Introduction
Benzo(a)pyrene (B(a)P) is a typical compound of polycyclic aromatic hydrocarbons present in industrial pollution, auto exhaust, tobacco smoke and coal tar (Boström et al, 2002). B(a)P have been proven to be a complete carcinogen in many animal models (reviewed in Rubbin, 2001). A generally accepted concept is that B(a)P-induced genetic mutation is a crucial initiating step during carcinogenesis (Hornberg et al, 1996; Jeffy et al, 2002). Nevertheless, other evidence indicates that cell cycle is regulated by oncogenes and tumor suppressor genes (Schreiber et al. 1999), and cell cycle alterations occur in the response of cells to various carcinogens( Khan and Dipple, 2000; Sherr, 1996), suggesting that cell cycle control is necessary for carcinogenic activity of B(a)P.
Progression through the cell cycle is controlled by a set of proteins, including the different cyclins, cyclin-dependent kinases (cdks) and their inhibitors (Bakiri, 2000). The regulation of mammalian cell proliferation by mitogen and stress occurs largely during the G1 phase of the cell cycle (Schreiber et al, 1999). B(a)P has been documented to accelerate cell cycle progression from G1 phase to S phase and induce cell proliferation in human embryo lung fibroblasts (Du et al, 2006; Jia et al, 2006; Gao et al, 2006a). However, the molecular mechanisms involved in the cell cycle alternations upon B(a)P exposure are still largely unknown. Thus, the identification of signaling molecule and related pathways involved in B(a)P-induced cell cycle alterations, not only contributes to our knowledge of signaling transduction processes, but also is essential for understanding the carcinogenic effect of B(a)P.
Previous studies have shown that transcription factor c-Jun plays a role in the regulation of normal cell cycle progression (reviewed in Weiss and Bohmann, 2004; Wisdom et al, 1999). There are growing evidence that c-Jun is implicated in chemicals-induced tumor and malignant transformation (Eferl et al, 2003; Huang et al, 1996, 1998). Although much is known about c-Jun involvement in cell cycle control, the precise mechanisms of its activation and contribution in B(a)P-induced cell cycle alternations remain unclear. Our recent results have shown that B(a)P stimulated the transactivation of activator factor 1 (AP-1) (Gao et al, 2006b), suggesting that c-Jun, acting as a primary member of AP-1, might play a role in the regulation of cell cycle upon B(a)P exposure. In view of above data, we sought to extend these earlier observations in the present study and, therefore, investigated the potential role of c-Jun in B(a)P-induced alternations of cell cycle as well as the likely interaction of several signaling pathways on the regulation of c-Jun activation.
Materials and Methods
Reagents and plasmids
B(a)P was purchased from Sigma Chemical Co (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) at 2 mmol/L stock concentration. RPMI1640 medium was obtained from Gibcol Co (Gibco BRL, NY, USA). The p53-specific inhibitor pifithrin-α was obtained from Calbiochem (San Diego, CA, USA). Transfectam reagent and the luciferase assay substrate were bought from Promega (Madison, WI, USA). The phospho-specific Rb antibody at Ser780 was bought from Cell Signaling Biotechnology (Beverly, MA, USA). The other antibodies used in the studies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). CMV-neo vector plasmid, c-Jun dominant negative mutant plasmid (TAM67) and cyclin D1-luciferase reporter plasmid were described in previous studies (Ding et al, 2006; Huang et al, 1999; Ouyang et al, 2004).
Cell culture
Human embryo lung fibroblasts (HELF) were purchased from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. HELF-sip53 cells, HELF-AP-1/vector cells, HELF-AP-1-DN-Akt cells and HELF-AP-1-DN-Δp85 cells were described previously (Gao et al, 2007; Wang et el, 2005). HELF cells and their derivations were cultured in RPMI-1640 medium with 10% heat-inactivated fetal bovine serum (FBS), 2 mmol/L glutamine, 50 mg/ml gentamycin sulfate at 37°C in a humidified atmosphere of 5% CO2. The cells were detached with trypsin and transferred to 75-cm2 culture flasks two to three times per week.
Stable transfection
HELF cells were cultured in a six-well plate until they reached 60-70% confluence. Fifteen microlitre of Transfectam reagent mixed with 15 μg of plasmids (1 μg of CMV-neo vector, 2 μg of cycline D1-luciferase reporter plasmid, and 12 μg of TAM67 or vector control) were used to transfect each well in the absence of serum. Transfected cells were selected in the presence of 400 μg/ml G418 for 2 weeks. The expression of TAM67 in the transfectant cells was detected by Western blot with an antibody that recognizes the COOH terminus of c-Jun. The stable transfectants were identified by measuring both the basal level of luciferase activity and the inhibition of c-Jun phosphorylation.
Growth curves
HELF-cyclin D1-TAM67 cells, HELF-cyclin D1/vector cells and parent HELF cells were seeded in 6-well plates (1.5×104/well). Cell viability was measured at daily intervals by counting the number of trypan blue-excluding intact cells. Cell number was determined daily in triplicate for 6 days and mean values were plotted into growth curves.
Cell proliferation assay (MTT)
Cells were seeded in 96-well plates (1×104/well) at 37°C overnight, and then exposed to B(a)P at various concentrations of 0.5-16 μmol/L. Following treatment for 24 h and 48 h, the culture medium was removed and replaced with a medium containing 0.5 mg of MTT dissolved in PBS (pH 7.2). After 4 h, the formed crystals were dissolved with 200 μl DMSO. The intensity of the color in each well was measured at a wavelength of 490 nm using a Dynex Technologies Microplate Reader.
Cell cycle analysis
Cells were cultured in 75-cm2 culture flasks to 80–85% confluence, and then serum-starved with 0.5% FBS RPMI-1640. After 24 h, cells were untreated or treated with B(a)P for 24 and 48 h. The cells were harvested and fixed with ice cold 70% ethanol overnight at 4°C. The fixed cells were incubated with 1mg/ml RNase A for 30 min at 37°C, and then stained with 1 mg/ml propidium iodide (PI) for 40 min at 4°C without light. The DNA content was determined by flow cytometry (Beckman Coulter, San Diego, CA) and EXPO 32 software.
Reporter gene assay
HELF-cyclin D1 cells (2×105) were seeded in each well of a 6-well plate. After being serum-starved for 24 h, the cells were exposed to B(a)P at the concentrations and time periods as indicated in the figure legends. The cultures were extracted with lysis buffer and luciferase activity was measured using luciferase assay reagent with a luminometer (TD-20/20, Turner Designs Instrument). The results are expressed as cyclin D1 induction (relative cyclin D1 induction).
Western blot
Whole-cell extracts were prepared by lysing the cells directly in SDS sample buffer. After sonication, protein samples (10-20 μl) were separated on SDS-polyacrylamide gels and electroblotted onto nitrocellulose membrane. Membranes were blocked with 5% (w/v) nonfat dry milk in Tris-buffered saline, pH 7.6, 0.1% (w/v) Tween-20 (TBST) for 1 h at room temperature (RT) and incubated with primary antibodies (1:1000) overnight at 4°C. After several washes with TBST, blots were incubated with the peroxidase-conjugated secondary antibody (1:2000) for 1 h at RT and developed with an enhanced chemical luminescence (ECL) detection system according to manufacture's instructions.
Indirect immunofluorescence
Cells were fixed with 2% paraformaldehyde (PFA) in phosphate-buffered saline (PBS), washed with PBS and permeabilized with 0.1% Triton X-100 in PBS on ice. The fixed cells were blocked with 5% goat serum for 1 h, and then incubated with primary antibodies in 5% goat serum at 4°C overnight. Antibodies included a monoclonal antibody against phosphorylated c-Jun at position Ser63 or a monoclonal antibody against cyclin D1. Bound antibodies were detected using FITC- or TRITC-conjugated secondary antibodies for 1 h at room temperature. After several washes, samples were examined using an OLYMPUS fluorescence microscope.
