Abstract
Serotonergic cells are located in a restricted number of brainstem nuclei, send projections to virtually all parts of the central nervous system, and are critical to normal brain function. They discharge tonically at a rate modulated by sleep/wake cycle and, in the case of medullary serotonergic cells in raphe magnus and the adjacent reticular formation (RM), are excited by cold challenge. Yet, beyond behavioral state and cold, endogenous factors that influence serotonergic cell discharge remain largely mysterious. The present study in the anesthetized rat investigated predictors of serotonergic RM cell discharge by testing whether cell discharge correlated to three rhythms observed in blood pressure recordings that averaged >30 minutes in length. A very slow frequency rhythm with a period of minutes, a respiratory rhythm, and a cardiac rhythm were derived from the blood pressure recording. Cross correlations between each of the derived rhythms and cell activity revealed that the discharge of 38 of the 40 serotonergic cells studied was significantly correlated to the very slow and/or respiratory rhythms. Very few serotonergic cells discharged in relation to the cardiac cycle and those that did, did so weakly. The correlations between serotonergic cell discharge and the slow and respiratory rhythms cannot arise from baroreceptive input. Instead we hypothesize that they are by-products of on-going adjustments to homeostatic functions that happen to alter blood pressure. Thus, serotonergic RM cells integrate information about multiple homeostatic activities and challenges and can consequently modulate spinal processes according to the most pressing need of the organism.
INTRODUCTION
Serotonergic neurons comprise less than a quarter of the cells in the rat medullary raphe magnus and adjacent reticular formation (collectively referred to as RM here), but are the major source of serotonin in the spinal and medullary dorsal horns (Dahlstrom and Fuxe 1964; Oliveras et al. 1977; Potrebic et al. 1994). Serotonergic RM cells have been implicated in a number of physiological processes including pain modulation, sleep-wake control, and cold defense. However, no clear picture has emerged of serotonergic cell functions in part because the endogenous factors that influence the discharge rate of serotonergic RM cells remain largely unknown.
The discharge of presumed serotonergic RM cells is related to thermoregulatory status, with elevated discharge rates elicited by central or peripheral cold challenge (Dickenson 1977; Nason and Mason 2006). Recordings from presumed serotonergic cells in awake cats suggest that serotonergic raphe cells in both the midbrain and medulla discharge at higher rates during waking than during slow wave sleep and are nearly silent during the atonia of paradoxical sleep, reflecting a strong influence of state and motor activity upon serotonergic cell discharge (Fornal et al. 1985; Heym et al. 1982; Trulson and Jacobs 1979). More recently, evidence from rat studies has raised the intriguing possibility that serotonergic dorsal raphe cells have diverse relationships to the sleep-wake cycle (Kirby et al. 2003; Urbain et al. 2006). However, signals beyond cold and state/motor activity that influence serotonergic cell discharge have not been identified. Recently, we noticed that some RM serotonergic cells in anesthetized rats noticeably vary their discharge in relation to changes in blood pressure that have a period of many minutes. No cardiovascular afferent senses blood pressure changes over the time scale of minutes, making it unlikely that a reflex arc mediates such slow changes in blood pressure. Instead the brain may generate these slow pressure rhythms as a by-product of a homeostatic action such as cutaneous vasomotion (Cowley 1992; Osborn et al. 2005).
The present study was initially designed to rigorously test whether a correlation exists between the discharge of serotonergic RM cells and slow spontaneous changes in blood pressure. In addition, because it was computationally straightforward to extend our study to analyze potential correlations between serotonergic cell discharge and respiratory and cardiac cycles, we have done so.
METHODS
Surgical preparation
Male Sprague-Dawley rats (240–400 g; Sasco, Madison, WI) were deeply anesthetized with 1.8–2.0% halothane (n=29) or isoflurane (n=7) in oxygen via a tracheal catheter. Transcutaneous needle electrodes were placed on either side of the thorax in order to record the electrocardiogram (EKG). A catheter was inserted into the brachial or femoral artery for recording of arterial blood pressure and a small craniotomy was made for the introduction of recording microelectrodes. pCO2 was not measured. Core body temperature of all rats was maintained at 37–38°C by use of a water-perfused heating pad and a plastic cover over the rat.
Protocol
When the surgical preparation was complete, anesthetic concentration was decreased to 1.0–1.2% and the rat was allowed to equilibrate for 2–3 hours. This anesthetic concentration produced a light plane of anesthesia at which the rats were able to withdraw from a noxious stimulus but showed no gross purposeful movement in the absence of noxious stimulation. After, a glass micropipette was introduced into the region of the RM (P 1.5–2.6 mm from interaural zero, L 0–1.0 mm, V 9.0–10.5 mm from the cerebellar surface) and a spontaneously active unit was isolated.
Neuronal activity and arterial blood pressure were simultaneously recorded for 15–55 min in the absence of intentional peripheral stimulation and at a steady-state concentration of anesthesia. To confirm that each cell met physiological criteria previously established for serotonergic cells (Mason 1997), the mean (x) and standard deviation (SDISI) of the interspike intervals were calculated from the first 5 min of the recording. For each cell, the value of the function, y(x, SDISI) = 146 − x + 0.98 SDISI, was calculated, where x is the mean interspike interval (in ms) and SDISI is the standard deviation of the intervals (in ms). Cells were classified as p5HT if the function value was less than zero (Mason 1997).
