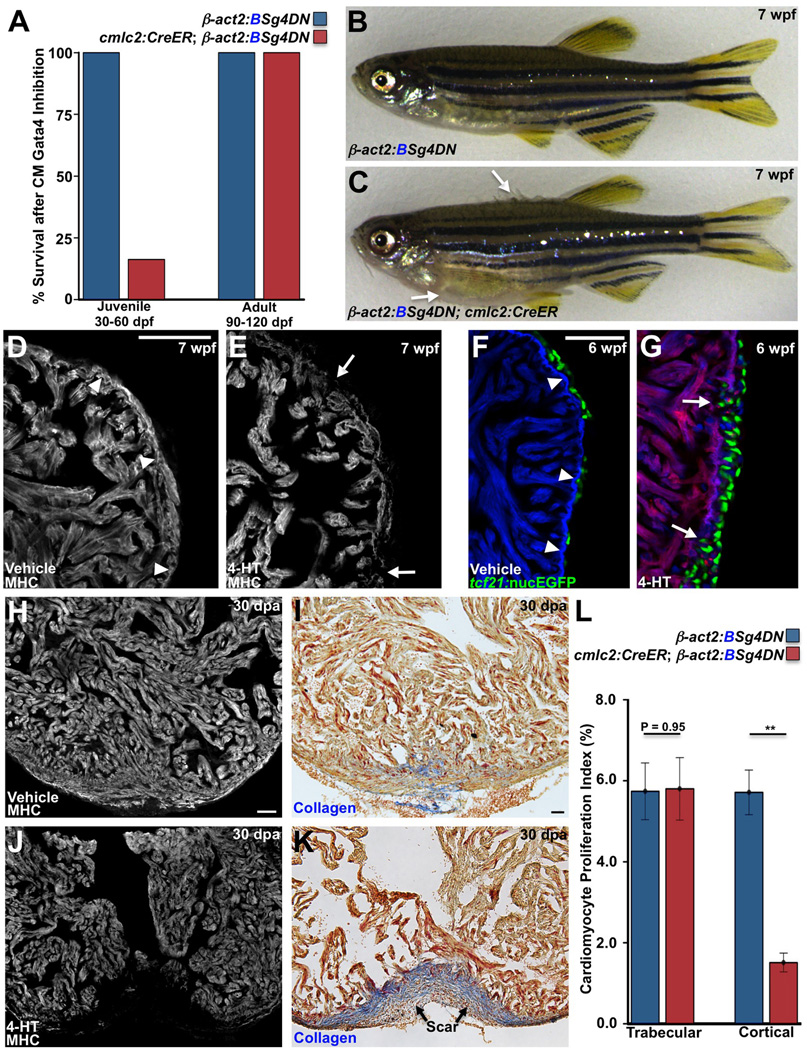
Figure 4. Gata4 Inhibition Blocks Juvenile Cortical Layer Formation and Adult Heart Regeneration.
(A) Survival of zebrafish after induction of a dominant-negative Gata4 construct (g4DN) for 30 days in cardiomyocytes at either 30 or 90 dpf. g4DN induction at 30 dpf sharply reduced survival of juveniles (n = 23–43), whereas g4DN induction at 90 dpf did not affect survival (n = 8–16).
(B) Cre (−) control animal exposed to 4-HT at 5 wpf, appearing normal at 7 wpf.
(C) A Cre (+) clutchmate exposed to 4-HT at 5 wpf, with flared scales indicative of edema (arrows).
(D and E) Sections of the 7 wpf ventricular wall after g4DN induction at 5 wpf, stained for Myosin heavy chain (MHC). Control animals have cortical muscle at the base of the heart, while the wall is thinned and disrupted after g4DN induction (n = 4).
(F and G) Maturing juvenile ventricles visualized for tcf21:nucEGFP+ epicardium (green), g4DN (red), and β-actin2:BFP (blue). The 6 wpf control ventricle (F) has an contiguous wall and normal epicardial cell distribution. g4DN induction at 5 wpf results in wall gaps (arrows) and increased epicardial cell presence at 6 wpf (G) (n = 6–9).
