Abstract
Background
Assay of T cell receptor excision circles (TRECs) in dried blood spots (DBS) obtained at birth permits population-based newborn screening (NBS) for severe combined immunodeficiency (SCID).
Objective
To report the first 2 years of TREC NBS in California.
Methods
Since August 2010, California has conducted SCID newborn screening. A high-throughput TREC qPCR assay using DNA isolated from routine DBS was developed. Samples with initial low TREC values had repeat DNA isolation with qPCR for TRECs and a genomic control, and immunophenotyping was performed within the screening program for infants with incomplete or abnormal results. Outcomes were tracked.
Results
Of 993,724 infants screened, 50 (1/19,900; 0.005%) had significant T cell lymphopenia. Fifteen (1/66,250) required hematopoietic cell or thymus transplantation or gene therapy; these infants had typical SCID (11), leaky SCID or Omenn syndrome (3), or complete DiGeorge syndrome (1). Survival to date in this group is 93%. Other T lymphopenic infants had variant SCID or combined immunodeficiency (6), genetic syndromes associated with T cell impairment (12), secondary T lymphopenia (9) or preterm birth (8). All T lymphopenic infants avoided live vaccines and received appropriate interventions to prevent infections. TREC test specificity was excellent: only 0.08% of infants required a second test and 0.016% required lymphocyte phenotyping by flow cytometry.
Conclusions
TREC NBS in California has achieved early diagnosis of SCID and other conditions with T lymphopenia, facilitating management and optimizing outcomes. Furthermore, NBS has revealed the incidence, causes and follow-up of T lymphopenia in a large, diverse population.
Keywords: Severe combined immunodeficiency (SCID), newborn screening (NBS), T cell receptor excision circle (TREC), T cell lymphopenia (TCL), DiGeorge syndrome
Introduction
Severe combined immunodeficiency (SCID), characterized by poor T cell production and failure of B cells to generate protective antibodies, is the most profound primary immunodeficiency and is fatal unless affected infants receive an adaptive immune system through allogeneic hematopoietic cell transplantation (HCT), adenosine deaminase (ADA) enzyme replacement, or gene therapy. Early detection of SCID is a key to successful treatment. In a retrospective series, infants diagnosed and treated with HCT in the first 3.5 months of life had improved survival and reduced morbidity.1 However, most infants with SCID lack a family history or clinical clues to permit diagnosis prior to the onset of infections, making this life-threatening condition a candidate for population-based newborn screening (NBS).2–5 With high burden on affected individuals, effective treatments, and availability of inexpensive high-throughput assay, SCID fits accepted criteria for population-based screening. The United States Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children recommended adding SCID to the uniform panel of NBS diseases; this recommendation was approved in May, 2010.6 Several states throughout the US now perform SCID NBS.7–9 California, the state with the most births and high ethnic diversity, began SCID NBS in August 2010.
Measurement of T-cell receptor excision circles (TRECs) to approximate thymic output was first used in HIV-1 infection10–12 and hematological malignancies.13 Adaptation for dried blood spotted onto filter paper made possible a TREC assay for SCID and other conditions with T cell lymphopenia (TCL),8,14 utilizing the dried blood spots (DBS) already universally obtained by heel-stick from infants in the first days of life to screen for metabolic diseases, hypothyroidism, hemoglobinopathies and cystic fibrosis. TREC copy number measured by a quantitative polymerase chain reaction (qPCR) is a biomarker for thymic output of naïve T cells.14,15 Low TRECs thus identify infants with SCID as well as other non-SCID immunodeficiencies in which there is a profound decrease in circulating naïve T cells.
Wisconsin started SCID NBS in 2008; that state’s findings, including 5 cases of SCID in >200,000 screened infants, have been published.9,16 Massachusetts has also instituted SCID NBS and reported cases.17 Here we summarize California’s first 2 years of screening nearly one million newborns. We present TREC data correlated with lymphocyte profiles and characteristics of 50 infants with T cell lymphopenia, including 15 cases of typical and leaky SCID and complete DiGeorge syndrome that required immune restoring therapy. We established the birth prevalence of SCID compared with other NBS diseases in California.
Methods
Specimens Tested
DBS specimens were collected from all infants born in California except those whose parents opted out for religious reasons and completed a form accepting responsibility for any harm coming to the child as a result of refusal to test. After completing other tests at regional laboratories, DBS were sent to the California Department of Public Health (CDPH) Genetic Disease Laboratory (GDL) (Richmond, CA) for TREC testing and archiving. An initial pilot was conducted under a waiver of review from the CDPH Committee for Protection of Human Subjects until SCID NBS was mandated by legislation, effective January, 2012. Specimens were tested a mean of 20 days after the infants’ birth, and non-normal results were usually reported when infants were 3–4 weeks of age. Follow-up complete and differential blood counts (CBC) and lymphocyte subset analyses were performed at a single site (Quest Diagnostics, Inc., San Juan Capistrano, CA) as a confirmatory test within the SCID NBS program. Lymphocyte phenotypes were interpreted by CDPH Immunology Consultants (JAC, SAM and JMP).
For details of DNA extraction and determination of TREC and control β-Actin gene copy number see the JACI Online Repository.
Statistical analysis
Infant ethnicity recorded from parental designation of 19 categories was used for calculations, grouping ethnicities to achieve large enough pools for analysis: Hispanic, White (non-Hispanic), Asian, Black, Native American or Other. Rates and projections from current data were performed using the normal approximation, except where low case numbers required use of the Poisson distribution.
