Abstract
RNF115, or Breast Cancer-Associated Gene 2 (BCA2), encodes a RING-finger ubiquitin E3 ligase, expression of which was associated with estrogen receptor (ER)-positive status in human breast tumors. Although the BCA2 promoter contains several estrogen response element (ERE) half-sites, the role of ER in the regulation of BCA2 transcription has not been reported. The aim of this study is to investigate the molecular mechanism by which estrogen regulates BCA2 transcription. BCA2 mRNA and protein levels were examined by RT-PCR and Western blot analysis, respectively, and localization was assessed by immunofluorescence. BCA2 promoter activity in response to E2 was tested by a dual luciferase reporter assay and ER binding to the BCA2 promoter was examined by chromatin immunoprecipitation assay. We found that BCA2 mRNA and protein levels are regulated by estrogen in ER-positive MCF7 breast cancer cells and MDA MB 231 cells stably transfected with ER. Estrogen treatment in hormonal depleted MCF7 and MDA MB 231/ER stably transfected cells resulted in increased nuclear ER and cytoplasmic and nuclear BCA2 staining. Cycloheximide is not able to inhibit BCA2 mRNA levels, suggesting potential BCA2 regulation at the transcriptional level. Anti-estrogens like tamoxifen and ICI 182 178 counteracted E2-induced BCA2 protein and knockdown of ER by ER siRNA resulted in a significant decrease in BCA2 protein and a lower nuclear expression pattern. Estrogen treatment lead to a significant increase in BCA2 promoter response, associated with increased binding of ER to the ERE region of the BCA2 promoter. BCA2 is therefore a newly identified transcriptional target of estrogen receptor.
Keywords: Breast cancer, Estrogen receptor (ER), Breast cancer-associated gene 2 (BCA2), Estrogen (E2), Estrogen response element (ERE), Tamoxifen and ICI 182 178 (ICI)
Introduction
Breast cancer is the second leading cause of cancer-related death in women, comprising about 23 % of all cancers diagnosed worldwide [1]. It has been postulated that long-term exposure to hormones, such as estrogen, contributes to breast cancer development and progression; therefore, understanding the involved molecular mechanism is currently a very attractive area of research. Estrogen hormones play a vital role in the development of both normal and malignant human breast epithelia with estrogen receptor alpha (ERα) being expressed in ~70 % of all breast cancers. ER functions as a major regulator of phenotypic properties in estrogen-responsive tumors by controlling transcription of different genes thereby influencing cell proliferation [2–4]. For example, expression of c-Myc [5, 6] and cyclin D [7], two target proteins of estrogen, are sufficient to induce cell cycle progression and cell division [8]. Antiapoptotic genes like EIT-6 and TIT-5 are also direct targets of ligand-mediated ER activity and were shown to promote colony growth in human breast cancer cells [9]. The sequence of events of ER transcriptional activity can be mapped out as follows: ER, a ligand inducible transcription factor, upon binding with 17β-estradiol (E2) changes its conformation, causing dimerization, and subsequently the ER dimer recognizes its cognate regulatory binding sites in the promoter and/or enhancer regions of target genes [10–12]. Recruitment of ER transcription factor to its response element enables enlisting of co-regulatory proteins, RNA polymerase II, and the basal transcription machinery, leading to the expression of a specific target gene in a single tissue type [13]. ER regulation of target gene expression is complex and involves different mechanisms, including both classical [14] and non-classical pathways [15]. Researchers have recently identified genome wide DNA binding regions of ER by using methods like chromatin immunoprecipitation (ChIP), microarray, and cloning–sequencing strategies [16]. These studies have provided much insight into the mechanism of action of ER and its role in estrogen-related diseases, including breast cancer.
We have previously described Breast Cancer-Associated Gene 2 (BCA2) as a RING-finger ubiquitin E3 ligase, identified from the invasive breast cancer cell line Hs578t by subtractive hybridization and differential display methods and the full length gene was isolated from MDA MB 468 cells [17, 18]. While the BCA2 gene was also identified in ER-negative cells, a large tumor tissue microarray study conducted to examine BCA2 expression in 945 invasive breast cancers indicated that BCA2 expression correlated with clinical variables such as lymph node status, regional recurrence and ER expression pattern [17, 18]. These findings lead us to examine the role of ER in BCA2 expression in the current study.
BCA2 is distributed in the cytoplasm and nucleus and its nuclear presence correlates with ER-positive status (p < 0.004) [17]. In silico analysis of the BCA2 gene using the TRANSFAC transcription factor binding site database revealed a canonical CAAT box (–737 to –734), reverse TATA box (–656 to –653), and several putative transcription factor binding sites in the promoter region. These include some general transcription factors and nuclear hormone receptors like the ER-binding estrogen response element (ERE) half-sites [17] at positions –129, –493, –1001, and –2407 base pairs upstream from the transcription start site. While BCA2 and ER were found to co-localize in the nucleus, whether or not, and how, ER regulates BCA2 expression and/or activity remains unknown.