(H–K) g4DN was induced in adults, and ventricular apices were resected, before analysis of regeneration at 30 dpa. Control animals (H and I) regenerated muscle with little or no scarring (blue indicates collagen), while g4DN induction blocked muscle regeneration (J) and induced scarring (K).
(L) Quantification of proliferation of trabecular and cortical cardiomyocytes in 7 dpa ventricles of g4DN-expressing animals versus controls (n = 12–15). **P < 0.005, Student’s t-test.
Scale bars = 50 µm.
To examine whether gata4+ cardiomyocytes display similar hierarchical proliferation dynamics during adult heart regeneration, we resected ventricular apices of adult gata4:ERCreER; priZm2 zebrafish and gave animals a 4-HT pulse at 5 dpa (Figure 1I). 4-HT-treated ventricles displayed no recombination in the absence of injury (Figure 1J). At 30 dpa, we observed many small clones in the regenerates and no obvious proliferation bias (Figure 1K and 1L). Within most sections through the regenerates, over 15 distinct clones could be identified. To confirm these results, we performed analogous experiments using a pan-cardiomyocyte labeling strategy. When recombination was initiated 5 days prior to injury in cmlc2:CreER; priZm animals, 18–30 distinct color clones were observed in serial sections of 60 dpa regenerates (Figure S1H–S1K). Thus, unlike initial cortical morphogenesis, cortical muscle regenerates through the similar proliferative activities of many gata4+ cardiomyocytes. The differences in proliferation dynamics during these events might be the result of different mitogenic signals, distributions of such signals, or a reflection of different patterns of cardiomyogenesis.
Experimental Injuries Stimulate Precocious Cortical Morphogenesis
Juvenile heart morphogenesis and adult regeneration are distinguished by their initiation, with the latter involving a major injury. To examine whether the emergence of gata4+ cortical myocytes in juveniles can be stimulated by injury in an analogous manner as regeneration, we pierced the ventral apical surface of 30 dpf ventricles with a fine tungsten needle. This region is not normally covered by cortical muscle until 8–9 wpf; however, we postulated that this micropuncture might encourage events that resemble natural wall breaching and cortical morphogenesis. We analyzed ventricular surfaces for precocious cortical muscle, which is distinguishable from primordial muscle by external location, cell shape, and subcellular striation pattern (Figure S2), at 6 wpf.
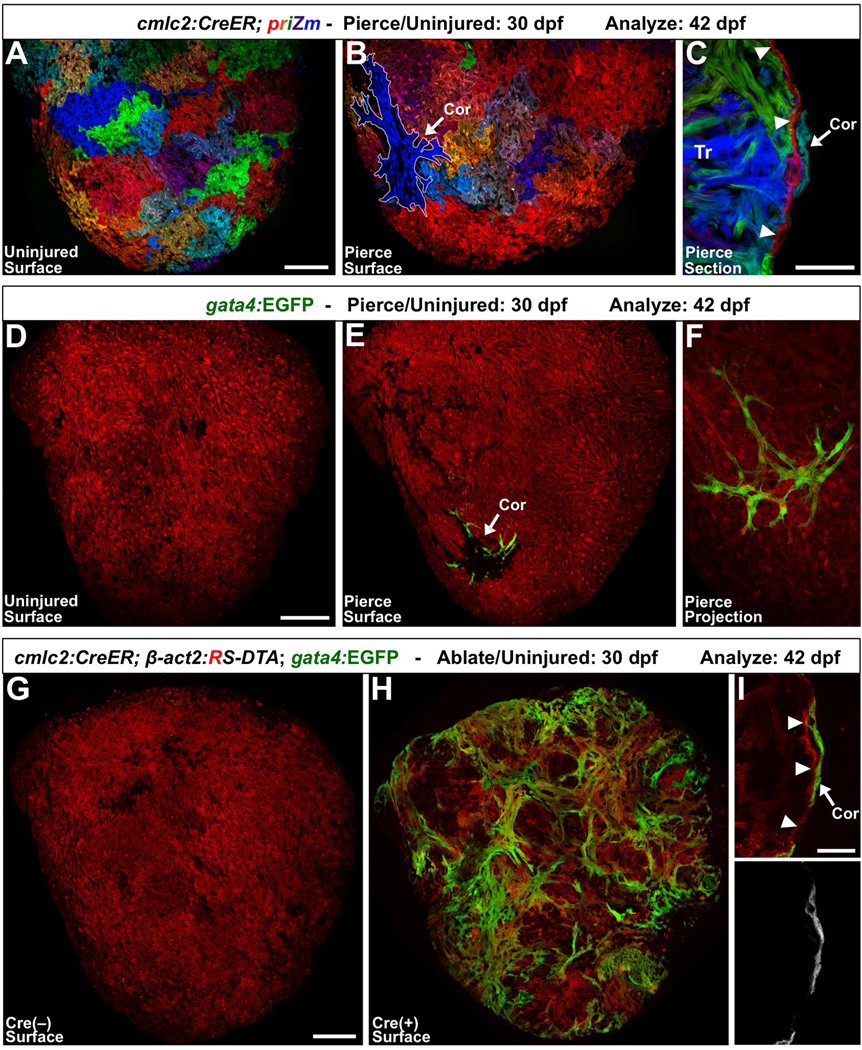