Results
California SCID NBS testing
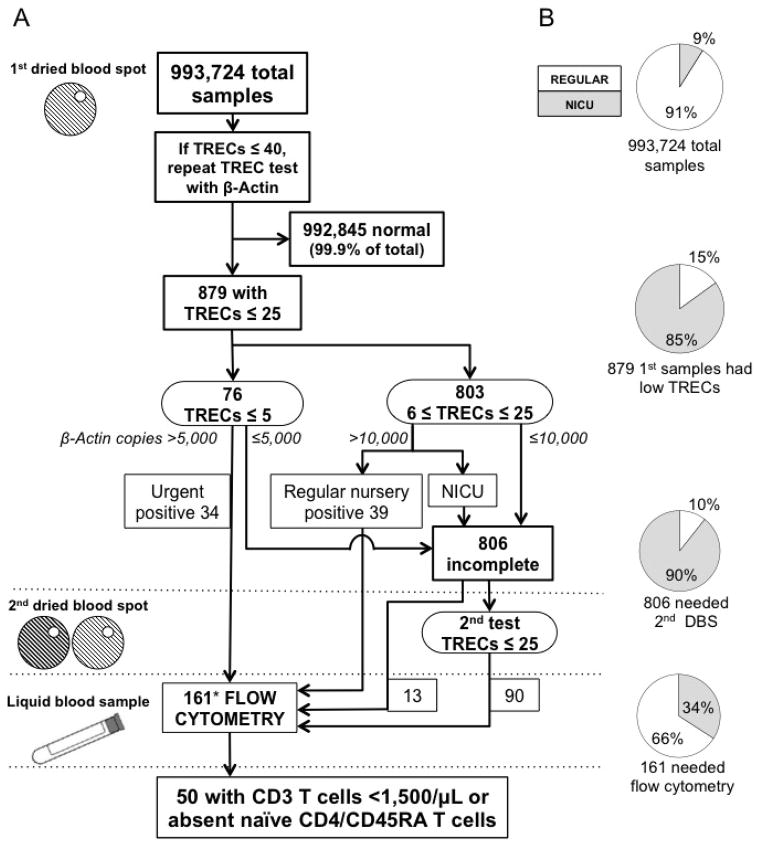
In 2 years, 993,724 infants were screened (Fig. 1; also see Figure E1 in the Online Repository). Numbers with each screening result are in Fig. 1A, while proportions from regular nurseries vs. neonatal intensive care units (NICUs) are in Fig. 1B. Only 879 infants (<0.1% of those screened) had an initial TREC value below the acceptable cutoff, with a disproportionate contribution of 85% from NICUs. Urgent positive samples, those with undetectable or 1–5 TRECs/μL of blood with normal control β-Actin copies, triggered immediate recall of 34 infants for flow cytometry, also indicated for 39 more infants with initial positive samples (6–25 TREC copies, normal β-Actin).
Figure 1.
A) California SCID NBS. Sample numbers are shown at each stage (boxes) according to TREC and β-Actin and nursery care as proxy for clinical status. [Also see Online Repository Fig. E1]. Infants with low TRECs on 2 DBS or positive initial results were recalled to receive flow cytometry. B) Distribution of regular nursery vs. neonatal intensive care unit (NICU) samples at each NBS stage.
*14 infants deceased, 1 lost to follow-up before flow cytometry.
A second DBS was requested from 806 infants (0.08% of all screened) because of initial samples with low TRECs, but also low β-Actin (designated “DNA amplification failure”), or low TRECs in NICU infants (designated “incomplete”, Fig. 1A, Fig. E1). Although providers were instructed to adopt immune system precautions pending further testing, the terms “DNA amplification failure” and “incomplete” were reported to indicate a likely technical problem with the sample or assay, rather than to imply that the infant was affected with an immune disorder at this stage of screening. As with initial non-normal samples, NICU samples predominated, accounting for 90% of requests for a second DBS (Fig. 1B). Only 11% of second samples, obtained when infants were between 3–4 weeks of age, were persistently abnormal. Infants with 2 DBS samples with low TRECs, irrespective of β-Actin copy number, were recalled for venous blood immunophenotyping (Fig. 1A, Fig. E1).
Thirteen infants had flow cytometry following only a single abnormal result due to clinical suspicion of an immune disorder. Eleven NICU infants with initial positive and 3 with repeated low TREC results died before flow cytometry could be performed, while one was lost to follow-up after 2 low TREC results; these 15 infants had serious medical conditions that could be associated with low T cell numbers, as discussed below. The 161 infants who underwent immunophenotyping by flow cytometry represented 1/6,200 births, with 106 (66%) from NICUs.
Lymphocyte profiles
Confirmatory CBC and lymphocyte subset analysis were an integral part of the SCID NBS program. Venous blood was obtained at a mean of 3–4 weeks after the initial DBS. TRECs increase during early postnatal life;14,16 thus this interval allowed for resolution of TCL in unaffected infants with little risk to SCID-affected infants, who are initially protected by transplacentally acquired maternal IgG.
Three pediatric immunologists interpreted all lymphocyte profiles for infants with abnormal TRECs. Although the normal range for newborns was 2,550–5,000 CD3 T cells/μL, we focused on those with either TCL of likely clinical significance, defined as <1,500 CD3 T cells/μL, or absence or marked reduction in CD4 naïve T cells (CD4/CD45RA), defined as <5% total CD3 T cells. Of infants classified “positive” by TREC test, 44% were abnormal by flow cytometry, as were 23% with “incomplete” TREC tests (Table I). False positives, samples that were “positive” or “incomplete” by TREC test but subsequently normal by flow cytometry (111 samples) made up only 0.01% of all the samples tested. Abnormalities in neutrophils, B cells and NK cells were noted when present.
Table I.
Non-normal TREC results and their associated flow cytometry results during the first 2 years of SCID NBS in California.
| Dried blood spot result | Liquid blood lymphocyte profile | ||
|---|---|---|---|
| Indicative of a T cell defect (T cells <1,500/μL or absent naïve CD4/CD45RA cells) | Not indicative of a significant T cell defect (T cells ≥1,500/μL; naïve T cells present) | Total | |
| Positive | 27 (includes all SCID cases) | 35 | 62a |
| Incomplete | 23 | 76 | 99b |
| Total | 50 | 111 | 161 |
11 infants died prior to flow cytometry, out of 73 infants initially identified as positive by TREC test (Fig. 1)
90 infants had persistent low TRECs; 3 died and 1 was lost to follow up prior to flow cytometry confirmation. Also included are 13 infants who proceeded to flow cytometry after only a single abnormal TREC test result, due to logistical reasons or physician requests (Fig. 1).
Categories of diagnosis among infants with T lymphopenia
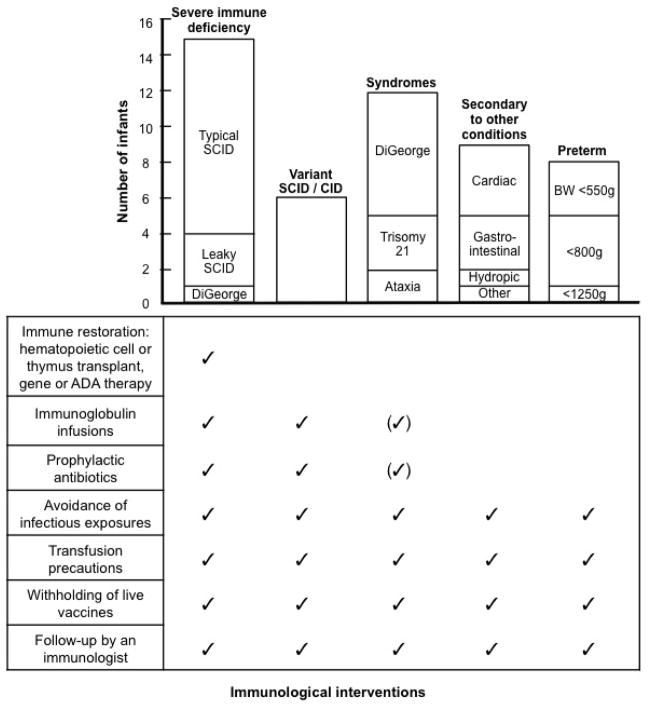
Of 161 liquid blood samples, 50 (31%) had TCL (T cells <1,500/μL), or 1/19,900 births. These infants had a spectrum of diagnoses (Table II, Table E2 in Online Repository). Typical SCID, leaky SCID and variant SCID had a combined incidence of 1 in 49,700 births, with individual incidences of 1/90,000, 1/331,000 and 1/166,000, respectively. Categories of severity were established reflecting the degree of lymphopenia and the extent of immunological intervention required (Fig. 2). As shown, all infants with TCL received immunological follow-up and instruction to avoid infectious exposures and live vaccines and to exercise transfusion precautions. Infants with multi-system congenital syndromes accompanied by TCL, as well as those with combined immunodeficiency (CID) or variant SCID, who had demonstrable T cell defects but no mutation in typical SCID genes, further required immunoglobulin infusions and antibiotic prophylaxis, initially or on a continuous basis, as dictated by their immunologic status.
