Abstract
Chronic obstructive pulmonary disease (COPD) is characterised by high morbidity and mortality. It remains unknown which aspect of lung function carries the most prognostic information and if simple spirometry is sufficient.
Survival was assessed in COPD outpatients whose data had been added prospectively to a clinical audit database from the point of first full lung function testing including spirometry, lung volumes, gas transfer and arterial blood gases. Variables univariately associated with survival were entered into a multivariate Cox proportional hazard model.
604 patients were included (mean±sd age 61.9±9.7 years; forced expiratory volume in 1 s 37±18.1% predicted; 62.9% males); 229 (37.9%) died during a median follow-up of 83 months. Median survival was 91.9 (95% CI 80.8–103) months with survival rates at 3 and 5 years 0.83 and 0.66, respectively. Carbon monoxide transfer factor % pred quartiles (best quartile (>51%): HR 0.33, 95% CI 0.172–0.639; and second quartile (51–37.3%): HR 0.52, 95% CI 0.322–0.825; versus lowest quartile (<27.9%)), age (HR 1.04, 95% CI 1.02–1.06) and arterial oxygen partial pressure (HR 0.85, 95% CI 0.77–0.94) were the only parameters independently associated with mortality.
Measurement of gas transfer provides additional prognostic information compared to spirometry in patients under hospital follow-up and could be considered routinely.
Short abstract
Transfer factor not GOLD stage is the most powerful predictor of survival in patients with COPD http://ow.ly/mGmjG
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem. Its prevalence, morbidity and mortality rates are increasing, and it is now the third leading cause of death worldwide [1]. However, survival rates vary widely between studies [2, 3], possibly due to different disease coding and the inclusion of COPD populations of varying severity. A range of pulmonary function tests is available to assess the severity of disease in COPD, including spirometry, gas transfer measurements, plethysmographic lung volumes and arterial blood gas analysis. The relative usefulness of these different measures remains unclear and in the context of finite healthcare resources it is important to establish whether they provide added value in the management of patients with this condition. In addition, where mortality is an end-point in clinical trials, it is important to be able to select or stratify patients appropriately in order that treatment effects can be determined accurately.
Several attempts have been made to identify potential predictors of survival among COPD patients. Since COPD has impacts beyond the lung [4, 5], the prognostic impact of both pulmonary and extrapulmonary factors has been investigated. Parameters of lung function and, particularly, forced expiratory volume in 1 s (FEV1) [6, 7], age [8], sex [6, 7], body mass index (BMI) [9, 10], quadriceps strength [11], arterial blood gases [12, 13], lung volumes [14], gas transfer [15, 16], severity of dyspnoea [17], anaemia [18, 19], presence of comorbidities [20] and reduced physical activity or exercise capacity [21] are only some of the factors that have been studied. However, several of these studies have been conducted in specific COPD populations, such as selected patients with severe emphysema [2], patients using long-term oxygen treatment (LTOT) or noninvasive mechanical ventilation [6, 22, 23], and patients during or immediately after hospitalisation for a COPD exacerbation [7, 12, 24]. A limitation of these studies has been the generalisability of the population and the fact that in most studies a full range of lung function measures has not been compared [25]. Thus it has not been possible to draw definitive conclusions about the key lung function prognostic factors in COPD.
Against this background, data from a large cohort of COPD outpatients, who had undergone comprehensive lung function assessment and who had been prospectively entered into a clinical audit database, were analysed to establish whether spirometry, gas transfer or lung volumes wielded the most predictive power for survival.
Methods
Study population
Data on outpatients attending the hospital COPD clinic were entered prospectively onto a clinical audit database. These included pulmonary function test results, anthropometrics, treatments received and exacerbation rate. Data were extracted for individuals who had had their first full lung function testing, including arterial blood gas analysis, performed between February 1996 and October 2010. COPD diagnosis was clinically based on symptoms, radiology appearances and spirometry criteria, using an FEV1 to forced vital capacity (FVC) ratio <0.7. Patients' treatment included inhaled β2-agonists, anticholinergics, oral theophyllines and inhaled steroids in various combinations. Exclusion criteria were evidence of chronic heart failure (New York Heart Association class III or IV), chronic renal failure, peripheral vascular disease or history of malignancy. The Royal Brompton, Harefield and National Heart and Lung Institute research ethics committee has ruled that ethical approval is not required for the retrospective analysis of routinely collected clinical data.