First, we labeled the cardiomyocytes of 2 dpf cmlc2:CreER; priZm embryos with diverse color tags [7]. We then applied the microinjury at 30 dpf. Nearly half of the ventricles acquired an ectopic surface cortical clone in the vicinity of the pierce by 42 dpf (15 of 33 pierced, vs. 0 of 39 uninjured; Figures 2A–2C). We repeated this procedure with gata4:EGFP animals and examined ventricular surfaces for ectopic gata4:EGFP+ cortical regions. Indeed, we found that gata4:EGFP specifically marked a small focus of cortical muscle in approximately half of the ventricles at the location of injury (12 of 23 pierced, vs. 0 of 20 uninjured; Figures 2D–2F). Notably, although triggered by injury, the cortical muscle observed in these experiments was not a result of regeneration, the replacement of lost tissue. Rather, cortical muscle was generated on the surface de novo and thus represents precocious morphogenesis.
Figure 2. Experimental Injuries Stimulate Ectopic Cortical Muscle Formation.
(A) cmlc2:CreER; priZm zebrafish were labeled at 2 dpf and assessed at 42 dpf, and indicate no signs of cortical cardiomyocytes on the ventral ventricular surface.
(B) When this region of the ventricle was pierced with a fine needle at 30 dpf, a small cortical clone (outlined in white; Cor) was detected at 42 dpf at the injury site in nearly half of the animals.
(C) Section indicating a cortical clone (arrow) at 42 dpf, in a ventricle that had been pierced at 30 dpf. Arrowheads indicate the single cardiomyocyte-thick primordial layer.
(D and E) 30 dpf priZm; gata4: EGFP ventricles were pierced as in (A–C), with β-actin2-expressing cells indicated in red. Uninjured animals had no gata4:EGFP+ cardiomyocytes on the ventral surface of the ventricle at 42 dpf (D). By contrast, approximately half of the animals pierced at 30 dpf displayed a focus of gata4:EGFP+ cortical muscle at the site of trauma (E, arrow).
(F) Higher magnification confocal projection of injury site in (E).
(G and H) Partial genetic ablation of cardiac muscle was initiated by brief 4-HT treatment at 30 dpf, and analyzed 12 days later. 4-HT-treated Cre(−) ventricles contained no gata4:EGFP+ surface cardiomyocytes (G). By contrast, the ventricular surfaces of 4-HT-treated Cre(+) animals contained large amounts of gata4:EGFP+ cortical muscle (H). (I) gata4:EGFP+ cortical cardiomyocytes (arrow) are located on the outside of the primordial layer (arrowheads).
Scale bars = 100 µm (A, B, D–H); 50 µm (C); 25 µm (I).
Second, we used a genetic Cre/loxP-based system to ablate cardiomyocytes at 30 dpf [14], before visualizing surface gata4:EGFP expression at 6 wpf. This injury model lesions trabecular and primordial muscle throughout the ventricle via stochastic induction of diphtheria toxin A expression. Nearly all animals treated with 4-HT exhibited large regions of ectopic gata4:EGFP+ cortical cardiomyocytes spanning both ventricular sides (15 of 16 animals, vs. 0 of 14 controls; Figures 2G–2I). These experiments revealed that injury can serve as a stimulus for cortical layer morphogenesis, as it does for adult heart regeneration.
