Abstract
This study was designed to retrospectively compare the impact of crude Sigma V collagenase (Sigma V, n=52) with high-purified Serva NB1 collagenase (Serva NB1, n=42) on human islet isolation outcomes. A three-step filtration was applied to the crude Sigma V to eliminate endotoxin contamination and impurities; in addition this process was used as a lot prescreening tool. Isolation outcomes were determined by digestion efficacy, islet yields, purity, viability, glucose-stimulated insulin release, and endotoxin content. The difference of the digestion efficacy between Sigma V and Serva NB1 was very small, however, statistically significant (Sigma V: 64.71% vs. Serva NB1: 69.71%, p <0.05). Islet yields were similar (Sigma V: 23422.58 vs. Serva NB1: 271097 IEq, p>0.05) between groups. No significant purity differences were observed for fractions with purities greater than 75%. Viability (Sigma V: 93.3% vs. Serva NB1: 94.8%, p>0.05), and stimulation indexes (Sigma V: 3.41 vs. Serva NB1: 2.74, p>0.05) were similar between the two groups. The impact of cold ischemia and age on the isolation outcome in the Sigma V group was comparable to the Serva NB1 group. However, we were intrigued to find that the endotoxin content of the final products in the filtered Sigma V group was significantly less than that in the high-purified Serva NB1 group (0.022 EU/ml vs. 0.052 EU/ml, p<0.05). In addition, we found that there was minimal lot to lot variation after filtration and no significant loss of enzymatic activity. These finding indicate that using this or other crude enzyme blends for research pancreata is warranted to reduce isolation costs and increase the amount of islets available for critical islet research. These findings also validate the need for a systematic enzyme analysis to resolve these inconsistencies in overall enzyme quality once and for all.
Keywords: Collagenase, Human islet isolation, Islet transplantation, Diabetes
INTRODUCTION
Human islet transplantation is an emerging therapy for Type I diabetes (8,19). One of the main limiting factors for wide-spread clinical application is inconsistent islet isolation outcomes. The quality of the enzyme used to dissociate the pancreas is of great importance for islet manufacturing. Significant global efforts to purify the components of collagenase and protease enzyme blends and to characterize the in vitro enzyme composition and digestion efficacy have been made; however, wide batch-to-batch, and even vial-to-vial, variability remains (16). This variability in enzyme blends has hindered the standardization of collagenase digestion in human islet isolation across centers and is associated with unpredictable islet isolation results (6,11).
The primary enzymes currently used for human islet isolation include Liberase HI and Serva NB1 collagenase (Serva NB1). Prior to 2007, Liberase HI was considered the gold standard enzyme for more than 10 years (9,14,15). Following disclosure that Liberase HI potentially contained bovine neural tissue contaminants, the islet community began to use Serva NB1 to reduce the risk of transmission of bovine spongiform encephalopathy. However, this enzyme change significantly increased islet isolation cost and decreased human islet isolation outcomes worldwide (1).
Before the introduction of Liberase HI, Sigma Collagenase V (Sigma V) and other crude Sigma collagenase blends were used for both human and other animal pancreatic islet isolations (21,22). However, these enzyme blends did not gain extensive application due to low digestion efficacy related to enzyme impurity, imbalanced combination of key active components, significant batch-to-batch and vial-to-vial enzyme variation, high endotoxin levels, and pigment contamination (12,13,21,22). Yet, most of the results regarding the use of the collagenase enzyme blends in human islet isolation were obtained long before the optimization of current standard isolation techniques (21). In this study, we investigated whether simple filtration of low-cost crude collagenase, Sigma V, combined with lot screening could represent an alternative to costly purified enzyme blends.
MATERIALS AND METHODS
Pancreas preservation and islet isolation
Human pancreata were obtained from organ procurement organizations following research consent. The pancreata were preserved, using either University of Wisconsin solution (UW) or Histidine-Tryptophan-Ketoglutarate (HTK), and transported to the cell isolation facility at the University of Illinois at Chicago. No donor randomization was applied.