Neurons that remained after the complete physiological protocol were intracellularly labeled with Neurobiotin which was subsequently visualized with a Texas Red fluorophore. These cells were then processed for serotonin immunofluorescence, using a Bodipy fluorophore, as previously described (Mason 1997). In the case of cells that were not intracellularly labeled, the recording site was marked by either iontophoresing Neurobiotin into the extracellular space or electrolytic lesion.
Data was acquired onto a computer attached to a Micro1401 A/D converter (CED, Cambridge, UK). The unit was acquired at 20 kHz, and the blood pressure at 250 Hz. Activity from single cells was discriminated off-line (Spike2, CED, Cambridge, UK).
Analysis
After decimating the blood pressure record by 5, the data consisted of blood pressure, sampled at 50 Hz, and the times of p5HT cell discharge, acquired with an accuracy of 5 μs. To work with the blood pressure and cell discharge data at the same time, it was necessary to transform the discrete list of firing times into some sort of a regularly sampled array. We considered three options: (1) assigning a frequency to each firing time and then interpolating to a signal sampled at 50 Hz, (2) modeling each spike as a smoothed bump function and constructing a waveform by adding these, or (3) rounding the time of each event to the nearest 1/50th of a second, and constructing an array of ones and zeroes, with the ones marking when a spike occurred. The first option was ruled out because, in addition to the possible artifacts introduced by the interpolation procedure, it was not clear how to correctly compute a frequency for each datum point. The second option seemed computationally complicated and involved needless processing when compared with the third option. The third option does indeed slightly distort the original data, by moving each event slightly in time. However, the rounding is done with no skew toward one direction or the other. Further, the error introduced with each approximation is at most 10 ms, whereas spikes occur at a mean interval of at least 300 ms. Being also straightforward to compute, we chose this method for turning the discrete nerve cell data into an array containing 50 samples/s.
In order to obtain frequency information regarding the blood pressure and cell, a Fourier transform was performed on each signal. A Fourier transform of the unit discharge revealed no obvious peaks. In contrast, upon applying a Fourier transform of the blood pressure, peaks were consistently found at about 5.5 Hz and 1.4 Hz, corresponding to cardiac and respiratory cycles, respectively (see Results). Beyond harmonics of these frequencies, no other peaks were found in the blood pressure signal. To extract cardiac, respiratory and slow rhythms from the blood pressure, three inverse transforms were applied. For instance, to extract the cardiac rhythm, all values in the transform outside of the cardiac frequency range were set to zero, and then an inverse transform was applied. A similar method was used to extract the respiratory rhythm from the blood pressure wave. Finally, to extract the slow component of the blood pressure signal, values in the transform greater than about 0.05 Hz were set to zero before applying an inverse transform. Linear trends were subtracted from each rhythm and all components were normalized to values averaging 3 and ranging between 1 and 5.
At this point of the analysis, there were three blood pressure arrays and one cellular discharge array. The same analysis was applied to each pair of one blood pressure array with the discharge array (A and B). Both arrays were of the same duration (=m in seconds) and sampling frequency (50 Hz) and therefore contained N (=50*m) points. To quantitatively analyze the similarity between the two signals, we performed a correlation between them. The output of this operation is an array (C) twice as long as the input arrays, indexed from −(N−1) to (N−1), whose values are computed as follows:
, the sum of the result of multiplying A and B point-wise. For n>0, ; this shifts the array B to the left n places, prior to multiplying A and B, and summing the products where the arrays overlap. Similarly, the array A is shifted to the left n places in .
The final step was to normalize the correlation wave C. The Cauchy-Schwarz inequality states that the sum of the products of two equal-length series is always less than or equal to the product of the root mean squares of the two series:
with equality holding if the series are linear scalars of each other or either series is equal to zero (not applicable here). Therefore each point in the correlation C(n) was divided by the product of the root mean squares of the subsets of the arrays A and B that were used to calculate C(n). This procedure normalized the scale of all correlations to a scale of 0 to 1, with 1 representing a perfect correlation (i.e. identity).
In order to determine the significance of each correlation, 100 “random” correlations were calculated. To calculate the random correlations, the interspike intervals present in the cell array were shuffled, 100 times, so that they were arranged in a random order. Since, the set of interspike intervals used were the same as those that were present in the true discharge record, this procedure produced a random, yet physiologically plausible, pattern of discharge. The correlation between each random pattern and the blood pressure record was calculated as described above. Then the average and standard deviation of these random correlations were computed. If the true correlation differed from the mean random correlation by more than three standard deviations, it was considered significantly different.
The respiratory and cardiovascular rhythms were converted to sawtooth functions by connecting sequential peaks and troughs. This sawtooth provided phase information from −π/2 to π/2. For each cell the timing of each spike was mapped onto these phase plots to determine the phase at which each spike occurred. Finally, two histograms were calculated to determine when in the respiratory or cardiac cycle a correlated cell fired preferentially.
All analyses were performed using Igor Pro 3.11 (WaveMetrics, Lake Oswego, OR). All values are reported as means ± SEM.
RESULTS
Cardiac, respiratory, and a very low frequency rhythm were derived from blood pressure
The discharge of all p5HT cells studied was obtained simultaneously with blood pressure (Figs. 1A–B, 2A) for a minimum of 15 minutes and an average of 32.9 ± 2.5 minutes. Although rats were acutely prepared for recording and held in a stereotaxic frame, they were never intentionally stimulated during recordings. A fast fourier transform of the cell discharge resulted in no peaks. However, a fast fourier transform of the blood pressure revealed peaks at mean frequencies of 5.5 ± 0.1 Hz and 1.4 ± 0.05 Hz, corresponding to cardiac and respiratory cycles, respectively (Fig. 1D). A very low frequency variation, with a period in the scale of minutes, was also present (Figs. 1A, 2A).