Accelerated Juvenile Growth Increases Mechanical Stress and Stimulates Ectopic Cortical Morphogenesis
Although experimental microinjuries induced gata4 and ectopic morphogenesis, they might not resemble endogenous influences. During juvenile growth, stress upon the heart is sensed as increased wall tension, caused by rising organismal mass and circulatory volume. Consistent with this, we observed relatively high expression of the cardiac stress and injury markers nppa and nppb [15–18] in growing juvenile zebrafish ventricles, compared to little or no detectable expression in adult ventricles possessing a complete cortical layer (Figures S3A–S3H). Expression of the retinoic acid-synthesizing enzyme raldh2, which is activated by injury in zebrafish and mammalian hearts [19–22], was also higher in growing juvenile ventricles than in adults (Figure S3I–S3O). Accordingly, we postulated that the biomechanical stress of growth is a natural parallel to the microinjuries described above, and that wall breaches and cortical emergences may be outcomes of this stress. To test this idea, we examined whether precocious cortical morphogenesis occurs in the context of physiologic increases in biomechanical stress.
First, in an attempt to increase biomechanical stress, we lowered aquarium density during growth, a procedure that accelerates animal growth [23]. As expected, we found that raising zebrafish at a density of one animal per 2 liters (accelerated growth; AG) elevated expression of nbbp at 5 wpf, compared to a standard density of 6 animals per 2 liters (normal growth; NG; Figures S3P and S3Q).
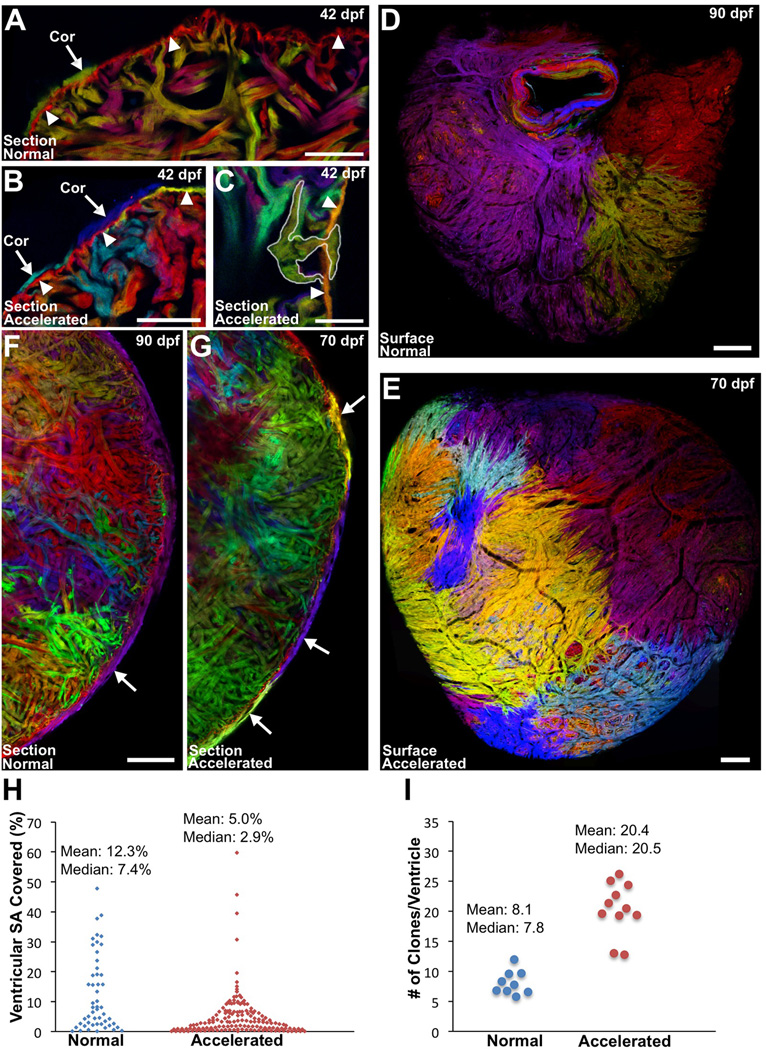
Next, we used multicolor clonal analysis to test whether these conditions altered breach patterns and cortical muscle morphogenesis. Previous findings indicated that each cortical clone represents a separate event in which a trabecular clone has breached the ventricular surface [7]. As such, the total number of cortical clones serves as a measure of the number of breaching events. After labeling cmlc2:CreER; priZm animals with 4-HT at 2 dpf and subjecting them to either NG or AG conditions, we collected ventricles at juvenile and adult stages. Histological analysis at 6 wpf revealed that NG animals had one or two detectable cortical clones at the ventricular base, whereas AG animals had more than triple the number of basal cortical clones, as well as clones near the chamber midpoint (4.1 ± 0.8 clones/heart AG vs. 1.3 ± 0.5 clones/heart NG; Figures 3A–3C). Clones were of comparable size within the two growth conditions. We also performed experiments in which we raised labeled cmlc2:CreER; priZm juveniles at an increased temperature, a treatment that raises the basal heart rate [24]. Similar to AG, 12 days of growth at 32°C (versus 25°C for control juveniles) caused ventricles to acquire more cortical clones at 6 wpf (4.0 ± 0.6 clones/ventricle at 32°C vs. 1.1 ± 0.4 clones/ventricle at 25°C; Figures S3R–S3U).