Table II.
Infants with low TRECs and T cell lymphopenia who required immune restoring therapy.
| SCID case # | Sex | Ethnicity | Nurserya | TREC copies | Lymphocyte phenotype | CD3 T cells | CD4 CD45RA T cells | Disease causing gene (mutations)b | Presenting clinical informationc |
|---|---|---|---|---|---|---|---|---|---|
| CA007 | M | Black | NICU | 0/μL | T− B+ NK− | 493/μL | <20/μL | IL2RG (R226C18) | 35 week gestation, prenatal diagnosis (older affected brother). Asymptomatic, infection-free; maternal engraftment present; PHA <10%. |
| CA053 | M | Hispanic | Regular | 0 | T− B+ NK− | <20 | <20 | IL2RG (M270fs) | Asymptomatic, infection-free, XY/XO mosaic; no maternal engraftment, PHA <10%. |
| CA096 | M | Asian | NICU | 0 | T− B+ NK− | 20 | <20 | IL2RG (W177X) | 1 of triplets, 33 week gestation, asymptomatic, infection-free; maternal engraftment present; PHA <10%. |
| CA125 | M | Hispanic | Regular | 0 | T− B+ NK− | 78 | 23 | IL2RG (L132R) | Asymptomatic, infection-free; maternal engraftment present; PHA <10%. |
| CA103 | F | Hispanic | Regular | 4 | T− B+ NK− | 1736 | <20 | JAK3 (L527_G528del) | Rash and cellulitis; maternal engraftment present; PHA not tested. |
| CA004 | F | Hispanic | Regular | 0 | T− B+ NK+ | 60 | 49 | IL7R (F71S, H180P) | Asymptomatic, infection-free; maternal engraftment present; PHA <10%. |
| CA005 | F | Hispanic | Regular | 0 | T− B+ NK+ | <20 | <20 | IL7R (I94fs, H180P) | Asymptomatic, infection-free, but acquired coxsackie virus at home pre-HCT; maternal engraftment present, PHA <10%. |
| CA067 | F | Hispanic | Regular | 0 | T− B+ NK+ | <20 | <20 | IL7R (G215V) | Asymptomatic, infection-free; maternal engraftment present, PHA <10%. |
| CA013 | M | Hispanic | Regular | 0 | T− B− NK+ | <20 | <20 | RAG1 (M661del) | Asymptomatic, infection-free, maternal engraftment not tested; PHA not tested. |
| CA027 | M | Hispanic | Regular | 0 | T− B− NK+ | 28 | <20 | RAG1 (R404Q19 R764P) | Rash coinciding with TREC report, deceased affected brother, infection-free; maternal engraftment present; PHA not tested. |
| CA177 | M | Hispanic | NICU | 0 | T− B+ NK+ | 80 | 3 | unknownd | 37 week gestation, maternal diabetes & hepatitis. Low glucose, respiratory distress, hepatomegaly, feeding difficulties; maternal engraftment present; PHA <10%. |
| CA024 | F | White | Regular | 20 | T− B− NK+ | 1583 | 24 | RAG1 (W522C20, N968K) | Asymptomatic, infection-free; no maternal cells; PHA not done. Omenn syndrome. |
| CA174 | F | Hispanic | Regular | 0 | T− B− NKlow | 51 | 3 | RAG1 (R474C21, I956T22) | Asymptomatic, infection-free, but acquired rhinovirus at home pre-HCT; no maternal cells; PHA 50%. Leaky SCID. |
| CA158 | F | White | Regular | 19 | T− B+ NK+ | 380 | 99 | RMRP I (r.211c>g, 262g>u)23 | Intrauterine growth retardation, short limbs; no maternal cells; PHA 40%. Leaky SCID, cartilage hair hypoplasia. |
| CA019 | F | Hispanic | NICU | 0 | T− B+ NK+ | <20 | <20 | 22q11 deletion | Complete DiGeorge syndrome with congenital heart disease. |
| SCID case # | Treatmente / donor (match) / cell type | Age at HCT(s) | Conditioningf | Graft vs. host disease prophylaxisg | Clinical courseh | Current status (age)i |
|---|---|---|---|---|---|---|
| CA007 | HCT, CD34-selected maternal cryopreserved mobilized peripheral blood | 3 wk | None | None | Acute skin graft vs. host disease (GvHD); T cell engraftment | Alive (28 mo), vitiligo, on IVIG |
| CA053 | HCT, MUD (10/10) bone marrow | 2.5 mo | G-CSF, Plerixafor | CsA | Slow T cell recovery, →boost | Alive (21 mo), on IVIG |
| Boost: 4 mo | None | CsA, MTX, tacrolimus | T cell engraftment | |||
| CA096 | HCT, maternal CD34-selected peripheral blood | 8 wk | None | None | Slow T cell recovery, →boost | Alive (15 mo), on scIG |
| Boost: 4 mo | None | CsA, methylprednisolone | T cell engraftment | |||
| CA125 | Autologous transduced CD34 cell gene therapy (trial #NCT01129544) | 4 mo | None | None | Failed T cell engraftment → unrelated donor cord blood HCT, T cell engraftment | Alive (13 mo), on IVIG, |
| CA103 | HCT, MUD (10/10) bone marrow | 4 mo | Flu, Bu, rATG, KGF | MTX, tacrolimus, methylprednisolone | T cell engraftment | Alive (14 mo), on IVIG |
| CA004 | HCT, maternal CD34-selected peripheral blood | 2 mo | Plerixafor | None | T cell engraftment and B cell function | Alive (28 mo), off IVIG, given live vaccines |
| CA005 | HCT, MUD (10/10) bone marrow | 4.5 mo | Flu, Bu, Cytoxan | Tacrolimus, MTX | T cell engraftment and B cell function | Alive (28 mo), off IVIG |
| CA067 | HCT, maternal CD34-selected peripheral blood | 1.5 mo | None | None | Slow T cell recovery, →boost | Alive (17 mo), on scIG |
| Boost: 5 mo | None | None | T cell engraftment | |||
| CA013 | HCT, matched sibling bone marrow | 3.5 mo | rATG x3 | Tacrolimus | Autologous recovery, →2nd HCT | Alive (27 mo), off IVIG |
| 2nd HCT: 9 mo | Flu, Bu, rATG | Tacrolimus | T cell engraftment | |||
| CA027 | HCT, unrelated (7/10) umbilical cord blood | 4 mo | Flu, Bu, rATG | Tacrolimus, methylprednisolone | Veno-occlusive disease (d+28), pulmonary hemorrhage | Died day +46 (at 5 mo) post transplant |
| CA177 | HCT, MUD (10/10) bone marrow | 4 mo | Flu, Bu, rATG, KGF | Tacrolimus, MTX, methylprednisolone | Acute skin and gut GvHD; T cell engraftment | Alive (7 mo), on IVIG |
| CA024 | HCT, MUD (9/10) T cell-depleted mobilized peripheral blood | 3.5 mo | Flu, Bu, Campath | CsA, MMF | Slow T cell recovery, →boost | Alive (25 mo), on IVIG |
| Boost: 7 mo | Flu, ATG | CsA, MMF | T cell engraftment | |||
| CA174 | HCT, MUD (9/10) bone marrow | 4.5 mo | Flu, Bu, Campath | MTX, CsA | T cell engraftment | Alive (7 mo), on IVIG |
| CA158 | HCT, MUD (10/10) bone marrow | 7 mo | Flu, Bu, Campath | MTX, CsA | T cell engraftment | Alive (9 mo), on IVIG |
| CA019 | Thymus transplant | 12 mo | Bu | None | Immune reconstitution; heart surgery after transplant | Alive (26 mo), off CsA, on IVIG |
NICU: neonatal intensive care unit.
