Abstract
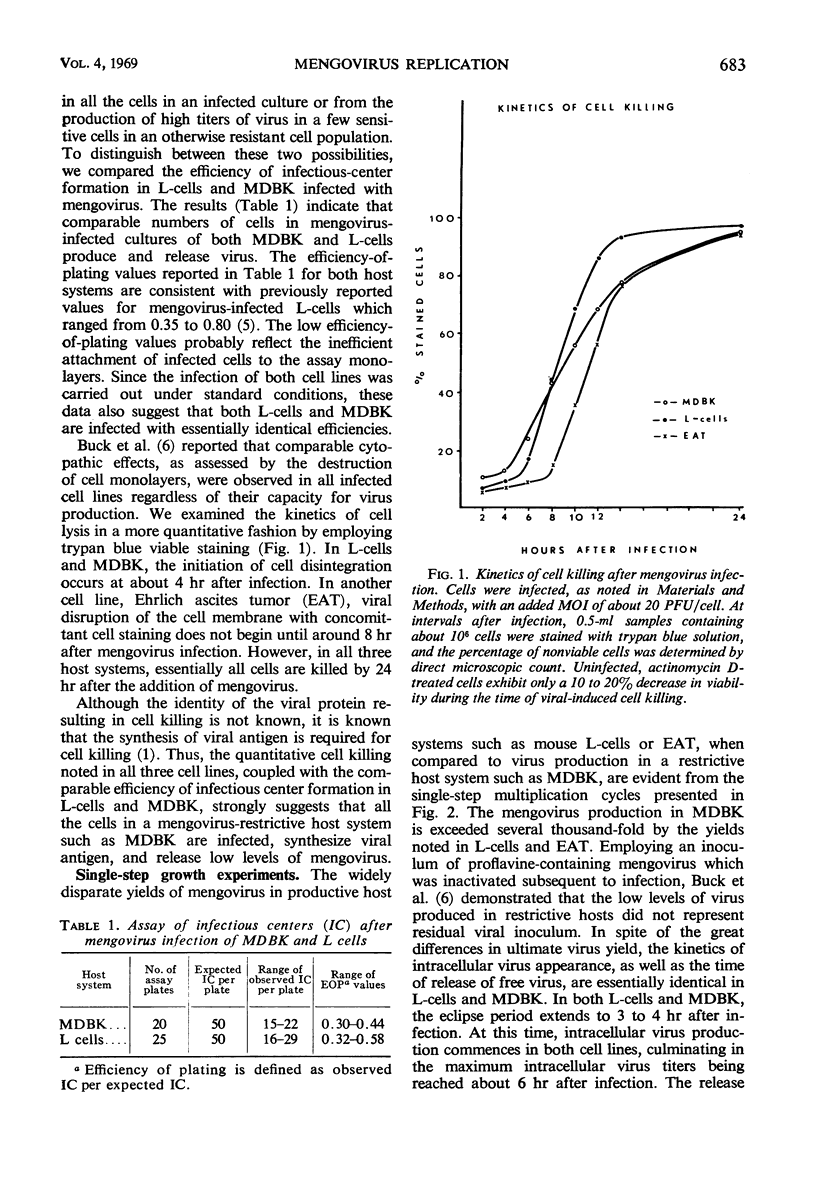
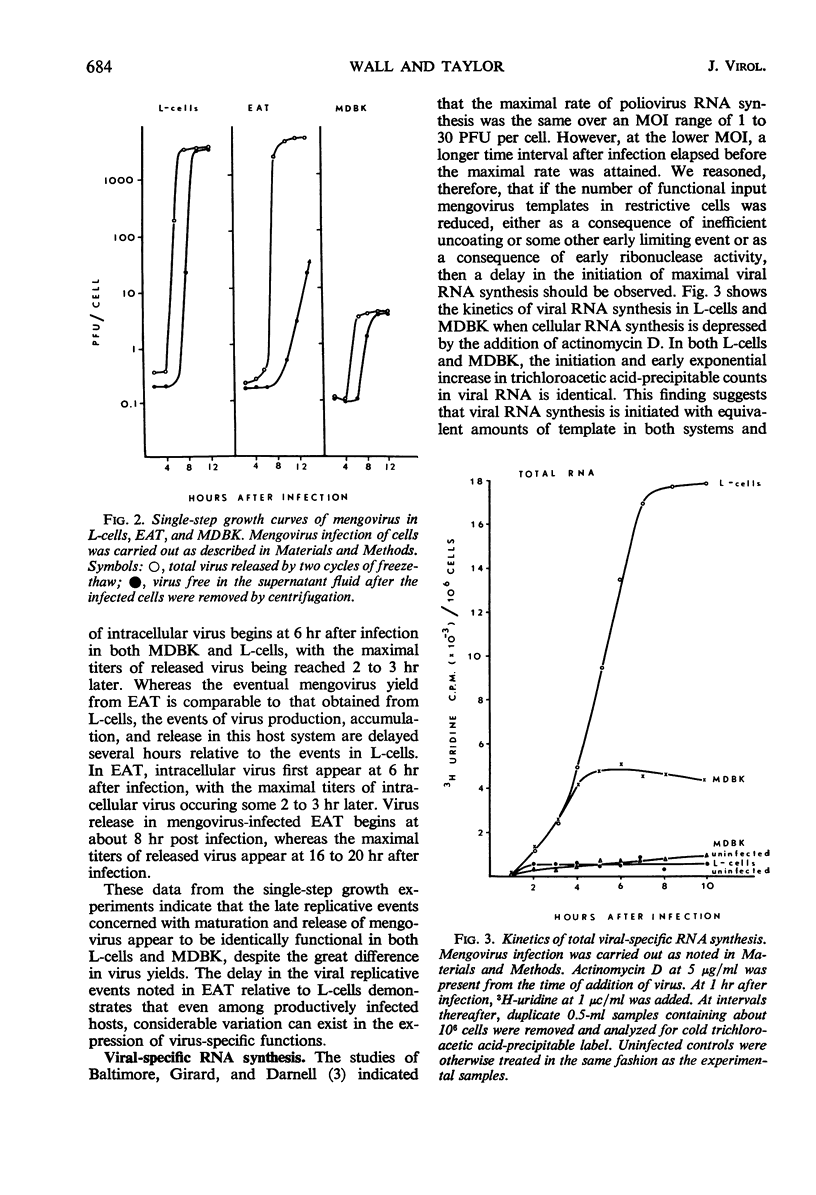
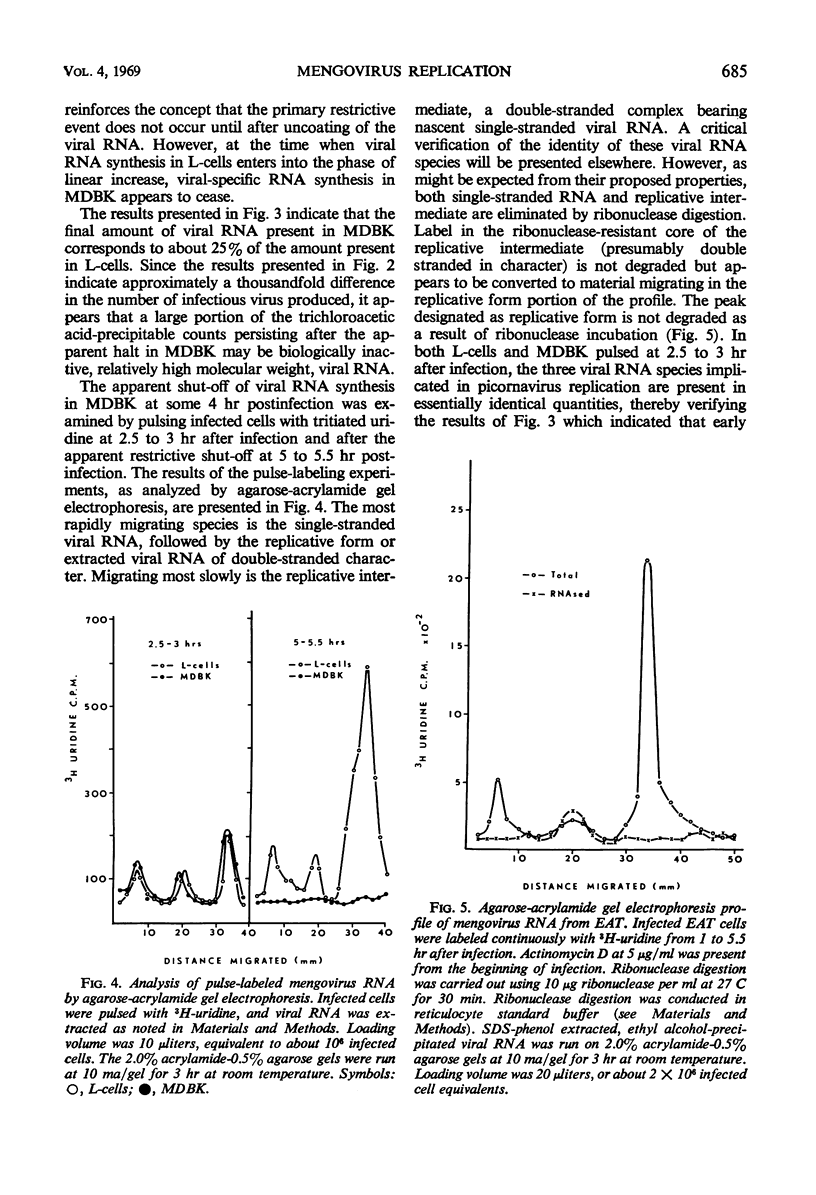
Mengovirus infection of a restrictive cell line, Maden's bovine kidney (MDBK), results in a virus yield 1,000-fold less than that obtained from productively infected cell lines such as L cells or Ehrlich ascites tumor cells (EAT). Cells of both types of host systems are infected with comparable efficiencies and are completely killed as a consequence of infection. Infective center assays, coupled with the observation of total cell killing, suggest that comparable numbers of cells synthesize viral antigen and release virus in both types of host system. Viral-specific ribonucleic acid (RNA) synthesis is initiated and proceeds in an identical fashion for approximately 4 hr after the infection of MDBK, EAT, or L-cells. At this time, viral RNA synthesis in MDBK ceases, whereas viral RNA synthesis in EAT and L-cells continues at a linear rate. These results indicate that none