Study measurements
Spirometry, gas transfer and lung volumes assessed by body plethysmography were measured using a CompactLab System (Jaeger, Hoechberg, Germany). Quality control followed accepted guidelines, with carbon monoxide transfer factor (TLCO) gas analyser calibrations prior to each session, volume calibrations prior to each patient test and biological calibrations daily. Predicted values used are those of the European Coal and Steel Community [26]. Arterial oxygen (PaO2) and carbon dioxide (PaCO2) partial pressures were measured in arterialised earlobe capillary samples and arterio-alveolar oxygen gradient calculated. Arterialised capillary blood sampling is carried out routinely by our clinical physiologists and is better tolerated than an arterial puncture, while giving equivalent results [27]. For every patient, FEV1, FVC, FEV1/FVC ratio, total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV) and inspiratory capacity (IC)/TLC ratio were recorded. The values of TLCO were adjusted for haemoglobin concentration, according to previously published equations [28].
The annual exacerbation rate referred to the 12-month period prior to the patient's clinic attendance and was derived from a detailed medical history using an event-based definition; an increase in the patient's dyspnoea, cough and/or sputum beyond normal day-to-day variations, which required treatment with antibiotics and or oral corticosteroids [29]. Survival, extracted from the central National Health Service, UK database was determined up to May 2011.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 17 for Windows XP. Data (including % predicted values) are presented as mean±sd. Exacerbation rate was treated a priori at the time of initial data entry as a categorical variable, separating the number of exacerbations into three groups: 0–1, 2–4 or >4 per year. Differences between survivors and non-survivors in demographic and clinical variables were assessed utilising the independent samples t-test or the Chi-squared model, where appropriate. The parameters found to be univariately associated with survival were subsequently entered in a multivariate Cox proportional hazard regression analysis model. Both FEV1 and TLCO were entered in the survival analysis as categorical variables (Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages and TLCO quartiles, respectively), as this methodology has been previously confirmed to better classify COPD patients with respect to survival [30, 31]. The Tukey–Hinges method was used to calculate the TLCO quartiles. Corresponding hazard ratios and 95% confidence intervals were calculated for each independent predictor. The median survival (with the corresponding 95% confidence intervals) and the overall survival rates were estimated using the Kaplan–Meier method. Correlation analysis was used to identify factors associated with PaO2. A level of p<0.05 was considered statistically significant.
Results
Study population
The study population consisted of 604 patients (380, or 62.9%, male and 224, or 37.1%, female), who had a mean age of 62 years. According to GOLD classification, most of the patients presented with severe or very severe disease (28.4% and 52.2%, respectively) and 17.1% of them were on LTOT. Approximately 85.5% of the patients were treated with inhaled steroids, 82.2% with long-acting inhaled β2-agonists and 63.4% with long acting anti-muscarinics. The demographic and clinical characteristics of the study population are presented in table 1.
Table 1– Characteristics of the study population.
| Age years | 61.9±9.7 |
| Sex % | |
| Male | 62.9 |
| Female | 37.1 |
| BMI kg·m−2 | 24.2±5.3 |
| FEV1 % pred | 37±18.1 |
| FVC % pred | 84.5±22.4 |
| FEV1/FVC | 34.7±12.3 |
| GOLD classification % | |
| Stage I | 2.3 |
| Stage II | 17.0 |
| Stage III | 28.4 |
| Stage IV | 52.2 |
| TLC % pred | 124.9±18.4 |
| RV % pred | 205.1±58.4 |
| IC/TLC % | 26.9±8.8 |
| FRC % pred | 172.7±38.5 |
| TLCO % pred | 40.8±18 |
| PaO2 kPa | 9.4±1.4 |
| PaCO2 kPa | 5.2±0.9 |
| Arterio-alveolar oxygen gradient kPa | 5.1±1.3 |
| LTOT % | 17.1 |
| Current smokers % | 13.2 |
| Smoking history pack-years | 43.8±23 |
| Exacerbations % | |
| 0–1 per year | 39.1 |
| 2–4 per year | 42.7 |
| >4 per year | 18.2 |
Data are presented as mean±sd, unless otherwise stated. n = 604. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GOLD: Global Initiative for Obstructive Lung disease; TLC: total lung capacity; RV: residual volume; IC: inspiratory capacity; FRC: functional residual capacity; TLCO: carbon monoxide transfer factor; PaO2: arterial oxygen partial pressure; PaCO2: arterial carbon dioxide partial pressure; LTOT: long-term oxygen treatment.
Mortality
The mean±sd follow-up for the total population was 80±49.8 months, while the median (95% CI) was 83.1 (6.6–210.8) months. 229 patients (37.9%) died during the follow-up period. Median (95% CI) survival for the total population was 91.9 (80.8–103) months and survival rates at 3 and 5 years were 0.83 and 0.66, correspondingly.