Single mutation: hemizygous in IL2RG, homozygous for other genes, heterozygous for 22q deletion. Two mutations: compound heterozygotes. Previously reported mutations are referenced with superscript numbers; others not published to date.
Proliferative response in vitro to phytohemagglutinin, <10% of healthy control range is a criterion for diagnosis of typical SCID; 10–50% consistent with leaky SCID.
No damaging variants found in ADA, AK2, CD3D, CD3E, CD3Z, DCLRE1C, IL2RG, IL7R, JAK3, LIG4, NHEJ1, PNP, PTPRC, RAC2, RAG1, RAG2, RMRP, ZAP70.
HCT, hematopoietic cell transplant; MUD, matched unrelated donor.
G-CSF, granulocyte colony stimulating factor; Flu, Fludarabine; Bu, Busulfan; rATG, rabbit anti-thymocyte globulin; KGF, keratinocyte growth factor.
CsA, cyclosporine A; MTX, methotrexate; MMF, mycophenolate mofetil.
No major infectious complications.
IVIG, intravenous immunoglobulin; scIG, subcutaneous immunoglobulin.
Figure 2.
T cell lymphopenia (TCL) by intervention and underlying condition. Cases requiring immune restoring therapy included 11 typical and 3 leaky SCID and 1 complete DiGeorge syndrome. There were 6 variant SCID, 12 congenital syndromes, 9 secondary TCL, and 8 preterm infants (weight ranges shown). All were advised to avoid live rotavirus and other live vaccines and to receive only CMV negative, irradiated blood products.
The 15 most severely affected infants required immune restoring therapies, including 11 with typical SCID, 3 with leaky SCID or Omenn syndrome, and one with complete DiGeorge syndrome. Diagnostic criteria of the Primary Immune Deficiency Treatment Consortium (PIDTC) were used for typical SCID, <300 autologous T cells/μL, often with maternally derived T cells, with in vitro responses to phytohemagglutinin (PHA) <10% of control; and leaky SCID/Omenn syndrome, no maternal cells, but autologous, oligoclonal, CD45RO memory phenotype T cells with PHA responses that could be >10%, but still below normal. In both typical and leaky SCID, specific gene mutations provided supporting evidence. Infants suspected to have typical or leaky SCID were immediately hospitalized at primary immunodeficiency centers and after further evaluation were treated with HCT, gene therapy or thymus transplantation (Fig. 2, Table II). Importantly, only one infant with typical SCID had a recognized family history that had led to prenatal diagnosis with immediate confirmation at birth (CA007, Table II); one other infant, CA027, had lost an older brother in infancy, whose retrospective diagnosis of SCID was recognized only after newborn screening identified our patient.
Specific diagnoses and treatment of infants identified by NBS
Table II summarizes information for the 15 infants who required immune restoring therapies. Within the 11 typical SCID diagnoses, 10 had undetectable TRECs, while one had 4 TRECs and extensive maternal engraftment with 1,736 T cells, but no naïve CD4/CD45RA T cells. Four males with a T−B+NK− phenotype had defects in the X-linked IL2RG gene encoding the cytokine receptor common γ chain. CA007 had a previously reported recurrent mutation (R226C),18 while previously unpublished mutations were found in CA053, CA096 and CA125, a frameshift (M270fs), nonsense (W177X), and predicted damaging missense mutation (L132R), respectively. CA103 with T−B+NK− SCID had an in-frame JAK3 deletion (L527_G528del) previously noted in an unrelated case (J. Puck, unpublished). Three females with T−B+NK+ SCID had homozygous or compound heterozygous mutations in IL7R encoding the IL-7 receptor α chain, including a frameshift (I94fs), and 3 novel missense mutations. Two males had RAG1 defects, one previously reported damaging (R404Q),19 one unreported but predicted damaging (R764P), and one novel homozygous deletion of a single codon (M661del). Infant CA177 had T−B+NK+ SCID with no molecular diagnosis.
Of the 3 infants with leaky SCID and Omenn syndrome, CA174 had undetectable TRECs and 50% of normal PHA response, while CA024 and CA158, the latter of whom also had dwarfism due to cartilage hair hypoplasia (CHH), had 20 and 19 TRECs, respectively. CA024 with Omenn syndrome was a compound heterozygote with one novel RAG1 defect and another RAG1 mutation previously associated with Omenn syndrome (W522C),20 with <5% naïve CD4/CD45RA T cells and no maternal engraftment. Infant CA174 had 51 T cells/μL, >10% of control responses to mitogens, and mutations of RAG1 (R474C, I956T) previously associated with leaky SCID or Omenn phenotypes.21,22 Infant CA158 had previously recognized heterozygous, single nucleotide mutations in RMRP causing CHH;23 she was the only infant in the severely affected group with any autologous naïve CD4/CD45RA T cells, underscoring the utility of combining TREC copy number, total CD3 T cell count and naïve phenotype markers on CD4 and CD8 T cells to characterize infants requiring urgent immune restoring therapy.
