Abstract
Objective
To demonstrate the importance of comorbid conditions in head and neck squamous cell carcinoma (HNSCC), we assessed the association between comorbidity and survival in an inner-city population of HNSCC patients.
Patients and Methods
Comorbid status at diagnosis was derived using medical records and the Adult Comorbidity Evaluation-27 (ACE-27) index on 288 patients with histologically confirmed HNSCC from Montefiore Medical Center in the Bronx (NY) between 2002 and 2011. The association between comorbidity, tumor human papillomavirus (HPV) status and overall and disease specific survival was assessed by Kaplan-Meier analysis and multivariable Cox regression adjusting for clinico-pathologic factors.
Results
The study population consisted of primary oropharyngeal (36%), laryngeal (33%) and oral cavity cancer patients (31%). Overall, 19% had no comorbidity, 43% mild comorbidity, 29% moderate comorbidity, and 9% severe comorbidity. The most common comorbid conditions were hypertension, diabetes mellitus, respiratory disease, other malignancies, and illicit drug use. Survival analyses revealed that increased comorbidity at diagnosis was significantly related to poorer overall survival (p=0.016), but not to cancer survival (p=0.369) or recurrence (p=0.652). Oropharyngeal cancer patients with HPV DNA positive tumors and lower levels of comorbidity had significantly better overall survival compared to patients with HPV negative tumors (hazard ratio = 0.2, 95%CI: 0.04–0.8), however there was no significant difference in overall (or disease specific) survival by HPV status among patients with higher levels of comorbidity at diagnosis (hazard ratio = 0.7, 95%CI: 0.2–2.8).
Conclusion
In an inner-city predominantly minority population, comorbidity at HNSCC diagnosis is relatively common and associated with poor overall survival, but not cancer survival or recurrence. Interestingly, the relationship between HPV and improved survival appears to be specific to patients with low comorbidity at diagnosis.
Keywords: ACE-27, comorbidity, head and neck, oropharyngeal, squamous cell carcinoma, survival, human papillomavirus
Introduction
Cancers of the head and neck constitute an anatomically heterogeneous group arising most often from the oral cavity, oropharynx, hypopharynx, and larynx. Standard practice for patients with head and neck malignancies is to stage them according to the tumor-node-metastasis (TNM) classification system. While appropriate for describing characteristics specific to tumors, more accurate prognostic information has been shown when TNM staging is combined with more patient-specific variables that bear more direct relation to survival.1 One such variable is comorbidity.
Comorbidity is defined as the presence of a disease or condition that is unrelated to the index disease.2 Head and neck cancer patients are unique in that they usually have histories of heavy tobacco and alcohol use, both of which contribute significantly to other pathologic conditions throughout the body. While these conditions are separate from the index cancer, they are important overall factors for the patient.3 Furthermore, in a subset of head and neck squamous cell carcinoma (HNSCC), growing epidemiologic evidence supports an etiologic role for human papillomaviruses (HPV), which also constitute a prognostic marker of improved survival and response to therapy.
A number of studies have demonstrated the increased prevalence of comorbidity in head and neck cancer patients,2, 4, 5 which can impact treatment selection6, 7 and prognosis.8–13 For example, patients with severe cardiovascular or respiratory comorbidity may be excluded from surgical treatment because of an increased potential for adverse treatment outcomes.14 The impact of comorbidity has been described for both short and long term survival, and over a variety of different treatment combinations.15–17
Given its demonstrated importance in patients with head and neck cancer, the American College of Surgeons, the American Cancer Society, and the International Head and Neck Scientific Group have advocated for the routine inclusion of comorbidity information in cancer registries.18 However, no studies have assessed the independent effect of comorbidity and HPV on survival in HNSCC. We sought to assess the degree of comorbidity at diagnosis in an inner-city population of HNSCC patients that is largely ethnic minority with high levels of drug use, HIV/AIDS, Hepatitis C, and other diseases.