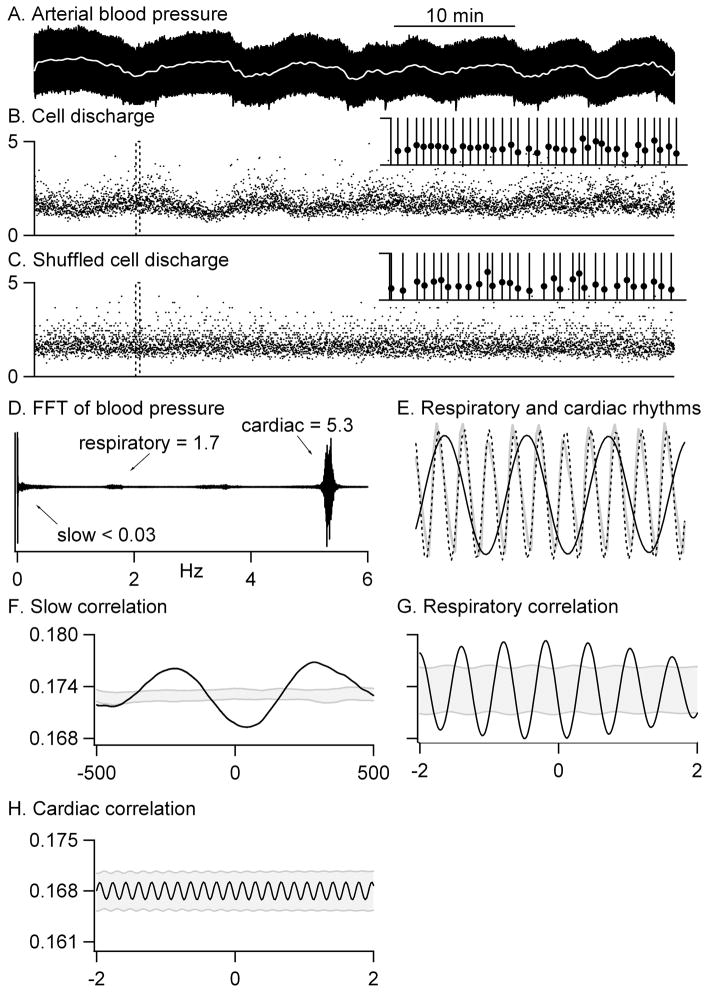
Figure 1.
The analysis procedure is illustrated. A–B: Blood pressure (A) and cell activity (B) were simultaneously recorded. The slow frequency-component of the blood pressure is shown by the white line in A. The cellular transform is illustrated in the inset in B for a short record (20 s) of activity (dashed box in B). Each spike was assigned to the nearest 20 ms bin as illustrated by the vertical lines. Superimposed on the transformed cell data is the instantaneous frequency (the reciprocal of the interspike interval) at every spike time (filled circles). C: All interspike intervals were shuffled 100 times; one such shuffled record is illustrated. The inset shows the cellular transform for this shuffled record with the same conventions as in B. D–E: A fast fourier transform of arterial blood pressure produced a spectrum with peaks at frequencies corresponding to respiratory and cardiac cycles. Inverse transforms of selected frequency ranges isolated rhythms corresponding to respiratory (solid black line in E) and cardiac (dashed line in E) activity as well as a very slow component (white line in A). The grey line in E is the raw blood pressure trace. F–H: Cross-correlations between the cell activity and each blood pressure component revealed a significant inverse correlation with the slow rhythm (F) and the respiratory rhythm (G) but no correlation to the cardiac rhythm (H). For respiration, upward corresponds to expiration and downward to inspiration. The grey stippled region marks 3 standard deviations on other side of the average correlation between shuffled cell records (n=100) and each blood pressure rhythm. The time scale in A applies to A–C. The duration of the records in E is 2 s. The ordinate scale in F applies to F and G.
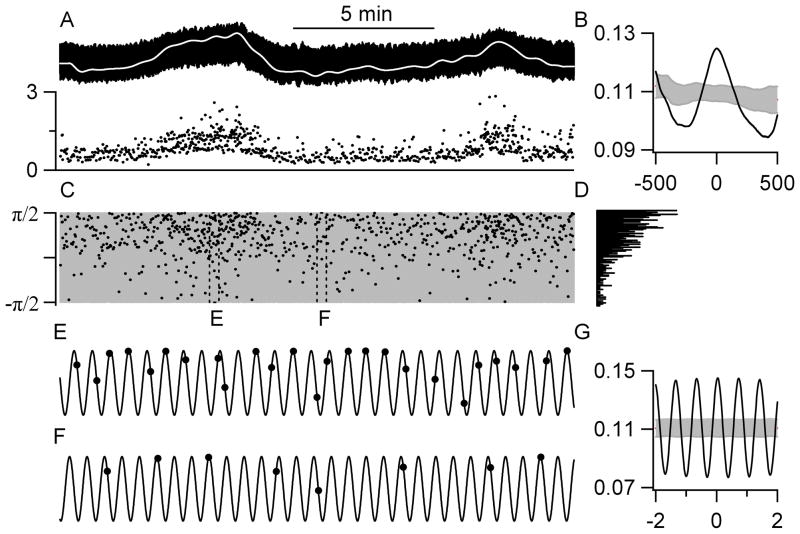
Figure 2.
