Abstract
Activity of locus coeruleus (LC) neurons and release of the peptide galanin (GAL), which is colocalized with norepinephrine (NE) in LC neurons, has been implicated in depression and, conversely, in antidepressant action. The present study examined the influence of chronic administration (for 14 days, via subcutaneously-implanted minipump) of antidepressant (AD) drugs representing three different classes (tricyclic [desipramine], selective serotonin reuptake inhibitor [SSRI] [paroxetine], and monoamine oxidase inhibitor [MAOI] [phenelzine]) on mRNA for GAL, GAL receptors (GalR1, R2, and R3), and tyrosine hydroxylase (TH), the rate-limiting enzyme for NE synthesis, in four brain regions – LC, A1/C1, dorsal raphe (DRN), and ventral tegmentum (VTA) of rats. Consistent with previous findings that chronic administration of AD drugs decreases activity of LC neurons, administration of AD drugs reduced mRNA for both GAL and TH in LC neurons. GAL and TH mRNA in LC neurons was highly correlated. AD drugs also reduced GAL and TH mRNA in A1/C1 and VTA but effects were smaller than in LC. The largest change in mRNA for GAL receptors produced by AD administration was to decrease mRNA for GalR2 receptors in the VTA region. Also, mRNA for GalR2 and GalR3 receptors was significantly (positively) correlated in all three predominantly catecholaminergic brain regions (LC, A1/C1, and VTA). Relative to these three brain regions, unique effects were seen in the DRN region, with the SSRI elevating GAL mRNA and with mRNA for GalR1 and GalR3 being highly correlated in this brain region. The findings show that chronic administration of AD drugs, which produces effective antidepressant action, results in changes in mRNA for GAL, GAL receptors, and TH in brain regions that likely participate in depression and antidepressant effects.
Keywords: galanin, norepinephrine, locus coeruleus, ventral tegmentum, antidepressant, desipramine, paroxetine, phenelzine
INTRODUCTION
The locus coeruleus (LC), the principal noradrenergic cell group in the brain, has been implicated in the pathophysiology of depression since the catecholamine hypothesis of depression was proposed in the 1960s (Bunney and Davis, 1965; Schildkraut, 1965; Schildkraut and Kety, 1967). Research suggests that activity of LC neurons is elevated in depression; evidence supporting this has been observed in clinical studies (e.g., Ordway et al., 1994; Wong et al., 2000; Ehnvall et al., 2003) as well as in preclinical investigations that have examined neurobiological changes in animal models of depression (Weiss, 1981; Simson and Weiss, 1988; Stone et al., 2009; 2011). Consistent with this, the converse also appears to be the case – namely, that LC activity is reduced when effective antidepressant (AD) therapy is applied. The data supporting this similarly derives from preclinical studies with animals (for a summary of effects of antidepressant treatments on electrophysiological measurement of LC activity, see Table 1 in West et al., 2009; also Nestler et al., 1990) as well as clinical studies that have observed reductions in the noradrenergic metabolite 3-methoxy-6-hydroxyphenylglycol (MHPG) in cerebrospinal fluid of patients undergoing AD treatment (for summary, see Table 4 in Grant and Weiss, 2001).
However, a notable inconsistency in relating heightened activity of LC neurons to depression (and, by extension, diminution of LC activity to recovery from depression) is that the most apparent consequence of heightened LC activity would be increased release of norepinephrine (NE) in the brain, and the evidence linking the behavioral changes seen in depression to perturbations of NE in the brain is tenuous. Reviewing basic research relating symptoms of depression to changes in noradrenergic activity in the brain reveals that experimental alterations in brain NE can affect behaviors such as motor activity, investigatory behavior, social interaction, and sleep (e.g., Stone and Medlinger, 1974; Carey, 1976; Delini-Stula et al., 1984; Eison et al., 1977; Kaitin et al., 1986), but the changes in these behaviors resulting from profound perturbations of NE are often small, variable, and depend upon specific testing conditions for changes to be seen (e.g., Amaral and Sinnamon, 1977; Carli et al., 1983; Crow et al., 1978; Robbins et al., 1989; Robinson et al., 1977). In contrast, alteration of brain dopamine (DA) appears to have much more impact on behaviors related to depression. Lesions of DA pathways in the brain and/or introduction of DA agonists and antagonists into DA-rich regions of the forebrain (i.e., striatum and nucleus accumbens), as well as measurement of DA metabolism in these brain regions, consistently shows that changes in DA have major effects on motor activity, alimentary behavior, and reward/hedonic processes (e.g., Pijnenburg and van Rossum, 1973; Mogenson and Nielsen, 1984; Museo and Wise, 1990; Ungerstedt, 1971; Stricker and Zigmond, 1984; Yokel and Wise, 1975; Stellar and Stellar, 1985; Robbins et al., 1989). Thus, basic research investigating the relationship between monoamines in the brain and behavioral responses relevant to depression points to DA as being potentially important in depression, and much more so than NE.
In view of the foregoing, the challenge in addressing the neurobiological basis for depression relative to LC could be viewed as the need to integrate elevated activity of LC neurons with dopaminergic mediation of the behavior affected in depression. A potential resolution can be derived from a report by Grenhoff, Svennson, and colleagues in 1993 (Grenhoff et al., 1993). These investigators presented data indicating that “burst” firing of LC neurons (i.e., rapid firing of LC) will release galanin (GAL) from terminals on axons of LC neurons projecting to the ventral tegmental area (VTA), and that the hyperpolarizing influence of GAL on DA cell bodies in the VTA will decrease the activity of these DA neurons. This finding gave rise to the formulation that the hyperactivity of LC neurons observed in depression might bring about such depression-related responses by decreasing the neural activity of dopaminergic cell bodies in the VTA as the result of GAL released from LC-derived terminals in the VTA (Weiss et al., 1996; 1998).
We have tested the formulation described just above in a number of ways. First, GAL microinfused into VTA of rats brought about a reduction in their motor activity in both the home cage and in a Porsolt-type swim test (Weiss et al., 1998; see also Kuteeva et al., 2008 and Mitsukawa et al., 2009). Second, after depressed motor activity was produced in rats by exposing them to a highly stressful event, blockade of GAL receptors in their VTA by microinfusion of the antagonist galantide into VTA hastened recovery from this stress-induced behavioral depression (Weiss et al., 2005). Third, and of most interest for the research presented here, we have tested the converse of the formulation described above. As indicated earlier, effective AD treatments decrease activity of LC neurons. If this reduction in LC activity indeed decreases GAL release from LC axon terminals in VTA and thereby releases VTA-DA neurons from GAL-mediated inhibition, then effective AD treatments should increase VTA-DA neuronal activity. We have recently reported (West and Weiss, 2011) that chronic administration of six AD drugs (two tricyclics, three SSRIs, one SNRI, and one NDRI) as well as electroconvulsive shock produces an increase in VTA-DA neuronal activity, with the most consistent effects on spontaneous firing rate but also increasing burst firing in some instances; only the monoamine oxidase inhibitor tested, which will, by its biochemical action, increase extracellular DA and thereby directly inhibit VTA-DA neuronal activity (e.g., Adell and Artigas, 2004), did not have this effect. An increase in VTA-DA neuronal activity resulting from chronic administration of DMI (Chiodo and Bunney, 1983) and SSRIs (Sekine et al., 2007) also has been reported previously.