Methods
Study Design
Our study population included HNSCC patients admitted to Montefiore Medical Center (MMC) in the Bronx (NY) between 2002 and 2011. The study was approved by the Institutional Review Boards at MMC and Albert Einstein College of Medicine. Standard histological confirmation of tumors was performed for all cases, with TNM staging based on the American Joint Committee on Cancer classification. Details on smoking history and alcohol consumption were collected by medical interview at enrollment. Additional clinico-pathologic factors collected included: age, gender, race, ethnicity, tumor anatomic site, primary treatment modality and detection of HPV16 DNA in the tumor (targeting the oncogenic type found in almost all HPV positive HNSCC) using previously described PCR protocols.19
Comorbidity Measures
Methods for assessing comorbidity vary, including patient interviews and patient chart reviews.20 A number of different indices exist that differ in the level of detail collected on the severity of comorbid conditions.11, 21–24 We employed the Adult Comorbidity Evaluation-27 (ACE-27), which includes 27 different comorbid elements and disease severity, as this has been shown to perform best in head and neck cancer patients.12, 25
A single trained individual (AAA) abstracted comorbidity information collected at diagnosis through retrospective review of patient medical records. Materials used included admission notes, routine laboratory assessments, and radiation oncology records. A score of 0, 1, 2, or 3 was assigned for each of 27 conditions assessed representing none, mild, moderate, or severe comorbidity, respectively. An overall comorbid score was then assigned according to the highest scoring single condition. In the event where there were two moderate level comorbidities, an overall score of severe was assigned.11, 24
Statistical Methods
Contingency tables with Chi-square or Fisher’s exact tests were used to describe the study population and the cross-sectional associations between comorbid conditions and disease characteristics at diagnosis. Patients were followed from time of diagnosis as part of an ongoing cohort study.26 Overall and disease specific survival were defined as time from diagnosis (in months) to death from all causes or head and neck cancer, respectively. Disease recurrence was defined as the time from treatment start to first incidence of local or regional recurrence or distant metastasis. Remaining subjects were censored at the time they were last known to be alive. Kaplan-Meier analyses and Cox proportional hazards regression were used to assess the relationship between comorbidity and overall survival, cancer survival or recurrence. The multivariate Cox regression analyses included an exhaustive search for significant covariate predictors, identified by univariate analyses. The potential for confounding was examined using a change-in-point estimate criterion in adjusted survival models incorporating covariates with the variable for comorbidity, and were subsequently controlled for in the final multivariable regression models.27 Covariates examined included all clinico-pathologic factors assessed at diagnosis, including: age, gender, race, ethnicity, smoking history, alcohol consumption, tumor anatomic site, TNM stage, and primary treatment modality, as well as HPV detection in the tumor (available on 151 patients). Proportional hazards assumptions were tested by Schoenfeld residuals. Evidence of statistical interaction (effect modification) by HPV status and tumor site was also assessed by testing for significant cross-product terms between these covariates and comorbid status in the final multivariate regression models. Inference was based on the Wald chi-square test statistic for two-way interaction. Statistical analyses were conducted with the STATA 12 software package (College Station, TX), and significance was based on a two-sided p-value of less than 0.05 or 95% confidence interval that does not include 1.0.
Results
Population Description
A total of 338 patients were identified for the current study. Of this group, 50 patients were excluded due to insufficient prior medical records, leaving a total of 288 patients. The mean age at diagnosis was 61.7 years (±11.6 standard deviation), ranging from 22–89. The study population was comprised of mostly men (72.2%), and consisted of 28.8% African-American and 27.4% Hispanic subjects. Of the various primary sites, 86 patients (30.6%) had SCC of the oral cavity, 92 patients (32.7%) of the larynx, and 103 patients (35.8%) of the oropharynx. Two-hundred and six patients (71.5%) had late stage disease (TNM stages III–IV). The remaining demographic, diagnostic and clinico-pathologic characteristics of the study population are shown in Table 1.
Table 1.
Patient characteristics at diagnosis
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 208 (72.2%) |
| Female | 80 (27.8%) |
| Race | |
| Asian | 9 (3.1%) |
| Black or African American | 79 (27.4%) |
| Unknown | 2 (0.7%) |
| White | 198 (68.8%) |
| Ethnicity | |
| Hispanic/Latino | 83 (28.8%) |
| Unknown | 3 (1.04%) |
| Non-Hispanic/Latino | 202 (70.1%) |
| Alcohol Consumption | |
| Never/Past | 210 (73%) |
| Current | 78 (27%) |
| Tobacco Smoking | |
| Never | 44 (15.28%) |
| Past | 130 (45.1%) |
| Current | 114 (39.6%) |
| Tumor Site | |
| Lip/Oral Cavity | 86 (29.9%) |
| Oropharynx | 103 (35.8%) |
| Larynx | 92 (32.7%) |
| Other | 7 (2.4%) |
| TNM Stage | |
| Early (I or II) | 73 (25.4%) |
| Late (III or IV) | 206 (71.5%) |
| Unknown | 9 (3.1%) |
| Nodal Stage | |
| NX | 11 (3.8%) |
| N0–N1 | 151 (52.4%) |
| N2–N3 | 127 (44.1%) |
| HPV Status | |
| HPV16 − | 121 (42.0%) |
| HPV16 + | 30 (10.4%) |
| Not tested | 137 (47.6%) |
Comorbidity in the Population
Of the 288 patients in the study sample, 55 (19.1%) had no comorbidity at the time of diagnosis; 125 (43.4%) had mild overall comorbidity, 81 (28.1%) had moderate comorbidity, and 27 (9.4%) had severe comorbidity. Table 2 details the distribution of comorbid scores in the population and the prevalence of some of the more common comorbid conditions.
Table 2.