Statistical Analysis
The significance of the difference between the treated and untreated groups was determined with the Student's t test. The results are expressed as mean±SD. The differences were considered significant at P < 0.05.
Results
B(a)P-induced c-Jun phosphorylation in HELF cells
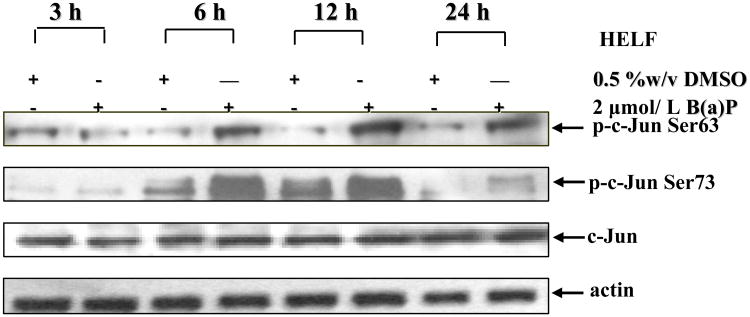
In response to various toxic stimuli, c-jun mRNA expression is rapidly induced several fold above the basal level in a wide variety of tissues and/or cell types (reviewed in Reddy and Mossman, 2002). In general, the mRNA levels of c-jun peak within 15–30 min of stimulation and return to basal level within 1–2 h, whereas c-Jun protein expression remains elevated above basal level for 2–24 h, depending upon the stimuli (Hazzalin et al, 2002). Consistent with this observation, under our experiment condition, B(a)P exposure markedly induced the increases in the phosphorylation of c-Jun at Ser63 and Ser73, but had no effect on total c-Jun expression as compared to those in cells of DMSO control (Fig.1A). The phosphorylation levels of c-Jun maximally occurred at 12 h and decreased at 24 h after exposure, paralleled the kinetics of AP-1 transactivation reported previously (Gao et al, 2006b).
Fig. 1. B(a)P enhanced c-Jun phosphorylation in HELF cells.
HELF cells were cultured until subconfluent (80-85 %) in 75-cm2 culture flasks with RPMI1640 containing 10% FBS, and then replaced with 0.5% FBS RPMI-1640. After being cultured for 24 h, the cells were exposed to 2 μmol/L B(a)P for various time points as indicated. The cells were then washed once with ice-cold PBS and extracted with SDS sample buffer. The cell extracts were separated on polyacrylamide-SDS gels, transferred, and probed with specific antibodies as indicated, and then detected with secondary antibody as indicated. The results were one representative data from three independent experiments.
B(a)P-induced c-Jun phosphorylation through PI-3K/Akt pathway
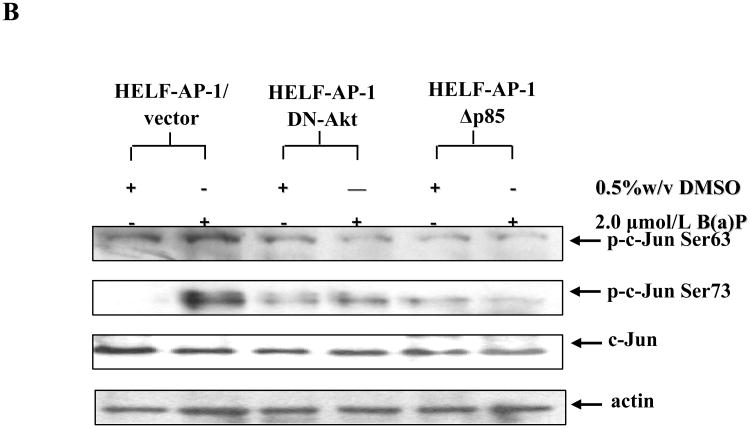
c-Jun, as a downstream transcription factor, is modulated by a variety of signaling pathways (reviewed in Weiss and Bohmann, 2004). We recently found that PI-3K/Akt/p70S6K were implicated in AP-1 transactivation induced by B(a)P (Gao et al., 2007), but their role in B(a)P-induced c-Jun activation has not been identified. To elucidate the role of PI-3K/Akt pathway in B(a)P-induced c-Jun phosphorylation, we investigated whether PI-3K and Akt were required for c-Jun activation caused by B(a)P exposure. Consequently, HELF-AP-1-Δp85 and HELF-AP-1-DN-Akt, which are well characterized transfectants in our prior study (Gao et al, 2007), were employed for this investigation. After B(a)P stimulation for 6 h, immunofluorescence assay was performed to compare c-Jun phosphorylation in transfected cells with that in control cells. As shown in Fig. 2A, vector control cells exhibited marked nuclear staining with phospho-specific c-Jun (Ser63) after B(a)P treatment (panel b), indicative of an increase in c-Jun phosphorylation. This effect was not seen in both HELF-AP-1-Δp85 cells (panel d) and HELF-AP-1-DN-Akt cells (panel f), indicating that disruption of either PI-3K or Akt could inhibit B(a)P induced c-Jun phosphorylation. Western blot analysis also showed that overexpression of dominant-negative mutant PI-3K potently blocked Akt activation (data not shown) and c-Jun phosphorylations in response to B(a)P (Fig. 2B). In contrast, B(a)P-induced c-Jun phosphorylation was suppressed partly in cells transfected with dominant negative mutant Akt (Fig. 2B). In addition, B(a)P treatment had little or no effect on c-Jun expression in any of cell lines tested. These observations reveal that B(a)P is able to induce c-Jun phosphorylation through PI-3K/Akt-dependent pathway.
Fig. 2. PI-3K/Akt pathway mediated B(a)P-induced c-Jun activation.
A, HELF-AP-1/vector (a and b), HELF-AP-1-DN-Akt (c and d) and HELF-AP-1- DN-Δp85 (e and f) were seeded into six-well plates respectively and cultured in 10% FBS RPMI 1640 medium at 37°C overnight. After being serum-starved for 24 h, cells were untreated (a, c and e) or treated (b, d and f) with 2 μmol/L B(a)P for 6 h, and then fixed. The fixed cells were subsequently blocked with 5% goat serum, incubated with phospho-specific c-Jun at Ser63 antibody, and detected by immunofluorescence assay with FITC-conjugated second antibody (green). B, The aforementioned cells were incubated with 2 μmol/L B(a)P for 12 h, and then extracted, sequentially detected by Western blot with specific antibodies as indicated.