To understand the mechanistic basis underlying ER and BCA2 association, in this study we have explored the possibility whether the BCA2 gene can be regulated by ER. Our results show that BCA2 is a transcriptional target of the estrogen-occupied ER. We found that estrogen treatment increased BCA2 mRNA and protein levels, which were inhibited by the ER antagonists tamoxifen and ICI 182 178 (ICI) in ER-positive MCF7 breast cancer cells and ER-negative MDA MB 231 stably transfected with ER. Based on these results, we hypothesized that stimulation of ER by E2 activates the BCA2 promoter via its ERE half-sites. To test this hypothesis, we transiently transfected HEK 293T, MCF7, and MDA MB 231 (parental and ER) cells with a reporter plasmid containing a 1 kb insert of the proximal promoter region of the human BCA2 gene promoter, inclusive of several ERE half-sites, and monitored the luciferase signal levels. Our data show that BCA2 promoter activity increases upon E2 induction, inhibitable by tamoxifen and ICI. Furthermore, ChIP results using MCF7 cells demonstrated an increase in ER binding to the BCA2 promoter upon E2 induction, again inhibitable by tamoxifen or ICI. Thus our results, for the first time, demonstrate that BCA2 is transcriptionally regulated by ER, at least in ER-positive MCF7 cells.
Materials and methods
Cell lines, culture medium and chemicals
MCF7, MDA MB 231, and HEK 293T cells were obtained from the American Type Culture Collection (Manassas, VA). The phenol red-free RPMI from Invitrogen, ICI 182 178 from Tocris, and tamoxifen and 17-β estradiol were obtained from Sigma-Aldrich (St. Louis, MO).
RNA extraction and RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN) and was reverse transcribed to complementary DNA (Two Step DNA kit, Invitrogen). The cDNA was amplified using primer pairs for BCA2 forward 5′-GGGGTCACCAGACTCACACT-3′ and reverse 3′-CAGGAAAAAGGGTGTGGAGA-5′ and for pS2 forward 5′-TTCTATCCTAATACCATCGACG-3′ and reverse 3′-TTTGAGTAGTCAAAGTCAGAGC-5′. The loading control HPRT primers: forward 5′-TGACACTGGCAAAACAATGCA-3′ and reverse 3′-GGTCCTTTTCACCAGCAAGCT-5′ were used.
Plasmid constructs and transient transfections
The full length human ERα (pCMV3) expression plasmid was generously provided by Dr. David Shapiro and all the plasmids were transfected using FuGENE transfection reagent. Custom made ER small interfering RNA (siRNA) was obtained from QIAGEN (sense strand 5′-GACUUGAAUUAAUAAGUGATT-3′ and antisense 5′-ACUUAUUAAUUCAAGUCTC-3′). The siRNA was transfected using the RNAifect Transfection Kit (QIAGEN). AllStars negative control siRNA from QIAGEN was used as a control for transfection which shows that the changes in phenotype or gene expressions are nonspecific.
Western blot assay
Exponentially growing cells were cultured in hormone-depleted media, and total protein was extracted using RIPA buffer, and Western blotting was performed as previously described [18].
BCA2 promoter luciferase reporter Assay
Cells were plated in 24-well plates and transfected with BCA2 promoter–luciferase vector (Switch Gear Genomics) and renilla vector (Promega). After 24 h, cells were treated with 10 nM E2, 100 nM ICI and/or 100 nM Tamoxifen. Luciferase activity was measured using the Dual Luciferase Reporter Assay Kit (Promega) and promoter activity was calculated as relative luciferase units (RLU).
Immunocytochemistry
Cells were treated with 10 nM E2 for 24 h and ER-specific siRNA for 72 h. Cells were fixed with methanol–acetone and then a primary BCA2 antibody, and a Cy3-conjugated anti-rabbit IgG secondary antibody were used for staining. A pre-diluted anti-mouse ER antibody (Invitrogen) was used as the primary and FITC-conjugated anti-mouse IgG as the secondary antibody.
Chromatin immunoprecipitation assay
MCF7 cells were treated with 10 nM estradiol for 12 h. Chromatin immunoprecipitation (ChIP) was performed using a ChIP assay kit (Upstate, Charlottesville, VA) and a ChIP-specific ER antibody (Sc-543, Santa Cruz). Normal rabbit IgG (5μg, Santa Cruz) was used as a control. The EpiTect ChIP qPCR primer assay kit for human RNF115 (NM_014455.2(−) 03Kb: GPH1000795(−)03A was obtained from SABio-sciences. These ChIP-qPCR assays are pre-designed and the RT-PCR assays optimized to measure genomic DNA promoter sequence enrichment within ChIP samples. The cycles used were 95 °C for 10 min, 40 cycles (95 °C, 15 s, 60 °C, 60 s). Normalization was done using the percent input method by using 1 % of the starting chromatin as input. A dilution factor of 100 or 6.644 cycles (log2 of 100) is subtracted from the Ct value of the diluted input. All ChIP experiments were run in triplicates and standard errors were calculated.