In the investigation reported here, we sought to further examine the formulation described above. Insofar as therapeutic administration of AD drugs (i.e., chronic treatment) decreases LC activity, the anticipated effect of this would be to decrease activity-dependent peptides in LC neurons, predominantly tyrosine hydroxylase (TH) and also GAL. A decrease in GAL in LC neurons would appear to subserve the function of decreasing GAL release in VTA to reduce an inhibitory influence on VTA-DA neurons. As a first step in assessing this, we report here measurement of mRNA for GAL and TH in LC neurons resulting from chronic administration (for 14 days) of three different AD drugs – a tricyclic (desipramine, DMI), an SSRI (paroxetine, PAR), and a monoamine oxidase inhibitor (phenelzine, PHE). Additionally, to possibly assess effects on stimulation of GAL receptors in VTA, mRNA for galanin type 1, 2, and 3 receptors was also measured. Measurement of these mRNAs was made in LC and VTA; however, these measures were also made in cells of the dorsal raphe (DRN) and ventrolateral medulla (A-1, C-1 cell body region) as these brain regions contain serotonergic and catecholaminergic cell bodies of interest. In all of these brain regions GAL is colocalized in neurons containing monoamine transmitters (Melander et al., 1986; Hökfelt et al., 1987; Xu and Hökfelt, 1997), and the three types of GAL receptor have also been identified in these brain regions (Smith et al., 1998; O’Donnell et al., 1999; Waters and Krause, 2000; Branchek et al., 2000).
MATERIALS AND METHODS
Subjects
Sprague-Dawley male rats were used for this study. A total number of 36 animals were used for this experiment. Subjects were mature adult rats aged 5-7 months at the time of the experiment. Prior to inclusion in the experiment and throughout the experimental period (i.e., drug administration), animals were group housed 2-3 per cage. They were maintained on a 12:12 light:dark cycle at a temperature of approximately 21°C with laboratory chow and water available ad libitum.
Groups and Drugs
Rats were allocated to four groups of eight rats each. Three groups each received a different AD drug. The three AD drugs represented three major types of AD medication: a monoamine oxidase inhibitor, phenelzine (PHE), a tricyclic AD, desipramine (DMI), and an SSRI, paroxetine (PAR). A fourth group (VEH), also with eight rats, was the non-drug group, and received the vehicle in which the drugs were dissolved. The rats in the four groups were matched for age and body weight. The mean body weight (and standard error) of the groups at the time drug administration began was: PHE, 710.6 ± 23.4 grams (g); DMI, 707.2 ± 27.7 g; PAR, 690.8 ± 33.0 g; and VEH, 710.1 ± 23.6 g. PHE and DMI were dissolved in distilled water. PAR was dissolved in 50% dimethyl sulfoxide (DMSO), 25% polyethylene glycol (PEG), and 25% distilled water due to its low solubility in water alone. For the VEH group, half of the rats received distilled water as the vehicle and half received the vehicle used for PAR (DMSO, PEG, and distilled water). Following sacrifice of all animals described above, four additional rats were added to the study when it became evident that some rats in the DMI group had experienced problems with drug delivery via the implanted minipump; three of these additional rats were given DMI dissolved in a 0.85% saline solution and one received the saline VEH. Drug doses used in this study were 5 mg/kg/day for PHE and 10mg/kg/day for both DMI and PAR. The doses were chosen based on previous studies that have shown both robust AD behavioral changes (e.g., West and Weiss, 2005) as well as effects on LC electrophysiology (West et al., 2009) by administration of these drugs at these doses given via minipump.
Osmotic Minipump Implantation
The drugs were administered via model 2ML2 Osmotic Minipumps (Durect Corp., Cupertino, CA). Osmotic minipumps were chosen for AD delivery because they slowly release drug over 14 days, thus eliminating the stress animals would experience by daily injections of the AD (Garabal et al., 1991). The surgical procedure was performed by guidelines set forth by the IACUC committee at Emory University. Animals were anesthetized by inhaling isoflurane (3%), and were maintained under isoflurane anesthesia through the minipump implantation procedure. The lower back was shaved and swabbed with 70% ethanol. A 1-2 cm incision was made in the lower back and the minipump containing either drug or vehicle was placed into the subcutaneous pocket. For the PAR group, the minipump was inserted subcutaneously and a tube leading from the outflow of the pump was then inserted into the intraperitoneal cavity through a small incision where it was fixed by sutures to the cavity wall. This procedure for PAR administration, which caused the drug to be extruded into the peritoneal cavity, was done to minimize the possibility that the pump outflow would become blocked by the somewhat viscous vehicle. Animals in the VEH group that received PAR vehicle underwent the same implantation procedure. After implantation of the pump, the opening in the skin was closed with wound clips and the animals were returned to their cages once they regained consciousness. Animals then remained in their home cages during the two-week period of drug administration.
Tissue Dissections
After 14 days of drug administration, the animals were anesthetized with isoflurane, decapitated, and the brain was quickly removed and sliced into 1.0 mm sections. The LC, VTA, A1/C1 cell-body region of the ventral brainstem (A1/C1), and DRN regions were then dissected from the brain slices (see Figure 1). Each tissue sample was immediately weighed and placed in dry ice. The samples were then stored at −80°C until RNA extraction was performed. The mean weight (and standard error) for each brain region were as follows: LC, 3.50 ± 0.01 mg; VTA, 6.43 ± 0.19 mg; A1/C1, 4.55 ± 0.18 mg; and DRN, 2.52 ± 0.086 mg. At the time of sacrifice, trunk blood was also collected so that plasma levels of the drug could be determined.
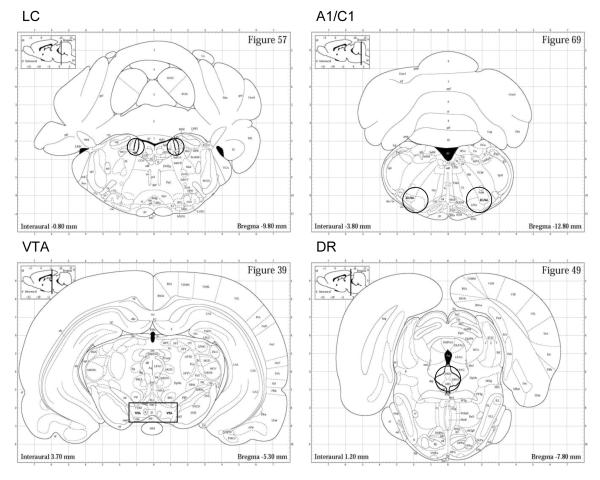
Figure 1.