An example of a cell with a significant correlation to the respiratory cycle. A: Blood pressure (top trace) and cell activity (bottom trace) were simultaneously recorded. The slow frequency-component of the blood pressure is shown by the white line. B: There was a significant correlation between this cell’s activity and the slow frequency-component of blood pressure. The same convention as in Figure 1. C: The arcsin of the respiratory rhythm (grey) maps phase from −π/2 (inspiration) to π/2 (expiration). The phase at which each action potential occurred is illustrated by the black dots. D: A histogram of the phase at which the cell discharged shows that the cell typically discharged during expiration. The 20-s periods framed by the dashed boxes marked E and F are blown up in panels E and F. E–F: This cell fired preferentially during expiration regardless of whether the cell was firing more (E) or less (F) than average. G: B: There was a significant correlation between this cell’s activity and the respiratory component of blood pressure.
To isolate each rhythm, the entire fast fourier transform was set to zero except for a region surrounding the cardiac (mean: 5.2–5.7 Hz) or respiratory (mean: 1.2–1.6 Hz) peak or a region below a mean cutoff frequency of 0.05 Hz. An inverse transform of each of these modified transforms produced isolated cardiac (Fig. 1E, dashed line), respiratory (Fig. 1E, black line), and slow (Figs. 1A and 2A, white lines) rhythms. For the respiratory rhythm, inspiration occurred at the troughs and expiration at the peaks. The record of each cell’s discharge, an array of time points, was then converted to a 50 samples/s array of zeroes (no firing) and ones (spike) as described in detail in the Methods and illustrated in the inset in Figure 1B. The results of cross correlations between cell discharge and each rhythm isolated from the blood pressure record are reported below.
Serotonergic cells were identified by their slow and steady discharge
Neurons were classified as p5HT (n=42) by use of a previously described algorithm (see Methods and Mason, 1997). Of the 8 intracellularly labeled p5HT cells, 6 contained serotonin immunoreactivity. The two cells that did not contain serotonin immunoreactivity were omitted from further analysis. For the remaining 40 p5HT cells, background discharge rates ranged from 0.4 to 4.8 Hz and averaged 1.7 ± 0.1 Hz. The mean coefficient of variation of the interspike interval (CV) was 0.39 ± 0.03 with a range of 0.19–0.93. Cells were located in raphe magnus (n = 23) or the adjacent nucleus reticularis magnocellularis pars α (n = 15) between the levels of the facial nucleus and the superior olivary complex. The locations of 2 cells were not recovered.
The discharge of most serotonergic cells (70%) correlated to slow changes in blood pressure
Most p5HT cells (n=28), including 5 immunochemically confirmed serotonergic neurons, discharged in correlation to slow changes in blood pressure (Figs. 1, 2). The mean period of the slow correlations was more than 11 minutes (667 ± 108 s). Of the 28 p5HT cells with discharge related to low frequency variations in blood pressure, 16 were negatively correlated (Fig. 1F) and 12 positively correlated (Fig. 2B). The value of the peak or trough closest to zero (in time) averaged 0.17 ± 0.01, suggesting that a correlation to slow blood pressure changes accounts for about 17% of the variability in cell discharge. The mean phase relationship was not different for cells that were negatively or positively correlated (p=0.20) and averaged a 1° advance with a range from a 113° lag to a 154° advance.
To determine whether cross correlations were significant, the interspike intervals were shuffled. An example of one such shuffling is illustrated in Figure 1C. Each of 100 shuffled cell records was then correlated to the slow, respiratory and cardiac rhythms and the average and standard deviation of the cross correlations to shuffled data calculated. Any cell – blood pressure pair with a cross correlation that was more than 3 standard deviations away from the mean shuffled correlation (the stippled region in Figs. 1F–H, 2 B, G) was considered to be significantly correlated. Thus the cell shown in Figure 1 was inversely correlated to the slow oscillations in blood pressure (Fig. 1F) and to the respiratory rhythm (Fig. 1G) but was not correlated to the cardiac cycle (Fig. 1H).
The discharge of most serotonergic cells (83%) correlated to a respiratory rhythm in the blood pressure
The spontaneous activity of 33 p5HT cells, including 5 immunochemically confirmed serotonergic neurons, was significantly correlated with the respiratory rhythm isolated from the blood pressure record (Figs. 1, 2). Of the 33 p5HT cells with respiratory rhythm-related discharge, 24 preferentially discharged during expiration (Fig. 2) and 9 during inspiration (Fig. 1G). The value of the peak or trough closest to zero averaged 0.17 ± 0.01. The mean phase relationship was a 1° lag but this was quite variable, ranging from a lag of 88° to an advance of 95°.
The mean interspike interval was 791 ± 81 ms was similar to the mean inter-breath interval of 739 ± 24 ms. However, the interspike interval was never equal to the inter-breath interval during any recording, differing by an average of 354 ± 63 ms. Thus, as seen in Fig. 2E–F, a cell with a tendency to discharge during expiration did not fire during each respiratory cycle, but when it fired it was most frequently during expiration and only rarely during inspiration. Further, the cell’s tendency to discharge during expiration was not different during periods of slower or faster discharge, related to the slow component of blood pressure (compare E to F in Fig. 2).