Results
BCA2 is a breast cancer-related, estrogen-dependent, gene
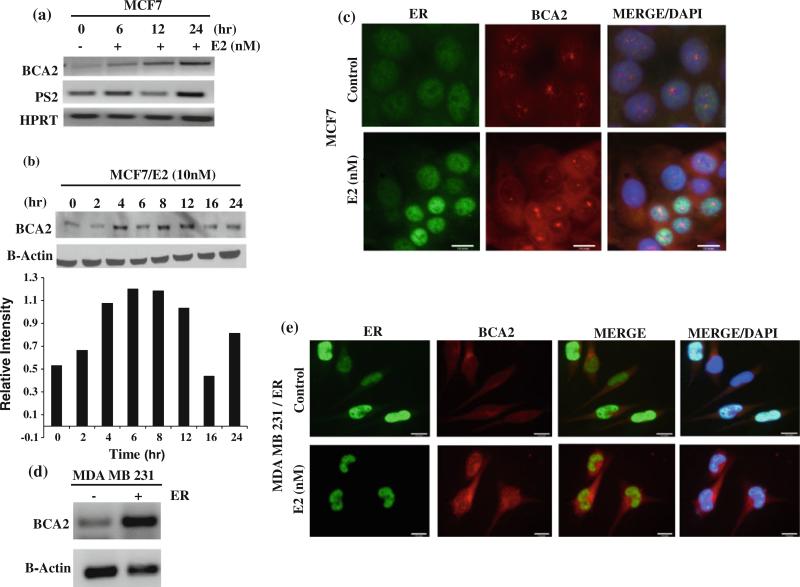
Previous northern blot analysis suggests that BCA2 mRNA expression is up regulated by estrogen [17]. However, whether or not BCA2 is transcriptionally regulated by ER has not been shown. In this study, we first tested the effect of estrogen on BCA2 gene expression in ER-positive MCF7 breast cancer cells. Cells were grown in hormone-depleted conditions for 48 h and treated with E2 for different time points. RT-PCR analysis revealed an increase in BCA2 mRNA expression level 6 h after treatment with 10 nM estradiol (E2), which further increased after 24 h (Fig. 1a). Expression of pS2 mRNA was used as a positive control for E2-induced ER transcriptional activity and HPRT as a loading control (Fig. 1a).
Fig. 1.
Regulation of BCA2 gene by estrogen. a. BCA2 mRNA expression in MCF-7 cells after treatment with 10 nM E2 for 6, 12, or 24 h. pS2 mRNA was used as a control for ER transcriptional activity and HPRT as an internal loading control. b. Up-regulation of BCA2 protein levels with E2 induction in a kinetic experiment. c. Immunofluorescence staining of ER and BCA2 in MCF7 cells with 10 nM E2 treatments. d. MDA MB 231 parental and ER stable cell lines used for BCA2 mRNA levels. e. Immunoflorescence staining of BCA2 and ER in MDA MB 231 parental and ER-transfected cell lines
Western blot analysis of these treatment conditions showed that BCA2 protein levels reach a maximum between 6 and 8 h after induction with E2 (Fig. 1b).
Furthermore, immunofluorescence analysis showed that when MCF7 cells were grown in hormone-depleted medium, ER is mostly localized to the cytoplasm; however, after treatment with E2, nuclear expression levels of ER increased significantly (Fig. 1c). Also, both ER and BCA2 were found to be localized to the nucleus upon E2 induction, and a distinct punctuated signal for nuclear BCA2 was observed (Fig. 1c).
In order to further evaluate E2-dependent BCA2 expression, we transfected ER-negative MDA MB 231 cells with ER plasmid, and found that BCA2 mRNA levels were significantly increased in ER-transfected cells, compared to the parental cells/vector control (Fig. 1d). Immunofluorescence staining of MDA MB 231/ER cells upon E2 induction also showed increased BCA2 expression in both the cytoplasm and nucleus, similar to that seen in MCF7 cells (Fig. 1e).
Effect of cycloheximide and anti-estrogens on BCA2 expression
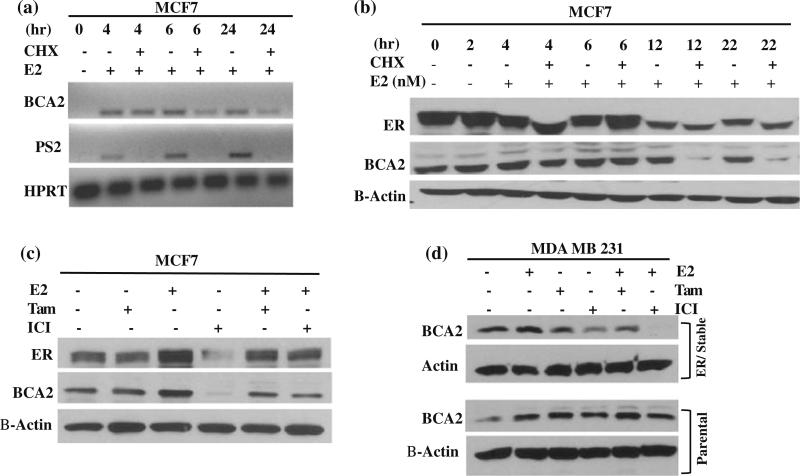
We next tested the effect of cycloheximide (CHX) on E2-induced BCA2 expression. The BCA2 mRNA levels were not significantly affected by CHX treatment under the tested conditions (Fig. 2a), while the mRNA levels of pS2 were decreased, indicating that BCA2 gene expression is primarily regulated by E2 at the transcriptional level. Our data under the experimental conditions tested suggests that, although pS2 mRNA expression is regulated by estrogen at the transcriptional level [19], its stability requires de novo protein synthesis. Furthermore, the protein levels of BCA2 were affected by CHX treatment from 12 to 22 h, indicating a half-life greater than 6 h (Fig. 2b). CHX treatment also had some effect on ER protein level (Fig. 2b).