In addition, female CA019 had complete DiGeorge syndrome with undetectable TRECs, absent thymus and absent T cells. Apart from this infant, who received a thymus transplant, and CA125, a male with X-linked SCID who initially received gene therapy, all others received allogeneic HCT according to protocols in place at the individual treatment centers. One infant, CA027, with RAG1 SCID, died 46 days post transplant of irreversible hepatic sinusoidal obstruction syndrome, a result of myeloablative conditioning with busulfan. All others are alive, engrafted and healthy. Four patients, 2 with X-linked SCID, 1 with IL-7Rα SCID and 1 with RAG1-deficient Omenn syndrome required booster infusions of donor cells, while infant CA125 did not engraft autologous gene-corrected HCT and required subsequent conditioning and an allogeneic cord blood transplant. While some of the patients are still early post-transplant and others continue to require immunoglobulin, 3 have had full B cell reconstitution and no longer receive immunoglobulin.
Infants assigned to the remaining categories of newborn TCL diagnoses are in Table E2. Six had variant SCID/combined immunodeficiency (CID), defined as TCL with functional T cell impairment for >3 months, without syndromic features or defects in a known SCID gene. Four had positive and 2 had incomplete TREC tests; their other immunologic features and treatments are listed. This category was unanticipated prior to NBS for SCID, but invites a search for new immunodeficiency diagnoses.
Also in Table E2 are 15 infants with congenital syndromes with variable degrees of T cell impairment; 8 had chromosome 22q11.2 interstitial deletion causing partial DiGeorge syndrome (with low but detectable T cells, in contrast to the complete DiGeorge patient discussed above). While 4 were recognized immediately after birth by their congenital cardiac malformations, one was diagnosed only after SCID NBS revealed his TCL. In addition, 4 infants had TCL associated with trisomy 21 and one had CHARGE (ocular coloboma, heart anomaly, choanal atresia, retardation, genital and ear anomalies) syndrome. While DiGeorge and CHARGE syndromes and trisomy 21 are often identified by their characteristic facies and clinical features, non-immunologists may fail to appreciate the degree of TCL immunodeficiency that may be present; thus NBS has identified the subset of infants with these conditions who were particularly affected by TCL and thus at risk for infections. Three of these infants died of complications not associated with the immune system prior to having lymphocyte enumeration.
NBS also flagged 2 apparently healthy infants with ataxia telangiectasia (AT) and newborn TCL, who were initially considered to have variant SCID.24 A study of newborn DBS archived from California cases of AT showed that >50% would have been identified by NBS with the TREC test as currently performed here.24 TCL was also found secondary to congenital or postnatal major congenital heart disease (6 cases), gastrointestinal malformations (3 cases), hydrops (2 cases), multiple anomalies without a unifying diagnosis (2 cases) and chylothorax (1 case). See Online Table E2 for further details.
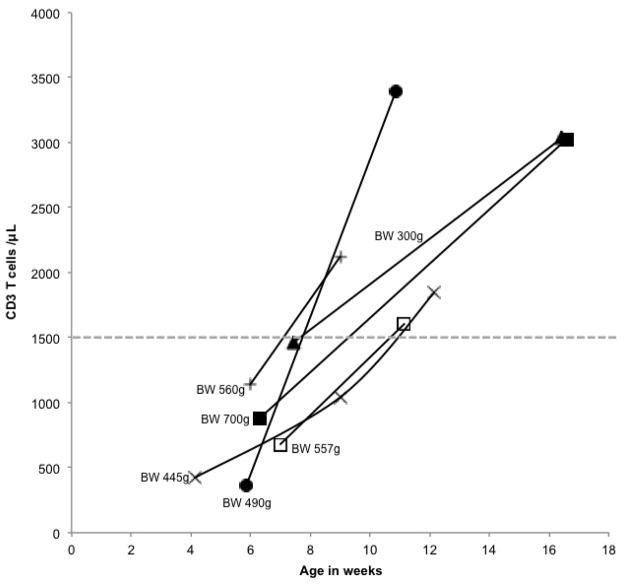
A final group of 8 infants with TCL were born at 24–27 weeks’ gestation with birth weights of 300–1200g (Table E2). All had incomplete TREC results. For 6 surviving infants subsequent lymphocyte profiles demonstrated improvement in T cell numbers over time (Fig. 3).
Figure 3.
T cell concentrations in infants with initial T cell lymphopenia associated with preterm birth, obtained at gestational ages on the X-axis. Birth weights are indicated. Subsequent T cell concentrations were >1,500 cells/μL, dotted line, the threshold set by California NBS program.
Demographics of T lymphopenias in California
TREC NBS data combined with self-reported ethnic origins permitted estimates of incidence of SCID and TCL for California’s ethnically diverse population. Using the pre-NBS SCID incidence estimate of 1/100,000 from the literature,14 California Hispanic and Asian populations had higher than expected numbers of SCID diagnoses (including typical, leaky and variant SCID), with respect to proportion of total births (p <0.001 and p <0.05 for Hispanics and Asians, respectively) (Table III). We also considered the incidence of non-SCID TCL secondary to clinical syndromes, congenital malformations or other non-immune conditions (excluding preterm birth alone in which TCL resolved with time), estimating incidence at 1 in 50,000 births; Asian, Black and Native American populations had higher numbers of TCL than expected, with respect to proportion of total births (p <0.01, p <0.002, and p <0.001, respectively) (Table III).
Table III.
Ethnic composition of infants with low TRECs and T cell lymphopenias.
| Ethnicity | SCID, Leaky SCID, Omenn Syndrome, Complete DiGeorge, and Variant SCID | Other T cell lymphopenias: Syndromes with T cell impairment and Secondary T cell lymphopenia excluding preterm birth alone | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number observed | Number expected with incidence 1/100,000 | p-valuea | Number observed | Number expected with incidence 1/50,000 | p-valuea | |
|
|
||||||
| Hispanic | 13 | 4.85 | <0.001 | 9 | 9.70 | NS |
| White | 4.5 | 2.52 | NS | 8 | 5.04 | NS |
| Asian | 2.5 | 0.81 | <0.05 | 5 | 1.63 | <0.01 |
| Black | 1 | 0.53 | NS | 6 | 1.06 | <0.0002 |
| Native American | 0 | 0.09 | NS | 2 | 0.18 | <0.001 |
| Other | 0 | 0.54 | NS | 1 | 1.08 | NS |
|
| ||||||
| Total | 21 | 29 | ||||
NS, not significant.