Brain sections showing representative dissections (punches or cuts) taken to obtain samples of the locus coeruleus (LC), A1/C1 brainstem (A1/C1), ventral tegmental (VTA), and dorsal raphe nucleus (DRN) brain regions. For LC, tissue was obtained by 1.0 mm diameter bilateral punches of three contiguous 1.0 mm thick sections; for A1/C1 and DRN, tissue was obtained by 1.5 mm diameter punches of two contiguous 1.0 mm thick sections on midline (DRN) and bilaterally (A1/C1); for VTA, a rectangular dissection on midline (as shown) was taken from two contiguous 1.0 mm thick sections. Brain sections shown are approximately the midpoint of regions dissected. Illustrations are taken from Paxinos and Watson, 1998.
Quantitative real time RT-PCR (qRT-PCR)
Total RNA in each tissue sample was extracted using the TRIzol Plus Purification kit (Invitrogen, Cat. No. 1218355) following the protocol included with the kit. The purity and quantity of the RNA was analyzed using a Nanodrop Spectrophotometer ND-1000. All 260/280 values were between 1.79-2.26, indicating that the RNA samples were pure. RNA was then stored in −80 °C until cDNA synthesis was performed.
The cDNA was made using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen, Cat. No. 11752-050) following the protocol included with the kit. The amount of RNA used per region for cDNA synthesis were 499.2 ng for the LC, 485.2 ng for the VTA, 347.7 ng for the A1/C1 region, and 240.0 ng for the DRN. At the conclusion of this step, the cDNA for the samples of each brain region was diluted (see below) and the set of samples for each dilution was then stored in a −20 °C freezer until it was used for the qRT-PCR.
We generated a qRT-PCR standard curve for each primer set. cDNA from the LC was used to make the standard curve for each assay because preliminary studies found that the LC expressed a reliable level of mRNA for each primer analyzed in this study. We then determined the appropriate dilutions of each cDNA so that the qRT-PCR determined cDNA concentration fell within the standard curve for each primer. The dilutions were as follows: LC [1:6 (cDNA to DEPC-treated water for all primers)]; VTA (Gal=1:2, TH=1:20, GalR1=1:6, GalR2=1:8, GalR3=1:4, and β-Actin =1:10); A1/C1 region (Gal=1:2, TH=1:1, GalR1, GalR2 and β-Actin = 1:8, GalR3=1:4); and DRN (Gal and GalR3=1:4, TH=1:2, GalR1=1:6, GalR2 and β-Actin =1:8)]. An equivalent amount of preliminary RNA was used to make the LC cDNA for the standard curves of the brain region being examined. For each sample of each brain region, a qRT-PCR was conducted using 2 μL from the cDNA dilution, 1 μL of each primer pair separately (GAL, TH, GalR1, GalR2, GalR3 and ß-Actin, a control housekeeping gene; primers were obtained from SuperArray Bioscience Corporation), 12.5 μL of Platinum SYBR Green qRT-PCR SuperMix-UDG (Invitrogen) and 9.5 μL of water. Two microliters of DEPC-water was used as a non-template control. The volume in each well totaled 25 μL. The BioRad iCycler IQ-5 Real Time PCR system was set to the following cycling parameters: 50 °C for 2 minutes and 95 °C (for 8.5 minutes for UDG inactivation) and DNA polymerase activation followed by 40 cycles of denaturation and annealing/extension (95°C for 15 seconds; 60 °C for 60 seconds, respectively).
Data and statistical Analysis
qRT-PCR results for each sample were produced by comparison of measures with a standard curve generated within the same assay for the primer being assessed. Each sample was run in duplicate, and an average of the two measures constituted the value of the sample used in analysis. In a small number of instances (5 samples out of 864 in the study), aberrant measures were encountered and discarded. A measure was considered aberrant if it was at least twice or half the duplicate and also was outside the range of all other measures for all samples in that group. Six additional samples in the DRN region were not included in the DRN analysis because a problem was occurred in the β-Actin assay of these samples. β-Actin mRNA was used as a standardizing tool to assess the relative amounts and quality of the mRNA in each sample. Following measurement of β-Actin, and determination that this mRNA in each brain region did not differ across the four groups (done by ANOVA; see below), the remaining mRNAs in each sample were then corrected for the β-Actin mRNA of that sample. Data for each of the different normalized mRNAs obtained for each brain region were analyzed separately by one-way analysis of variance (ANOVA) comparing the four groups in the experiment (one non-drug and three AD-treated groups). If a significant overall effect of group was found, post hoc comparison of the VEH group with each of the three drug groups was done using Dunnett’s test. In a few cases, additional t-tests were run to assess the difference between the VEH group versus all of the AD-treated animals grouped together.
RESULTS
Blood levels of drug and body weight loss
Measurement of blood levels of drug revealed that three animals receiving DMI had blood levels at sacrifice that were below the minimum value for a therapeutic effect of DMI in humans [75-300 ng/ml (compiled from Van Brunt, 1983; Orsulak, 1986; Preskorn, 1989; Gutteck and Rentsch, 2003)]; these three animals, which all had problems with their minipumps during the experiment, including one rat that extruded its pump several days before sacrifice, were excluded from the DMI group. The therapeutic blood level of PAR in humans ranges from 40-120 ng/ml (compiled from Preskorn, 1997; Devane, 1999; Rasmussen and Brøsen, 2000; Baumann et al., 2004); no animals were discarded from the PAR group for failing to reach the minimum therapeutic level. No assay for phenelzine was available. The mean (and standard error) of the blood levels of the included DMI and PAR rats at the time of the sacrifice were 1174.9 ± 348.7 μg/ml and 1559.5 ± 398.9 μg/ml, respectively. With respect to body weight, the drug-treated groups lost weight. As noted above, the fully mature male rats (5-7 months of age) used in this study were quite large at the beginning of treatment (approximately 700 grams body weight). Mean (and standard error) group body weight changes were: PHE, −104.0 ± 9.4 g; DMI, −71.3 ± 10.6 g; and PAR, −53.0 ± 7.3 g. In comparison, the non-drug-treated animals (VEH group) showed a small weight gain over the treatment time period (+4.6 ± 7.3 grams).
GAL, TH, GalR1, GalR2, GalR3, and β-Actin mRNA
Locus Coeruleus
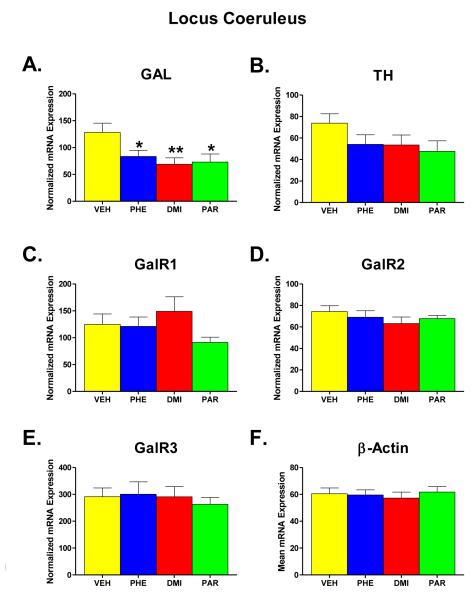
Figure 2 (part A-F) shows the results of administration of PHE, DMI, and PAR for two weeks on the expression levels of GAL, TH, GalR1, GalR2, GalR3, and β-Actin mRNA in the LC region. β-Actin was included as a standardizing measure for the level of expression of mRNA in each sample, having been pre-judged to likely be unaffected by the drug treatments. Part F of Figure 2 shows that this was indeed the case, as the treatment groups showed no significant differences between the groups in β-Actin mRNA levels when a one-way ANOVA was performed [F (groups) = 0.21, df = 3, 29, p = 0.891]. Therefore, each value obtained for GAL, TH, GalR1, GalR2, and GalR3 mRNA was normalized for the expression of β-Actin mRNA in that sample. The means of the normalized mRNA values for the different groups are shown in parts A-E.