Fifty-two isolations were performed using Sigma Collagenase V and forty-two isolations were performed using Serva NB1 purified enzyme blend. In order to reduce the variability due to differences in isolation procedures, only the isolations conducted in the period between June 2007 and December 2009 were compared. Serva NB1 (Premium and GMP grades, SERVA Electrophoresis GmbH, Heidelberg, Germany) was reconstituted with cold HBSS (Mediatech, VA), supplemented with 10 U/ml Heparin, and complemented with Neutral Protease (SERVA Electrophoresis GmbH, Heidelberg, Germany). Variable units of collagenase (1600-2057 units) and Neutral Protease (200-257 units) were used based on the pancreas weights. Sigma V, with an enzyme activity of FALGPA 1.0-3.0 mg/solid (Sigma, MO), was reconstituted with 350 ml of Perfusion solution (Mediatech, VA), which was supplemented with 20 mM of Hepes (Mediatech, VA) and 10 mM of glutamine (Invitrogen, CA), to a final concentration of 2.86 mg/ml. To reduce endotoxin and pigment levels, the reconstituted enzyme went through a three-step filtration process using decreasing pore size filters (0.8, 0.45 and 0.22 μm, Nalgene). The three-step filtration process was also used as a pre-screening tool. Lots that were difficult to filtrate through a 0.8 μm filter were discarded, since they contained too many impurities and would result in a loss of enzyme activity during the filtration process.
The isolation, purification, and culture procedures were performed as previously described (8,18,19). Briefly, the pancreata were trimmed and distended with either Serva NB1 or Sigma V enzymes and digested using a modified Ricordi semi-automatic method. The digestion phase was stopped between 10-20 min based on microscopic observation of islet cleavage (degree of islets released from exocrine tissue) and tissue volume by the same experienced personnel. Digested tissues were then collected and washed three times. The tissues were incubated with UW solution for 30 min prior to continuous density purification using the UIC-UB gradient (3) in a Cobe 2991 cell separator (Cobe 2991, Cobe, CO) and subsequently cultured in CMRL culture media (Mediatech, VA) at 37°C supplemented with ITS (Invitrogen, CA), Sodium bicarbonate (Sigma, MO), Hepes, Human Albumin (Grifols, CA) and Ciprofloxacin (Hospira INC., USA).
Islet quality score
Final quality of isolated human islets was scored using a standardized system based on size distribution, fragmentation, density, border sharpness and shape. Each of these criteria was scored from 0 to 2. Islets of maximal quality scored 10; islets of poorest quality scored 0.
Glucose-stimulated insulin secretion and viability assays
Static glucose incubations (GSI) were performed, as previously described, to evaluate islet physiology and potency (2). Briefly, 10 purified islets were hand-picked and incubated with Krebs-ringer buffer containing 1.67 (low) mM glucose and 20 mM Hepes for 1 hr. The islets were then transferred into new Krebs buffer containing 16.7 mM (high) glucose for 1 hr and insulin concentration was determined using a conventional enzyme-linked immunosorbent assay (ELISA, Mercodia, Sweden). The stimulation index (SI) was calculated by dividing insulin release during high glucose (16.7 mM) by insulin release during basal glucose (1.67 mM). The post-isolation islet viability was determined using fluorescent staining with Syto-Green (Invitrogen, CA) and Ethidium Bromide (Sigma, MO), as previously described (17,23).
Endotoxin measurement
Endotoxin content in the final islet preparation was measured by the Endosafe Portal Test System (PTS™, Charles River Laboratory). In brief, 1.0 ml of final islet product was spun down for 10 seconds, using a bench centrifuge at 1000 rpm. Supernatants (25 μl) were injected into cartridges provided by Charles River in triplicates. Readouts were expressed as EU/mL.
Statistical analysis
All results were expressed as either mean ± standard deviation or standard error (SD or SE). Differences between Sigma V and Serva NB1 were analyzed by paired or unpaired Student’s t tests and Chi-square tests. Statistical analysis for multiple comparisons between Sigma V lots were analyzed by one-way ANOVA. Level of statistical significance for most analyses was set at p<0.05; multiple comparisons using ANOVA were considered significant at p<0.01.
RESULTS
Human pancreata characteristics
Pancreas characteristics are shown in Table 1. Donor age, gender, weight and body mass index did not show any significant differences between the two groups. The percentage of organs preserved in UW or HTK solutions, which are used for organ flush and cold storage, was also similar between groups. The only significant difference observed was cold ischemia time, which was significantly higher for the Sigma V group (10.63 min ± 2.0 vs. Serva NB1 9.32 min ± 2.95 group, p=0.023).
Table 1. Donor characteristics of human pancreata.