Discussion
Newborn screening programs present a number of ethical and public health questions. Justifications offered in support of newborn screening include early diagnosis, improved treatments, and lives saved. Decisions to institute a new NBS test are tied to seriousness of the disorder, incidence, detectability by a sensitive and specific assay, treatment availability and effectiveness, and outcomes and costs of treatment in the presence vs. absence of screening. These factors must be judged to outweigh monetary costs, needless anxiety engendered by false positive results, and workload imposed on medical practices. In 2006, recommendations developed to bring uniformity to NBS led to a core panel of 29 diseases for NBS, widely adopted by the states.25 SCID was the first disease to be added to this federal uniform panel by evidence-based review and cost effectiveness analyses.6,26 At this time 10 states, Puerto Rico and the Navajo Nation screen all births for SCID, with several other states planning to add the TREC test to their NBS panels (A. Brower, NBSTRN, personal communication). Actual data on the performance of SCID NBS in large populations, such as that presented here, will inform decisions of additional programs regarding adoption of the TREC test.
SCID NBS with the TREC assay in California
In California, nearly one million infants were screened over 2 years. Tracking of cases encompassed both SCID disorders, the primary target, and clinically significant TCL with <1,500 autologous T cells/μL as a secondary target. The 2-year cohort included 50 infants with SCID and TCL, or 0.5/10,000 births. When compared to data for the 29 other core NBS conditions in California, all TCL disorders would have placed 8th in frequency (Table E3). Furthermore, the disorders that require urgent immune restorative therapies (SCID, leaky SCID, Omenn syndrome and complete DiGeorge syndrome) would have ranked 15th at 0.13/10,000 births (Table E3). Thus, SCID and TCL occur more frequently than half of the conditions originally accepted in the uniform federal panel for NBS.
The performance of the TREC test was also excellent in comparison with other NBS tests; <0.1% of infants screened were non-normal, 0.08% with incomplete results needing a repeat TREC test, and 0.016% needing venous blood for immunophenotyping. Positive predictive value was high, with 31% of flow cytometry tests confirming <1,500 T cells/μL. Infants in NICUs constituted 9% of total California births, but had disproportionately frequent non-normal TREC results (Fig. 1B). The preponderance of non-normal first tests in NICU infants has been noted by other states using the TREC test,16,27,28 but is also found for other NBS tests. Rather than collect a series of repeated DBS samples from NICU infants, we elected to perform lymphocyte enumeration after 2 non-normal DBS samples; this was cost-effective because of the timing of the 2 DBS samples roughly 3 weeks apart, the small overall number of venous blood samples needed, and having a single laboratory for flow cytometry.
We believe the designated TCL definition of <1,500 T cells/μL as a secondary target of TREC screening captures infants with significant immune compromise who should avoid live rotavirus vaccination and receive evaluation and management by pediatric immunologists on an individualized basis. Of the infants with non-SCID TCL confirmed by flow cytometry, 76% were cared for in NICUs, in contrast to the infants with typical SCID, leaky SCID and variant SCID, of whom 76% were in regular nurseries. Thus TCL was identified as a significant problem for NICU infants, often accompanying an underlying condition.
Advantages of early SCID diagnosis
Typical SCID, leaky SCID, Omenn syndrome and complete DiGeorge syndrome are life-threatening disorders that require emergent immune restorative treatment. However, only one infant with typical SCID in our series had a recognized positive family history (Table II), while 2 others had presented to a hospital with cellulitis or rash. Thus the early diagnosis of SCID through NBS allowed specific treatments (immunoglobulin and prophylactic antimicrobial agents) to be instituted early to avoid infections and maintain health, crucial to the success of all subsequent immune restoring therapies.
Admission to specialized SCID treatment centers, management of pre-HCT treatment and timing of HCT depended on family circumstances, identification of an appropriate donor and center-specific protocols. Through enrollment in current PIDTC protocols, our patients’ outcomes will help shape future clinical trials to determine optimal treatments for SCID diagnosed by newborn screening. HCT treatments ranged from an infusion of cryopreserved maternal CD34-enriched cells at 3 weeks of age with neither conditioning nor prophylaxis for graft versus host disease (GvHD) in the infant with X-linked SCID and an affected brother, to a female infant who received conditioning and GvHD prophylaxis for a matched unrelated donor bone marrow transplant at 7 months of age for leaky SCID secondary to cartilage hair hypoplasia. One infant received experimental gene therapy for X-linked SCID due to an IL2RG mutation, though he required a subsequent cord blood transplant due to failure of autologous transduced cells to engraft. One infant with complete DiGeorge syndrome received a thymus transplant at 12 months of age.
Fourteen of the 15 infants who required immune restoration are currently alive, engrafted and healthy at 4 to 27 months after their most recent treatment. Survival of these infants after detection by NBS has been 93%, comparable to retrospective studies reporting increased survival of SCID infants detected at birth due to a prior positive family history.3–5 Although the Duke University retrospective data for SCID HCT indicated best survival for infants transplanted before the age of 3.5 months,7 our infants identified by newborn screening have excellent survival at older transplant ages because of prompt institution of measures to avoid infections.
Advantages of diagnosis of T cell lymphopenias
TREC newborn screening revealed a wide spectrum of non-SCID disorders with TCL as a neonatal feature (Fig. 2). The California cutoff values of <40 TRECs/μL for having the qPCR re-run, and ≥25 TRECs/μL on a repeat determination with normal β-Actin were selected by testing known positive cases and anonymous DBS from different nursery settings. Similarly, 1,500 T cells/μL was below the reference lower limit of 2,550 T cells/μL for term healthy newborns, but was chosen as a prudent value to capture significant TCL without incurring a large burden of NICU infants without true immune compromise. These parameters have allowed infants at the most T lymphopenic end of the spectrum of syndromic disorders to be identified, whether they had underlying DiGeorge syndrome or an unanticipated number of other syndromes including trisomy 21, ataxia telangiectasia,24 cartilage hair hypoplasia, or CHARGE syndrome. Also unexpected were the congenital defects (cardiac and gastrointestinal malformations, hydrops, chylothorax) that resulted in T cell lymphopenias, though in these cases the T cell numbers improved when the defects were repaired, suggesting either suppression of lymphopoiesis due to illness or intrinsically normal rates of thymic T cell production but increased loss or destruction peripherally. Taken together, these cases highlight the importance of TCL as a signal of infection risk and a critical feature of a broad range of genetic syndromes and congenital malformations. Live vaccine avoidance and infection prophylaxis for these infants believed prudent at this time can be provided when they are identified by TREC NBS.
Furthermore, discovery of previously unsuspected cases of TCL in otherwise healthy infants has made possible investigation of heretofore unknown cases, designated as variant SCID. Eight infants without mutations in known SCID genes were initially allocated to this group, with 2 reclassified when found to have ataxia telangiectasia. Longitudinal follow-up of the remaining 6 has revealed persistent TCL with variable degrees of impairment of lymphocyte proliferation to mitogen or antigen stimulation, B lymphocyte production and antibody responses to vaccine challenge. Deep sequencing of DNA from patients and unaffected family members may identify their molecular defects.