Distribution of comorbid conditions by overall comorbid score
| Characteristic | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Overall ACE-27 Score | 55 (19.1%) | 125 (43.4%) | 81 (28.1%) | 27 (9.4%) |
| System | ||||
| Cardiovascular | ||||
| HTNa | 151 (52.4%) | 128 (44.4%) | 9 (3.1%) | 0 (0.0%) |
| Angina/CADa | 264 (91.7%) | 23 (8%) | 1 (0.3%) | 0 (0.0%) |
| CHFa | 286 (99.3%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Myocardial Infarction | 281 (97.6%) | 1 (0.4%) | 6 (2.1%) | 0 (0.0%) |
| Arrhythmia | 281 (97.6%) | 1 (0.4%) | 6 (2.0%) | 0 (0.0%) |
| Venous Disease | 286 (99.3%) | 1 (0.4%) | 1 (0.4%) | 0 (0.0%) |
| Peripheral Artery Disease | 282 (97.9%) | 4 (1.4%) | 2 (0.7%) | 0 (0.0%) |
| Respiratory Disease | 253 (88.9%) | 32 (11.1%) | 1 (0.4%) | 2 (0.7%) |
| Hepatic Disease | 255 (88.5%) | 30 (10.4%) | 2 (0.7%) | 1 (0.5%) |
| Diabetes | 246 (85.4%) | 26 (9.0%) | 12 (4.2%) | 4 (1.4%) |
| Psychiatric Disease | 265 (92.0%) | 22 (7.6%) | 1 (0.4%) | 0 (0.0%) |
| Illicit Substance Abuse | 259 (90.0%) | 24 (8.3%) | 5 (1.7%) | 0 (0.0%) |
| AIDS | 273 (94.8%) | 10 (3.5%) | 5 (1.7%) | 0 (0.0%) |
| Stroke | 274 (95.1%) | 8 (2.8%) | 6 (2.1%) | 0 (0.0%) |
| Solid Tumor Including | 249 (86.5%) | 14 (4.9%) | 25 (8.7%) | 0 (0.0%) |
| Melanoma | ||||
Hypertension (HTN); Coronary Artery Disease (CAD); Congestive Heart Failure (CHF)
Patients with laryngeal SCC were significantly more likely to have severe comorbidity when compared to either oral cavity or oropharyngeal SCC patients (p = 0.002; Table 3). Smokers, including past and current, were significantly more likely to have mild (51.5% and 38.9%), moderate (29.2% and 28.3%) or severe comorbidity (6.2% and 15.9%) compared to non-smokers (31.8%, 25%, and 2.3%, respectively, p = 0.001). With respect to age, patients under 60 years of age had significantly less comorbidity than those 60 or over (p = 0.001). Gender, TNM stage, nodal stage, race, and ethnicity were not related to increased comorbidity.
Table 3.
Distribution of overall comorbid scores by patient characteristics and tumor site
| Characteristic | None | Mild | Moderate | Severe | P valuea |
|---|---|---|---|---|---|
| Tumor Site | |||||
| Oral Cavity | 26 (30.2%) | 31 (36.1%) | 23 (26.7%) | 6 (7%) | |
| Oropharynx | 18 (17.5%) | 53 (51.5%) | 24 (23.3%) | 8 (7.8%) | P = .110 |
| Larynx | 9 (9.8%) | 37 (40.2%) | 34 (37%) | 12 (13%) | P = .005 |
| Smoking | |||||
| All Sites | |||||
| Never | 18 (40.9%) | 14 (31.8%) | 11 (25%) | 1 (2.3%) | |
| Past | 17 (13.1%) | 67 (51.5%) | 38 (29.2%) | 8 (6.2%) | P = .001 |
| Current | 19 (16.8%) | 44 (38.9%) | 32 (28.3%) | 18(15.9%) | P = .001 |
| Larynx | |||||
| Never | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 0 (0%) | |
| Past | 2 (4.2%) | 23 (47.9%) | 18 (37.5%) | 5 (10.4%) | P = .209 |
| Current | 6 (14.6%) | 13 (31.7%) | 15 (36.6%) | 7 (17.1%) | P = .768 |
| Oropharynx | |||||
| Never | 7 (41.2%) | 7 (41.2%) | 3 (17.7%) | 0 (0%) | |
| Past | 8 (18.6%) | 23 (53.5%) | 10 (23.3%) | 2 (4.7%) | P = .282 |
| Current | 3 (7%) | 23 (53.5%) | 11 (25.6%) | 6 (14%) | P = .009 |
| Oral Cavity | |||||
| Never | 10 (41.7%) | 6 (25%) | 7 (29.2%) | 1 (4.2%) | |
| Past | 6 (16.7%) | 19 (52.8%) | 10 (27.8%) | 1 (2.8%) | P = .105 |
| Current | 9 (36%) | 6 (24%) | 6 (24%) | 4 (16%) | P = .591 |
| Age (years) | |||||
| All Sites | |||||
| <60 | 36 (29%) | 46 (37.1%) | 30 (24.2%) | 12 (9.7%) | |
| 60+ | 19 (11.6%) | 79 (48.2%) | 51 (31.1%) | 15 (9.2%) | P = .002 |
| Larynx | |||||
| <60 | 6 (15.4%) | 14 (35.9%) | 13 (33.3%) | 6 (15.4%) | |
| 60+ | 3 (5.7%) | 23 (43.4%) | 21 (39.6%) | 6 (11.3%) | P = .222 |
| Oropharynx | |||||
| <60 | 14 (29.8%) | 20 (42.6%) | 10 (21.3%) | 3 (6.4%) | |
| 60+ | 4 (7.1%) | 33 (58.9%) | 14 (25.0%) | 5 (8.9%) | P = .027 |
| Oral Cavity | |||||
| <60 | 15 (41.7%) | 12 (33.3%) | 7 (19.4%) | 2 (5.6%) | |
| 60+ | 11 (22.0%) | 19 (38.0%) | 16 (32.0%) | 4 (8.0%) | P = .239 |
| HPV Status | |||||
| All Sites | |||||
| HPV16 − | 19 (15.7%) | 51 (42.2%) | 39 (32.2%) | 12 (9.9%) | |
| HPV16 + | 8 (26.7%) | 15 (50.0%) | 6 (20.0%) | 1 (3.3%) | P = .251 |
| Larynx | |||||
| HPV16 − | 4 (9.5%) | 15 (35.7%) | 19 (45.2%) | 4 (9.5%) | |
| HPV16 + | 1 (50.0%) | 0 (0.0%) | 1 (50.0%) | 0 (0.0%) | P = .371 |
| Oropharynx | |||||
| HPV16 − | 2 (6.5%) | 18 (58.1%) | 7 (22.6%) | 4 (12.9%) | |
| HPV16 + | 7 (28.0%) | 12 (48.0%) | 5 (20.0%) | 1 (4.0%) | P = .159 |
| Oral Cavity | |||||
| HPV16 − | 13 (27.1%) | 18 (37.5%) | 13 (27.1%) | 4 (8.3%) | |
| HPV16 + | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | 0 (0.0%) | P = .576 |
P value by chi-square or Fisher’s exact test comparing comorbid scores between rows using the first row as the reference group. Row percentages shown.