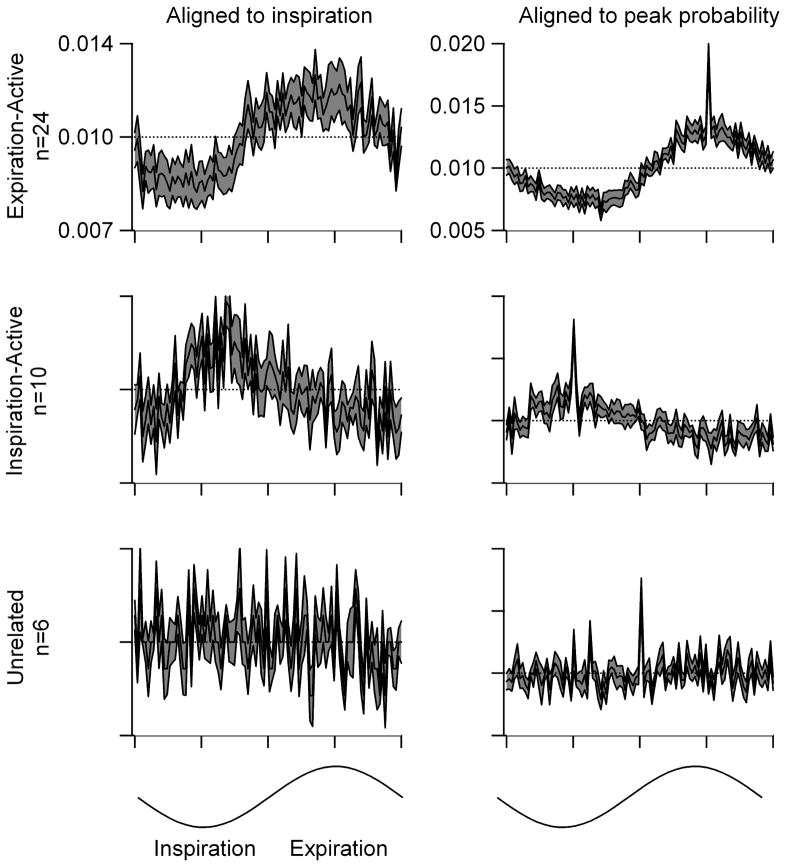
Figure 3 illustrates the mean firing probabilities across the respiratory cycle for cells that were preferentially active during expiration, preferentially active during inspiration and for cells whose discharge was not significantly related to breathing. For expiration-active cells, the probability of firing in any 3.6° of the respiratory cycle was 1.44 ± 0.22 times greater during expiration than during inspiration. For inspiration-active cells, the probability of firing was 1.11 ± 0.00 times greater during inspiration than during expiration, a value that was not significantly different from that for expiration-active cells (t-test, p=0.15).
Figure 3.
Histograms of cell discharge probability across the respiratory cycle are shown for cells that preferentially discharged during expiration (expiration-active, top), for those that preferentially discharged during inspiration (inspiration-active, middle), and for those whose discharge was unrelated to breathing (unrelated, bottom). Individual histograms were aligned either to the inspiratory onset (left column; ordinate scale shown at top left) or to the peak in discharge probability (right column; ordinate scale shown at top right). For the latter, the peak in discharge probability was placed at 270° for expiration-active cells (top), at 90° for inspiration-active cells (middle), and at 180° for cells without any relation to respiratory cycle (bottom). In each panel the average histogram is shown by the solid black line. One standard error on either side of the average is shown in the stippled gray. The respiratory cycle was divided into 100 bins so that each bin covers 3.6° and an average probability of firing in each bin of 1.0%; the latter is marked by a dashed line in each graph. The phase assignments are illustrated by the sin waves at the bottom.
The discharge of a minority of serotonergic cells (22%) correlated with the cardiac cycle
The spontaneous activity of 9 p5HT cells, including 3 immunochemically confirmed serotonergic neurons, was significantly correlated with the cardiac cycle. Most cells with cardiac-related discharge (n=5) preferentially discharged during diastole while 3 discharged preferentially during systole. The cross correlation peak for one cell with cardiac-related discharge was exactly midway between diastole and systole. Cells never fired during each cycle. The mean phase relationship was an advance of 2° but varied from a lag of 90° to an advance of 72°.
The relationship between cardiac cycle and cell discharge was noticeably weaker than that between respiratory cycle and cell discharge and, unlike the correlation between the other two rhythms and cell discharge, was not apparent to the naked eye in a phase plot such as that shown in Figure 2C. Corresponding to this, the ratio of firing during the preferred phase to firing during the non-preferred phase was 1.03 ± 0.02 for diastole-active cells and 0.99 ± 0.02 for systole-active cells. In sum, few cells discharged in relation to cardiac cycle and those that did, did so weakly.
The discharge of most cells correlated to multiple rhythms derived from blood pressure
Almost all cells studied (38/40) discharged in relation to the slow rhythm or the respiratory rhythm with most cells’ discharge (23/40, 58%) correlated to both respiratory and slow rhythms. For cells whose discharge correlated to both respiratory and slow rhythms, almost half (10/23) were correlated to both rhythms when blood pressure was either high (direct correlation to the slow rhythm and correlation to expiration) or low (indirect correlation to the slow rhythm and correlation to inspiration). The remainder (13/23) correlated to the two rhythms in opposing directions with respect to blood pressure. The discharge of 10 cells correlated to respiration but not to the slow pressure component while that of 5 cells correlated to the slow component but not to respiration. All cells with cardiac-related discharge also exhibited respiratory-related discharge with a preference for firing during expiration (9/9).
DISCUSSION
The present results demonstrate that very slow (period of >10 mins) and respiratory-related changes in blood pressure each predict about 17% of the variability in the discharge of most serotonergic RM cells. Blood pressure- and respiratory-related discharge may inform serotonergic RM cells of homeostatic adjustments generated by the central nervous system. Such an input would allow RM cells to evaluate new threats to safety and homeostasis in light of on-going activities to maintain the same.