PI-3K/Akt pathway modulated B(a)P-induced c-Jun phosphorylation via the activation of ERK but not JNK
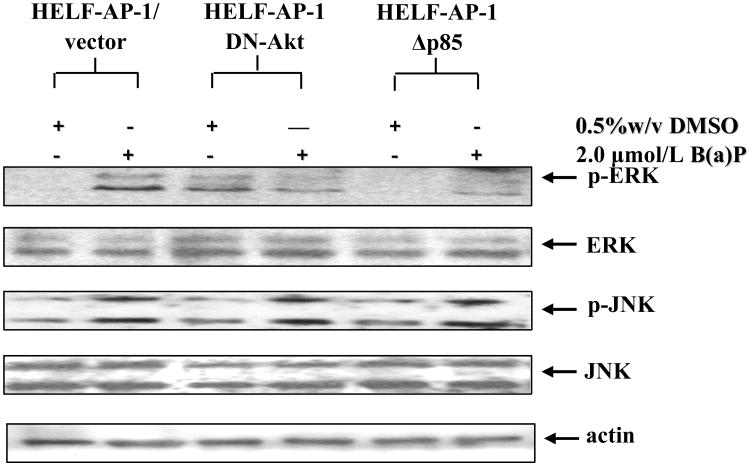
The conventional position of the c-Jun protein in the signaling transduction cascades is close to mitogen activated protein kinase (MAPK) family (Kennedy and Davis, 2003). We hypothesized that PI-3K/Akt might mediate B(a)P-induced c-Jun phosphorylation through MAPK pathway based on our recent observation that two subgroups of the MAPK family, extracellular signaling regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK), were involved in B(a)P-induced c-Jun activation (Jiao et al, 2007). To test it, the effect of B(a)P on the phosphorylation levels of ERK and JNK were analyzed in the cells transfected with dominant-negative mutants of PI-3K (Δp85) or Akt. Figure 3 showed that exposure of HELF cells to B(a)P led to an increase in the phosphorylations of ERK and JNK. Overexpression of dominant negative mutant PI-3K specially inhibited B(a)P-induced phosphorylations of ERK but not JNK (Fig. 3). Identical effects were observed in HELF-AP-1-DN-Akt cells (Fig. 3). These results clearly indicate that PI-3K/Akt pathway regulates B(a)P-induced c-Jun activation specially via ERK, but not JNK.
Fig. 3. ERK activation was essential for PI-3K/Akt pathway mediating B(a)P-induced c-Jun activation.
HELF-AP-1/vector, HELF-AP-1-DN-Akt and HELF-AP-1-DN-Δp85 cells were treated with or without 2 μmol/L B(a)P for 12 h. The cells were then washed once with ice-cold PBS and extracted with SDS sample buffer. The cell extracts were separated on polyacrylamide-SDS gels, transferred, and probed with specific antibodies as indicated, and then detected with secondary antibody. The results were one representative data from three independent experiments.
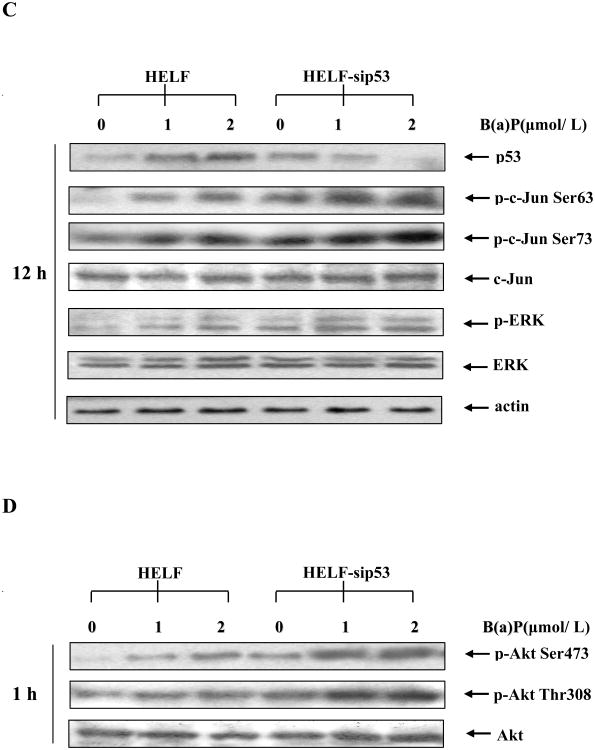
p53 mediated B(a)P-induced c-Jun phosphorylation through PI-3K/Akt/ERK pathway
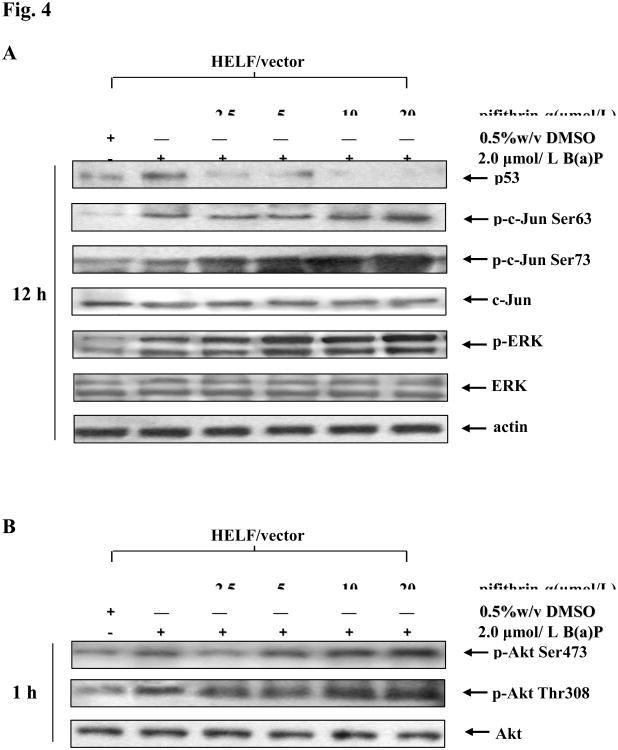
Previous studies have indicated that p53 protein functions as a down-regulator in AP-1 activity (Wang et al, 2005; Wu, 2004). Other studies have identified that p53 can suppress cell transformation caused by oncogene activation (Lin and Lowe, 2001). Together, these observations suggest that p53 might provide an inhibitory signaling for B(a)P-induced c-Jun activation. To test such a hypothesis, HELF cells was pretreated with pifithrin-α, a p53 inhibitor, for 30 min and then exposed to B(a)P Pretreatment of cells with pifithrin-α led to dramatic inhibition of B(a)P-induced p53 overexpression, whereas it markedly increased B(a)P-induced c-Jun phosphorylation (Fig. 4A). Because it has been reported recently that pifithrin-α is also able to inhibit the activity of cytochrome P450 1B1 (Sparfel et el, 2006), p53 siRNA was further used to address the causality between loss of p53 function and elevation of c-Jun activation. The results indicated that p53 siRNA can inhibit the expression of p53, which subsequently leads to the increase in phosphorylation of c-Jun induced by B(a)P (Fig. 4C). More importantly, knockdown of p53 expression in HELF cells by either its chemical inhibitor or specific siRNA caused the elevation of B(a)P-induced the phosphorylation of ERK (Fig. 4A and 4C) and Akt (Fig. 4B and 4D). Based on the above data, we anticipate that basal level of p53 expression might inhibit B(a)P-induced c-Jun activation by regulating the activity of Akt and ERK.
Fig. 4. p53 inhibition increase B(a)P-induced c-Jun phosphorylation through the activation of PI-3K/Akt/ERK pathway.
A, B, After pretreatment with the various concentrations of p53 inhibitor pifithrin-α for 30 min, HELF cells were incubated with or without 2 μmol/L B(a)P for 12 h (A) and 1 h (B). The cells were then extracted with SDS sample buffer and separated on polyacrylamide-SDS gels, and detected with specific antibodies as indicated. C, D, HELF and HELF-sip53 cells were stimulated with several concentrations of B(a)P for 12 h (C) or 1 h (D). The cells were then extracted and analyzed by Western blot with specific antibodies as indicated. The results were one representative data from three independent experiments.