When separated by tumor site, oropharyngeal SCC patients showed significant associations between smoking status and comorbidity, and age and comorbidity (Table 3). HPV test results were available on a subset (N = 151) of HNSCC cases. HPV positive oropharyngeal SCC patients had only somewhat less comorbidity than HPV negative patients (p = 0.159) and not significantly, whereas HPV positive oral and laryngeal SCC patients showed no difference in comorbidity by HPV status.
Comorbidity and Survival
The impact of comorbidity on survival was first examined using Kaplan-Meier curves collapsing the overall ACE-27 scores into two groups: none-mild, and moderate-severe in accordance with prior studies (Figure 1). HNSCC patients with moderate-severe comorbidity at diagnosis were found to have significantly poorer overall survival than patients with mild or no comorbidity (log rank p = 0.016), whereas significant associations were not found for head and neck cancer survival (p = 0.369) or recurrence (p = 0.652).
Figure 1.
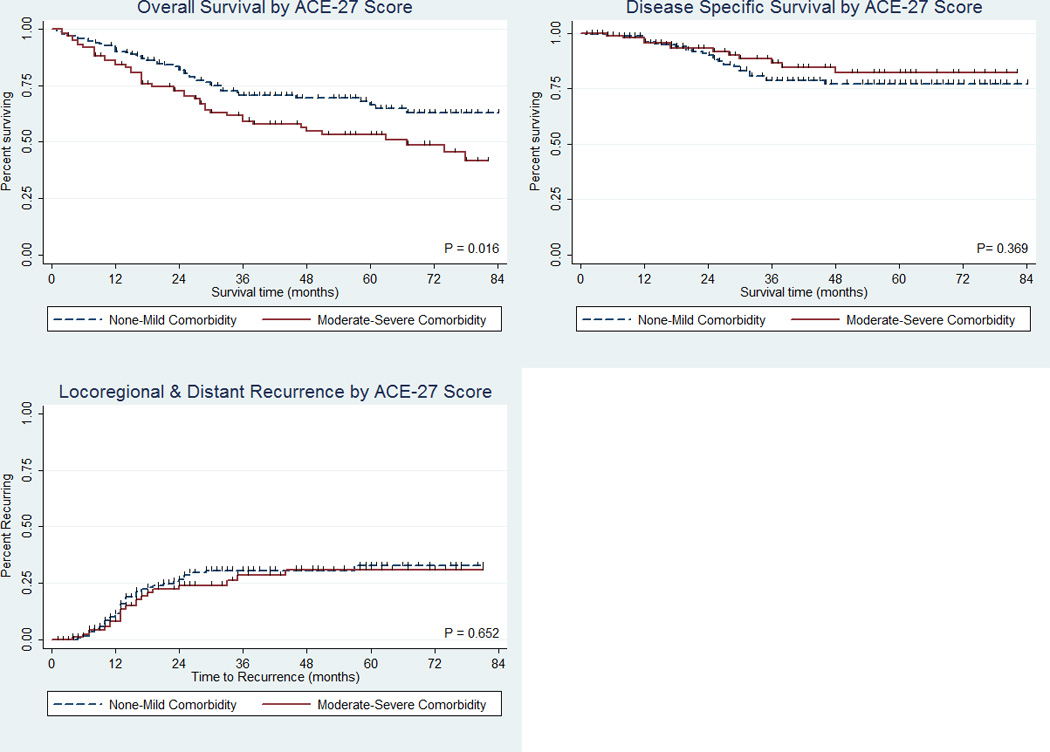
Kaplan Meier plots for overall survival (top left), disease specific survival (top right), and disease recurrence (bottom left) by overall comorbidity
The effect of comorbidity on survival was also explored using Cox proportional hazard regression. Univariate analyses showed significant associations between clinical outcome and HPV, comorbidity, tumor stage, nodal stage and primary treatment regimen. Multivariate analyses were conducted controlling for all significant prognostic factors and tumor site, which was a confounding factor. Tumor stage and nodal stage showed collinearity, so the final regression models were adjusted for nodal stage only, which showed the strongest association with survival. Patients with mild or no comorbidity were found to have better overall survival compared to patients with moderate to severe, although this was not significant after adjusting for tumor site, primary treatment, nodal status, gender and HPV status (adjusted hazard ratio [aHR] = 0.7, 95% confidence interval [CI]: 0.5–1.2). When restricted on tumor site, decreased comorbidity showed a similar, albeit non-significant, association with survival in laryngeal SCC patients (aHR = 0.6, 95%: 0.3–1.2), whereas no decrease was found in oral cavity SCC patients (aHR = 1.2, 95%: 0.6–2.7). Oropharyngeal SCC patients with mild or no comorbidity were also found to have better overall survival (aHR = 0.6, 95% CI: 0.3–1.3), albeit not significantly after adjusting for nodal stage, primary treatment, gender and HPV status, which itself was significantly associated with better survival (aHR = 0.3, 95% CI: 0.1–0.9).