of the early viral events leading to the initiation of viral-specific RNA synthesis constitutes the primary site of mengovirus restriction in MDBK. Rather it appears that the cessation of viral RNA synthesis in restrictive cells constitutes the primary limiting event. Based on its delayed interaction with mengovirus RNA synthesis, it appears that the host-related restrictive agent is initially compartmentalized and then released as a consequence of infection subsequent to those early events in mengovirus infection leading to the initiation and continued synthesis of viral RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Granger G. A., Taylor M. W., Holland J. J. Efficient, inefficient, and abortive infection of different mammalian cells by small RNA viruses. Virology. 1967 Sep;33(1):36–46. doi: 10.1016/0042-6822(67)90091-8. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- GINZBURG-TIETZ Y., KAUFMANN E., TRAUB A. STUDIES ON NUCLEAR RIBOSOMES. II. TRANSFER OF RIBOSOMES FROM NUCLEUS TO CYTOPLASM IN THE EARLY STAGES OF VIRAL INFECTION. Exp Cell Res. 1964 Apr;34:384–395. doi: 10.1016/0014-4827(64)90373-8. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- Holland J. J. Virus-directed protein synthesis in different animal and human cells. Science. 1968 Jun 21;160(3834):1346–1348. doi: 10.1126/science.160.3834.1346. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloviev V. D., Krispin T. I., Zaslavsky V. G., Agol V. I. Mechanism of resistance to enteroviruses of some primate cells in tissue culture. J Virol. 1968 Jun;2(6):553–557. doi: 10.1128/jvi.2.6.553-557.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub A., Teitz Y., Kaufmann E. A temporal and structural relationship between viral RNA polymerase and nicotinamide adenine dinucleotide pyrophosphorylase in the cytoplasm of Krebs-2 ascites cells infected with encephalomyocarditis virus. J Mol Biol. 1968 Sep 28;36(3):371–385. doi: 10.1016/0022-2836(68)90162-9. [DOI] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]