Fig. 2.
BCA2 regulation by cycloheximide and anti-estrogens. a. Hormone-depleted MCF7 cells were treated with E2 and/or CHX for different time points as indicated and used for measurement of BCA2 mRNA levels. b. Western blot analysis of BCA2 in MCF7 cells after CHX and/or E2 treatments for the different time points tested. c. Effect of ICI, tamoxifen and E2 treatments on BCA2 protein levels in MCF7 cells. d. MDA MB 231 vector control (parental) and ER-stable transfected cells were treated with 10 nM E2, 100 nM ICI and/or 100 nM tamoxifen for 24 h, followed by analysis for BCA2 protein levels. Actin was used as loading control
To study ER dependency on BCA2 expression, anti-estrogens, tamoxifen, and ICI 182 178, were used. Again, E2 treatment induced levels of ER and BCA2 proteins in MCF7 cells, both of which were inhibited by addition of either tamoxifen or ICI (Fig. 2c). In this experiment, the basal levels of ER and BCA2 proteins were inhibited by addition of ICI but not Tamoxifen (Fig. 2c). When MDA MB 231 vector control cells were treated with E2, induction of BCA2 protein was observed, which seems to be not inhibitable by the anti estrogen treatments (Fig. 2d). Slight induction of BCA2 protein was found in MDA MB 231/ER-transfected cells treated with E2, which was inhibited by anti-estrogens (Fig. 2d). Therefore, it appears that anti-estrogens were able to reduce BCA2 protein expression in ER-positive cell lines.
Effect of ER knock down on BCA2 expression levels
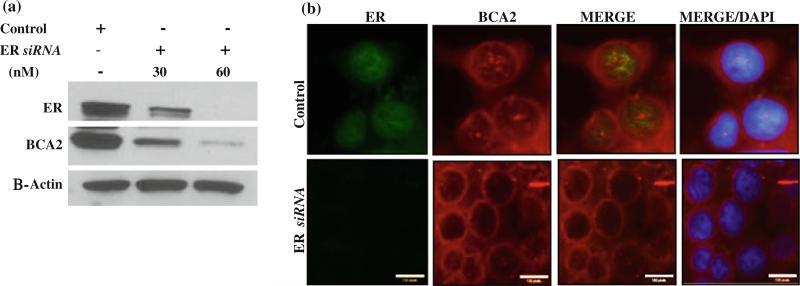
To provide direct evidence for the functional role ER has on BCA2 expression, we used small interfering RNAs (siRNAs) to knock down ER in MCF7 cells. A single treatment with ER siRNA decreased ER transcripts within 48 h, and this effect lasted for at least 2 days after single transfection (data not shown). When ER expression was knocked down in MCF7 cells using ER siRNA at concentrations of 30 and 60 nM, BCA2 protein levels were decreased significantly when compared to control treated cells (Fig. 3a). Immunoflorescence staining of cells treated with ER siRNA also showed lower expression levels of ER and BCA2 (Fig. 3b). Interestingly, even though BCA2 protein expression predominantly decreased in the ER-knockdown cells, its expression was localized near the nuclear membrane region and cytoplasm, with significantly impaired nuclear expression. These results suggest that ER influences BCA2 protein expression.
Fig. 3.