Predictors of survival
table 2 presents the differences in patient characteristics between survivors and non-survivors. Survivors were younger (p = 0.024) with less severe disease (according to GOLD classification), presented with higher BMI (p = 0.004), FEV1 % pred (p<0.001), FVC % pred (p<0.001), FEV1/FVC ratio (p<0.001), TLCO % pred (p = 0.015), IC/TLC % (p<0.001) and PaO2 at room air (p = 0.001), and a lower proportion received LTOT (p<0.001), compared to non-survivors. Conversely, TLC % pred (p = 0.015), FRC % pred (p<0.001), RV % pred (p<0.001) and PaCO2 (p = 0.003) were lower among the patients who were still alive at the end of the follow-up period. The following parameters were not associated with survival differences: sex, H+ concentration, arterio-alveolar gradient, smoking status and history (pack-years), and exacerbation rate.
Table 2– Differences between survivors and non-survivors.
| Survivors | Non-survivors | p-value | |
| Subjects n | 375 | 229 | |
| Age years | 61.1±10.1 | 63±9.2 | 0.024 |
| Sex % | |||
| Male | 62.4 | 63.8 | ns |
| Female | 37.6 | 36.2 | |
| BMI kg·m−2 | 24.7±5.3 | 23.4±5.2 | 0.004 |
| FEV1 % pred | 40.6±18.8 | 30.9±15 | <0.001 |
| FVC % pred | 88.4±22 | 78.2±21.6 | <0.001 |
| FEV1/FVC | 36.4±12.8 | 31.8±11 | <0.001 |
| GOLD classification % | <0.001 | ||
| Stage I | 3.2 | 0.9 | |
| Stage II | 22.7 | 7.4 | |
| Stage III | 33 | 20.5 | |
| Stage IV | 41.1 | 71.2 | |
| TLCO % quartiles | <0.001 | ||
| Best quartile (>51) | 31.3 | 13.9 | |
| Quartile 2 (51–37.3) | 29.1 | 18.3 | |
| Quartile 3 (37.3–27.9) | 22.2 | 29.3 | |
| Quartile 4 (<27.9) | 17.4 | 38.5 | |
| TLCO % pred | 44.7±18.4 | 34.1±15.2 | <0.001 |
| TLC % pred | 123.4±17 | 127.5±20.5 | 0.015 |
| FRC % pred | 166.1±35.3 | 184.4±41.1 | <0.001 |
| RV % pred | 195.6±53.2 | 220.9±63.1 | <0.001 |
| IC/TLC % | 28.8±8.6 | 23.5±8 | <0.001 |
| PaO2 kPa | 9.6±1.3 | 9.2±1.6 | 0.001 |
| PaCO2 kPa | 5.1±0.7 | 5.4±1 | 0.003 |
| Arterio-alveolar oxygen gradient kPa | 5±1.3 | 5.1±1.4 | ns |
| LTOT % | 11.0 | 27.1 | <0.001 |
| Smoking status % | |||
| Never or ex-smokers | 88.4 | 83.4 | ns |
| Current smokers | 11.6 | 16.6 | |
| Smoking history pack-years | 45.6±25.1 | 41.1±19.4 | ns |
| Exacerbations % | |||
| 0–1 per year | 40.3 | 37.1 | |
| 2–4 per year | 40 | 47.2 | ns |
| >4 per year | 19.7 | 15.7 |
Data are presented as mean±sd, unless otherwise stated. BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GOLD: Global Initiative for Obstructive Lung disease; TLCO: carbon monoxide transfer factor; TLC: total lung capacity; FRC: functional residual capacity; RV: residual volume; IC: inspiratory capacity; PaO2: arterial oxygen partial pressure; PaCO2: arterial carbon dioxide partial pressure; LTOT: long-term oxygen treatment; ns: nonsignificant.
When these univariate predictors were entered in a proportional Cox hazard model, the parameters which were found to predict mortality in the COPD population were age, TLCO % pred quartiles and PaO2 (table 3). When the lowest TLCO quartile (<27.9% pred) was used as reference, patients with TLCO >51% pred and those with TLCO between 51 and 37.3% pred had significant lower mortality risk than those with TLCO <27.9% pred. In addition, table 4 presents these results with TLCO % pred and FEV1 % pred treated as continuous variables. figure 1 presents the Kaplan–Meier survival curves, adjusted for age, PaO2, GOLD stages, FVC % pred, FEV1/FVC, TLC % pred, FRC % pred, IC/TLC, RV % pred, BMI and PaCO2 for the four population groups, categorised by TLCO % pred quartiles. By contrast, figure 2 presents the Kaplan–Meier survival curves, adjusted for age, PaO2, TLCO % pred quartiles, FVC % pred, FEV1/FVC, TLC % pred, FRC % pred, IC/TLC, RV % pred, BMI and PaCO2 for the four population groups categorised by GOLD stages. Figure E1 in the online data supplement shows receiver operating characteristic curves for survival prediction comparing TLCO % pred, FEV1 % pred, IC/TLC and FEV1/FVC ratio. TLCO % pred had the most predictive power with areas under the curve of 0.69, 0.67, 0.64 and 0.62 respectively.