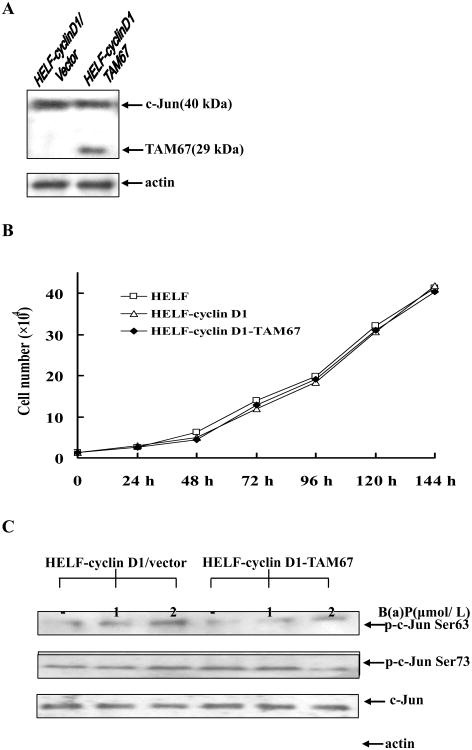
B(a)P-induced cell proliferation was inhibited in HELF by overexpression of TAM67
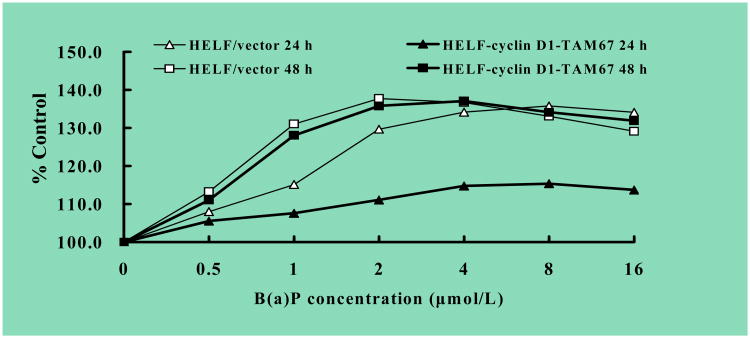
To test potential role of c-Jun phosphorylation in B(a)P-induced cell proliferation, we established a stably transfectant expressing dominant negative mutant of c-Jun (TAM67) in HELF (HELF-cyclin D1-TAM67). As shown in Fig.5A, Western blot showed a 29 kDa TAM67 protein expression as compared with vector control transfectant (Fig. 5A). TAM67 transfectant displayed growth rate identical to that for control cells (Fig. 5B). We further showed that overexpression of TAM67 prominently repressed phosphorylation of c-Jun at Ser63 and Ser73 as compared with that of control cells (Fig. 5C. To determine the effect of TAM67 on B(a)P-induced cell proliferation, HELF cells was treated with B(a)P at various concentrations of 0.5-16 μmol/L for 24 h and 48 h, and then MTT assay was performed. As shown in Fig. 6 that overexpression of TAM67 significantly hampered B(a)P-induced cell proliferation by >50% at 24 h after treatment, whereas it only showed a slight inhibition of B(a)P-induced cell proliferation at 48 h after B(a)P exposure. These results clearly indicate that c-Jun activation is only involved in the regulation of cell proliferation upon B(a)P exposure at early phase rather than laster phase.
Fig. 5. Characteristics of TAM67-transfected cells.
A, HELF cells were stably transfected with TAM67 as described in Materials and Methods, and extracts were subjected to immunoblotting with an antibody to the C terminus of c-Jun to detect TAM67 at 29 kDa (left). Endogenous c-Jun proteins appeared at the upper region of the gel at about 40 kDa B, TAM67 stable transfectant (HELF-cyclin D1-TAM67 cells), vector transfectant (HELF-cyclin D1 cells) and parent HELF cells were seeded in 6-well plates (1×104/well). Cell viability was measured at daily intervals by counting the number of trypan blue-excluding intact cells. Cell number was determined daily in triplicate for 6 d, and mean values were plotted. C, HELF-cyclin D1/vector or HELF-cyclin D1-TAM67 cells were stimulated with several concentrations of B(a)P for 12 h as indicated. The cells were then extracted and analyzed by Western blot with specific antibodies.
Fig. 6. B(a)P induced cell proliferation in HELF-cyclin D1/vector but not in TAM67-expressing cells.
HELF-cyclin D1/vector (open) and HELF-cyclin D1-TAM67 (filled) cells were seeded in 96-well plates (5 ×103/ well) at 37°C overnight, and then exposed to B(a)P at various concentrations of 0.5-16 μmol/L. Following treatment for 24 h (triangles) and 48 h (squares), MTT assay was performed as described in materials and methods respectively. All experiments were done in triplicate, and proliferation rate was expressed as a percentage of the control.
B(a)P-induced cell cycle alternations was reversed in HELF-cyclin D1-TAM67 cells
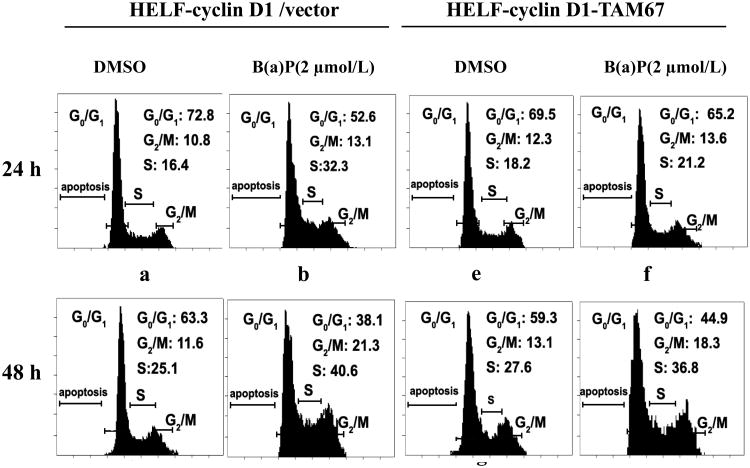
Numerous published reports have shown that c-Jun is required for progression through the G1 phase of the cell cycle in fibroblasts (Schreiber et al, 1999; Wisdom, et al, 1999). In view of these, together with recent finding that B(a)P-induced cell proliferation is accompanied by increased G1/S transition (Gao et al, 2006a; Jia et al, 2006), it was of interest to investigate whether TAM67 had a suppressive function on alternations of the cell cycle caused by B(a)P Thus, cell cycle distribution was determined in HELF-cyclin D1/vector cells and HELF-cyclin D1-TAM67 cells after a 24- and 48- h incubation of 2 μmol/L B(a)P Figure 7 showed exposure of cells to B(a)P evoked a dramatic alteration in cell cycle distribution with a decrease in the fraction of cells in G1 phase and a corresponding increase in the fraction of cells in S phase, the percentages in S phase were 30.4% and 41.5% after B(a)P treatment for 24 h and 48 h, respectively (panel b and d). In contrast, TAM67 overexpression dramatically reduced those alternations (panel f and h), which were consistent with cell proliferation assay. These data strongly indicate that c-Jun activation is essential for cell cycle alternations elicited by B(a)P.
Fig. 7. Overexpression of TAM67 abrogated B(a)P-induced cell cycle alternations.
HELF-cyclin D1/vector and TAM67-transfected cells were culture in 75-cm2 culture flasks with RPMI1640 containing 10% FBS at 37°C overnight. After being cultured in 0.5% FBS RMPI1640 for 24 h, cells were unstimulated or stimulated with 2 μmol/L B(a)P, as indicated, for 24 h and 48 h. Cells were sequentially fixed with 70% ice-cold ethanol overnight at 4°C, and then incubated with RNase A (1 mg/ml) for 30 min at room temperature. DNA was stained with propidium iodide (50 μg/mL) at 4°C for 1 h. Cell cycle distribution was determined by flow cytometry. The results were one representative data from three independent experiments.