Further investigating the association with comorbidity by HPV status, we found HNSCC patients with HPV positive tumors and lower levels of comorbidity had significantly better overall survival compared to patients with HPV negative tumors, whereas those with HPV positive tumors and moderate to severe comorbidity showed little to no difference in overall survival compared to patients with HPV negative tumors, regardless of comorbid status (Table 4, Figure 2).
Table 4.
Adjusted associations between clinical outcome, comorbidity and HPV
| HPV × Comorbidity | All HNSCC | Oropharyngeal SCC | ||||
|---|---|---|---|---|---|---|
| Overall survival | HRa | 95% CI | P valueb | HRa | 95% CI | P valueb |
| HPV16−/Moderate-Severe | 1.0 | (referent) | 1.0 | (referent) | ||
| HPV16−/None-Mild | 1.0 | 0.6 – 1.6 | 0.88 | 0.9 | 0.3 – 2.5 | 0.91 |
| HPV16+/Moderate-Severe | 1.1 | 0.3 – 4.1 | 0.83 | 1.0 | 0.2 – 4.6 | 0.95 |
| HPV16+/None-Mild | 0.2 | 0.1 – 0.8 | 0.02 | 0.1 | 0.03–0.7 | 0.02 |
| Not tested for HPV | 0.5 | 0.3 – 0.8 | 0.01 | 0.3 | 0.1 – 0.8 | 0.02 |
| Disease specific survival | ||||||
| HPV16−/Moderate-Severe | 1.0 | (referent) | 1.0 | (referent) | ||
| HPV16−/None-Mild | 2.2 | 0.8 – 5.5 | 0.11 | 0.6 | 0.1 – 3.4 | 0.60 |
| HPV16+/Moderate-Severe | 3.7 | 0.6 –22.3 | 0.15 | 1.6 | 0.2 –11.9 | 0.64 |
| HPV16+/None-Mild | 0.3 | 0.04–2.8 | 0.31 | 0.2 | 0.02–1.6 | 0.12 |
| Not tested for HPV | 1.1 | 0.4 – 2.9 | 0.91 | 0.3 | 0.1 – 1.7 | 0.17 |
| Locoregional & Distant recurrence | ||||||
| HPV16−/Moderate-Severe | 1.0 | (referent) | 1.0 | (referent) | ||
| HPV16−/None-Mild | 1.7 | 0.8–3.8 | 0.18 | 0.6 | 0.1 – 2.9 | 0.49 |
| HPV16+/Moderate-Severe | 3.2 | 0.6 –16.6 | 0.16 | 1.9 | 0.3 – 12.3 | 0.51 |
| HPV16+/None-Mild | 0.8 | 0.2 – 3.0 | 0.71 | 0.3 | 0.05–1.8 | 0.19 |
| Not tested for HPV | 1.7 | 0.8 – 3.7 | 0.18 | 0.9 | 0.2 – 3.6 | 0.93 |
Hazard ratio (HR) and 95% confidence interval (CI) by Cox proportional hazards regression mutually adjusting for variables shown, nodal status, primary treatment, gender, and tumor site (where applicable).
Adjusted P-values by Wald test.
Figure 2.