Regulation of BCA2 protein by ER. a. MCF-7 cells were treated with different concentrations of ER siRNA, followed by detection of BCA2 and ER protein levels. ER knockdown with siRNA decreased BCA2 protein levels and β actin was used as a loading control. b. Immunoflorescence analysis of MCF7 cells after treatment with ER siRNA
BCA2 proximal promoter is responsive to estrogen
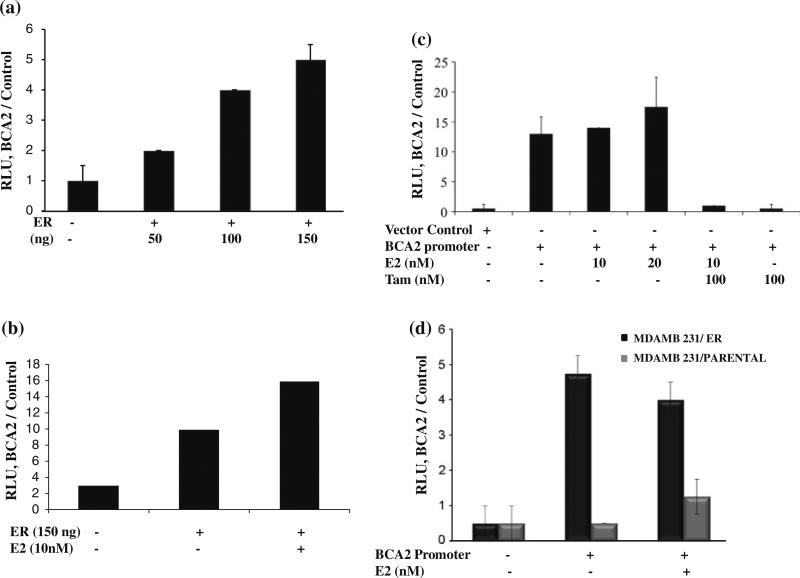
We next investigated the molecular mechanism by which estrogen regulates BCA2 expression. Since estrogen regulates its target gene expression by binding to an ERE, we therefore investigated whether the BCA2 promoter is also activated by such a mechanism. In silico analysis revealed several ER-binding half-sites [17] in the BCA2 promoter region at positions –129, –493, –1001, and –2407 base pairs upstream from the transcription start site along with other transcription factor-binding sites. The BCA2 promoter was cloned into a promoterless luciferase plasmid and initial transient transfections were carried out in HEK293T cells along with renilla and ER luciferase plasmids at different ratios of pGL4 promoter to pCDNA3 ER expression vectors to define the optimal levels for luciferase expression. BCA2 promoter activity increased with increasing concentrations of ER (Fig. 4a) in HEK293T cells and this signal was further increased with E2 treatments (Fig. 4b). When these experiments were carried out in MCF7 cells, E2 treatment only slightly increased BCA2 promoter activity which, however, was completely abolished by tamoxifen co-treatments (Fig. 4c). The basal levels of the BCA2 promoter activity were also inhibitable by either tamoxifen (Fig. 4c) or ICI (data not shown).
Fig. 4.
Luciferase reporter activity of the BCA2 promoter. a. Representative results of BCA2 promoter activity with ER plasmid when transfected into HEK293T cells. Renilla luciferase was used as internal control for transfection efficiency. Values shown are representative of four separate experiments. b. The promoter activity tested with different concentration of E2 in HEK 293 cells. The promoter and renilla plasmid without ER plasmid were used as control. c. BCA2 promoter activity with E2 induction and antiestrogen treatments in MCF7 cells. p values were calculated by two tailed t test. p < 0.239 was obtained for bars 3 and 4 and p < 0.005 for bars 5 and 6. d. Reporter assay of MDA MB 231 vector control (parental) and ER-transfected cells with estrogen treatments. p < 0.2 was obtained for MDA MB 231 vector control and p < 0.09 for MDA MB 231/ER transfected
To further define the role of ER in BCA2 transcription, we tested the promoter activity in control vector- and ER-stably transfected MDA MB 231 cells in the luciferase reporter assay. The control cell line showed minimal luciferase activity, while the ER-transfected cells exhibited almost a ninefold increase in luciferase signal when compared to the non-promoter controls (Fig. 4d). Taken together, these data suggest that that both E2 and ER control BCA2 promoter activity, and consequently the levels of BCA2 mRNA and protein.
ER is recruited to the BCA2 promoter upon E2 induction
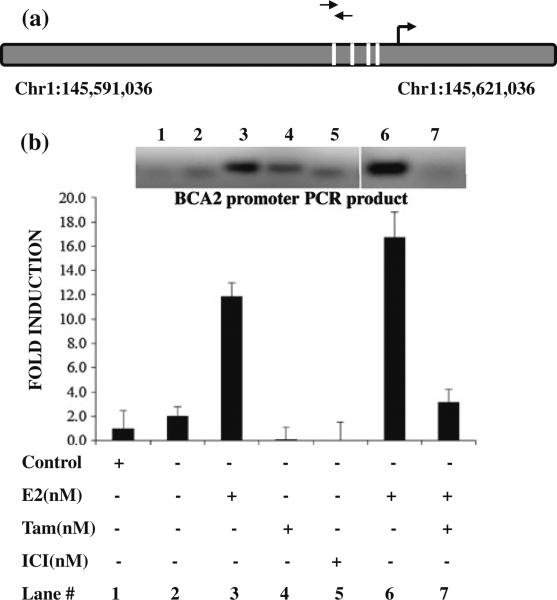
We next performed a ChIP experiment to investigate whether ER is recruited to the BCA2 promoter upon induction with E2. The DNA–protein complex was immunoprecipitated using an anti-ER antibody and was then analyzed by quantitative PCR with primers specific for the BCA2 proximal promoter ERE region (Fig. 5a) with binding positions near Chr1:145608166–145608185 regions on the plus strand and binding to the sequence 5′-GGCAGGTGGTGGTGGCCCAG-3′. The qPCR data shown in Fig. 5b depicts recruitment of ER to BCA2 promoter in MCF7 cells after treatment with E2. An increase in ER binding to the BCA2 promoter is evident and this increase was quantified to be sixfold over the non-induced control sample. The increase in qPCR signal provides direct evidence of ER recruitment to the BCA2 promoter region upon E2 treatment in MCF7 cells. Treatment with tamoxifen inhibits binding of ER to the BCA2 promoter induced by E2 (Fig. 5b). Tamoxifen or ICI treatment alone also inhibited the basal levels of ER binding to the BCA2 promoter (Fig. 5b). These results confirm E2-induced recruitment of ER to the BCA2 promoter, supporting our conclusion that ER induces BCA2 expression in ER-positive breast cancer cells.