Table 3– Predictors of mortality in the total chronic obstructive pulmonary disease population, according to the multivariate Cox regression analysis.
| HR (95% CI) | p-value | |
| Age years | 1.034 (1.009–1.059) | 0.007 |
| PaO2 kPa | 0.860 (0.755–0.979) | 0.023 |
| TLCO % pred | 0.006 | |
| Best quartile (>51) | 0.332 (0.172–0.639) | 0.001 |
| Quartile 2 (51–37.3) | 0.515 (0.322–0.825) | 0.006 |
| Quartile 3 (37.3–27.9) | 0.711 (0.477–1.059) | 0.093 |
| Quartile 4 (<27.9) | Reference | |
| GOLD classification | 0.125 | |
| Stage I | 0.506 (0.087–2.94) | 0.448 |
| Stage II | 0.407 (0.176–0.939) | 0.035 |
| Stage III | 0.639 (0.408–1.002) | 0.053 |
| Stage IV | Reference | |
| FVC % pred | 0.989 (0.969–1.010) | 0.250 |
| FEV1/FVC | 1.015 (0.994–1.037) | 0.170 |
| RV % pred | 0.988 (0.975–1.051) | 0.079 |
| FRC % pred | 1.023 (0.999–1.048) | 0.057 |
| TLC % pred | 1.008 (0.976–1.041) | 0.633 |
| IC/TLC % | 2.15 (0.37–3.293) | 0.418 |
| PaCO2 kPa | 0.985 (0.796–1.218) | 0.887 |
| BMI kg·m−2 | 1.009 (0.970–1.036) | 0.903 |
PaO2: arterial oxygen partial pressure; TLCO; carbon monoxide transfer factor; GOLD: Global Initiative for Obstructive Lung disease; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; RV: residual volume; FRC: functional residual capacity; TLC: total lung capacity; IC: inspiratory capacity; PaCO2: arterial carbon dioxide partial pressure; BMI: body mass index.
Table 4– Predictors of mortality in the total chronic obstructive pulmonary disease population, according to the multivariate Cox regression analysis.
| HR (95% CI) | p-value | |
| Age years | 1.030 (1.004–1.056) | 0.021 |
| PaO2 kPa | 0.851 (0.748–0.969) | 0.015 |
| TLCO % pred | 0.971 (0.957–0.984) | <0.001 |
| FEV1 % pred | 1.016 (0.974–1.060) | 0.463 |
| FVC % pred | 0.977 (0.950–1.004) | 0.090 |
| FEV1/FVC | 0.988 (0.944–1.033) | 0.582 |
| RV % pred | 0.987 (0.975–1.001) | 0.055 |
| FRC % pred | 1.021 (0.998–1.045) | 0.078 |
| TLC % pred | 1.011 (0.979–1.044) | 0.507 |
| IC/TLC % | 2.009 (0.378–3.451) | 0.628 |
| PaCO2 kPa | 0.991 (0.798–1.230) | 0.932 |
| BMI kg·m−2 | 1.006 (0.973–1.041) | 0.710 |
Carbon monoxide transfer factor (TLCO) % pred and forced expiratory volume in 1 s (FEV1) % pred entered the analysis as continuous variables. PaO2: arterial oxygen partial pressure; FVC: forced vital capacity; RV: residual volume; FRC: functional residual capacity; TLC: total lung capacity; IC: inspiratory capacity; PaCO2: arterial carbon dioxide partial pressure; BMI: body mass index.
Figure 1–
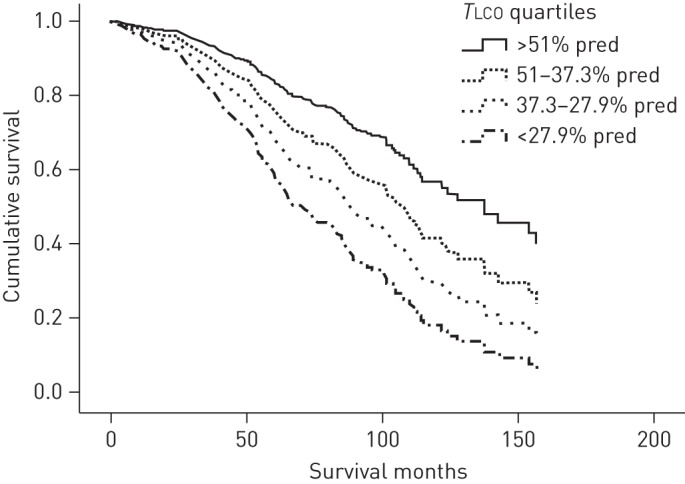
The Kaplan–Meier survival curves, adjusted for age, arterial oxygen partial pressure, Global Initiative for Chronic Obstructive Lung Disease classification, forced vital capacity (FVC) % pred, forced expiratory volume in 1 s to FVC ratio, total lung capacity (TLC) % pred, functional residual capacity % pred, inspiratory capacity to TLC ratio, residual volume % pred, body mass index and arterial carbon dioxide partial pressure for the four population groups, categorised by carbon monoxide transfer factor (TLCO) % pred quartiles.