Data are expressed as mean ± SD and analyzed by unpaired student t-test by two-tail distribution. BMI=body mass index. CIT=cold ischemia time.
| Variables | Sigma V | Serva NB1 | p value |
|---|---|---|---|
| N | 52 | 42 | |
| Donor age (year) | 47.38 ± 12.87 | 48.46 ± 12.55 | 0.68 |
| Gender (%) | 53.8 (M) /46.2(F) | 54.8(M)/46.3(F) | 0.45 |
| Donor weight (kg) | 82.28 ± 19.54 | 88.82 ± 19.90 | 0.11 |
| BMI (kg/m2) | 27.34 ± 5.76 | 28.95 ± 6.63 | 0.21 |
| Preservation solutions (%) |
50(UW)/50(HTK) | 46(UW)/54(HTK) | 0.57 |
| CIT (hr) | 10.63 ± 2.39 | 9.32 ± 2.95 | 0.023 |
| Pancreas weight (g) | 94.32 ± 28.86 | 93.30 ± 30.18 | 0.87 |
Islet isolation outcomes
A comparison of outcome variables from human islet isolations using either the Sigma V or the Serva NB1 enzymes is summarized in Table 2. The time required to free the majority of the islets from the surrounding exocrine tissue was not different between the Sigma V and the Serva NB1 groups. Enzyme digestion efficacy was calculated by dividing the weight of the digested tissue by total pancreas weight. The observed difference in digestion efficacy between Serva NB1 and Sigma V was very small, however, statistically significant (69.71 % ± 23.74 vs. 64.71% ± 19.12, p <0.05). When we compared pre and post purification yields, the mean islet equivalents (IEq) of the Sigma V group was very similar to yields of the Serva NB1 group. Also pre and post-purification islet yield per gram of pancreas were comparable among the groups.
Table 2. Isolation outcomes of human pancreata.
Data are expressed as mean ± SD and analyzed by unpaired student t-test by two-tail distribution. IEq= islet equivalent.
| Variables | Sigma V | Serva NB1 | p value |
|---|---|---|---|
| Digestion time (min) | 14.36 ± 4.22 | 14.11 ± 3.76 | 0.78 |
| Digestion rate (%) | 64.71 ± 19.12 | 69.71 ± 23.74 | 0.0014 |
| Pre-purification yield (IEq) |
307317.4 ± 155714.3 |
336312.4 ± 156379 | 0.37 |
| Free islet percentage of Pre-Purification (%) |
73.06 ± 11.49 | 66.71 ± 24.9 | 0.34 |
| Post-purification yield (IEq) |
234227.58 ± 129728.42 |
271097.1 ± 157723.64 |
0.23 |
| Purity of post- purification (%) |
82.1 ± 13.6 | 87.3 ± 7.58 | 0.0492 |
| Purification recovery rate (%) |
82.01 ± 41.70 | 78.65 ± 36.73 | 0.82 |
| IEq/gram pre- purification |
3536.72 ± 1991.45 | 3820.42 ± 1775.21 | 0.48 |
| IEq/gram post- purification |
2628.12 ± 1458.8 | 2980.54 ± 1534.91 | 0.27 |
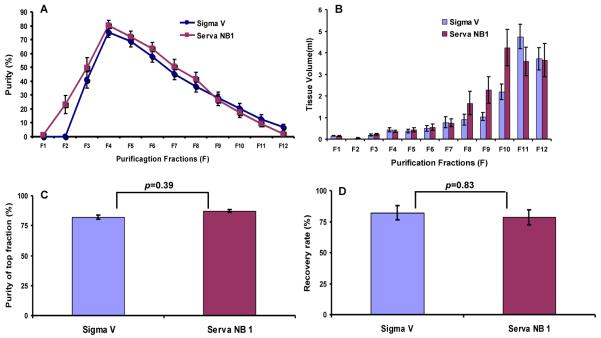
Successful separation of islets from exocrine tissue using a continuous density gradient depends on several factors, such as the percentage of free islets and the difference in cell density between islets and exocrine cells. The percentage of free islets in the Sigma V group after digestion tended to be higher than in the Serva NB1 group, but the difference did not reach statistical significance. In addition, we further compared the purification outcomes by evaluating the distribution of islet purity and tissue volume. Each collection fraction showed a similar distribution pattern and volume between the two groups (Figure 1A and 1B). With regard to differences in purity of the fractions, only the purest fraction displayed a lower value in the Sigma V group compared to the Serva NB1 group (82.1 % ± 13.6 vs.87.3 % ± 7.58, p<0.05), while all the remaining fractions presented similar purity. After purification, islets were divided into three groups accordingly to purity. The “high purity group” had purity greater or equal to 75, the “middle purity group” had purity between 74-40, and the “low purity group” had purity less than 40%. No significant difference was observed for fractions with purities greater than 75% (Figure 1C). Overall, these data indicated that the purity differences did not affect the islet recovery rate during the purification process. The islet recovery rate was calculated by dividing the pre-purification by the post-purification yield and was 82.01 % ± 41.70 in the Sigma V group and 78.65 % ± 36.73 in the Serva NB1 group (p>0.05, Figure 1D).