Fig. 5.
Chromatin Immunoprecipitation. a. Schematic representation of the ERE half-sites in the proximal promoter region of the BCA2 promoter and primer positions used to amplify ER-binding site. Primer pairs indicated in arrows bind to the position 145608166–145608185 in the plus strand of chromosome 1 and the binding sequence is GGCAGGTGGTGGTGGCCCAG. b. ChIP-qPCR data of the MCF7 cells with E2, tamoxifen and/or ICI treatments along with IgG control. Data were presented as relative amount of immunoprecipitated DNA normalized to input as measured by qPCR assay. The inset shows EhBr staining of the PCR product (size of 100 base pairs, upper band) of the BCA2 promoter amplified region specific to the promoters. The lower band represents primer dimers. This gel represents single well, while the bar graph is representative of average of three experiments
Discussion
In this study, we have identified BCA2 as a target of ER transcriptional activity in ER-positive breast cancer cells (MCF-7 and MDA MB 231/ER) and have shown that BCA2 gene up-regulation is hormone dependent. We have shown that the BCA2 promoter is activated by hormone-induced ER, where ER is recruited to the BCA2 promoter ERE half-sites. This study confirms that the up-regulation of BCA2 mRNA and protein expression by hormone is largely due to transcriptional activation. To our knowledge, this is the first report describing hormonal regulation of BCA2 transcription.
The conventional model of ER binding suggests that the ER dimer binds to a consensus or pseudopalindromic ERE. Each ER monomer binds to a half-site major groove with a spacer in between and the size of which influences dimer stability, target specificity, and ERE/ER complexation [20]. However, studies have shown that ER can activate transcription of genes containing nonconventional elements like ERE half-sites [14, 21–23]. There is a vast amount of literature available confirming that ER requires only minimal recognition of some element, a half ERE, for transcriptional activation of a particular gene. The basic structural nature of ER and ERE complexes is dependent on ERE as shown by many protease digestion profiles for different ER/ERE complexes [24]. It is well documented that for a number of genes, the minimal recognition element for transcriptional activation of ER is HERE half ERE (HERE) (5′-GGTCA-3′) [25, 26]; although, there is some controversy over whether ER can bind to an ERE half-site as a homo-dimer [27–29] or either as a monomer or heterodimer [30]. Studies of ER interaction with the promoter of lactoferin have shown that this promoter contains an SF-1 response element upstream of an imperfect ERE and that one ER dimer binds to this SF-1 response element [31]. Furthermore, the rat prolactin gene promoter binds ER as a homo-dimer to an SF-1 response element and ERE half-site [32]. The DNA foot printing studies of the well-established ER-regulated gene pS2 promoter in MCF7 breast cancer cells showed that it has an imperfect ERE half-site in its promoter [33, 34]. Studies of another widely studied estrogen-responsive gene, progesterone receptor, showed that ER interacts with an ERE half-site located 4 base pairs upstream to the adjacent Sp1 binding sites in its promoter region and that ER can increase its Sp1-DNA binding ability [35–37]. Thus, a half-site ERE consensus sequence is adequate to bind ER with an affinity sufficient enough for transcriptional activation, suggesting that these simple elements, residing on complex promoters, are able to enhance transcriptional activity with the help of multiple cellular factors and co-activators which function to stabilize ER binding by protein–protein interactions [38, 39].
Whole genome analysis of transcription factors provided us with an unbiased perspective of their regulatory dynamics. Studies of genome wide analysis of ERα DNA binding sites in the MCF breast cancer cell lines by Lin et.al [40] identified 1,234 high probability binding sites for ER, out of which 94 % were validated by the standard ChIP assays. Of these 94 % validated binding sites, 25 % of them were solely ERE half-motifs.