Figure 2–
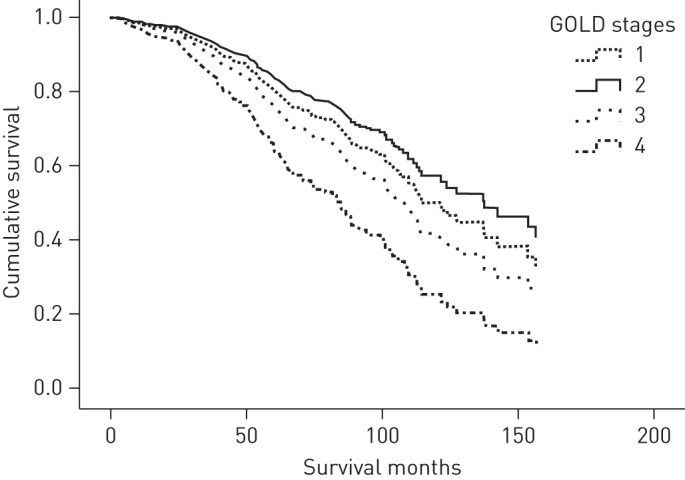
The Kaplan–Meier survival curves, adjusted for age, arterial oxygen partial pressure, carbon monoxide transfer factor % pred quartiles, forced vital capacity (FVC) % pred, forced expiratory volume in 1 s to FVC ratio, total lung capacity (TLC) % pred, functional residual capacity % pred, inspiratory capacity to TLC ratio, residual volume % pred, body mass index and arterial carbon dioxide partial pressure for the four population groups, categorised by Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages. Survival only differed significantly between GOLD stage II and IV (p = 0.04).
Because oxygen therapy has an impact on survival in COPD, additional analyses were performed excluding patients on long-term oxygen. These are available in an online data supplement (tables E1 and E2). The results in this population were similar, with TLCO % pred again the strongest predictor of survival.
Factors associated with PaO2
All recorded demographic and clinical parameters were investigated to identify potential determinants of PaO2. Correlation analysis revealed positive, but weak associations between PaO2 and FEV1 %pred (r = 0.25, p<0.001), TLCO % pred (r = 0.29, p<0.001) and IC/TLC (r = 0.23, p<0.001), and a moderate negative correlation between PaO2 and PaCO2 (r = -0.47, p<0.001). When these variables were entered in a multivariate regression analysis model, only PaCO2 (beta = -0.45, p<0.001) and TLCO % pred (beta = 0.28, p<0.001) were retained together explaining only 26% of the variance in PaO2 values in the study population: PaO2 = -0.719 (PaCO2)+0.018 (TLCO % pred)+12.431.
Discussion
The main finding of the present study was that, in stable outpatients with COPD, TLCO % pred and PaO2, as well as younger age, were the only variables independently associated with survival. Staging according to the original GOLD criteria, other lung function parameters, anthropometric measures and exacerbation history did not wield any additional prognostic information.
Significance of the findings
The present paper compares different lung function parameters directly in a group of comprehensively assessed COPD patients. COPD is characterised by airflow obstruction due to a mixture of small airways disease and lung parenchymal destruction [29]. FEV1 and FEV1/FVC reflect the severity of airflow obstruction, while plethysmographic lung volumes represent the consequences of gas trapping and lung hyperinflation due to airflow obstruction and decreased lung elastic recoil. As the product of the transfer coefficient and alveolar volume, TLCO reflects both changes in functional lung volume and gas transport across the alveolar–capillary membrane and thus reflects the degree of parenchymal destruction and loss of pulmonary capillary bed, due to emphysema. In addition, the presence of processes that increase pulmonary venous pressure, such as in left heart failure, pulmonary oedema and mitral stenosis, could also result in a reduction in TLCO [32]. These effects of both pulmonary circulation abnormalities and possibly subclinical cardiac pathology on TLCO values could partially explain its superiority as a prognostic factor compared to FEV1 or plethysmographic lung volumes.