Figure 1. Purification outcomes of human pancreata.
(A) Purified islet distribution over a density gradient of 1.066-1.078 g/cm3. (B) Tissue volume collected on continuous gradient. (C) Purity of top fraction. (D) Purification recovery rate. Data are expressed as mean ± SE and analyzed by unpaired student t-test by two-tail distribution.
In general, the typical size of a human islet of Langerhans is 50-400 μm. Variations in islet size depend on many physiological and pathological factors, such as age, body size, and metabolic requirements. It has been shown that the type of collagenase and enzymatic activity during the isolation process significantly impacts the size of the isolated islets, secondary to fragmentation (11). Both groups represented a similar size distribution (Figure 2A). The ratio of actual islet number (AIN) to islet equivalent number (IEN), an indicator of islet fragmentation, was also assessed to compare islet preparations digested with Sigma V or Serva NB1. This analysis revealed a similar ratio between the two groups (0.91 ± 0.03 of Sigma V vs. 0.89 ± 0.04 of Serva NB 1, p>0.05, Figure 2B).
Figure 2. The size characteristics of isolated human islets.
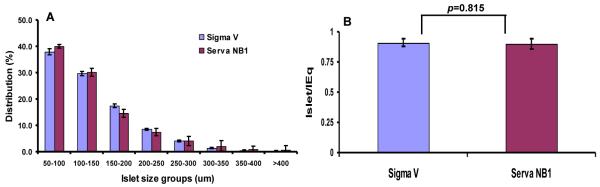
(A) Size distribution. (B) Ratio of actual islet number vs. IEq. Data are expressed as mean ± SE and analyzed by unpaired student t-test by two-tail distribution. IEq=islet equivalent.
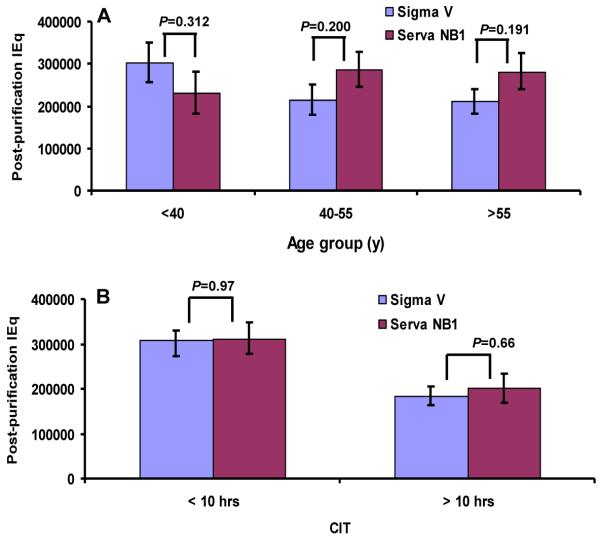
Tissue-specificity and age difference are also important factors in determining the efficacy of collagenase digestion-dissociation. Comparison of post-purification yields of three age groups revealed no significant difference between Sigma V and Serva NB1 (Figure 3A). The association of cold ischemia and isolation outcomes was compared between the Sigma V and the Serva NB1 groups and no significant difference was observed in either short or prolonged ischemia groups (Figure 3B).
Figure 3. Impact of age and cold ischemia on the isolation outcomes of Sigma V.
(A) Age impact on isolation outcomes of Sigma V. (B) CIT impact on isolation outcomes of Sigma V. Data are expressed as mean ± SE and analyzed by unpaired student t-test by two-tail distribution. CIT=cold ischemia time. IEq=islet equivalent.