B(a)P up-regulation of cyclin D1 pathway was blocked by overexpression of TAM67
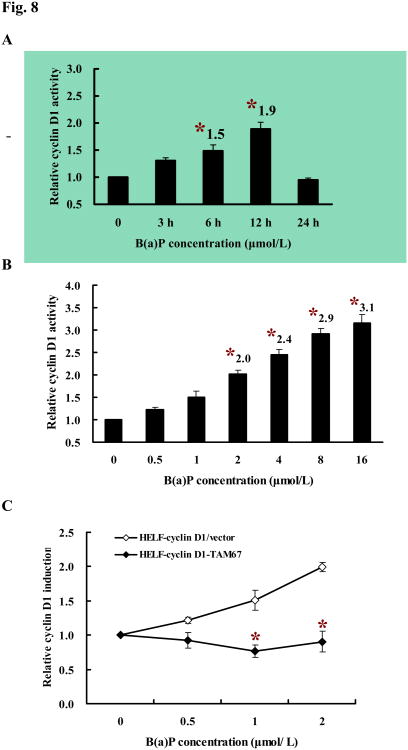
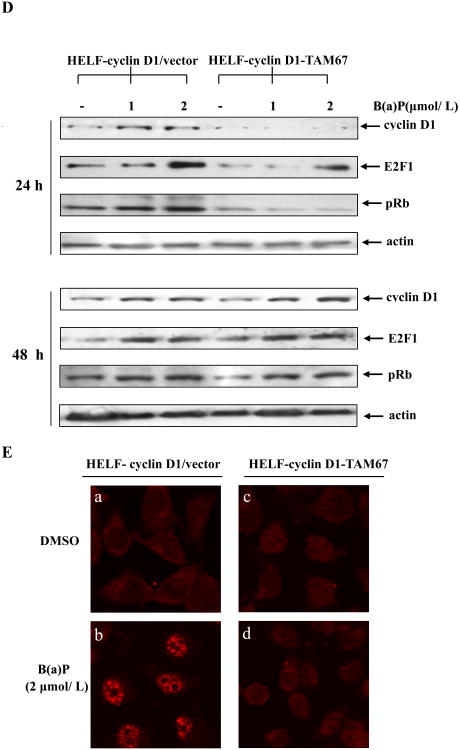
Cyclin D1 serves as a key sensor and integrator of extracellular signals of cells in early to mid-G1 phase, mediating its function through binding both the CDKs and other signaling molecules to modulate the genes that are involved in regulation of cell proliferation and differentiation (Neumeister et al, 2003; Wang et al, 2004). We have shown previously that B(a)P exposure induces cyclin D1 expression in human embryo lung fibroblasts (Du et al, 2006). Consistently, under the same condition, exposure of HELF-cyclin D1/vector cells to B(a)P obviously induced cyclin D1 transcription in a dose- (Fig. 8A) and time-dependent manners (Fig. 8B). c-Jun has been linked directly to transcriptional regulation of cyclin D1 in many experimental models (Hennigan and Stambrook, 2001; Wisdom et al, 1999). To determine whether c-Jun regulates cyclin D1 induction by B(a)P, reporter gene assay was performed to examine the induction of cyclin D1 transcription after B(a)P stimulation for 12 h. As indicated in Fig. 8C, B(a)P-induced cyclin D1 transcription was dramatically blocked in TAM67-transfected cells as compared to vector control transfectant. Western blot analysis also showed that overexpression of TAM67 significantly inhibited the cyclin D1 protein expression by B(a)P (Fig. 8D). Identical result was obtained from the immunofluorescence assay (Fig. 8E). Together, these findings mentioned above suggest that c-Jun activation is required for the induction of cyclin D1 by B(a)P.
Fig. 8. Requirement for c-Jun activation for up-regulation of G1regulators in HELF cells.
A, HELF-cyclin D1/vector cells (2×105) were seeded in each well of a 6-well plate. After being serum-starved for 24 h, the cells were exposed to 2 μmol/L B(a)P for the time points as indicated, The luciferase activity was then measured, and the results are presented as relative cyclin D1 induction. Columns, means of triplicate assay wells; bars, standard deviation. The asterisk indicates a significant increase from medium control (P < 0.05). B, HELF-cyclin D1/vector cells were treated with various concentrations of B(a)P as indicated for 12 hours. The luciferase activity was measured as described in Materials and Methods. C, HELF-cyclin D1/vector (open) and HELF-cyclin D1-TAM67 (filled) cells were subjected to reporter gene assay after treatment with the indicated concentrations of B(a)P for 12 h. All treatments were performed in triplicate and the results were expressed as cyclin D1 induction relative to control. The asterisk indicates a significant decrease from HELF-cyclin D1/vector cells (P < 0.05). D, HELF-cyclin D1/vector cells and HELF-cyclin D1-TAM67 cells were untreated or treated with the indicated concentrations of B(a)P for 24 h and 48 h, and then extracted and detected by Western blot with special antibodies as indicated. E, HELF-cyclin D1/vector (a and b) or HELF-cyclin D1-TAM67 cells (c and d) were subjected to incubate with special anti-cyclin D1 antibody and detect by immunofluorescence assay with TRITC-conjugated second antibody (red).
Cyclin D1 is the regulatory subunit of the cyclin D1-CDK4 complex that phosphorylates and inactivates the cell-cycle inhibiting function of the retinoblastoma protein (Rb) (Andrysík et al, 2007). Rb is thought to silence specific genes that are active in the S phase of the cell cycle through repression of E2F transcriptional activity (Stacey, 2003). Cyclin D1, pRb and E2F comprise a pivotal signaling pathway in medicating cell cycle from G1 to S. To further evaluate the role of c-Jun in the control of cell cycle regulators, phosphorylation of Rb and expression of E2F1 were analyzed by Western blot after B(a)P treatment. Figure 8D showed that B(a)P led to obvious increases in E2F1 expression and Rb phosphorylation at Ser780, and suppression of c-Jun phosphorylation by using TAM67 potently impaired B(a)P-induced overexpression of E2F1 and hyperphosphorylation of Rb. Taken together, the aforementioned observations provide compelling evidence that c-Jun participates in the modulation of G1 regulators - cyclin D1, pRb and E2F1, and in turn, medicates cell cycle alterations induced by B(a)P.
Discussion
Cell cycle regulation has been increasingly recognized as one of the important mechanisms required for the carcinogenic effect of B(a)P during the past several years, but the molecular basis of this is still obscure. Here, we focused on the role and related activating pathways of c-Jun in B(a)P-induced cell cycle alternations. Our current findings suggest that c-Jun is an important player for mediating B(a)P-induced cell cycle alternations through p53-dependent PI-3K/Akt/ERK signaling pathway. These data provide the evidence that activation of c-Jun is involved in B(a)P-induced cell cycle alternations, and reinforce the concept that signaling mechanisms for c-Jun activation are cell-type and inducer specific.
c-Jun, acting as immediate-early genes and early response proto-oncogenes, is activated by a broad range of physiological and pathological stimuli, including cytokines, growth factors, stress signals and infections, as well as toxic stimuli (Hoffer et al, 1996; Jochen et al, 2004). Aberrant expression and/or activation of c-Jun are often associated with many diseases, especially cancer (Jochum et al, 2001; Zhou and Thompson, 1996). In this study, exposure of HELF cells to B(a)P evoked c-Jun phosphorylation at Ser63 and Ser73, which is known to extend half-life of the protein and improve its transactivation potential (Weiss and Bohmann, 2004). Consistently, treatment of cells to benzo(a)pyrene-7,8-diol-9,10-epoxide (BPDE), ultimate metabolite of B(a)P, also led to marked increases in c-Jun phosphorylation (data not shown). Similarly, exposure of mouse epidermal Cl41 cells to BPDE induces significant increases in phosphorylation and expression of c-Jun (Li et al, 2004; Ding et al, 2006; Huang, et al, 2006). Additionally, it has been reported that aryl hydrocarbon receptor (AhR) could increase the mRNA and protein levels of c-Jun, stimulating AP-1 expression (Kizu et al. 2003). Induction of c-Jun depends on the medication of AhR through activation of p38 MAPK signaling pathway (Weiss et al. 2005). Therefore, B(a)P might enhance c-Jun activity by AhR-dependent mechanism, which is also involved in metabolic activation of B(a)P. Since B(a)P is identified as a complete carcinogen in animal carcinogenesis models, our results strongly suggest that c-Jun activation is one of the events necessary for the tumor promotion of B(a)P.