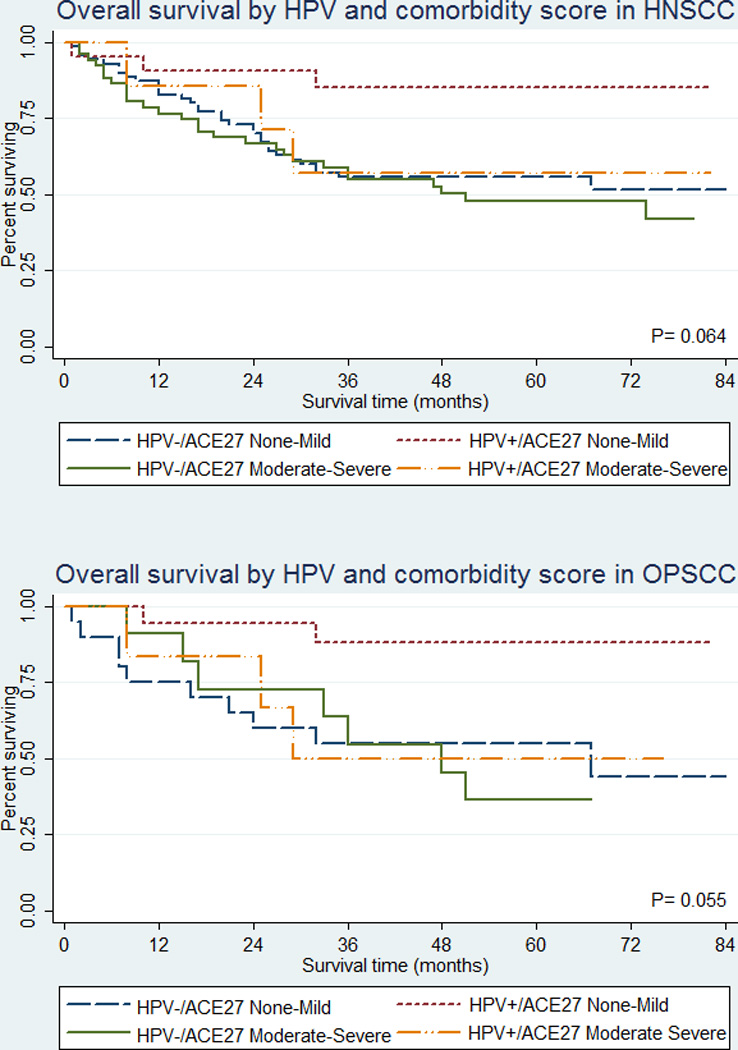
Kaplan Meier plots for overall survival by HPV status and comorbidity for HNSCC patients combined (top) and oropharyngeal SCC patients only (bottom)
The differential relationship was also observed among oropharyngeal SCC patients. When stratified by comorbid status, we observed a significant association with overall survival by HPV status among oropharyngeal SCC patients with mild or no comorbidity at diagnosis (aHR = 0.2, 95%CI: 0.04–0.8) but not among patients with moderate to severe comorbidity (aHR = 0.7, 95%CI: 0.2–2.8). Testing for statistical interaction revealed borderline significant evidence of effect modification by overall comorbid score on the association between HPV and overall survival in oropharyngeal SCC patients alone (p = 0.077) and HNSCC sites combined (p = 0.080).
We observed similar, albeit not significant, relationships between HPV and disease specific survival (aHR = 0.3, 95%CI: 0.03–3.2) and decreased disease recurrence (aHR = 0.5, 95%CI: 0.1–3.3) among oropharyngeal SCC patients with mild to no comorbidity at diagnosis, but not among patients with moderate to severe comorbidity (aHR = 1.0, 95%CI: 0.1–7.5 and aHR = 1.4, 95%CI: 0.2–9.7, respectively). Among patients with increased comorbidity, those with HPV16 positive tumors (N=7) were more likely to have smoked compared to (N=51) patients with HPV16 negative tumors (100% vs. 86%), and were more likely to have a history of moderate to severe hypertension (29% vs. 8%), myocardial infarction (14% vs. 2%), arrhythmia (14% vs. 4%), angina/coronary artery disease (14% vs. 6%), and severe diabetes (14% vs. 2%), although these differences were not significant. In contrast, the same conditions and behaviors were less likely to be detected among mildly comorbid patients with HPV16 positive tumors (N=21) compared to those with HPV16 negative tumors (N=70), including smoking (66% vs. 84%), again not significant. Adjusting for smoking history did not affect the observed associations with HPV and comorbidity, and the differences between individual comorbid groups and HPV status were also not significant.
Discussion
In this inner-city population of HNSCC patients including oral cavity, oropharyngeal and laryngeal cancers, we found a high prevalence of comorbidity with 81% of patients having at least one comorbid condition and 38% with multiple conditions, representing a moderate to severe level of comorbidity at the time of presentation. This level of comorbidity is higher than previously reported in other head and neck cancer patient populations. In an earlier study of 341 patients with head and neck cancer, Piccirillo showed a lower rate of moderate to severe comorbidity (21%) that was second only to lung and colorectal cancer.3 Paleri et al. also demonstrated a lower prevalence of comorbidity in laryngeal cancer patients where 64% of patients had some kind of concurrent comorbidity and 25% had multiple coexisting comorbidities.5
Differences were also observed with respect to the distribution of types of comorbid conditions. For example, Piccirillo et al. found that hypertension (28%), respiratory disease (13%), other solid tumors (11%), diabetes (10%) and angina (10%) were the most prevalent conditions in a separate cohort of 1,200 patients with HNSCC.7 Datema et al. similarly showed an increased prevalence of cardiovascular (32%), respiratory (6%) and gastrointestinal disorders (7%) in their cohort of 1,371 head and neck cancer patients.15 While we also found hypertension, respiratory disease and other solid malignancies to be the most prevalent comorbid conditions in our population, other conditions detected included diabetes mellitus, illicit drug use, hepatic disease, and psychiatric illness, which have been associated with short-term mortality.15 Bronx County, where MMC is located, has one of the highest rates of poverty in the United States. In 2008, only 2.9% of Bronx County adults had health insurance.28 The consequent barriers to health care access experienced in this population may explain the higher prevalence of severe comorbidity and multiple different conditions observed in our population compared to others.4, 5, 7,