Figure 2.
Effects on mRNA levels of galanin (GAL), tyrosine hydroxylase (TH), galanin receptors type 1, 2, and 3 (GalR1, GalR2, GalR3), and β-Actin (Parts A-F) in the locus coeruleus region (LC) of the brain following chronic administration (for two weeks) of one of three AD drugs -- phenelzine (PHE), desipramine (DMI), and paroxetine (PAR) -- or vehicle (VEH). The mRNA values for GAL, TH, GalR1, GalR2, and GalR3 mRNA in each animal were expressed as percent of the β-Actin mRNA found in the sample for that animal; this is referred to as normalized mRNA expression. Shown is the mean ±SEM for each group. Doses of the ADs given were 5.0 mg/kg/day for PHE and 10.0 mg/kg/day for DMI and PAR. Number of animals in each group were VEH n=9; PHE n=8; DMI n=8; and PAR n=8. Statistically significant differences are noted as follows: * = significantly (p<0.05) lower than VEH; ** = significantly (p<0.01) lower than VEH.
The most notable differences observed in the LC were seen with respect to the effect of the drug treatments on GAL mRNA (part A). For GAL mRNA, an overall significant effect of group was found in the one-way ANOVA comparing the treatment groups (F = 3.69, df = 3, 29, p = 0.023). Comparison of individual groups with the VEH control group revealed a significant difference from the PHE group (p = 0.046), the DMI group (p = 0.009), and the PAR group (p = 0.0145). For the other mRNA measures (parts B-E) in this brain region, no other significant treatment tended to have a similar effect on TH mRNA as was observed for GAL mRNA; that is, all three AD drugs tended to reduce TH mRNA in the LC. While the overall effect of group in the one-way ANOVA for this measure did not reach significance [F (groups) = 1.631, df = 3, 29, p = 0.204], a t-test comparing all of the drug-treated animals (n=24) with the VEH control group (n=9) revealed a statistically significant difference (t=2.21, df= 31, p <0.02 one tailed), thereby indicating that treatment with AD drugs significantly reduced TH mRNA in the LC.
Ventral Tegmental Area
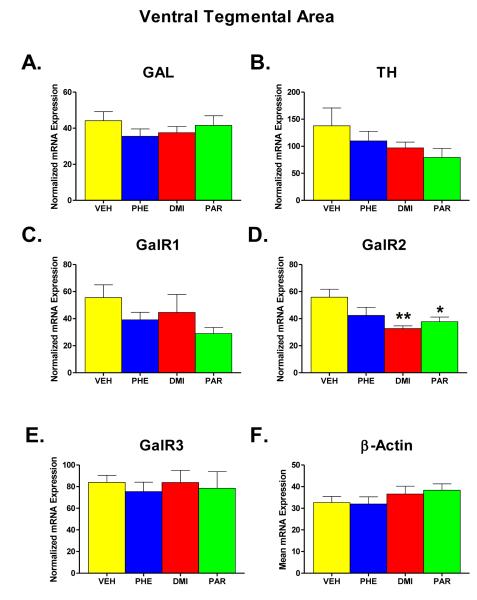
Figure 3 (part A-F) shows the results of administration of PHE, DMI, and PAR on the expression levels of GAL, TH, GalR1, GalR2, GalR3, and β-Actin mRNA in the VTA region. As was the case in LC, β-Actin mRNA was unaffected by the drug treatments, which can be seen in Part F of Figure 3. The effect of treatment group on the level of β-Actin mRNA expression was not significant in a one-way ANOVA (F = 0.926, df = 3, 29, p = 0.441). Therefore, each value obtained for GAL, TH, GalR1, GalR2, and GalR3 mRNA was again normalized for the expression of β-Actin in that sample, and the means of the normalized mRNA values for the different groups are shown in parts A-E.
Figure 3.
Effects on mRNA levels of galanin (GAL), tyrosine hydroxylase (TH), galanin receptors type 1, 2, and 3 (GalR1, GalR2, GalR3), and β-Actin (Parts A-F) in the ventral tegmental region (VTA) of the brain following chronic administration (for two weeks) of one of three AD drugs -- phenelzine (PHE), desipramine (DMI), and paroxetine (PAR) -- or vehicle (VEH). All other details as described in legend for Figure 2.
In the VTA, the most notable effect was seen with respect to the expression of GalR2 mRNA (Part D of Figure 3). A one-way ANOVA showed a significant effect of group for this measure (F = 4.651, df = 3, 29, p = 0.009). Comparison of the individual drug-treated groups with the VEH revealed that both the DMI and PAR groups each differed significantly from the VEH group (p = 0.004 and 0.027, respectively). For the other mRNA measures (parts A, B, C, and E), no other significant overall effects of group were found. However, inspection of Figure 5 reveals that other mRNAs showed a tendency to be lower in the drug-treated rats than in the VEH condition. When t-tests were carried out comparing all of the drug-treated rats (n=24) with the VEH group (n=9), rats treated with AD drugs showed lower expression of Gal R1 mRNA (t=1.81, p = 0.039 one-tailed) and also TH mRNA (t=1.72, p = 0.047 one-tailed).
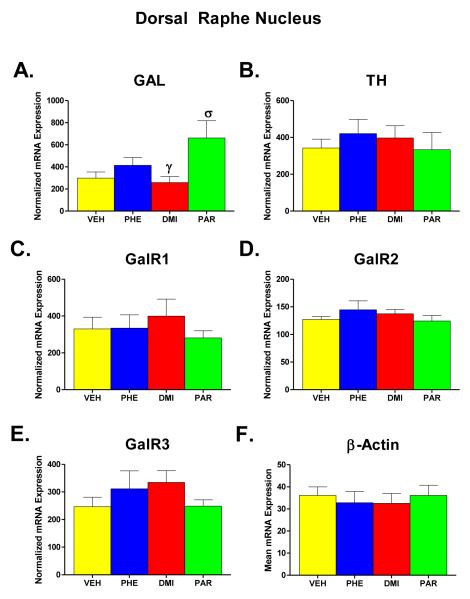
Figure 5.