Islet quality
A comparison of islet quality among the Sigma V and Serva NB1 groups is summarized in Table 3. These results show similar values for stimulation indexes, as measured by static glucose incubations, and viability assessments, as measured with inclusion and exclusion dyes. However, the endotoxin level of the final product in the Sigma V group was significantly lower than the Serva NB1 group (0.022 EU/ml ± 0.026 vs.0.052 EU/ml ± 0.006, p<0.003) (Table 3). This is a remarkable observation, since Serva NB1 is supposed to be a highly purified enzyme blend, produced in cGMP conform facilities.
Table 3. Islet quality-related characteristics.
Data are expressed as mean ± SD and analyzed by unpaired student t-test by two-tail distribution. SI=stimulation index.
| Variables | Sigma V | Serva NB1 | p value |
|---|---|---|---|
| Viability (%) | 93.28 ± 3.98 | 94.84 ± 2.93 | 0.061 |
| SI | 3.41 ± 2.34 | 2.74 ± 1.74 | 0.187 |
| Endotoxin (EU/ml) | 0.022 ± 0.026 | 0.052 ± 0.006 | 0.003 |
| Quality score | 5.85 ± 1.63 | 5.78 ±1.26 | 0.81 |
Lot-to-lot variation of Sigma V performance on isolation outcomes
Table 4 summarizes the isolation outcomes of four different lots of Sigma V. After our filtration process and lot screening, we only observed minimal lot-to-lot differences.
Table 4. Lot-to-lot variation of Sigma V.
Data are expressed as mean ± SD and analyzed by one-way ANOVA. IEq=islet equivalent. SI=stimulation index. Multiple comparisons using ANOVA were considered significant at p<0.01.
| Variables |
Lot A:
077K8628 |
Lot B:
047K7681 |
Lot C:
077K8629 |
Lot D:
026K8640 |
P value |
|---|---|---|---|---|---|
| N | 24 | 10 | 6 | 6 | |
| Pancreas weight(g) | 95.13 ± 28.01 | 99 ± 20.27 | 90.66 ± 19.36 | 114.00± 44.24 | 0.294 |
| Digestion time(m) | 15,22 ± 2.76 | 15,11 ± 5.63 | 12.83 ± 2.73 | 14.20 ± 1.30 | 0.172 |
| Digestion percentage (%) | 65.47 ± 16.95 | 53.66 ± 25.97 | 62.66 ± 27.77 | 69.17 ± 13.14 | 0.222 |
| Pre-purification IEq | 3621366.6 ± 173610.8 |
232418 ± 138778.2 |
202285.5 ± 120189.2 |
317356.3 ± 66904.6 |
0.084 |
| Post-purification IEq | 248662.9 ± 133587.4 |
220136.5 ± 129539.8 |
205890.3 ± 138822.7 |
278854.5 ± 161982.1 |
0.645 |
| Purity of post-purification (%) |
83.76 ± 12.65 | 76.25 ± 20.48 | 87.5 ± 7.07 | 78.33 ± 15.06 | 0.441 |
| Purification recovery rate (%) | 68.82 ± 23.82 | 77.37 ± 22.40 | 88.16 ± 10.64 | 97.83 ± 75.22 | 0.179 |
| IEq /gram pre-purification | 4048.13 ± 2031.83 |
2108 ± 1045.1 |
2247.67 ± 1102.53 |
3229.29 ± 1603.03 |
0.067 |
| IEq/gram post-purification | 2782.21 ± 1535.32 |
2096.57 ± 1311.85 |
2306.33 ± 1454.03 |
2701.24 ± 1743.10 |
0.751 |
| SI | 4.18 ± 4.34 | 2.81 ± 1.71 | 2.09 ± 1.09 | 3.42 ± 1.28 | 0.465 |
| Viability (%) | 93.31 ± 4.84 | 93.88 ± 2.20 | 91.25 ± 2.96 | 93.27 ± 5.06 | 0.467 |
| Endotoxin (EU/ml) | 0.020 ± 0.03 | 0.016 ± 0.01 | 0.015 ± 0.01 | 0.02 ± 0.01 | 0.749 |
| Quality score | 5.58±1.63 | 6.38 ±1.74 | 5.63±1.48 | 6.72±1.69 | 0.091 |
DISCUSSION
In this study we evaluated the use of crude collagenase for human islet isolation as an alternative to expensive, highly purified enzyme blends. With a simple three-step filtration-purification approach, and a lot screening based on the ease of filtration, we were able to achieve comparable results in terms of islet yields and quality.