Figure 3. Accelerating Growth Leads to Increased Contributions to the Cortical Layer.
(A–C) Sections of 6 wpf ventricles of cmlc2:CreER; priZm zebrafish that were labeled at 2 dpf and grown at normal (A) or accelerated growth conditions (B and C). NG fish contained one or two small cortical clones at the base of the ventricle (A, arrow) outside the primordial layer (arrowheads). Multiple small cortical clones were seen in AG (B, arrows), including those breaching (C, outlined) the primordial layer (B and C, arrowheads).
(D and E) Surface muscle of adult cmlc2:CreER; priZm ventricles after normal (D) or accelerated growth (E) conditions. More cortical clones are generated during AG.
(F and G) Confocal slices through ventricles after normal (F) or accelerated (G) growth, indicating a higher number of cortical clones (arrows) after accelerated growth.
(H) Percentage surface area (SA) occupied by clones after normal (51 clones, 9 ventricles) or accelerated growth (153 clones, 11 ventricles).
(I) Extrapolated number of surface clones per ventricle from data in (H).
Scale bars = 100 µm (C–G); 50 µm (A and B); 25 µm (C).
We then examined ventricles from AG and NG groups at 70 and 90 dpf, respectively, ages at which animal size was comparable. We calculated the areas covered by clones in ventricles with recombined (non-red) clones comprising greater than 50% of the total surface area, as previously defined [7]. In each group, about half met this criterion (9 of 18 ventricles, NG; 11 of 20, AG). Extrapolating for unrecombined cortical muscle, there was an average of 8.1 ± 0.7 clones comprising the cortical layer in NG animals, with each ventricle containing one or two clones larger than 20% of the surface area (Figures 3D, 3F, 3H, and 3I). By contrast, AG ventricles had a larger average number of clones, 20.4 ± 1.3 clones per animal (Figures 3E, 3G, 3I, and Figure S3V–S3Y). These clones were smaller on average (89037 µm2 AG vs. 173569 µm2 NG; p < 0.05); only 4 of 11 AG ventricles contained a clone larger than 20% (Figure 3H). We found that the total surface area from ventricles of each group was not significantly different, indicating that the difference in clones was not an effect of ventricular size (1716390 µm2 AG vs. 1392466 µm2 NG; p = 0.28). These findings reveal that conditions of rapid organismal growth, accompanied by a physiologic increase in biomechanical stress, boost breaching events and clonal contributions to the cortical layer. Remarkably, although animals under the two growth conditions initiated cortical layer development with a ~2.5-fold difference in the number of source cardiomyocytes, plasticity in the system generated similar myocardial structures.