Effects on mRNA levels of galanin (GAL), tyrosine hydroxylase (TH), galanin receptors type 1, 2, and 3 (GalR1, GalR2, GalR3), and β-Actin (Parts A-F) in the dorsal raphe region (DRN) of the brain following chronic administration (for two weeks) of one of three AD drugs -- phenelzine (PHE), desipramine (DMI), and paroxetine (PAR) -- or vehicle (VEH). The number of animals in each group after excluding six samples that were measured incorrectly were VEH n=7; PHE n=7; DMI n=7; and PAR n=6. All other details as described in legend for Figure 2, except for the statistical comparisons for which groups receiving different AD drugs were also compared with each other. Statistically significant differences are noted as follows: σ = significantly (p<0.05) higher than VEH. γ = significantly (p < 0.05) lower than PAR.
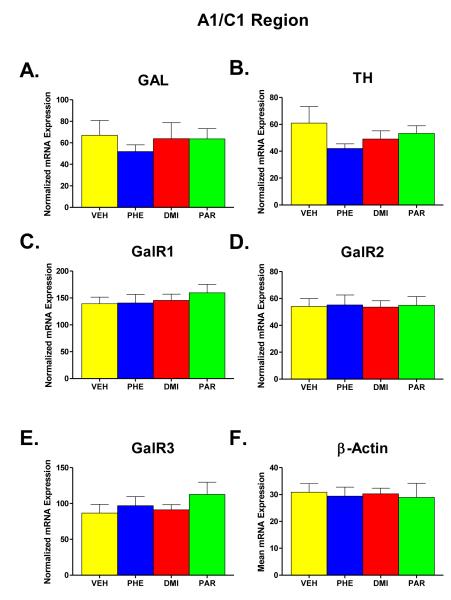
A1/C1 Region
Figure 4 (part A-F) shows the results of administration of PHE, DMI, and PAR administration on the expression levels of GAL, TH, GalR1, GalR2, GalR3, and β-Actin mRNA in the A1/C1 region. β-Actin was again unaffected by the drug treatments, as can be seen in Part F of Figure 4. The effect of treatment group on the level of β-Actin mRNA expression was not significant in a one-way ANOVA (F = 0.058, df = 3, 29, p = 0.981). Therefore, each value obtained for GAL, TH, GalR1, GalR2, and GalR3 mRNA was normalized for the expression of β-Actin in that sample, and the means of the normalized mRNA values for the different groups are shown in parts A-E.
Figure 4.
Effects on mRNA levels of galanin (GAL), tyrosine hydroxylase (TH), galanin receptors type 1, 2, and 3 (GalR1, GalR2, GalR3), and β-Actin (Parts A-F) in the A1/C1 cell-body region in the ventral brainstem following chronic administration (for two weeks) of one of three AD drugs -- phenelzine (PHE), desipramine (DMI), and paroxetine (PAR) -- or vehicle (VEH). All other details as described in legend for Figure 2.
One-way ANOVAs for each of the other mRNA measures (parts A-E) showed no significant effect of group in any case. The largest effect seen in this brain region was a tendency for TH mRNA expression to be lower in drug-treated animals, but even here, a t-test comparing all of the drug-treated animals (n=24) with the VEH group (n=9) only approached, but did not reach, statistical significance (t=1.43, p = 0.081 one–tailed).
Dorsal Raphe Nuclei
Figure 5 (parts A-F) shows the results of administration of PHE, DMI, and PAR on the expression levels of GAL, TH, GalR1, GalR2, GalR3, and β-Actin mRNA in the DRN. β-Actin was again unaffected by the drug treatments, as can be seen in Part F of Figure 5. The effect of treatment group on the level of β-Actin mRNA expression was not significant in a one-way ANOVA (F = 0.191, df = 3, 23, p = 0.902). Consequently, each value obtained for Gal, TH, GalR1, GalR2, and GalR3 was normalized for the expression of β-Actin in that sample, and the means of the normalized mRNA values for the different groups are shown in parts A-E.
In the DRN, GAL mRNA (Part A) was differentially affected by the drug treatments. A significant effect of group was found in a one-way ANOVA for this measure (F = 4.06, df = 3, 23, p = 0.019). Comparison of individual groups with the VEH control group revealed that GAL mRNA expression of the PAR group was significantly higher than GAL mRNA expression of VEH controls (p = 0.022) and also the DMI group (p = 0.011). No other significant differences were seen in this brain region.
Correlations between mRNAs in the four brain regions
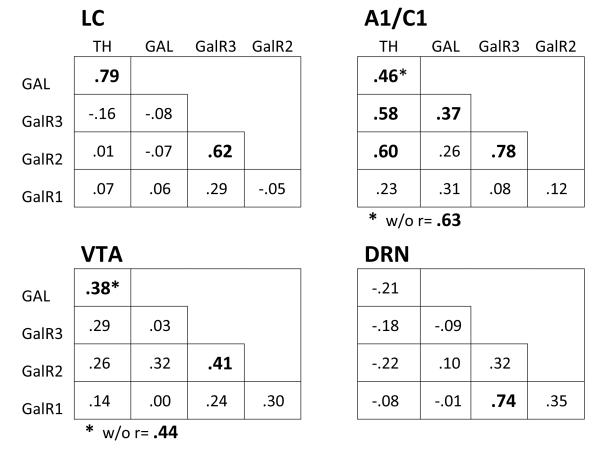
Figure 6 shows correlation coefficients between the different mRNAs assessed in this experiment; coefficients observed within each of the four brain regions are shown. The highest correlation in the study was seen between the mRNA for TH and GAL in the LC. Figure 7 presents the scatterplot of all points in this correlation, showing that the positive correlation between TH and GAL mRNA is present not only across all animals in the study but is evident within each of the four treatment groups as well. A significant correlation between these two mRNAs was also seen in the A1/C1 region. Also, in all three catecholaminergic cell-body regions (LC, A1/C1, and VTA), the mRNA for GalR2 and GalR3 was significantly correlated. In DRN, the profile of correlations differed from that seen in the other brain regions, with TH mRNA in this brain region being uncorrelated or showing small negative correlations with the other mRNAs, and the mRNA for GalR1 and GalR3 being highly correlated, which was unique to this brain region.
Figure 6.
Shown are the correlation coefficients between the normalized mRNA values for tyrosine hydroxlase (TH), galanin (GAL), and galanin receptor types 1, 2, and 3 (Gal R1, Gal R2, and GalR3) within samples of locus coeruleus (LC), A1/C1 ventral brain region (A1/C1), ventral tegmental region (VTA), and dorsal raphe region (DRN). Number of subjects was n=33 for LC, A1/C1, and VTA; n=27 for DRN. Coefficients in boldface are statistically significant (p<.05 or less). Two coefficients indicated by * also have noted the correlation when a single discrepant point is removed from the correlation; these correlations are shown below each matrix indicated by “w/o” meaning “without single discrepant point.”