To the authors' knowledge, this is the first study identifying TLCO % pred as an independent predictor of survival in a large, unselected general population of COPD outpatients. Although abnormal pulmonary function has long been considered as a predictor of mortality [3, 14, 25, 33–35], most studies in COPD have not assessed the impact of TLCO [18, 23, 36–39]. Two smaller studies have previously reported a correlation between transfer factor and survival in stable COPD patients but only in univariate and not multivariate analysis [15, 16]. Martinez et al. [2] studied COPD patients who were randomised to the medical arm of the National Treatment Emphysema Trial (NETT) and found no association between TLCO % pred and survival. Although this was a larger population, it had been highly selected to meet the trial inclusion criteria for lung volume reduction surgery eligibility. Apart from these negative results, transfer factor has been identified as an independent predictor of survival in specific COPD subpopulations, such as patients receiving LTOT or noninvasive mechanical ventilation [40, 41].
Arterial oxygen partial pressure breathing room air was also a strong predictor of survival in this cohort. This is consistent with the findings of several previous studies, in which PaO2 values were positively associated with survival, both in COPD patients who were on LTOT [18, 23, 42] and those who were not [42]. Although some authors have failed to identify such an association in the past [8, 22], most of the latter studies were conducted in smaller, often non-general COPD populations and PaO2 was sometimes measured on supplementary oxygen, which could explain discrepant findings. In the present dataset, only 26% of the variance in PaO2 was explained by other lung function parameters. This supports the concept that PaO2 reflects an integration of factors, including ventilation perfusion mismatch and cardiac output, neither of which are well captured by conventional lung function measurements (airflow, lung volumes and gas transfer) but which may have implications for survival since cardiovascular morbidity and mortality is common in COPD [5].
Several parameters were not independently associated with survival in the current study. FEV1 was higher among survivors, but this difference was significant only in the univariate analysis, indicating that TLCO is a better prognostic indicator. Although there are previous studies where FEV1 was identified as a predictor of survival in COPD [3, 6, 43], several others have had findings similar to ours, since they failed to find such an association [9, 12, 24]. Furthermore, a recent review concluded that FEV1 can probably predict mortality better as a component of the BODE (BMI, airflow obstruction, dyspnoea and exercise tolerance) index, than as an individual parameter [33]. Casanova et al. [14] identified the IC/TLC ratio (or “inspiratory fraction”) as a predictor of mortality in a study which included a large number of COPD outpatients; however, TLCO was not measured in that cohort. In the only study in which the prognostic value of IC/TLC was assessed in combination with transfer factor, TLCO and not the inspiratory fraction was identified as an independent predictor of mortality [44], a finding which is confirmed by our results.
As in previous studies, older age was a predictor of reduced survival [11]. With a few exceptions [15, 22], this has been a consistent finding among both general COPD populations and COPD patients receiving LTOT [2, 17, 33, 40], though recently, of COPD phenotypes identified by cluster analysis, the one with the worst survival was younger patients with severe airflow limitation, a low BMI, frequent exacerbations and low rates of cardiovascular morbidities [45]. However, the most important finding regarding age in this study is that it can predict survival independently from the stage of the disease judged by the severity of airflow obstruction.
The present findings in COPD patients can also be considered in the light of data available from the general population suggesting that vital capacity rather than airflow obstruction is associated with mortality [46]. In a large population of people undergoing lung function for a wide range of indications, TLCO was the most powerful predictor of survival [47]. This study also observed some variation in the predictive power of lung function parameters depending on the equation used to generate predicted values, though it is unlikely that this would have significantly altered the present findings.
Methodological issues
The study was based on prospectively collected comprehensive clinical data from patients under hospital care for management of their COPD. Other studies in this area have tended to measure only a limited selection of lung function tests (e.g. in the ECLIPSE trial gas transfer was not measured) [25, 35], to have had highly selected populations (e.g. the “medical” arm of the NETT trial) [2], or to include small cohorts only [11, 44]. The single centre approach has minimised bias regarding different patient populations and various measurement techniques that might have occurred in multicentre cohorts. Usual clinical practice was for patients to undergo routine comprehensive lung function assessment, including blood gas analysis, so it is unlikely that the results are biased by any specific indication for lung function testing.
The use of the UK National Health Service central data spine allowed us to establish the vital status of all participants. Data on cause of death were not available which might have allowed us to refine causal hypotheses linking lung function to mortality. As described above, an issue in previous studies of factors associated with survival in COPD has been one of the generalisability of the population. We studied a hospital clinic population likely to have a predominance of emphysema. This might have influenced the association between gas transfer measurements and survival, since there is an established correlation between emphysema severity and TLCO. Some caution is needed in extrapolating outcomes to patients with milder disease, but the findings are likely to be applicable to secondary care patients in general.