Gata4 Inhibition Blocks Cortical Layer Formation and Regeneration
To determine the significance of the injury-related gata4 response in zebrafish cardiomyocytes, we constructed a transgenic line that enabled inducible, tissue-specific expression of a dominant-negative Gata4 (g4DN) cassette (Tg(bactin2:loxP-mTagBFP-STOP-loxP-mCherry-2a-sr-gata4)pd62; referred to as β-act2:BSg4DN; Figure S4A). This g4DN protein blocks wild-type Gata4 from interacting with its target sequences. We found that myocardial g4DN overexpression in early zebrafish embryos caused heart looping defects, a phenotype that mirrors cardiac defects caused by an antisense morpholino against gata4, but not defects caused by morpholinos against gata5 or gata6 (Figure S4F) [25]. We crossed this line to cmlc2:CreER to allow 4-HT-inducible, Cre-mediated g4DN expression in cardiomyocytes.
To test late myocardial requirements of Gata4, we pulsed zebrafish with 4-HT at juvenile (30 dpf) and adult (90 dpf) stages. g4DN expression for 30 days did not impact the survival or gross appearance of adult fish. By contrast, when g4DN expression was induced in 30 dpf juveniles, just prior to gata4+ cortical cardiomyocyte emergence, survival dropped to 73% at 7 wpf, and to just 16.3% after 30 days of g4DN expression (Figure 5A). Within 2–3 weeks of 4-HT treatment, most juvenile animals developed overt signs of heart failure, including lethargy, gasping behavior, and edema (Figures 4B and 4C; Movies S2 and S3).
Notably, histological examination revealed no evidence of cortical muscle in juvenile cmlc2:CreER; β-act2:BSg4DN animals. While g4DN expression did not increase indicators of apoptosis, the ventricular wall was thinned with many areas of discontinuity (Figures 4D and 4E; Figures S4G and S4H). Visualization of epicardial cells indicated a heightened response, with areas of epicardial penetration into gaps within the wall (Figures 4F and 4G). We also observed particularly strong nppb expression compared to controls, consistent with increased biomechanical stress (Figures S4B and S4C). These data indicate that juvenile zebrafish require myocardial Gata4 activity to form the ventricular cortical layer and sustain cardiac function during growth.
To examine Gata4 function in the injured adult zebrafish heart, we induced g4DN expression, partially resected ventricles, and assessed regeneration 30 days later. Strikingly, g4DN expression blocked muscle regeneration and caused severe scarring at the injury site (Figures 4H–4K). The proliferation of trabecular myocytes was not significantly different between experimental groups; yet, animals with cardiomyocyte g4DN expression displayed a ~73% reduction in cortical cardiomyocyte proliferation (Figure 4L; Figures S4D and S4E). These data indicate that the Gata4 requirement for cardiomyocyte proliferation during regeneration is not pan-myocardial, but is preferential to cortical muscle, analogous to its requirement in juvenile cortical layer formation.
Conclusions
Regeneration links an injury stimulus with developmental programs used earlier in life. Here, we examined juvenile zebrafish heart morphogenesis to determine if gene expression and function during this phase might be recalled for adult cardiac regeneration. Our study identifies a Gata4-driven program that is specifically activated in cardiomyocytes forming the cortical layer, and is required for both cortical layer morphogenesis and adult regeneration. Cortical layer formation occurs in the context of biomechanical stress and, like regeneration, can be stimulated by injury.
Juvenile growth programs can involve genes first used during embryogenesis, but in a physiologic context more similar to adult tissue. Our evidence indicates that a cardiomyogenic Gata4 program can be attenuated and re-induced multiple times during the lifetime of an animal for distinct morphogenetic events like looping, cortical wall formation, and cardiac regeneration. It is possible that the reiteration of juvenile programs during adult tissue regeneration is a recurring theme. In support of this idea, a recent study showed that the transcription factor and satellite cell marker Pax7 is required for skeletal muscle regeneration in neonatal mice, but is dispensable for regeneration during juvenile and adult stages [26].