In this study, we have shown that ER activation by E2 caused an induction of BCA2 mRNA and protein expression levels in the ER-positive MCF7 breast cancer line and MDA MB 231/ER stably transfected cell line. Consistent with this, treatment of these cells with ER antagonists ICI and tamoxifen significantly decreased the BCA2 expression levels. Confocal microscopy suggested that E2 induced a relatively significant BCA2 signal in both the cytoplasm and nucleus. When hormone-depleted MCF7 cells were treated with ER siRNA, the knockdown caused a significant decrease (about 63 %) in BCA2 protein levels and its localization was limited to the cytoplasm and nuclear membrane regions. Interestingly, genome wide ER-binding studies suggest that the promoter region of BCA2 consists of an ER-binding peak detected upon induction. Bioinformatics studies of the BCA2 promoter revealed four ERE half sites in the proximal promoter region. BCA2 promoter luciferase assays confirmed the role of ER in BCA2 promoter activation. ER antagonists clearly showed inhibitory effects in the reporter assays in all of the cell lines tested, either with endogenous ER or exogenously transfected ER. The specificity of BCA2 promoter response to E2 is demonstrated by the lack of reporter activity in its absence. The E2 induced reporter activity was reduced to the basal level with excess of tamoxifen used to treat ER-positive breast cancer patients [41, 42]. Recruitment of ER to the BCA2 promoter with E2 induction in our ChIP analysis is direct evidence of ER activation of the BCA2 gene, but further analysis of the exact factors involved in this molecular mechanism have yet to be established. We also found the transcriptional regulation of BCA2 by ER in another ER-positive cell line T47D (data not shown). Although the basal transcription of this gene may also be regulated by other transcription factors, especially in ER-negative cell lines, all of the experimental evidence discussed in this article confirms that BCA2 is an ER-regulated gene at least in the ER-positive MCF7 breast cancer cell line. More work is needed to extend this study to many other ER-positive breast cancer cell lines and tissues.
BCA2 is an E3 ligase whose activity might be important for the ubiquitination of proteins essential in the pathogenesis of breast cancer. BCA2 expression correlates with ER status in breast cancer patients and is predicted to be oncogenic in hormone-dependent breast cancers. Therefore, the mechanism by which BCA2 is regulated might be important in the understanding of breast cancer development and metastasis. Thus, targeting BCA2 ligase activity with small molecule inhibitors may be useful in the inhibition of breast cancer development and progression.
Acknowledgments
This study was partially supported by the National Cancer Institute at the National Institutes of Health (5R01CA127258-05, 1R01CA120009, and 3R01CA120009-04S1) to QPD and Postdoctoral Training Grant by Susan Komen Foundation (KG101529) to FRK; the Wayne State University; Karmanos Cancer Center, Detroit, MI.
Footnotes
In memory of Professor Angelika Burger who initiated this project. Professor Burger's research focused on the identification and validation of new molecular targets for the treatment of breast cancer. Dr. Burger's particular interest was in the investigation of the role of the ubiquitin E3 ligase, BCA2, in breast cancer development and to further design and develop small molecular inhibitors targeting BCA2. Dr. Burger was a very gifted researcher, who lost her personal battle with cancer in May, 2011. This article is dedicated to her as a token of our appreciation of a truly outstanding researcher.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. doi:10.10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gruvberger S, et al. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001;61(16):5979–5984. [PubMed] [Google Scholar]
- 3.Oh DS, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24(11):1656–1664. doi: 10.1200/JCO.2005.03.2755. doi:10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Nat Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubik D, Dembinski TC, Shiu RP. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987;47(24 Pt 1):6517–6521. [PubMed] [Google Scholar]
- 6.Lang JC, et al. Transcriptional regulation of the human c-myc gene. Br J Cancer Suppl. 1988;9:62–66. [PMC free article] [PubMed] [Google Scholar]
- 7.Altucci L, et al. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12(11):2315–2324. [PubMed] [Google Scholar]
- 8.Prall OW, et al. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18(8):4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth P, et al. Novel estrogen and tamoxifen induced genes identified by SAGE (serial analysis of gene expression). Onco-gene. 2002;21(5):836–843. doi: 10.1038/sj.onc.1205113. [DOI] [PubMed] [Google Scholar]
- 10.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez R, et al. Diversity in the mechanisms of gene regulation by estrogen receptors. BioEssays. 2002;24(3):244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- 12.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108(4):465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 13.Katzenellenbogen BS, et al. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res. 2000;55:163–193. discussion 194–195. [PubMed] [Google Scholar]
- 14.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucl Acids Res. 2001;29(14):2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushner PJ, et al. Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp. 2000;230:20–26. doi: 10.1002/0470870818.ch3. discussion 27–40. [DOI] [PubMed] [Google Scholar]