We chose to define exacerbations as episodes of worsening of disease sufficient to cause patients to seek medical assistance and receive a prescription for antibiotics. This definition can, to some extent, be criticised as dependent on behaviour but has the merit of incorporating an element of “clinical significance”. Techniques such as diary cards have been developed and this remains an area of controversy [48] but, to date, no consensus exists in the literature as to which is the “gold standard”. It seems unlikely that a different definition would have caused any systematic difference in the results obtained.
Patients were diagnosed with COPD on clinical grounds, including an FEV1/FVC ratio of <0.7. The lower limit of normal for this ratio is now considered to be a more reliable threshold for diagnosing airflow obstruction. However, since the fixed ratio was the one that was used when patients were being diagnosed initially and entered onto the COPD database it would be inappropriate to change this retrospectively. In addition, the relatively small number of patients with mild airflow obstruction, where the risk of misclassification is highest, makes this unlikely to jeopardise the findings of the study.
The present study compares the prognostic power of different lung function tests in predicting mortality. The Medical Research Council dyspnoea score was not recorded systematically so it is not possible to calculate the ADO (age, dyspnoea and obstruction) score or BODE score (the latter also requires a walking distance to be measured) and direct comparison is not possible [49].
In conclusion, in a large, general COPD outpatient population where a complete range of lung function testing had been applied, gas transfer provided the best prediction of survival, together with age and PaO2. Spirometry, although easy to perform and low-cost, cannot offer as much information as gas transfer, which is also superior to plethysmographic lung volume measurements. An argument can therefore be made for performing measures of gas transfer in routine practice for patients with COPD to provide them with the best prognostic information, and future classifications of COPD should include TLCO and not rely merely on the severity of airflow obstruction. The results may also inform trial design, suggesting that gas transfer should also be used for stratification where therapies that are intended to influence mortality are to be tested.
Acknowledgments
We would like to thank the clinical physiologists in the lung function department at Royal Brompton Hospital, London, UK who performed the tests described in this paper.
Footnotes
This article has supplementary material available from www.erj.ersjournals.com
Support statement: The study was supported by NIHR Respiratory Biomedical Research Unit at Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London, UK.
Conflict of interest: None declared.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173: 1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Clin Chest Med 1990; 11: 555–569 [PubMed] [Google Scholar]
- 4.Kelly JL, Elkin SL, Fluxman J, et al. Breathlessness and skeletal muscle weakness in patients undergoing lung health screening in primary care. COPD 2013; 10: 40–54 [DOI] [PubMed] [Google Scholar]
- 5.Shrikrishna D, Hopkinson NS. Chronic obstructive pulmonary disease: consequences beyond the lung. Clin Med 2012; 12: 71–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonelli Incalzi R, Fuso L, De Rosa M, et al. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997; 10: 2794–2800 [DOI] [PubMed] [Google Scholar]
- 7.Gudmundsson G, Gislason T, Lindberg E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res 2006; 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133: 14–20 [DOI] [PubMed] [Google Scholar]
- 9.Schols AM, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1791–1797 [DOI] [PubMed] [Google Scholar]
- 10.Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest 2003; 123: 1460–1466 [DOI] [PubMed] [Google Scholar]
- 11.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62: 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003; 124: 459–467 [DOI] [PubMed] [Google Scholar]
- 13.Yildiz OA, Onen ZP, Sen E, et al. Predictors of long-term survival in patients with chronic obstructive pulmonary disease. Saudi Med J 2006; 27: 1866–1872 [PubMed] [Google Scholar]
- 14.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 591–597 [DOI] [PubMed] [Google Scholar]
- 15.Solanes I, Casan P, Sangenis M, et al. Factores de riesgo de mortalidad en la EPOC [Risk factors for mortality in chronic obstructive pulmonary disease.]. Arch Bronconeumol 2007; 43: 445–449 [DOI] [PubMed] [Google Scholar]
- 16.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest 2010; 138: 635–640 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440 [DOI] [PubMed] [Google Scholar]
- 18.Chailleux E, Fauroux B, Binet F, et al. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR Observatory. Chest 1996; 109: 741–749 [DOI] [PubMed] [Google Scholar]