c-Jun, also referred to as downstream transcription factor, is regulated in a given cell by a various of signaling pathways including ras/ MAPK (Davis, 2000; Hibi et al, 2003), PI-3K/Akt (Shahabi et al. 2006), IKK/NF-κB (Fujioka et al, 2004) and p53/p21 (Huang et al, 2003). Here, we found that PI-3K/Akt has been implicated in c-Jun activation. Using dominant negative mutants of either PI-3K (Δp85) or Akt substantial inhibited c-Jun phosphorylation elicited by B(a)P This is consistent with our recently observation that PI-3K/Akt/p70S6k is involved in AP-1 transactivation by B(a)P (Gao et al, 2007). PI-3K signaling pathway also plays a crucial role during tumorgenesis. Compelling data have shown that PI-3K activation occurs in the development of many cancers (Phillip, 2006). Studies from our group have confirmed that PI-3K/Akt/p70S6k pathway medicates B(a)P-induced cell cycle alternations (Gao et al, 2007), clearly indicating that PI-3K signal pathway is associated with the carcinogenic effects of B(a)P.
The link between PI-3K and MAPK in response of cells to B(a)P has been suggested in many experimental models. Previous studies have demonstrated that PI-3K/Akt regulates B(a)PDE-induced AP-1 transactivation specifically through JNK-dependent pathway (Li et al, 2004; Huang et al. 2005). In the current work, we found that PI-3K/Akt pathway mediated c-Jun phosphorylation selectively through activation of ERK, but not JNK. It is well known that serum and growth factors activate c-Jun transcriptional activity mainly through ERK, whereas cytokines and genotoxic stress is mostly through activating JNK cascades (Reddy and Mossman, 2002). In light of our observation that B(a)P exposure induce cell proliferation, our results indicate that in this context, B(a)P might act as a proliferative signal, inducing c-Jun phosphorylation through the activation of ERK pathway. Nonetheless, the PI-3K-dependent activation of c-Jun by enhancing ERK activity, might be an incomplete picture of PI-3K interaction with MAPK. Analogous to the foregoing observations, one might postulate that PI-3K/Akt pathway phosphorylates and activates other member of MAPK, such as JNK and p38, defined by different cell type and/or extracellular stimuli. The correlation between PI-3K and MAPK is still under investigation in our laboratory.
Transcription factor p53 is one of the classic tumor suppressor genes that interfere with cancer development (Appella, 2001). It is known that p53 is the most commonly (>50%) mutated gene associated with human tumors (Reid et al, 2004). Previous findings show that p53 can even induce expression of AP-1 components such as Fos (Elkeles et al., 1999), suggesting that p53 activity can act upstream of AP-1 signaling. Similarly, our results from the pifithrin-α, to certain degree, suggests that p53 might provide an inhibitory signaling for B(a)P-induced c-Jun activation, although it has been shown that pifithrin-α not only has anti-p53 function (Komarova and Gudkov, 2000; Chramostova et al, 2004), but also in some cases has inhibitory effect towards cytochrome P450 1 isoforms (Solhaug et al, 2005; Sparfel et el, 2006). Furthermore, our data shown here indicate that inhibition of p53 by its siRNA induces the significant increases in the phosphorylation of c-Jun, Akt and ERK. Taken together with the evidence that and PI-3K and MAPK signaling pathways play critical roles in turmorgenesis (Dai et al, 2005; Hay, 2005; Johnson and Nakamura, 2007), these results further substantiate that inhibitory effect of p53 on c-Jun activation might be due to its specific inhibition of PI-3K/Akt/ERK pathway.
Considerable studies support a crucial role of c-Jun in cell cycle regulation (Eferl et al. 1999; Schreiber et al. 1999). In our previous studies, we found that exposure of cells to B(a)P induced cell cycle alternations accompanied with AP-1 transactivation (Gao et al. 2006b), suggesting that c-Jun might be involved in B(a)P-induced cell cycle alternations in AP-1-dependent manner. However, since c-Jun has been indicated to be involved in pro-survival or pro-apoptosis under different circumstances, and the function of c-Jun varies according to the cellular or microenvironment, in a certain context c-Jun might complement or oppose, even might act as a bystander with no direct effects on B(a)P-induced cell cycle alternations in HELF cells, the actual role of c-Jun in B(a)P-induced cell cycle alternations has not been ascertained. To answer this question, the cells stably expressing dominant negative mutant c-Jun (TAM67) were established, termed as HELF-cyclin D1-TAM67. Consistent with numerous observations in a wide variety of cell types, we found that TAM67 leads to a marked inhibition of AP-1 transactivation in B(a)P-stimulated cells (data not shown). The expression of TAM67 was well tolerated in untreated cells, and most of the cell lines generated displayed normal growth kinetics and essentially unaltered basal cyclin D1 transactivity. HELF-cyclin D1-TAM67 cell lines therefore provided a comparatively stable model system in which to investigate the role and regulatory mechanism of c-Jun in B(a)P-induced cell cycle alternations.
The present study indicated that exposure of HELF cells to 0.5∼2 μmol/L B(a)P evoked the increase in the percentage of cells in S phase, and induced cell proliferation. Inconsistent with this notion, 1 mmol/L B(a)P is able to induce S-phase delay in HELF cells, which is related to formation of DNA adducts (Binkova et al, 2000). Additionally, it has been reported that 2 μmol/L B(a)P induces G1 arrest in Swiss 3T3 cells (Khan and Dipple, 2000), whereas the same concentration elicits significant apoptosis in human carcinoma cells (Yoshii et al. 2001). These apparent contradictions might be primarily due to different cell types and/or stimuli concentration. Anyway, in this context, B(a)P induced an increase in G1-S transition. Overexpression of TAM67 was able to significantly block G1-S transition after treatment with B(a)P for 24 h. However, a slight alternation was observed at 48 h in TAM67-expressing cells after B(a)P treatment. Consistent with this result, TAM67 was able to inhibit cell proliferation when TAM67-transfected cells were incubated with B(a)P for 24 hours but only showing a little effect at 48 hours after B(a)P exposure. Moreover, similar results were obtained when the effect of TAM67 on cell cycle regulators, cyclin D1, E2F1 and Rb, was analyzed by Western blot. These observations suggest that the effect of TAM67 might be adapted by HELF-cyclin D1-TAM67 cells after long-term of B(a)P exposure, or there might be some other pathways involved in cell cycle alternations stimulated by B(a)P.
It is noteworthy that numerous genes coding for cell cycle machinery (cyclin, CDKs, etc) contain putative c-Jun/AP-1 binding sites in their promoters (Wisdom et al, 1999). cyclin D1 is a prototype of such a gene that is implicated in G1 progression (Fu et al, 2004). Thus, in this experiment using the cyclin D1 luciferase reporter, transactivation of cyclin D1 was stimulated after exposure HELF-cyclin D1 cells to B(a)P for 12 h. In contrast, in HELF-cyclin D1-TAM67 cells, the effect of B(a)P on cyclin D1 was completely blocked after treatment with B(a)P for 12 h. The role of TAM67 was further checked by applying western blot and immunofluorescence assay. Paralleled the aforementioned results of cell cycle, B(a)P-induced cyclin D1 overexpression was recovered in HELF-cyclin D1-TAM67 cells exposed to B(a)P for 48 h. TAM67 also showed similar inhibitory effects on hyperphosphorylaton of pRb and overexpression of E2F1 induced by B(a)P. These findings clearly show that c-Jun is essential for the activation of regulators of G1 phase, including cyclin D1, E2F1 and pRb, and consequently promote G1-S transition.