Currently, the availability of reliable enzymes for pancreas digestion is the limiting factor in islet manufacturing. The lack of lot-to-lot consistency and unpredictable in-process enzyme activity continues to be the Achilles heel of clinical islet transplantation.
The herein presented study was not randomized, but the donor and organ characteristics between the two tested groups were comparable for all the variables known to impact human islet isolation outcomes. Cold ischemia time was found to be significantly longer for the organs tested with the Sigma V enzyme; however, it is unlikely that one hour of ischemia time is of clinical relevance.
Islet isolations performed with Sigma V achieved similar outcomes compared to isolations using Serva NB1. We observed similar purity and islet size distribution in the final product, indicating that neither cellular edema nor fragmentation was increased by the use of the crude collagenase Sigma V. Both enzymes performed similarly across pancreata of various ischemia times and donor age groups.
Despite the use of costly manufacturing methods and a cGMP conformed manufacturing environment, the endotoxin levels in islet preparations processed with the Serva NB1 were higher than in the Sigma V group. This is remarkable, since only a simple three-step filtration process was used to purify the Sigma V. Recent studies have demonstrated that endotoxin contamination of enzymes and the materials used during the islet isolation procedure play an important role in inflammation-induced functional stunning, destruction of islets, and amplification of the auto-immune and allo-immune reactions (4,5,7). One recent study demonstrated that endotoxin level in the lyophilized Sigma V blend was as high as 6.9 ng/mg (10) without filtration. However, our data demonstrates that implementation of a simple filtration process can decontaminate the Sigma V blend, without causing significant loss of digestion efficacy.
Lot-to-lot variation is a major concern for almost every blend of collagenase used in human and rodent islet isolations. The effect of enzymatic composition variation on isolation outcome is augmented by other factors, such as isolation technique and isolation team experience. Enzyme lot testing can only be completed on human pancreata during islet isolation and, therefore, is extremely expensive. This inherently limits enzyme development for human islet isolation. We developed a prescreening method using our three-step filtration, in which only those lots that pass easily through the three-step filtration process were tested in human islet isolations. As a result, Sigma V lot-to-lot variation was not as large in our experience, as compared to other studies (22).
Each year there are approximately 6,000 organ donors available in the United States, though less than 1,500 are used for either whole pancreas transplantation or islet transplantation (20). Thousands of pancreata are not used because of poor donor characteristics and/or economic concern for isolation cost. Some studies indicate that Liberase HI and Serva NB1 are superior over crude enzyme blends (13), however, the monetary cost of these enzymes are very high. Since cost burden is one of the primary factors contributing to the low number of isolations performed annually in the United States, it is important to maximize the utilization of pancreatic donors, not only for clinical transplantation but also for research and drug development. Therefore, if a crude and primitive enzyme blend can perform at least equally as well as a highly purified enzyme, then using this crude blend for research pancreata is justified.
This study demonstrates that by filtering the low-cost, Sigma Collagenase V, we can obtain human islet isolation outcomes comparable to those obtained with the expensive, highly purified Serva NB1. These results indicate that the current methods for enzyme purification and blending lack a scientific base. A systematic analysis of essential enzyme components for successful human pancreas dissociation is necessary to ultimately deliver a well-defined enzyme blend for successful human islet isolation.
ACKNOWLEDGEMENTS
The authors thank the member of the UIC isolation team for islet preparations, quality assessment, and data collection. This study was partially supported by NIH Center for Research Resources (Islet Cell Resources) NIH Clinical Islet Consortium (CIT).