- 19.Boutou AK, Karrar S, Hopkinson NS, et al. Anemia and survival in chronic obstructive pulmonary disease: a dichotomous rather than a continuous predictor. Respiration 2013; 85: 126–131 [DOI] [PubMed] [Google Scholar]
- 20.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 155–161 [DOI] [PubMed] [Google Scholar]
- 21.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD. Chest 2011; 140: 331–342 [DOI] [PubMed] [Google Scholar]
- 22.Gray-Donald K, Gibbons L, Shapiro SH, et al. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 961–966 [DOI] [PubMed] [Google Scholar]
- 23.Dallari R, Barozzi G, Pinelli G, et al. Predictors of survival in subjects with chronic obstructive pulmonary disease treated with long-term oxygen therapy. Respiration 1994; 61: 8–13 [DOI] [PubMed] [Google Scholar]
- 24.Almagro P, Calbo E, Ochoa de Echaguen A, et al. Mortality after hospitalization for COPD. Chest 2002; 121: 1441–1448 [DOI] [PubMed] [Google Scholar]
- 25.Celli BR, Locantore N, Yates J, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 185: 1065–1072 [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J 1993; 6: Suppl. 16, 5–40 [PubMed] [Google Scholar]
- 27.Zavorsky GS, Cao J, Mayo NE, et al. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol 2007; 155: 268–279 [DOI] [PubMed] [Google Scholar]
- 28.Clark EH, Woods RL, Hughes JM. Effect of blood transfusion on the carbon monoxide transfer factor of the lung in man. Clin Sci Mol Med 1978; 54: 627–631 [DOI] [PubMed] [Google Scholar]
- 29.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–555 [DOI] [PubMed] [Google Scholar]
- 30.Miller MR, Pedersen OF. New concepts for expressing forced expiratory volume in 1 s arising from survival analysis. Eur Respir J 2010; 35: 873–882 [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Pedersen OF, Dirksen A. A new staging strategy for chronic obstructive pulmonary disease. Int J Chron Obstr Pulmon Dis 2007; 2: 657–663 [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes JMB, Pride NB. Examination of the carbon monoxide diffusing capacity (DlCO) in relation to its Kco and Va components. Am J Respir Crit Care Med 2012; 186: 132–139 [DOI] [PubMed] [Google Scholar]
- 33.Celli BR, Cote CG, Lareau SC, et al. Predictors of survival in COPD: more than just the FEV1. Respir Med 2008; 102: Suppl. 1, S27–S35 [DOI] [PubMed] [Google Scholar]
- 34.Celli BR. Predictors of mortality in COPD. Respir Med 2010; 104: 773–779 [DOI] [PubMed] [Google Scholar]
- 35.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 2008; 31: 869–873 [DOI] [PubMed] [Google Scholar]
- 36.Budweiser S, Jorres RA, Riedl T, et al. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest 2007; 131: 1650–1658 [DOI] [PubMed] [Google Scholar]
- 37.Bang KM, Gergen PJ, Kramer R, et al. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest 1993; 103: 536–540 [DOI] [PubMed] [Google Scholar]
- 38.Coleta KD, Silveira LV, Lima DF, et al. Predictors of first-year survival in patients with advanced COPD treated using long-term oxygen therapy. Respir Med 2008; 102: 512–518 [DOI] [PubMed] [Google Scholar]
- 39.Lima DF, Dela Coleta K, Tanni SE, et al. Potentially modifiable predictors of mortality in patients treated with long-term oxygen therapy. Respir Med 2011; 105: 470–476 [DOI] [PubMed] [Google Scholar]
- 40.Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004; 23: 28–33 [DOI] [PubMed] [Google Scholar]
- 41.Dubois P, Machiels J, Smeets F, et al. CO transfer capacity as a determining factor of survival for severe hypoxaemic COPD patients under long-term oxygen therapy. Eur Respir J 1990; 3: 1042–1047 [PubMed] [Google Scholar]
- 42.MacNee W. Predictors of survival in patients treated with long-term oxygen therapy. Respiration 1992; 59: Suppl. 2, 5–7 [DOI] [PubMed] [Google Scholar]
- 43.Domingo-Salvany A, Lamarca R, Ferrer M, et al. Health-related quality of life and mortality in male patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 680–685 [DOI] [PubMed] [Google Scholar]
- 44.Moore AJ, Soler RS, Cetti EJ, et al. Sniff nasal inspiratory pressure versus IC/TLC ratio as predictors of mortality in COPD. Respir Med 2010; 104: 1319–1325 [DOI] [PubMed] [Google Scholar]
- 45.Burgel PR, Roche N, Paillasseur JL, et al. Clinical COPD phenotypes identified by cluster analysis: validation with mortality. Eur Respir J 2012; 40: 495–496 [DOI] [PubMed] [Google Scholar]
- 46.Burney PGJ, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax 2011; 66: 49–54 [DOI] [PubMed] [Google Scholar]
- 47.Ward H, Cooper B, Miller MR. Validation of lung function prediction equations from patient survival data. Eur Respir J 2012; 39: 1181–1187 [DOI] [PubMed] [Google Scholar]
- 48.Quint JK, Donaldson GC, Hurst JR, et al. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J 2011; 37: 501–507 [DOI] [PubMed] [Google Scholar]
- 49.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009; 374: 704–711 [DOI] [PubMed] [Google Scholar]