In summary, we presented previously data supporting the hypothesis that PI-3K signaling pathway was necessary for AP-1 transactivation and cell cycle alternations induced by B(a)P (Gao et al, 2007). We now extend this hypothesis to suggest that, collectively, c-Jun activation by p53-dependent PI-3K/Akt/ERK pathway contributes to B(a)P-caused increased G1-S transition via cell cycle regulators such as cyclin D1, E2F1, and pRb in HELF cells. In light of the key role of alterations of cell cycle machinery in tumorgenesis, these findings will help us to understand the signal transduction mechanisms involved in the carcinogenic effects of B(a)P.
Acknowledgments
Grant information: This work was supported by grants of National Natural Science Foundation of China (30671747, 30371206), 973 National Key Basic Research and Development Program (2002 CB 512905), and supported in part by Philip Morris USA Inc, Philip Morris International and NIH/NIEHS (ES012451).
Abbreviations
- B(a)P
Benzo(a)pyrene
- DMSO
dimethyl sulfoxide
- HELF
human embryo lung fibroblast
- HELF-AP-1/vector
human embryo lung fibroblast transfected with AP-1 luciferase reporter plasmid
- HELF-AP-1-DN-Akt
human embryo lung fibroblast cotransfected with AP-1 luciferase reporter plasmid and dominant negative mutant plasmid of Akt
- HELF-AP-1-DN-Δp85
human embryo lung fibroblast stably cotransfected with AP-1 luciferase reporter plasmid and dominant negative mutant plasmid of PI-3K (Δp85)
- HELF-cyclin D1/vector
human embryo lung fibroblast stably transfected with cyclin D1 luciferase reporter plasmid
- HELF-cyclin D1-TAM67
human embryo lung fibroblast stably cotransfected with cyclin D1 luciferase reporter plasmid and dominant negative mutant plasmid of c-Jun (TAM67)
- HELF-sip53 cells
human embryo lung fibroblast stably transfected with p53 siRNA
- p-Akt
phosphorylation of Akt
- p-c-Jun
phosphorylation of c-Jun
- p-ERK
phosphorylation of ERK
- p-JNK
phosphorylation of JNK
- pRb
phosphorylation of retinoblastoma protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrysík Z, Vondrácek J, Machala M, Krcmár P, Svihálková-Sindlerová L, Kranz A, Weiss C, Faust D, Kozubík A, Dietrich C. The aryl hydrocarbon receptor-dependent deregulation of cell cycle control induced by polycyclic aromatic hydrocarbons in rat liver epithelial cells. Mutat Res. 2007;615:87–97. doi: 10.1016/j.mrfmmm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M. Cell cycle-dependent variations in c-Jun and Jun B phosphorylation: a role in the control of cyclin D1 expression. EMBO J. 2000;19:2056–5068. doi: 10.1093/emboj/19.9.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvis M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, David JP, Möhle-Steinlein U, Tronche F, Schetüz G, Wagner EF. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002;21:1782–1790. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chramostova K, Vondracek J, Sindlerova L, Vojtesek B, Kozubik A, Machala M. Polycyclic aromatic hydrocarbons modulate cell proliferation in rat hepatic epithelial stem-like WB-F344 cells. Toxicol Appl Pharmacol. 2004;196:136–148. doi: 10.1016/j.taap.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK Group of MAP kinases. Cell 2000. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Ding J, Li J, Chen J, Chen H, Ouyang W, Zhang R, Xue C, Zhang D, Amin S, Desai D, Huang C. Effects of polycyclic aromatic hydrocarbons (PAHs) on vascular endothelial growth factor induction through phosphatidylinositol 3-kinase/AP-1-dependent, HIF-1alpha-independent pathway. J Biol Chem. 2006;281:9093–9100. doi: 10.1074/jbc.M510537200. [DOI] [PubMed] [Google Scholar]
- Du H, Tang N, Liu B, You B, Shen F, Ye M, Gao A, Huang C. Benzo(a)pyrene-induced cell cycle progression is through ERKs/cyclin D1 pathway and requires the activation of JNKs and p38 mapk in human diploid lung fibroblasts. Mol Cell Biochem. 2006;287:78–89. doi: 10.1007/s11010-005-9073-7. [DOI] [PubMed] [Google Scholar]
- Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development, c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-κB and AP-1 connection: Mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Gao A, Liu B, Shi X, Huang C, Jia X, You B, Ye M, Shen F, Du H. Vitamin C inhibits benzo(a)pyrene-induced cell cycle changes partly via cyclin D1/E2F pathway in human embryo lung fibroblasts. Biomed Environ Science. 2006a;19:239–244. [PubMed] [Google Scholar]
- Gao A, Liu B, Huang C, Shi X, Jia X, You B, Ye M. ERK and JNK/AP-1 pathways involved in benzo(a)pyrene induced cell cycle changes in human embryo lung fibroblasts. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006b;24:72–76. [PubMed] [Google Scholar]
- Gao A, Liu B, Shi X, Jia X, Ye M, Jiao S, You B, Huang C. Phosphatidylinositol-3 kinase/Akt/p70S6K/AP-1 signaling pathway mediated benzo(a)pyrene-induced cell cycle alternation via cell cycle regulatory proteins in human embryo lung fibroblasts. Toxicol Lett. 2007;170:30–41. doi: 10.1016/j.toxlet.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch. Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- Hennigan RF, Stambrook PJ. Dominant negative c-jun inhibits activation of the cyclin D1 and cyclin E kinase complexes. Mol Biol Cell. 2001;12:2352–2363. doi: 10.1091/mbc.12.8.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Science. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Hibi M, Davis RJ, McLaren A, Cohen P. A reinvestigation of the multisite phosphorylation of the transduction fator c-Jun. EMBO J. 2003;22:3875–3886. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer A, Chang CY, Puga A. Dioxin induces transcription of fos and jun genes by Ah receptor-dependent and -independent pathways. Toxicol Appl Pharmacol. 1996;141:238–247. doi: 10.1006/taap.1996.0280. [DOI] [PubMed] [Google Scholar]
- Hornberg C, Maciuleviciute L, Seemayer NH. Sister chromatid exchanges in rodent tracheal epithelium exposed in vitro to environmental pollutants. Toxicol Lett. 1996;88:45–53. doi: 10.1016/0378-4274(96)03717-4. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Song L, Zhang D, Tong Q, Ding M, Bowman L, Aziz R, Stoner GD. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res Jan. 2006;66:581–587. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P cells. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma WY, Dong Z. The extracellular-signal-regulated protein kinases (ERKs) are required for UV-induced AP-1 activation in JB6 cells. Oncogene. 1999;18:2828–2835. doi: 10.1038/sj.onc.1202639. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Young MR, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci USA. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Shu L, 1 Dilling MB, Easton J, Harwood FC, Ichijo H, Houghton PJ. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21Cip1. Mol Cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Hosokawa Y, Yoshimoto K, Schipani E, Mallya S, Papanikolaou A, Kifor O, Tokura T, Sablosky M, Ledgard F, Gronowicz G, Wang TC, Schmidt EV, Hall C, Brown EM, Bronson R, Arnold A. Primary hyperparathyroidism caused by parathyroid-targeted overexpression of cyclin D1 in transgenic mice. J Clin Invest. 2001;107:1093–1102. doi: 10.1172/JCI10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffy BD, Chirnomas RB, Chen EJ, Gudas JM, Romagnolo DF. Activation of the aromatic hydrocarbon receptor pathway is not sufficient for transcriptional repression of brca-1: requirements for metabolism of Benzo[a]pyrene to 7r,8t-dihydroxy-9t,10- epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Cancer Res. 2002;62:113–121. [PubMed] [Google Scholar]