Limitations of the TREC test
Although TCL is a feature of many immunodeficiencies, screening with the TREC assay cannot identify all infants with serious defects of T cell function. In disorders such as MHC II deficiency, ZAP70 deficiency, and others in which T cell receptor rearrangement is intact, but more distal pathways in maturation and function are deficient, TRECs are not expected to be decreased. Consistent with this expectation, during the 2 years of California’s TREC NBS program, 2 cases of MHC II deficiency,29 2 of Wiskott Aldrich syndrome, and 1 of delayed-onset adenosine deaminase (ADA) SCID were not detected by TREC screening because their newborn TREC numbers were normal. In addition, defects limited to humoral immunity or neutrophils are not detected. Testing for insufficient TRECs, a biomarker of numerically adequate naïve T cells, will detect only SCID diseases with impaired T cell production and secondary conditions that drain naïve T cells from peripheral blood. Importantly, a negative TREC test does not rule out the possibility of primary immunodeficiency. While SCID NBS with TRECs has raised awareness of SCID among physicians and the public, continued educational efforts are important to make sure immune disorders are sought when clinical features suggest.
Incidence of SCID and related disorders in the diverse California population
The genotypes of patients with typical SCID are consistent with prior reports, with the most frequent genotype being X-linked IL2RG. The ethnic variation observed in the incidence of SCID and non-SCID TCL warrant further investigation; data will be forthcoming as cases accumulate. There are reports of differing SCID incidences according to immune deficiency registries from different countries,30–35 with autosomal recessive SCID being more prevalent in cultures in which consanguineous marriage is common.36 As TREC screening continues in the ethnically diverse state of California, underlying ethnic patterns of SCID incidence will be revealed. Furthermore, children from parents of discordant ethnic backgrounds may have a lower than average population incidence of SCID caused by autosomal recessive mutations, as they are unlikely to harbor common founder alleles. In contrast, X-linked SCID incidence should be independent of ethnicity due to the substantial proportion of newly arising mutations.
Incidence of conditions with TCL and proportion of patients with defined syndromes who have significant immune compromise can also be determined in an unbiased, prospective manner. TREC NBS in California combined with retrospective analysis of newborn dried blood spots from patients with ataxia telangiectasia led to a new incidence estimate of one case per 200,000 births.24 Similarly, using published incidence estimates of around 1/5,000 births for chromosome 22q11 deletion, the most frequent human interstitial deletion syndrome,37 California should have 100 births each year with this cytogenetic abnormality. In our 2 years of screening, 9 infants, or 5% of the expected number of births with DiGeorge/22q11 deletion, were identified to have <1,500 T cells/μL. While our TCL cutoff is somewhat arbitrary, this is the first unbiased estimate of T lymphopenia of this degree in this condition.
Conclusion
The California experience lends insight to the logistics, testing algorithms and results of the TREC test as applied to newborn screening for SCID and related TCL disorders. The specificity and sensitivity of the screening test and the advantage of centralized incorporation of confirmatory lymphocyte profiling by flow cytometry add to available experience to date. Furthermore, our large population gives the new, accurate estimates of birth prevalence of SCID and non-SCID TCL, justifying inclusion of SCID screening in the uniform core panel. There is great variety in the clinical, genotypic and demographic characteristics of SCID and TCL infants in California, and continued screening and monitoring will reveal the full phenotypic spectrum. To complement SCID NBS, we need to continue to improve rapid, high throughput genotyping to delineate the underlying genetic defects in SCID and TCL patients, in order to tailor appropriate genotype-specific treatment strategies to optimize their treatment and outcomes. TREC screening will also facilitate understanding of syndromes and conditions that present with TCL, making possible standardization and improvement of their immunologic management.
Clinical Implications.
TREC newborn screening permits early diagnosis and treatment of SCID and other T lymphopenic disorders, thus revealing new information about their incidence and clinical spectrum while making optimal outcomes possible.
Acknowledgments
The authors thank Ajit Bhandal for expert assistance with methodology and TREC testing, and Leslie Gaffney and Renna Killen for help in assembling data.
Funding
Support for the initial phase of CA SCID screening was provided by contract HHSN267200603430C to New York State from the Eunice Kennedy Shriver Institute of Child Health and Development, The Jeffrey Modell Foundation, and Perkin Elmer Genetics. Analysis of SCID cases was supported by the Primary Immune Deficiency Treatment Consortium, NIH AI U54 082973, with case development criteria facilitated by NIH R13 AI094943 from the NIH Office of Rare Disease Research of the National Center for Advancing Translational Sciences (NCATS). JMP received support from the UCSF CTSI, NIH NCATS 1UL1 RR024131, RO3 HD 060311 and RO1 AI 078248; AK was supported by an HCA International Foundation Travelling Scholarship.
Abbreviations
- ADA
adenosine deaminase
- AT
ataxia telangiectasia
- CDPH GDL
California Department of Public Health Genetic Disease Laboratory
- CHARGE
coloboma (ocular), heart anomaly, atresia (choanal), retardation, genital and ear anomalies
- CHH
cartilage hair hypoplasia
- CID
combined immunodeficiency
- CMV
cytomegalovirus
- DAF
DNA amplification failure
- DBS
dried blood spot
- HCT
hematopoietic cell transplant
- NBS
newborn screening
- NBSTRN
Newborn Screening Translational Research Network
- NICU
neonatal intensive care unit
- PCR
polymerase chain reaction
- qPCR
quantitative PCR
- PHA
phytohemagglutinin
- PIDTC
Primary Immune Deficiency Treatment Consortium
- TCL
T cell lymphopenia
- TREC
T cell receptor excision circle
- SCID
severe combined immunodeficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Railey MD, Lokhnygina Y, Buckley RH. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. The Journal of pediatrics. 2009;155(6):834–40. e1. doi: 10.1016/j.jpeds.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeli MM, Buckley RH. Why newborn screening for severe combined immunodeficiency is essential: a case report. Pediatrics. 2010;126(2):e465–9. doi: 10.1542/peds.2009-3659. [DOI] [PubMed] [Google Scholar]
- 3.Chan A, Scalchunes C, Boyle M, Puck JM. Early vs. delayed diagnosis of severe combined immunodeficiency: a family perspective survey. Clin Immunol. 2011;138(1):3–8. doi: 10.1016/j.clim.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117(11):3243–6. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- 5.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–8. doi: 10.1182/blood.v99.3.872. [DOI] [PubMed] [Google Scholar]
- 6.Howell RR. Report on Newborn Screening for Severe Combined Immunodeficiency. 2011 [cited; Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children]. Available from: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/severeimmunodeficiency.pdf.