In addition to the high degree of comorbidity in our patient population, we also found a significant relationship between increased comorbidity and poorer overall survival. This was consistent with previous findings that showed increased comorbidity to be independently correlated with decreased survival using older comorbidity indices such as the Modified Medical Comorbidity Index and the Washington University Head and Neck Comorbidity Index.3, 11 Utilizing the ACE-27, Piccirillo also showed that increased overall comorbidity correlated with decreased survival in cohorts of 1,086 and 1,201 HNSCC patients, respectively.13, 7 Similar relationships were also reported for the ACE-27 in laryngeal and pharyngeal cancer patients,9,16,29 and HNSCC patients undergoing microvascular reconstruction.8
Given the improved survival rates reported for HPV positive HNSCC,30 we hypothesized that there would be decreased comorbidity in patients with HPV positive tumors, but found no relationship between overall comorbidity and HPV status. However, we did observe that while both comorbidity and HPV status were related to overall survival, the association with HPV was specific to patients with mild or no comorbidity at diagnosis. In particular, HPV16 detection in oropharyngeal tumors was significantly associated with improved disease specific survival and recurrence among patients with little or no comorbidity but not moderate to severe comorbidity. Moreover, whereas there was a 40% improvement in hazards for disease specific survival and recurrence for HPV16 negative oropharyngeal SCC patients with mild or no comorbidity compared to moderate or severe comorbidity, the difference was greater among HPV16 positive patients. Although smoking was correlated with HPV detection among patients with moderate to severe comorbidity, it was not significantly associated with survival and did not affect the observed interactive effect of HPV and comorbidity on cancer progression in our multivariate models.
This study has both strengths and limitations. Among the former, we were able to show that HNSCC with multiple comorbid conditions had poorer overall survival independent of other clinico-pathologic factors like nodal stage and HPV. However, comorbidity alone was not associated with disease specific survival or recurrence. To our knowledge, this is the first study of comorbidity in head and neck cancer to incorporate HPV results into its survival models. However, HPV status was not available for all tumors. Therefore, we cannot be certain that the observed associations by HPV status are generalizable. Secondly, we were not able to assess relationships with individual comorbid conditions by HPV status among HNSCC due to small numbers. Thirdly, given that comorbid conditions can develop over time, we cannot rule out possible correlations with disease specific events or residual confounding by changes in treatment.25 Yung et al. reported that patient comorbidity at diagnosis is significantly related with 5-year survival but that one’s comorbid burden changes over time.31 The severity of comorbidities caused by long-term smoking and alcohol can also change as age-related organ dysfunction adds to the burden of disease. These temporal effects, however, will require repeated assessment and long term follow-up. Lastly, measures of socioeconomic status were not collected in this study, although this is unlikely to have affected survival, as the majority of patients treated had health insurance.
Conclusion
Comorbidity at diagnosis was prevalent in this inner-city patient population of primary HNSCC patients, and differs by tumor site, age and smoking status. As also reported in other studies, increased comorbidity was associated with poorer overall survival but not disease specific survival or recurrence. Although not significant, we did find evidence of interaction between HPV status and comorbidity whereby the association between HPV and survival was only significant in those with low comorbid states at time of diagnosis. Further study is needed, however, to assess how changes in comorbidity over time relate to HPV status, smoking and response to different treatment regimens in HNSCC patients.
Acknowledgments
We thank the participants of this study, Christian Keller for his help with pathological staging, and Gregory Rosenblatt for his assistance with data management.
Sources of support
This project is supported in part by NIH grant CA115243 (to NFS) and the Department of Otorhinolaryngology-Head & Neck Surgery, Albert Einstein College of Medicine/Montefiore Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors report no conflicts of interest in this study.