Multiple studies have implicated patterns of organ stress in the construction of vertebrate organs [27–29]. Our study supports a model in which cortical muscle emergence during juvenile growth functions as a stochastic repair mechanism to reinforce possible weak points on the single cardiomyocyte-thick wall, relieving wall stress. After breaching, gata4+ cortical cardiomyocytes proliferate on the ventricular surface to form muscle patches, and individual surface clones merge into a contiguous layer that fortifies the wall. As might be predicted by this model, growing juvenile animals under myocardial Gata4 blockade fail to form cortical muscle and develop heart failure. Adult zebrafish, upon cardiac injury, appear to repurpose key aspects of this injury-activated juvenile morphogenesis program to enact heart regeneration.
Supplementary Material
Highlights.
-
-
A Gata4-dependent program creates the cortical layer during juvenile growth
-
-
Multicolor clonal analysis defines contributions of individual gata4+ cardiomyocytes
-
-
Cortical layer morphogenesis can be activated by injury
-
-
Heart regeneration reiterates key aspects of the juvenile Gata4 program
Acknowledgments
We thank K. Kikuchi, J. Wang, A. Wills, and S. Singh for making transgenic animals; J. Burris, A. Eastes, P. Williams, and N. Blake for zebrafish care; M. Bagnat and Poss lab members for comments on the manuscript; and T. Slotkin for help with statistics. This work was supported by a National Heart, Lung, and Blood Institute (NHLBI) Medical Scientist Training Program supplement (2T32-GM007171-38 S1 to V.G.); a predoctoral fellowship from the American Heart Foundation (AHA; to M.G.); an AHA Fellow-to-Faculty Award (12FTF11660037 to R.K.); a training grant (T32HD060600 to G.E.R); and grants from NHLBI (HL111400 to T.E.; HL081674 to K.D.P.) and AHA (K.D.P.). K.D.P. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental information includes 4 figures, Supplemental Experimental Procedures, and 3 movies, and can be found with this article online at
References
- 1.Alvarado AS. Regeneration in the metazoans: why does it happen? BioEssays. 2000;22:12. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nacu E, Tanaka EM. Limb regeneration: a new development? Ann. Rev. Cell Dev. Biol. 2011;27:409–440. doi: 10.1146/annurev-cellbio-092910-154115. [DOI] [PubMed] [Google Scholar]
- 4.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta V, Poss KD. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature. 2012;484:479–484. doi: 10.1038/nature11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P, He A, Pu WT. Regulation of GATA4 transcriptional activity in cardiovascular development and disease. Curr. Top. Dev. Biol. 2012;100:143–169. doi: 10.1016/B978-0-12-387786-4.00005-1. [DOI] [PubMed] [Google Scholar]
- 9.Charron F, Nemer M. GATA transcription factors and cardiac development. Semin. Cell Dev. Biol. 1999;10:85–91. doi: 10.1006/scdb.1998.0281. [DOI] [PubMed] [Google Scholar]
- 10.Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin. Cell Dev. Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development. 2011;138:3421–3430. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braunwald E. Biomarkers in heart failure. N. Engl. J. Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 16.Liang F, Gardner DG. Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism. J. Clin. Invest. 1999;104:1603–1612. doi: 10.1172/JCI7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houweling AC, van Borren MM, Moorman AF, Christoffels VM. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc. Res. 2005;67:583–593. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Becker JR, Robinson TY, Sachidanandan C, Kelly AE, Coy S, Peterson RT, MacRae CA. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc. Res. 2012;93:463–470. doi: 10.1093/cvr/cvr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science. 2012;338:1599–1603. doi: 10.1126/science.1229765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135:183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
- 24.Barrionuevo WR, Burggren WW. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am. J. Physiol. 1999;276:R505–R513. doi: 10.1152/ajpregu.1999.276.2.R505. [DOI] [PubMed] [Google Scholar]
- 25.Torregroza I, Holtzinger A, Mendelson K, Liu TC, Hla T, Evans T. Regulation of a vascular plexus by gata4 Is mediated in zebrafish through the chemokine sdf1a. PLoS One. 2012;7:e46844. doi: 10.1371/journal.pone.0046844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 28.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson CM, Gleghorn JP. Sculpting organs: mechanical regulation of tissue development. Ann. Rev. Biomed. Eng. 2012;14:129–154. doi: 10.1146/annurev-bioeng-071811-150043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.