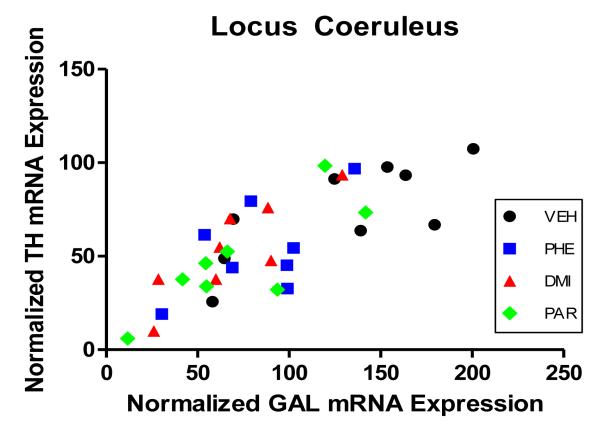
Figure 7.
Shown is the correlation of mRNA for tyrosine hydroxylase (TH) and galanin (GAL) in the LC region. For each subject in the study is shown its normalized mRNA value for TH plotted against its normalized mRNA value for GAL. Different treatments received by the subjects, indicated by the shape of the point in the figure, were vehicle (VEH), phenelzine (PHE), desipramine (DMI), or paroxetine (PAR). Dose/duration of drug treatment and number of animals per group is described in legend for Figure 2. The overall correlation for these points is r = .79 (p<0.001).
DISCUSSION
When three AD drugs with different pharmacologic actions – a tricylic (DMI) which primarily blocks norepinephrine reuptake, an SSRI (PAR) which primarily blocks serotonin reuptake, and a monoamine oxidase inhibitor (PHE) which retards the catabolism of all monoamines – were administered to rats for two weeks, certain changes in mRNA for TH, GAL, and GAL receptors were observed in different brain regions. Although the doses of these drugs that were used were standard in rat studies, the blood levels that were measured in this study were higher than normally encountered in therapeutic ranges, probably due to our having used fully mature male rats. However, dose-response analyses of several AD drugs that were carried out in our laboratory measuring effects on LC electrophysiological activity have shown that high blood levels of AD drugs are associated with similar effects on LC activity (i.e., inhibition of LC activity) to what is seen at lower therapeutic levels except that the change may be somewhat larger (West et al., 2009); therefore, effects on mRNA reported here should be representative of what the drugs will produce at lower blood levels.
Salient changes were seen in the brain area that was the principal focus of this study, the LC. In LC cells, chronic administration of each of the three AD drugs significantly reduced GAL mRNA. The mRNA for TH in LC also was reduced by the three drugs; this effect was significant when the three ADs were considered together and compared with the vehicle-infused group. A similar finding in regard to the influence of chronic administration of AD drugs on the mRNA for TH in LC was previously reported in a well-known study by Nestler and colleagues (Nestler et al., 1990). The findings reported here are consistent with the known effects of chronic administration of AD drugs on activity of LC neurons, which is to decrease their activity (for summary, see West et al., 2009). Insofar as both TH and GAL are activity-dependent peptides – that is, their synthesis increases when cell bodies in which they are synthesized are active, and their synthesis decreases when these cell bodies are inactive – it would be expected that the inhibitory influence of AD drugs on the activity of LC neurons might well be reflected in decreased synthesis, reflected in decreased mRNA, for TH and GAL in these cells. That synthesis of both TH and GAL in LC is activity-dependent is supported by the finding that the mRNA for TH and for GAL in LC was highly correlated, as shown in Figure 7. The correlations carried out used all of the animals in the study, thereby including vehicle-infused rats whose LC activity would have been higher than that of the animals infused with AD drugs; thus, the correlation shows, appropriately, a relationship seen across differing levels of LC activity. However, as also can be seen in Figure 7, the positive relationship between TH and GAL mRNA was evident within each of the four groups in the study.
In the VTA, both GAL and TH mRNA showed the tendency to be reduced by AD treatment; these effects, however, neither of these effects reached two-tailed statistical significance. Nevertheless, such findings do not offer support for the theoretical interpretation presented in the introduction. This formulation proposed that effective AD treatment will, by decreasing LC activity, decrease release of GAL in VTA, thereby increasing VTA-DA neuronal activity. The findings reported here point to the opposite effect. When Razani et al. (2000) examined changes in DRN produced by infusion of GAL into the ventricular system of the rat brain, they found that this infusion decreased GAL mRNA in DRN. Such findings indicate that increased extracellular levels of GAL (via the infusion) caused a decrease in GAL mRNA in the cell bodies of DRN that presumably were affected by the GAL infusion. While the Razani et al study did not assess changes in VTA, the findings would seem applicable to VTA-DA neurons in that extracellular GAL exerts an inhibitory (hyperpolarizing) influence on both VTA-DA and DRN neurons (refs). Thus, decreased mRNA for both TH and GAL mRNA in VTA resulting from AD treatment suggests that AD treatment may have decreased VTA-DA activity, perhaps by increasing extracellular GAL in VTA or by some other mechanism. However, this conclusion is tempered by the fact that DA neurons in the VTA region represent a relatively small number interdigitated amongst other neurons that synthesize different transmitters, and, consequently, it is unclear as to which neurons contributed to the mRNA measured in the VTA sample. While the TH-containing neurons in VTA are catecholaminergic, the same cannot be said for GAL. Without in situ determination, which was not performed, a firm conclusion as to what neurons in this region contributed to GAL mRNA is not possible.
The largest change in mRNA seen in the VTA region was a decrease in mRNA for GalR2 receptors, which is particularly interesting in view of the report by Kuteeva et al. (2008) suggesting that stimulation of GalR2 receptors mediates antidepressant action. If this is correct, decreased synthesis of Gal R2 receptors in the VTA region could point to antidepressant activity taking place, with the decrease in mRNA for this receptor resulting either from negative feedback on synthesis of this receptor because such action is occurring or from direct stimulation of GalR2 receptors by GAL to bring about an antidepressant effect.
The other two brains regions examined were A1/C1 and DRN. In the A1/C1 region, AD treatment showed a tendency to decrease TH and GAL mRNA, so that this region looked similar to LC and VTA in this regard, but this effect in the A1/C1 region did not reach statistical significance. In the DRN, however, a markedly different pattern for GAL mRNA was seen in relation to the AD treatment than was evident in the other three brain regions. GAL mRNA, which was also the only mRNA in DRN to be significantly affected by different drug treatments, was markedly increased in the animals that had received PAR. This increase was distinctly different from what was seen in all other groups, and significantly different from VEH- and DMI-treated animals. The study by Razani et al. (2000) clearly demonstrates an interaction between GAL and 5-HT1A receptors in DRN neurons, suggesting that action of 5-HT and GAL is linked in these cells. The results shown here reinforce this interaction, and, moreover, indicate that activation of 5-HT1A receptors, as will occur with PAR treatment, profoundly affects GAL mRNA in DRN neurons. Additionally, insofar as the effect of PAR treatment on GAL mRNA in DRN differed for that of DMI treatment, the results presented here point to an effect of a 5-HT-reuptake-blocking drug on GAL that is not shared by ADs that do not possess this capacity.