References
- 1.Piccirillo JF, Feinstein AR. Clinical symptoms and comorbidity: significance for the prognostic classification of cancer. Cancer. 1996;77(5):834–842. [PubMed] [Google Scholar]
- 2.Paleri V, Wight RG. Applicability of the adult comorbidity evaluation - 27 and the Charlson indexes to assess comorbidity by notes extraction in a cohort of United Kingdom patients with head and neck cancer: a retrospective study. J Laryngol Otol. 2002;116(3):200–205. doi: 10.1258/0022215021910528. [DOI] [PubMed] [Google Scholar]
- 3.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Landis SH, El-Haririy IA, van Herk-Sukel MP, van den Haak P, Janssen-Heijnen ML, Penning-van Beest FJ, et al. Prevalence and incidence of acute and chronic comorbidity in patients with squamous cell carcinoma of the head and neck. Head Neck. 2011;34(2):238–244. doi: 10.1002/hed.21720. [DOI] [PubMed] [Google Scholar]
- 5.Paleri VR, Narayan R, Wight RG. Descriptive study of the type and severity of decompensation caused by comorbidity in a population of patients with laryngeal squamous cancer. J Laryngol Otol. 2004;118(7):517–521. doi: 10.1258/0022215041615281. [DOI] [PubMed] [Google Scholar]
- 6.Derks W, de Leeuw RJ, Hordijk GJ. Elderly patients with head and neck cancer: the influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head Neck Surg. 2005;13(2):92–96. doi: 10.1097/01.moo.0000156169.63204.39. [DOI] [PubMed] [Google Scholar]
- 7.Piccirillo JF, Vlahiotis A. Comorbidity in patients with cancer of the head and neck: prevalence and impact on treatment and prognosis. Curr Oncol Rep. 2006;8(2):123–129. doi: 10.1007/s11912-006-0047-z. [DOI] [PubMed] [Google Scholar]
- 8.Borggreven PA, Kulik DJ, Langendijk JA, Doornaert P, de Bree R, Leemans CR. Severe comorbidity negatively influences prognosis in patients with oral and oropharyngeal cancer after surgical treatment with microvascular reconstruction. Oral Oncol. 2005;41(4):358–364. doi: 10.1016/j.oraloncology.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Homma A, Sakashita T, Oridate N, Suzuki F, Suzuki S, Hatakeyama H, et al. Importance of comorbidity in hypopharyngeal cancer. Head Neck. 2010;32(2):148–153. doi: 10.1002/hed.21158. [DOI] [PubMed] [Google Scholar]
- 10.Paleri V, Wight RG, Davies GR. Impact of comorbidity on the outcome of laryngeal squamous cancer. Head Neck. 2003;25(12):1019–1026. doi: 10.1002/hed.10333. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128(10):1172–1179. doi: 10.1001/archotol.128.10.1172. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo JF, Spitznagel EL, Jr, Vermani N, Costas I, Schnitzler M. Comparison of comorbidity indices for patients with head and neck cancer. Med Care. 2004;42(5):4820–4826. doi: 10.1097/01.mlr.0000124254.88292.a1. [DOI] [PubMed] [Google Scholar]
- 13.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 14.Ferrier MB, Spuesens EB, Le Cessie S, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg. 2005;131(1):27–32. doi: 10.1001/archotol.131.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Datema FR, Ferrier MB, van der Schroeff MP, Baatenburg de Jong RJ. Impact of comorbidity on short-term mortality and overall survival of head and neck cancer patients. Head Neck. 2010;32(6):728–736. doi: 10.1002/hed.21245. [DOI] [PubMed] [Google Scholar]
- 16.Peters TT, Langendijk JA, Plaat BE, Wedman J, Roodenburg JL, van Dijk BA, Sluiter WJ, van der Laan BF, Halmos GB. Co-morbidity and treatment outcomes of elderly pharyngeal cancer patients: A matched control study. Oral Oncol. 2011;47(12):1159–1164. doi: 10.1016/j.oraloncology.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Singh B, Bhaya M, Zimbler M, Stern J, Roland JT, Rosenfeld RM, et al. Impact of comorbidity on outcome of young patients with head and neck squamous cell carcinoma. Head Neck. 1998;20(1):1–7. doi: 10.1002/(sici)1097-0347(199801)20:1<1::aid-hed1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Morrow M, Sylvester J, DesHarnais S. CoC and NCDB to examine approaches to classifying comor- bidities: news from the Commission on Cancer. J Am Coll Surg. 2000;11:2–3. [Google Scholar]
- 19.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukerji SS, Duffy SA, Fowler KE, Khan M, Ronis DL, Terrell JE. Comorbidities in head and neck cancer: agreement between self-report and chart review. Otolaryngol Head Neck Surg. 2007;136(4):536–542. doi: 10.1016/j.otohns.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluatin the outcome of diabetes mellitus. J Chronic Dis. 1974;27(7–8):387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Bang DH, Piccirillo JF, Littenberg B, Johnson A. The Adult Comorbidity Evaluation-27 (ACE-27) Test -- A New Comorbidity Index for Patients with Cancer. Proc Am Soc Clin Oncol. 2000;19:1701. [Google Scholar]
- 25.Paleri V, Wight RG, Silver CE, Haigentz M, Jr, Takes RP, Bradley PJ, Rinaldo A, Sanabria A, Bien S, Ferlito A. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol. 2010;46(10):712–719. doi: 10.1016/j.oraloncology.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Belbin TJ, Bergman A, Brandwein-Gensler M, Chen Q, Childs G, Garg M, et al. Head and neck cancer: reduce and integrate for optimal outcome. Cytogenet Genome Res. 2007;118(2–4):92–109. doi: 10.1159/000108290. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Robins JM. Identifiability, exchangeability and confounding revisited. Epidemiol Perspect Innov. 2009;6:4. doi: 10.1186/1742-5573-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New York State DOH [Internet] [July 10, 2012];Socio-Economic Status Indicators - Bronx County. 2011 Nov; http://www.health.ny.gov/statistics/chac/chai/docs/ses_bronx.htm.
- 29.Peters TT, van der Laan BF, Plaat BE, Wedman J, Langendijk JA, Halmos GB. The impact of comorbidity on treatment-related side effects in older patients with laryngeal cancer. Oral Oncol. 2011;47(1):56–61. doi: 10.1016/j.oraloncology.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 30.O'Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Yung KC, Piccirillo JF. The incidence and impact of comorbidity diagnosed after the onset of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(10):1045–1049. doi: 10.1001/archotol.134.10.1045. [DOI] [PubMed] [Google Scholar]