- 7.Buckley RH. The long quest for neonatal screening for severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2012;129(3):597–604. doi: 10.1016/j.jaci.2011.12.964. quiz 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: The winner is T-cell receptor excision circles. The Journal of allergy and clinical immunology. 2012;129(3):607–16. doi: 10.1016/j.jaci.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbsky J, Thakar M, Routes J. The Wisconsin approach to newborn screening for severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2012;129(3):622–7. doi: 10.1016/j.jaci.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nature medicine. 2000;6(9):1036–42. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 11.Di Mascio M, Sereti I, Matthews LT, Natarajan V, Adelsberger J, Lempicki R, et al. Naive T-cell dynamics in human immunodeficiency virus type 1 infection: effects of highly active antiretroviral therapy provide insights into the mechanisms of naive T-cell depletion. Journal of virology. 2006;80(6):2665–74. doi: 10.1128/JVI.80.6.2665-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Barfield R, Benaim E, Leung W, Knowles J, Lawrence D, et al. Prediction of T-cell reconstitution by assessment of T-cell receptor excision circle before allogeneic hematopoietic stem cell transplantation in pediatric patients. Blood. 2005;105(2):886–93. doi: 10.1182/blood-2004-04-1405. [DOI] [PubMed] [Google Scholar]
- 14.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. The Journal of allergy and clinical immunology. 2005;115(2):391–8. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Morinishi Y, Imai K, Nakagawa N, Sato H, Horiuchi K, Ohtsuka Y, et al. Identification of severe combined immunodeficiency by T-cell receptor excision circles quantification using neonatal guthrie cards. The Journal of pediatrics. 2009;155(6):829–33. doi: 10.1016/j.jpeds.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Chase NM, Verbsky JW, Routes JM. Newborn screening for SCID: three years of experience. Annals of the New York Academy of Sciences. 2011;1238:99–105. doi: 10.1111/j.1749-6632.2011.06241.x. [DOI] [PubMed] [Google Scholar]
- 17.Hale JE, Bonilla FA, Pai SY, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, et al. Identification of an infant with severe combined immunodeficiency by newborn screening. The Journal of allergy and clinical immunology. 2010;126(5):1073–4. doi: 10.1016/j.jaci.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 18.Puck JM, Middelton L, Pepper AE. Carrier and prenatal diagnosis of X-linked severe combined immunodeficiency: mutation detection methods and utilization. Human genetics. 1997;99(5):628–33. doi: 10.1007/s004390050418. [DOI] [PubMed] [Google Scholar]
- 19.Noordzij JG, de Bruin-Versteeg S, Verkaik NS, Vossen JM, de Groot R, Bernatowska E, et al. The immunophenotypic and immunogenotypic B-cell differentiation arrest in bone marrow of RAG-deficient SCID patients corresponds to residual recombination activities of mutated RAG proteins. Blood. 2002;100(6):2145–52. [PubMed] [Google Scholar]
- 20.Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood. 2001;97(1):81–8. doi: 10.1182/blood.v97.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Gruber TA, Shah AJ, Hernandez M, Crooks GM, Abdel-Azim H, Gupta S, et al. Clinical and genetic heterogeneity in Omenn syndrome and severe combined immune deficiency. Pediatric transplantation. 2009;13(2):244–50. doi: 10.1111/j.1399-3046.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 22.Sobacchi C, Marrella V, Rucci F, Vezzoni P, Villa A. RAG-dependent primary immunodeficiencies. Human mutation. 2006;27(12):1174–84. doi: 10.1002/humu.20408. [DOI] [PubMed] [Google Scholar]
- 23.Ridanpaa M, Sistonen P, Rockas S, Rimoin DL, Makitie O, Kaitila I. Worldwide mutation spectrum in cartilage-hair hypoplasia: ancient founder origin of the major70A-->G mutation of the untranslated RMRP. European journal of human genetics : EJHG. 2002;10(7):439–47. doi: 10.1038/sj.ejhg.5200824. [DOI] [PubMed] [Google Scholar]
- 24.Mallott J, Kwan A, Church J, Gonzalez-Espinosa D, Lorey F, Tang LF, et al. Newborn Screening for SCID Identifies Patients with Ataxia Telangiectasia. Journal of clinical immunology. 2012 doi: 10.1007/s10875-012-9846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newborn screening: toward a uniform screening panel and system. Genetics in medicine : official journal of the American College of Medical Genetics. 2006;8 (Suppl 1):1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID) Molecular genetics and metabolism. 2011;104(3):383–9. doi: 10.1016/j.ymgme.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011) Journal of clinical immunology. 2012;32(1):82–8. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 28.Comeau AM, Hale JE, Pai SY, Bonilla FA, Notarangelo LD, Pasternack MS, et al. Guidelines for implementation of population-based newborn screening for severe combined immunodeficiency. Journal of inherited metabolic disease. 2010;33(Suppl 2):S273–81. doi: 10.1007/s10545-010-9103-9. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CY, Chase J, Lloret MG, Stiehm ER, Moore T, Aguilera MJ, et al. Newborn screening for severe combined immunodeficiency does not identify bare lymphocyte syndrome. The Journal of allergy and clinical immunology. 2013 doi: 10.1016/j.jaci.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. The Journal of pediatrics. 1993;123(4):564–72. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 31.Ryser O, Morell A, Hitzig WH. Primary immunodeficiencies in Switzerland: first report of the national registry in adults and children. Journal of clinical immunology. 1988;8(6):479–85. doi: 10.1007/BF00916954. [DOI] [PubMed] [Google Scholar]
- 32.Fasth A. Primary immunodeficiency disorders in Sweden: cases among children, 1974–1979. Journal of clinical immunology. 1982;2(2):86–92. doi: 10.1007/BF00916891. [DOI] [PubMed] [Google Scholar]
- 33.Stray-Pedersen A, Abrahamsen TG, Froland SS. Primary immunodeficiency diseases in Norway. Journal of clinical immunology. 2000;20(6):477–85. doi: 10.1023/a:1026416017763. [DOI] [PubMed] [Google Scholar]
- 34.Baumgart KW, Britton WJ, Kemp A, French M, Roberton D. The spectrum of primary immunodeficiency disorders in Australia. The Journal of allergy and clinical immunology. 1997;100(3):415–23. doi: 10.1016/s0091-6749(97)70257-4. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa H, Iwata T, Yata J, Kobayashi N. Primary immunodeficiency syndrome in Japan. I. Overview of a nationwide survey on primary immunodeficiency syndrome. Journal of clinical immunology. 1981;1(1):31–9. doi: 10.1007/BF00915474. [DOI] [PubMed] [Google Scholar]
- 36.Suliaman F, Al-Ghonaium A, Harfi H. High Incidence of Severe Combined Immune Deficiency in the Eastern Province of Saudi Arabia. Pediatr Asthma Allergy Immunol. 2006;19(1):14–8. [Google Scholar]
- 37.McDonald-McGinn DM, Emanuel BS, Zackai EH. 22q11.2 Deletion Syndrome. GeneReviews™ 1999 [Updated 2005 Dec 16] [cited 1993-; Internet]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1523/