REFERENCES CITED
- 1.Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 2.Barbaro B, Kuechle J, Salehi P, Rodriguez L, Qi M, Gangemi A, Benedetti E, Oberholzer J. Increased albumin concentration reduces apoptosis and improves functionality of human islets. Artif Cells Blood Substit Immobil Biotechnol. 2008;36(1):74–81. doi: 10.1080/10731190701857819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbaro B, Salehi P, Wang Y, Qi M, Gangemi A, Kuechle J, Hansen MA, Romagnoli T, Avila J, Benedetti E. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation. 2007;84(9):1200–1203. doi: 10.1097/01.tp.0000287127.00377.6f. others. [DOI] [PubMed] [Google Scholar]
- 4.Berney T, Molano RD, Cattan P, Pileggi A, Vizzardelli C, Oliver R, Ricordi C, Inverardi L. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation. 2001;71(1):125–132. doi: 10.1097/00007890-200101150-00020. [DOI] [PubMed] [Google Scholar]
- 5.Brandhorst H, Brandhorst D, Brendel MD, Hering BJ, Bretzel RG. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998;7(5):489–495. doi: 10.1177/096368979800700508. [DOI] [PubMed] [Google Scholar]
- 6.Bucher P, Mathe Z, Morel P, Bosco D, Andres A, Kurfuest M, Friedrich O, Raemsch-Guenther N, Buhler LH, Berney T. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation. 2005;79(1):91–97. doi: 10.1097/01.tp.0000147344.73915.c8. [DOI] [PubMed] [Google Scholar]
- 7.Eckhardt T, Jahr H, Federlin K, Bretzel RG. Endotoxin impairs the engraftment of rat islets transplanted beneath the kidney capsule of C57BL/6-mice. J Mol Med. 1999;77(1):123–125. doi: 10.1007/s001090050318. [DOI] [PubMed] [Google Scholar]
- 8.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. others. [DOI] [PubMed] [Google Scholar]
- 9.Gill JF, Chambers LL, Baurley JL, Ellis BB, Cavanaugh TJ, Fetterhoff TJ, Dwulet FE. Safety testing of Liberase, a purified enzyme blend for human islet isolation. Transplant Proc. 1995;27(6):3276–3277. [PubMed] [Google Scholar]
- 10.Jahr H, Pfeiffer G, Hering BJ, Federlin K, Bretzel RG. Endotoxin-mediated activation of cytokine production in human PBMCs by collagenase and Ficoll. J Mol Med. 1999;77(1):118–120. doi: 10.1007/s001090050316. [DOI] [PubMed] [Google Scholar]
- 11.Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007;7(5):1233–1241. doi: 10.1111/j.1600-6143.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 12.Lakey JR, Cavanagh TJ, Zieger MA, Albertson TE, Dwulet F, Wright MJ, Fetterhoff TJ. Evaluation of a purified enzyme blend for the recovery and in vitro function of isolated canine islets. Transplant Proc. 1998;30(2):590–591. doi: 10.1016/s0041-1345(97)01419-x. [DOI] [PubMed] [Google Scholar]
- 13.Lakey JR, Cavanagh TJ, Zieger MA, Wright M. Evaluation of a purified enzyme blend for the recovery and function of canine pancreatic islets. Cell Transplant. 1998;7(4):365–372. doi: 10.1177/096368979800700404. [DOI] [PubMed] [Google Scholar]
- 14.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46(7):1120–1123. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- 15.Linetsky E, Lehmann R, Li H, Fernandez L, Bottino R, Selvaggi G, Alejandro R, Ricordi C. Human islet isolation using a new enzyme blend. Transplant Proc. 1997;29(4):1957–1958. doi: 10.1016/s0041-1345(97)00178-4. [DOI] [PubMed] [Google Scholar]
- 16.Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, Marzorati S, Aldrighetti L, Freschi M, Grochowiecki T. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48(5):906–912. doi: 10.1007/s00125-005-1725-3. others. [DOI] [PubMed] [Google Scholar]
- 17.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJ, Socci C, Alejandro R. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185–195. doi: 10.1007/BF02581331. others. [DOI] [PubMed] [Google Scholar]
- 18.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 20.UNOS 2008 annual report of the United States Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation. 2009 available at http://www.unos.org/data/anrpt.main.htm.
- 21.van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267(1):139–146. doi: 10.1007/BF00318700. [DOI] [PubMed] [Google Scholar]
- 22.Wolters GH, Vos-Scheperkeuter GH, van Deijnen JH, van Schilfgaarde R. An analysis of the role of collagenase and protease in the enzymatic dissociation of the rat pancreas for islet isolation. Diabetologia. 1992;35(8):735–742. doi: 10.1007/BF00429093. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Acker J, Chen A, McGann L. In situ assessment of cell viability. Cell Transplant. 1998;7(5):443–451. doi: 10.1177/096368979800700503. [DOI] [PubMed] [Google Scholar]