An interesting aspect of the present findings is the ability to examine intercorrelations of the various mRNAs within brain regions. As described earlier, TH and GAL mRNA were highly correlated in the LC, consistent with the idea that (1) these peptides are found within the same cells in this region/sample/(presumably LC neurons), and (2) the mRNAs are highly correlated because the two peptides are both activity dependent. Similarly, in the A1/C1 and VTA regions, although colocalization of GAL and TH in catecholaminergic neurons in these brain regions is much less extensive or evident than in LC (e.g., Levin et al., 1987; Skofitsch and Jacobowitz, 1985), TH and GAL mRNA was also significantly correlated. In contrast, in the DRN region GAL and TH mRNA were not correlated (in fact, were somewhat negatively correlated). In DRN, unlike the other brain regions examined in this study, the mRNA for TH and much of the mRNA for GAL is clearly localized in different neurons in the sample – TH would be found in a small number of dopaminergic cells in this region designated as A10c and A10dc (Hökfelt et al., 1984) whereas much of the GAL mRNA would be colocalized in the more numerous serotonergic cells in DRN (e.g., Melander et al., 1986; Xu and Hökfelt, 1997); thus, it is perhaps not surprising that the DRN does not show the same relationship between mRNA for GAL and TH evident in the LC and in the two other catecholaminergic regions.
Also of interest, correlations between mRNAs for the three types of GAL receptors (GalR1, GalR2, and GalR3) indicated that these were associated differently in the brain regions examined. In the catecholaminergic cell-body regions (LC, A1/C1, and VTA), the mRNA for GalR2 and GalR3 was significantly correlated (r=.62, .78, and .41 respectively) whereas mRNA for GalR1 did not correlate with either of these. In contrast to this, in DRN GalR1 mRNA correlated highly with GalR3 mRNA (r=.74). This suggests that synthesis of GalR2 receptors is associated, or co-varies, with synthesis of GalR3 receptors in the catecholaminergic cell-body regions studied, whereas synthesis of GalR1 receptors is strongly associated, or co-varies, with synthesis of GalR3 receptors in the DRN region.
In summary, chronic administration of AD drugs that represented three classes of such drugs (i.e, tricyclic, SSRI, and MAOI) affected mRNA for GAL, GAL receptors, and TH in four brain regions studied. In the cell bodies of LC neurons, GAL and TH mRNA were decreased; insofar as GAL and TH are activity-dependent peptides, this effect is consistent with decreases in LC activity that are produced by chronic administration of these AD drugs. In the A1/C1 region and in VTA, GAL and TH mRNA also tended to be decreased. In VTA, the most marked change in GAL receptor mRNA produced by AD administration was a decrease in mRNA for GAL2 receptors. In DRN, the pattern of mRNA changes differed from that seen in the other brain regions studied, both in effects on GAL mRNA and with regard to intercorrelations of GAL receptor mRNA changes. These results confirm that, in brain regions that appear to play an important role in depression, mRNA for GAL, its receptors, and TH are altered with chronic administration of AD drugs that produces therapeutic effects of these drugs.
ACKNOWLEDGEMENTS
This research was supported in part by Public Health Service grants MH065737 and MH079794.
Sources of Support: Public Health Service Grants MH065737 and MH079794
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Sinnamon HM. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol. 1977;9:147–196. doi: 10.1016/0301-0082(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr., Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Effects of selective forebrain depletions of norepinephrine and serotonin on the activity and food intake effects of amphetamine and fenfluramine. Pharmacol Biochem Behav. 1976;5:519–523. doi: 10.1016/0091-3057(76)90262-8. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction task in rats; implication for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Deakin JFW, File SE, Longden A, Wendlandt S. The locus coeruleus noradrenergic system -- evidence against a role in attention, habituation, anxiety and motor activity. Brain Res. 1978;155:249–261. doi: 10.1016/0006-8993(78)91021-1. [DOI] [PubMed] [Google Scholar]
- de Weille JR, Fosset M, Schmid-Antomarchi H, Lazdunski M. Galanin inhibits dopamine secretion and activates a potassium channel in pheochromocytoma cells. Brain Res. 1989;485:199–203. doi: 10.1016/0006-8993(89)90685-9. [DOI] [PubMed] [Google Scholar]
- Delini-Stula A, Mogilnicka E, Hunn C, Dooley DJ. Novelty-oriented behavior in the rat after selective damage of locus coeruleus projections by DSP-4, a new noradrenergic neurotoxin. Pharmacol Biochem Behav. 1984;20:613–618. doi: 10.1016/0091-3057(84)90312-5. [DOI] [PubMed] [Google Scholar]
- Devane CL. Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cell Molec Neurobiol. 1999;19:443–466. doi: 10.1023/a:1006934807375. [DOI] [PubMed] [Google Scholar]
- Ehnvall A, Sjogren M, Zachrisson C.g., Agren H. Lifetime burden of mood swings and activation of brain norepinephrine turnover in patients with treatment-refractory depressive illness. J Affect Disord. 2003;74:185–189. doi: 10.1016/s0165-0327(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Eison MS, Stark AD, Ellison G. Opposed effects of locus coeruleus and substantia nigra lesions on social behavior in rat colonies. Pharmacol Biochem Behav. 1977;7:87–90. doi: 10.1016/0091-3057(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ögren SO, Jansson A, Cintra A, Härfstrand A, Agnati LF. Intraventricular injections of galanin reduces 5-HT metabolism in the ventral limbic cortex, the hippocampal formation and the fronto-parietal cortex of the male rat. Acta Physiol Scand. 1988;133:579–581. doi: 10.1111/j.1748-1716.1988.tb08444.x. [DOI] [PubMed] [Google Scholar]
- Garabal MF, Nunez MJ, Balboa JL, Suarez JA, Belmonte A. Effects of alpraxolam on the development of MTV-induced mammary tumors in female mice under stress. Cancer Lett. 1991;62:185–189. doi: 10.1016/0304-3835(92)90094-c. [DOI] [PubMed] [Google Scholar]
- Gopalan C, Tian Y, Moore KE, Lookingland KJ. Neurochemical evidence that the inhibitory effect of galanin on tuberoinfundibular dopamine neurons is activity dependent. Neuroendocrinology. 1993;58:287–293. doi: 10.1159/000126552. [DOI] [PubMed] [Google Scholar]
- Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biol Psychiatry. 2001;49:117–129. doi: 10.1016/s0006-3223(00)00936-7. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH. Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect. 1993;93:11–25. doi: 10.1007/BF01244934. [DOI] [PubMed] [Google Scholar]
- Gutteck U, Rentsch KM. Therapeutic drug monitoring of 13 antidepressant and five neuroleptic drugs in serum with liquid chromatography-electrospray ionization mass spectrometry. Clin Chem Lab Med. 2003;41:1571–1579. doi: 10.1515/CCLM.2003.240. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Mårtensson R, Björklund A, Kleinau S, Goldstein M. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier Science Publishers B.V.; Amsterdam: 1984. pp. 277–379. [Google Scholar]
- Hökfelt T, Millhorn D, Scroogy K, Tsuruo Y, Ceccatelli S, Lindh B, Meister B, Melander T, Schalling M, Bartfai T, Terenius L. Coexistence of peptides with classical neurotransmitters. Experientia. 1987;43:768–780. doi: 10.1007/BF01945354. [DOI] [PubMed] [Google Scholar]