Very low frequency variations in blood pressure do not have a known function
Very slow variations in blood pressure, similar to those observed here, have previously been described in the anesthetized rat (Leung and Mason 1996) and the awake dog (Persson et al. 1990). In awake cat, variations with periods of 10–13 min are present and are enhanced by baroreceptor denervation (Di Rienzo et al. 1991), suggesting that the baroreceptor reflex typically dampens these very low frequency changes in blood pressure. Since the baroreceptor reflex is depressed during anesthesia (Duke et al. 1977), slow changes in blood pressure may be most apparent in this preparation. This is in contrast to Mayer waves which are most prominent in awake animals and have a frequency of about 0.4 Hz in the rat, much faster than the slower frequency variations observed in this study (Julien 2006).
As mentioned above, no known cardiovascular circuit or mechanism mediates blood pressure changes over the time scale of minutes (see more below). Instead slow changes in blood pressure likely arise from the brain as a by-product of either a homeostatic action or somatomotor activity (Cowley 1992; Osborn et al. 2005). As an example of the former, activation of a central autonomic nucleus that elicits cutaneous vasoconstriction would incidentally alter blood pressure (at least in the anesthetized animal). It is also possible that the increase in firing rate observed in medullary serotonergic cells during somatomotor activity (Veasey et al. 1995) is actually secondary to the correlation between these cells’ and cardiorespiratory activities. Indeed, blood pressure changes occur across the sleep/wake cycle and different thermoregulatory states, during eating or exercise, in situations of painful attack or sexual activity, and in a myriad of other situations. Combinations of additional physiological parameters with these slow changes in blood pressure may then map out nuanced differences in the integrative state of the animal, splitting the broad categories of waking, sleeping, and anesthetized into the innumerable and varied conditions of mammalian life.
Cells identified as serotonergic in this study likely contain serotonin
All p5HT cells were characterized using a previously described algorithm developed from an analysis of physiologically characterized, intracellularly labeled, and immunocytochemically tested cells (Mason 1997). Of 46 cells that have been physiologically characterized as p5HT and tested for serotonin content since the original derivation of the classification algorithm, 43 have contained serotonin immunoreactivity (Mason 2001). Furthermore, the background discharge pattern, response to noxious stimulation and nuclear location of immunochemically-untested p5HT cells are similar to those of immunochemically-confirmed serotonergic cells in this and previous studies. It is therefore likely that all or nearly all of the RM neurons studied contained serotonin. Recent findings show that some serotonergic neurons are not tonically active when recorded in vitro (Zhang et al. 2006). If similar results obtain in vivo, then it should be recognized that our search strategy would preclude us from recording from such cells.
Discharge correlations to slow and respiratory variations in blood pressure are distinct phenomena
Serotonergic cell discharge was related to extremely slow variations in blood pressure as well as to the respiratory cycle revealed within the blood pressure record. One possibility is that a common mechanism underlies both correlations. However, 14 cells discharged in relation to only one of these two rhythms. Further, for the 24 cells that discharged in relationship to both rhythms, there was no consistent relationship between the sign of the two correlations. Thus, the correlations between serotonergic RM cell firing and the respiratory and slow rhythms appear distinct phenomenologically.
Baroreceptors are not the input source for the serotonergic cell correlations to slow and respiratory rhythms
Blood pressure-related information could reach serotonergic cells from peripheral baroreceptors via the nucleus of the tractus solitarius (NTS). Although NTS neurons do not project directly to RM, they do project to the ventrolateral medulla (VLM), the parabrachial nuclei, the hypothalamus, amygdala and the midbrain periaqueductal gray (PAG), all of which in turn project to RM (Berk and Finkelstein 1982; Gang et al. 1990; Holstege 1988, 1987; Vertes and Crane 1996; Vertes and Kocsis 1994; Zagon 1993). Nonetheless it is unlikely that beat-to-beat baroreceptor input plays a major role in the correlation of serotonergic neurons’ discharge with the slow or respiratory changes in blood pressure because: 1) most serotonergic neurons do not discharge in correlation with the cardiac cycle or do so very weakly (this study); 2) most serotonergic cells that discharge in relation to either the slow or respiratory rhythms do not discharge in relation to cardiac cycle (this study), a defining feature for central neurons that receive baroreceptor input; and 3) baroreceptive input to serotonergic cells is uncommon and weak when present (Gao and Mason 2001a). In conclusion, peripheral baroreceptor input may underlie the weak connection between serotonergic RM cell firing and cardiac cycle seen in a minority of cells but not the more robust correlations between cell discharge and either the slow rhythm or respiration.
A caveat worth mentioning here is that central neurons, perhaps in the NTS, could low-pass filter incoming baroreceptive input. If such neurons existed, they could provide the input needed for the correlation of serotonergic neurons’ discharge with the slow or respiratory changes in blood pressure. While this is a possibility, no neurons with the appropriate physiological characteristics have been observed.
Discharge in relation to slow variations in blood pressure likely reflects input from a central source
Baroreceptors, the only known afferents sensitive to blood pressure, operate on a short time scale and elicit corrective reflexes at latencies well under a second. At the other extreme of the time scale, the kidneys regulate blood pressure across the life span (Cowley 1992). For blood pressure changes over intermediate time scales, such as those termed slow in this paper, no cardiovascular afferent, circuit, or mechanism has been identified. Instead, as mentioned above, such changes are likely to be baroreceptor-independent and generated as by-products of efferent commands from central homeostatic control regions (Cowley 1992; Osborn et al. 2005). For example, vasomotion at thermoneutral temperatures cycles between vasoconstriction and vasodilation with a period of many minutes in the awake rat (Romanovsky et al. 2002). If blood pressure changes accompany such a redistribution of blood volume, then efference copy related to vasomotion could be the critical input for the oscillations in serotonergic cell discharge observed here.