- 16.Carroll JS, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 17.Burger AM, et al. Role of the BCA2 ubiquitin E3 ligase in hormone responsive breast cancer. Open Cancer J. 2010;3(1):116–123. doi: 10.2174/1874079001003010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burger AM, et al. A novel RING-type ubiquitin ligase breast cancer-associated gene 2 correlates with outcome in invasive breast cancer. Cancer Res. 2005;65(22):10401–10412. doi: 10.1158/0008-5472.CAN-05-2103. [DOI] [PubMed] [Google Scholar]
- 19.Cavailles V, Garcia M, Rochefort H. Regulation of cathepsin-D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol Endocrinol. 1989;3(3):552–558. doi: 10.1210/mend-3-3-552. [DOI] [PubMed] [Google Scholar]
- 20.Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994;15(3):391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- 21.Kato S, et al. A far upstream estrogen response element of the ovalbumin gene contains several half-palindromic 5′-TGACC-3′ motifs acting synergistically. Cell. 1992;68(4):731–742. doi: 10.1016/0092-8674(92)90148-6. [DOI] [PubMed] [Google Scholar]
- 22.O'Lone R, et al. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18(8):1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 23.Aumais JP, et al. Function of directly repeated half-sites as response elements for steroid hormone receptors. J Biol Chem. 1996;271(21):12568–12577. doi: 10.1074/jbc.271.21.12568. [DOI] [PubMed] [Google Scholar]
- 24.Walker P, et al. Sequence homologies in the region preceding the transcription initiation site of the liver estrogen-responsive vitellogenin and apo-VLDLII genes. Nucl Acids Res. 1984;12(22):8611–8626. doi: 10.1093/nar/12.22.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Marzouk S, et al. The plasticity of estrogen receptor-DNA complexes: binding affinity and specificity of estrogen receptors to estrogen response element half-sites separated by variant spacers. J Steroid Biochem Mol Biol. 2008;110(1–2):186–195. doi: 10.1016/j.jsbmb.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Ellerman JE, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13(10):2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 27.Klinge CM, et al. Cooperative estrogen receptor interaction with consensus or variant estrogen responsive elements in vitro. Cancer Res. 1992;52(5):1073–1081. [PubMed] [Google Scholar]
- 28.Ludwig LB, et al. A microtiter well assay for quantitative measurement of estrogen receptor binding to estrogen-responsive elements. Mol Endocrinol. 1990;4(7):1027–1033. doi: 10.1210/mend-4-7-1027. [DOI] [PubMed] [Google Scholar]
- 29.Klinge CM, et al. Comparison of tamoxifen ligands on estrogen receptor interaction with estrogen response elements. Mol Cell Endocrinol. 1998;143(1–2):79–90. doi: 10.1016/s0303-7207(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 30.Furlow JD, Murdoch FE, Gorski J. High affinity binding of the estrogen receptor to a DNA response element does not require homodimer formation or estrogen. J Biol Chem. 1993;268(17):12519–12525. [PubMed] [Google Scholar]
- 31.Zhang Z, Teng CT. Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. 2000;275(27):20837–20846. doi: 10.1074/jbc.M001880200. [DOI] [PubMed] [Google Scholar]
- 32.Anderson I, Gorski J. Estrogen receptor alpha interaction with estrogen response element half-sites from the rat prolactin gene. Biochemistry. 2000;39(13):3842–3847. doi: 10.1021/bi9924516. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, et al. Regulation of the estrogen-responsive pS2 gene in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol. 2000;74(4):157–168. doi: 10.1016/s0960-0760(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 34.Balleine RL, Clarke CL. Expression of the oestrogen responsive protein pS2 in human breast cancer. Histol Histopathol. 1999;14(2):571–578. doi: 10.14670/HH-14.571. [DOI] [PubMed] [Google Scholar]
- 35.Lee YJ, Gorski J. Estrogen-induced transcription of the progesterone receptor gene does not parallel estrogen receptor occupancy. Proc Nat Acad Sci USA. 1996;93(26):15180–15184. doi: 10.1073/pnas.93.26.15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneko KJ, Gelinas C, Gorski J. Activation of the silent progesterone receptor gene by ectopic expression of estrogen receptors in a rat fibroblast cell line. Biochemistry. 1993;32(32):8348–8359. doi: 10.1021/bi00083a039. [DOI] [PubMed] [Google Scholar]
- 37.Petz LN, Nardulli AM. Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Mol Endocrinol. 2000;14(7):972–985. doi: 10.1210/mend.14.7.0493. [DOI] [PubMed] [Google Scholar]
- 38.Tyulmenkov VV, Klinge CM. Estrogen receptors alpha and beta exhibit different estradiol and estrogen response element binding in the presence of nonspecific DNA. Arch Biochem Biophys. 2001;390(2):253–264. doi: 10.1006/abbi.2001.2382. [DOI] [PubMed] [Google Scholar]
- 39.Porter W, et al. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 1997;11(11):1569–1580. doi: 10.1210/mend.11.11.9916. [DOI] [PubMed] [Google Scholar]
- 40.Lin CY, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3(6):e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan VC. Tamoxifen: toxicities and drug resistance during the treatment and prevention of breast cancer. Annu Rev Pharmacol Toxicol. 1995;35:195–211. doi: 10.1146/annurev.pa.35.040195.001211. [DOI] [PubMed] [Google Scholar]
- 42.Jordan VC. Third annual William L. McGuire Memorial Lecture. “Studies on the estrogen receptor in breast cancer”—20 years as a target for the treatment and prevention of cancer. Breast Cancer Res Treat. 1995;36(3):267–285. doi: 10.1007/BF00713399. [DOI] [PubMed] [Google Scholar]