- Jia X, Liu B, Shi X, Gao A, You B, Ye M, Jiao S, Huang C. Inhibition of Benzo(a)pyrene-induced cell cycle progression by all-trans retinoic acid partly through cyclin D1/E2F-1 pathway in human embryo lung fibroblasts. Cell Biology International. 30:183–189. doi: 10.1016/j.cellbi.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jiao S, Liu B, Shi X, Huang C, Gao A, Ye M, Shen F, Du H. JNK and ERK signaling pathways regulate Benzo(a)pyrene-induced c-Jun activation in human embryo lung fibroblasts. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2006;25:385–388. [PubMed] [Google Scholar]
- Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Nakamura K. The c-Jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- Khan QA, Dipple A. Diverse chemical carcinogens fail to induce G1 arrest in MCF-7 cells. Carcinogenesis. 2000;21:1611–1618. [PubMed] [Google Scholar]
- Lin AW, Lowe SW. Oncogenic ras /activates the ARFp53 pathway to suppress epithelial cell transformation. Proc Natl Acad Sci USA. 2001;98:5025–5030. doi: 10.1073/pnas.091100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, Burnstein KL, Hayakawa K. A role of aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Arch Toxicol. 2003;77:335–343. doi: 10.1007/s00204-003-0454-y. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Gudkov AV. Suppression of p53: a new approach to overcome side effects of antitumor therapy. Biochemistry (Mosc) 2000;65:41–48. [PubMed] [Google Scholar]
- Li J, Chen H, Ke Q, Feng Z, Tang MS, Liu B, Amin S, Costa M, Huang C. Differential effects of polycyclic aromatic hydrocarbons on transactivation of AP-1 and NF-kappaB in mouse epidermal cl41 cells. Mol Carcinog. 2004;40:104–115. doi: 10.1002/mc.20020. [DOI] [PubMed] [Google Scholar]
- Maeno K, Melendez-Colon VJ, Luch A, Seidel A, Baird WM. Cancer initiation by polycyclic aromatic hydrocarbons results from formation of stable DNA adducts rather than apurinic sites. Carcinogenesis. 1999;20:1885–1891. doi: 10.1093/carcin/20.10.1885. [DOI] [PubMed] [Google Scholar]
- Morton S, Davis RJ, McLaren A, Cohen P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 2003;22:3876–3886. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER, Pestell RG. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olga P, Subarna B, Dan M, Holbrook NJ. Protective role for c-Jun in the cellular response to DNA damage. j biol chem. 2001;276:28546–28553. doi: 10.1074/jbc.M102075200. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Ma Q, Li J, Zhang D, Liu Z, Rustgi AK, Huang C. Cyclin D1 induction through IKB kinase B/nuclear factor-κB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res. 2005;65:9287–9293. doi: 10.1158/0008-5472.CAN-05-0469. [DOI] [PubMed] [Google Scholar]
- Reddy SPM, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung Am. J Physiol Lung Cell Mol Physiol. 2002;283:L1161–L1178. doi: 10.1152/ajplung.00140.2002. [DOI] [PubMed] [Google Scholar]
- Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspecaive with updates. Carcinogenesis. 2001;22:1903–1930. doi: 10.1093/carcin/22.12.1903. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Kolbus A, Piu F, Szabowski A, Möhle-Steinlein U, Tian J, Karin M, Angel P, Wagner EF. Control of cell cycle progression by c-Jun is p53 dependent. Genes & Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahabi NA, McAllen K, Sharp BM. δ opioid receptors stimulate Akt-dependent phosphorylation of c-jun in T cells. J Pharmacol Exp Ther. 2006;316:933–939. doi: 10.1124/jpet.105.091447. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: Cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller Lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- Solhaug A, Ovrebo S, Mollerup S, Lag M, Schwarze PE, Nesnow S, Holme JA. Role of cell signaling in B[a]P-induced apoptosis: characterization of unspecific effects of cell signaling inhibitors and apoptotic effects of B[a]P metabolites. Chem Biol Interact. 2005;151:101–119. doi: 10.1016/j.cbi.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sparfel L, Van Grevenynghe J, Le Vee M, Aninat C, Fardel O. Potent inhibition of carcinogen-bioactivating cytochrome P450 1B1 by the p53 inhibitor pifithrin alpha. Carcinogenesis. 2006;27:656–663. doi: 10.1093/carcin/bgi256. [DOI] [PubMed] [Google Scholar]
- Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15:158–163. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Ethier SP, Burchiel SW. Carcinogenic polycyclic aromatic hydrocarbons increase intracellular Ca2+ in primary human mammary epithelial cells. Carcinogenesis. 1997;18:1177–1182. doi: 10.1093/carcin/18.6.1177. [DOI] [PubMed] [Google Scholar]
- Tannheimer SL, Ethier SP, Caldwell KK, Burchiel SW. Polycyclic aromatic hydrocarbon-induced alterations in tyrosine phosphorylation and insulin-like growth factor signaling pathways in the MCF-10A human mammary epithelial cell line. Carcinogenesis. 1998;19:1291–1297. doi: 10.1093/carcin/19.7.1291. [DOI] [PubMed] [Google Scholar]
- Wang C, Li Z, Fu M, Bouras T, Pestell RG. Signal transduction mediated by cyclin D1: from mitogens to cell proliferation: a molecular target with therapeutic potential. Cancer Treat Res. 2004;119:217–237. doi: 10.1007/1-4020-7847-1_11. [DOI] [PubMed] [Google Scholar]
- Wang C, Pattabiraman N, Zhou JN, Fu M, Sakamaki T, Albanese C, Li Z, Wu K, Hulit J, Neumeister P, Novikoff PM, Brownlee M, Scherer PE, Jones JG, Whitney KD, Donehower LA, Harris EL, Rohan T, Johns DC, Pestell RG. Cyclin D1 repression of peroxisome proliferator-activated receptor expression and transactivation. Mol Cell Biol. 2003;23:6159–6173. doi: 10.1128/MCB.23.17.6159-6173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang Z, Tong Q, He J, Huang C. Loss of tumor suppressor p53 decreases PTEN expression and enhances signaling pathways leading to activation of activator protein 1 and nuclear factor κB induced by UV radiation. Cancer Res. 2005;65:6601–6611. doi: 10.1158/0008-5472.CAN-04-4184. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bohmann D. Deregulated repression of c-Jun provides a potential link to its role in tumorigenesis. Cell cycle. 2004;2:111–113. [PubMed] [Google Scholar]
- Weiss C, Faust D, Du H, Kolluri SK, Pelzer A, Schneider S, Dietrich C, Oesch F, Göttlicher M. TCDD induces c-jun expression via a novel Ah (dioxin) receptor-mediated p38-MAPK-dependent pathway. Oncogene. 2005;24:4975–4983. doi: 10.1038/sj.onc.1208679. [DOI] [PubMed] [Google Scholar]
- Wisdom R, Johnson RS, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther. 2004:3156–3161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- Yoshii S, Tanaka M, Otsuk Y, Fujiyama T, Kataoka H, Arai H, Hanai H, Sugimura H. Involvement of alpha-PAK-interacting exchange factor in the PAK1–c-Jun NH2-terminal kinase 1 activation and apoptosis induced by Benzo[a]pyrene. Mol Cell Biol. 2001;21:6769–6807. doi: 10.1128/MCB.21.20.6796-6807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]