Which central regions are candidate sources of input carrying a slow rhythmic signal to serotonergic cells? The list is familiar as the same structures that play critical roles in maintaining homeostasis also receive baroreceptive input from NTS and project to RM (see above) – the parabrachial nuclei, hypothalamus, amygdala, PAG and VLM. Thus the correlation to slow blood pressure changes likely reflects an efference copy (aka corollary discharge) input from the amygdala, hypothalamus, PAG, parabrachial nuclei, or VLM to serotonergic RM neurons.
The source of respiratory-related inputs to serotonergic cells is unclear
Most of the serotonergic RM cells examined had respiratory-related discharge. The respiratory-related discharge in serotonergic RM cells was striking in its prevalence and magnitude. As with baroreceptors, pulmonary stretch receptors project to the NTS from where they are distributed to the ventral and dorsal respiratory groups, PAG, parabrachial nuclei and hypothalamus. Regions that receive NTS input also participate in the central control of respiration and they all project to RM. The serotonergic cell correlation with respiratory rhythm may stem from either afferent information or efference copy; no data exist to distinguish between these two possibilities.
There is one oddity about the respiratory-related rhythm that is worth noting. The axons of serotonergic RM cells are unmyelinated and project variable distances throughout the spinal cord (Basbaum et al. 1988; Skagerberg and Bjorklund 1985). This means that phase information associated with particular spikes will be jumbled upon reaching the collective sites postsynaptic to serotonergic RM cells. The clear implication of this is that the phase association of serotonergic cell discharge is simply a by-product of the afferent limb but does not evoke phase-locked effects.
Serotonergic RM cells may modulate blood pressure control circuitry
The slow discharge rate of serotonergic cells and the slow pace of the cardiovascular system preclude a definitive identification of RM as the cause of changes in blood pressure but a strong possibility is that serotonergic RM cells modulate sympathetic, including cardiovascular, output. In support of this idea, activation of RM neurons evokes sympathoexcitatory changes in blood pressure, heart rate, and thermoregulation (Cao and Morrison 2003; Gilbey et al. 1981; Haselton et al. 1988b; Nason and Mason 2004). Some serotonergic RM cells project directly to preganglionic sympathetic neurons in the intermediolateral cell column and may influence sympathetic tone via this connection (Bacon et al. 1990; Haselton et al. 1988a). Within the thoracic spinal cord, serotonin depolarizes preganglionic sympathetic neurons (Coote et al. 1981; Kadzielawa 1983; Lewis and Coote 1990) whereas serotonin receptor antagonists block the pressor response evoked by medullary raphe stimulation (McCall 1984). A serotonin receptor agonist that presumably inhibits serotonergic cells blocks leptin-induced activation of brown adipose tissue, suggesting that serotonergic RM cells mediate food-induced thermogenesis, a process that is accompanied by elevations in blood pressure (Morrison 2004).
RM serotonergic cells also project to the ventrolateral medulla (Gao and Mason 2001b, 1997), a region where serotonin is tonically released (Gao et al. 1991) and where microinjection of serotonin or serotonin receptor agonists elicits a decrease in cardiovascular tone along with cutaneous vasodilation (Key and Wigfield 1994; Lovick 1989; Mandal et al. 1990). These results suggest that serotonergic RM cells could influence cardiovascular tone or thermoregulation through both a direct excitation of preganglionic sympathetic neurons and an indirect inhibitory effect mediated by inhibition of sympathoexcitatory cells in the ventrolateral medulla (Lewis and Coote 1993; Wang and Lovick 1992a, 1992b). It may be that serotonergic neurons that discharge in direct correlation with blood pressure preferentially target the thoracic intermediolateral cell column while serotonergic neurons that discharge in indirect correlation with blood pressure target sympathoexcitatory neurons in VLM. Alternatively, RM cells with discharge that is either directly or indirectly related to slow changes in systemic blood pressure may target the same postsynaptic cells and function antagonistically.
Serotonergic RM cells may modulate respiratory control circuitry
Electrical stimulation of or glutamate microinjection into RM in the anesthetized rat decreases respiratory rate, even causing apnea in some cases, evidence for RM’s participation in respiratory modulation (Cao et al. 2006a; Verner et al. 2004). While the findings that hypercapnia and, to a lesser degree, hypoxia evoke c-fos immunoreactivity in RM cells (Erickson and Millhorn, 1994; Teppema et al. 1997) are interpretable in many ways, RM appears to play a direct role in sensing hypercapnia. Dialyzing artificial cerebrospinal fluid with elevated [CO2] into the medullary raphe of awake rats evokes tachypnea, evidence that this region is chemosensitive (Nattie and Li 2001).
Since only about 20% of RM cells contain serotonin, evidence beyond that stated above is needed to implicate a role for serotonergic RM cells, specifically, in respiratory modulation. Serotonergic neurons from the medullary raphe studied in vitro are chemosensitive (Wang et al. 1998) and presumed inhibition of serotonergic neurons by microinjection of 8-OH-DPAT into RM attenuated the ventilatory reflex evoked by hypercapnia (Taylor et al. 2005). While these studies suggest that serotonergic medullary raphe cells sense hypercapnia, this conclusion has recently been challenged by a demonstration that none of 37 serotonergic cells studied in vivo were excited by elevated [CO2] (Mulkey et al. 2004), in contrast to the more than 20% of serotonergic cells that respond in vitro (Wang et al. 1998). Anatomical studies make clear that serotonergic cells in the medullary raphe project to the phrenic motor nucleus and that motor neurons there receive serotonergic afferents (Cao et al. 2006b; Holtman et al. 1984, 1990; Pilowsky et al. 1990). As is true for preganglionic sympathetic motor neurons and other somatic motor neurons, phrenic motor neurons are excited by serotonin application (Schmid et al. 1990). Finally, 8-OH-DPAT, a serotonergic receptor agonist that is thought to decrease serotonergic cell firing, increases respiratory rate when applied to the medullary ventrum (Holtman and King 1994; King and Holtman 1990).