- Kaitin KI, Bliwise DL, Gleason C, Nino-Murcia G, Dement WC, Libet B. Sleep disturbance produced by electrical stimulation of the locus coeruleus in a human subject. Biol Psychiatry. 1986;21:710–716. doi: 10.1016/0006-3223(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundström L, Sollenberg U, Langel Ü, Hökfelt T, Ögren SO. Differential role of galanin receptors in the regulation of depression-like behavior and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology. 2008;33:2573–2585. doi: 10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- Levin MC, Sawchenko PE, Howe PRC, Bloom SR, Polak JM. Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol. 1987;261:562–582. doi: 10.1002/cne.902610408. [DOI] [PubMed] [Google Scholar]
- Melander T, Hökfelt T, Rökaeus Å, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Bidirectional regulation of stress responses by galanin in mice: Involvement of galanin receptor subtype 1. Neuroscience. 2009;160:837–846. doi: 10.1016/j.neuroscience.2009.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen M. A study of the contribution of hippocampal-accumbens-subpallidal projections to locomotor activity. Behav Neural Biol. 1984;42:38–51. doi: 10.1016/s0163-1047(84)90412-6. [DOI] [PubMed] [Google Scholar]
- Museo E, Wise RA. Microinjections of a nicotinic agonist into dopamine terminal fields: Effects on locomotion. Pharmacol Biochem Behav. 1990;37:113–116. doi: 10.1016/0091-3057(90)90050-r. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, McMahon A, Sabban EL, Tallman JF, Duman RS. Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci USA. 1990;87:7522–7526. doi: 10.1073/pnas.87.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom O, Melander T, Hokfelt T, Bartfai T, Goldstein M. Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci Lett. 1987;73:21–26. doi: 10.1016/0304-3940(87)90024-3. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: Distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- Ordway GA, Smith KS, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J Neurochem. 1994;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- Orsulak PJ. Therapeutic monitoring of antidepressant drugs: Current methodology and applications. J Clin Psychiatry. 1986;47:39–50. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pijnenburg AJJ, van Rossum JM. Stimulation of locomotor activity following injection of dopamine into the nucleus accumbens. J Pharm Pharmacol. 1973;25:1003–1005. doi: 10.1111/j.2042-7158.1973.tb09995.x. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxdative drug metabolism. Clin Pharmacokinet. 1997;32:1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. Tricyclic antidepressants: the whys and hows of therapeutic drug monitoring. J Clin Psychiatry. 1989;50:34–42. [PubMed] [Google Scholar]
- Rasmussen BB, Brøsen K. Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interactions of the selective serotonin reuptake inhibitors? [Review] Ther Drug Monit. 2000;22:143–154. doi: 10.1097/00007691-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Razani H, Diaz-Cabiale Z, Fuxe K, Ögren SO. Intraventricular galanin produces a time-dependent modulation of 5-HT1A receptors in the dorsal raphe of the rat. NeuroReport. 2000;11:3943–3948. doi: 10.1097/00001756-200012180-00008. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Vanderwolf CH, Pappas BA. Are the dorsal noradrenergic bundle projections from the locus coeruleus important for neocortical or hippocampal activation? Brain Res. 1977;138:75–98. doi: 10.1016/0006-8993(77)90785-5. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ, Kety SS. Biogenic amines and emotion. Science. 1967;156:21–37. doi: 10.1126/science.156.3771.21. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR., Jr. Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: An in vivo electrophysiological study. Synapse. 2007;61:72–77. doi: 10.1002/syn.20349. [DOI] [PubMed] [Google Scholar]
- Seutin V, Verbanck P, Massotte L, Dresse A. Galanin decreases the activity of locus coeruleus neurons in vitro. Eur J Pharmacol. 1989;164:373–376. doi: 10.1016/0014-2999(89)90481-0. [DOI] [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Altered activity of the locus coeruleus in an animal model of depression. Neuropsychopharmacology. 1988;1:287–295. [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao W-J, Vaysse PJJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors: Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Stellar E. The Neurobiology of Motivation and Reward. Springer-Verlag; New York: 1985. [Google Scholar]
- Stone EA, Mendlinger S. Effect of intraventricular amines on motor activity of hypothermic rats. Res Commun Chem Pathol Pharmacol. 1974;7:549–556. [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. Marked behavioral activation from inhibitory stimulation of locus coeruleus α1-adrenoceptors by a full agonist. Brain Res. 2009;1291:21–31. doi: 10.1016/j.brainres.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67:193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker EM, Zigmond MJ. Brain catecholamines and the central control of food intake. Intl J Obesity. 1984;8:39–50. [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiologica Scandinavica Supplementum. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Van Brunt N. The clinical utility of tricyclic antidepressant blood levels: A review of the literature. Ther Drug Monit. 1983;5:1–10. doi: 10.1097/00007691-198303000-00001. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of Galanin-1, -2 and -3 receptor messenger RNAS in central and peripheral rat tissues. Neurosci. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CHK. Galanin: A significant role in depression? In: Hökfelt T, Bartfai T, Crawley J, editors. Annals of the New York Academy of Sciences. New York Academy of Sciences; New York: 1998. pp. 364–382. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Boss-Williams KA, Moore JP, West CHK. Testing the hypothesis that locus coeruleus hyperactivity produces depression-related changes via galanin. Neuropeptides. 2005;39:281–287. doi: 10.1016/j.npep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Demetrikopoulos MK, West CHK, Bonsall RW. Hypothesis linking the noradrenergic and dopaminergic systems in depression. Depression. 1996;3:225–245. [Google Scholar]
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of the rat brain [Review] Brain Res Rev. 1981;3:167–205. [Google Scholar]
- West CH, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on activity of dopaminergic neurons in the ventral tegmentum. Int J Neuropsychopharmacol. 2011;14:201–210. doi: 10.1017/S1461145710000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CHK, Ritchie JC, Boss-Williams KA, Weiss JM. Antidepressant drugs with differing pharmacological actions decrease activity of locus coeruleus neurons. Int J Neuropsychopharmacol. 2009;12:627–641. doi: 10.1017/S1461145708009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CHK, Weiss JM. A selective test for antidepressant treatments using rats bred for stress-induced reduction of motor activity in the swim test. Psychopharmacology. 2005;182:9–23. doi: 10.1007/s00213-005-0048-x. [DOI] [PubMed] [Google Scholar]
- Wong M-L, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: Relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQD, Zhang X, Pieribone VA, Grillner S, Hökfelt T. Galanin-5-hydroxytryptamine interactions: Electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience. 1998;87:79–94. doi: 10.1016/s0306-4522(98)00151-1. [DOI] [PubMed] [Google Scholar]
- Xu Z-QD, Hökfelt T. Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat. 1997;13:169–187. doi: 10.1016/s0891-0618(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: Implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]