Serotonergic RM cell activity are heterogeneous and may act either in concert, in parallel or in opposition
The serotonergic neurons studied here differed in the relationship of their discharge to slow, respiratory and cardiac cycles. This heterogeneity adds to previously reported differences in physiology and co-transmitter content within the serotonergic RM cell population of the rat (Gao and Mason 2001a, 2001b, 2000; Kachidian et al. 1991; Zhang et al. 2006). Different types of serotonergic cells may act in concert as, for example would occur if cells with opposing relations to blood pressure differentially facilitate preganglionic sympathetic neurons and suppress sympathoexcitatory cells in the brainstem (see above). Alternatively, subpopulations of serotonergic cells may share postsynaptic targets upon which they act antagonistically. Finally, different serotonergic cell subsets may act on entirely separate systems without any interaction. Unfortunately, the answer to this puzzle remains unclear.
Information about on-going homeostatic activities allows serotonergic RM cells to judge whether or not to interrupt these activities to respond to a new challenge
Serotonergic RM neurons project promiscuously within the spinal cord to contact cells with sensory, motor and autonomic functions and thereby modulate a plethora of spinal processes including cardiovascular, themoregulatory, respiratory, and nociceptive ones. Consistent with serotonergic RM cells’ having multi-system effects, microinjection of 8-OH-DPAT into RM, which presumably decreases serotonergic RM cell firing, lowers body temperature, increases arousal, and depresses the hypercapnic ventilatory response (Taylor et al. 2005). The present results, showing that the discharge of most serotonergic RM cells correlates with a slow-frequency component in blood pressure, is then evidence that serotonergic cell modulation is itself modulated by the particular state of the animal. Since all behaviors require a sensory, autonomic and motor platform that is capable of supporting them, a coordinated regulation of spinal processes is critical.
Like their non-serotonergic neighbors, at least some serotonergic RM neurons respond to challenges to safety such as unexpected and alerting external stimuli (Auerbach et al. 1985; Gao and Mason 2000; Leung and Mason 1999). The present results, while correlative, provide clear evidence that serotonergic RM cells also receive information about slow changes in blood pressure and respiration that we have argued here serve as proxies for on-going homeostatic activities. By receiving input related to existing as well as new and unexpected challenges to homeostasis, serotonergic RM cells are in an excellent position to determine whether to continue the current homeostatic activity or to respond to a novel challenge. Suggesting that serotonergic RM cell activity is needed for a full reaction to a novel challenge, 8-OH-DPAT microinjection into RM attenuates the tachycardia and pressor responses elicited by noxious stimulation, 5 minutes of “air-jet stress,” or lipopolysaccharide administration in the rabbit (Nalivaiko et al. 2005). Thus, serotonergic RM cells may be able to evaluate whether new challenges to homeostasis merit interruption of existing homeostatic functions and then coordinate the modulation of spinal sensory transmission, autonomic control and motor function in accordance with the verdict.
Acknowledgments
This research was supported by NINDS, the Pritzker Medical School (JRG), and the Brain Research Foundation. The authors thank Scott Mendelson, Drs. Kevin Hellman and Madelyn Baez and the SIDS group at Children’s Hospital in Boston for helpful comments, Dr. Dottie Hanck for help with the analytical methods, and Matthew Gealy and Jeff Streets for programming assistance.
References
- Auerbach S, Fornal C, Jacobs BL. Response of serotonin-containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. doi: 10.1016/0014-4886(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Zagon A, Smith AD. Electron microscopic evidence of a monosynaptic pathway between cells in the caudal raphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res. 1990;79:589–602. doi: 10.1007/BF00229327. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Zahs K, Lord B, Lakos S. The fiber caliber of 5-HT immunoreactive axons in the dorsolateral funiculus of the spinal cord of the rat and cat. Somatosens Mot Res. 1988;5:177–185. doi: 10.3109/07367228809144625. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 1982;8:511–526. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Disinhibition of rostral raphe pallidus neurons increases cardiac sympathetic nerve activity and heart rate. Brain Res. 2003;980:1–10. doi: 10.1016/s0006-8993(03)02981-0. [DOI] [PubMed] [Google Scholar]
- Cao Y, Fujito Y, Matsuyama K, Aoki M. Effects of electrical stimulation of the medullary raphe nuclei on respiratory movement in rats. J Comp Physiol A. 2006a;192:497–505. doi: 10.1007/s00359-005-0087-0. [DOI] [PubMed] [Google Scholar]
- Cao Y, Matsuyama K, Fujito Y, Aoki M. Involvement of medullary GABAergic and serotonergic raphe neurons in respiratory control: electrophysiological and immunohistochemical studies in rats. Neurosci Res. 2006b;56:322–331. doi: 10.1016/j.neures.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Coote JH, Macleod VH, Fleetwood WS, Gilbey MP. The response of individual sympathetic preganglionic neurones to microelectrophoretically applied endogenous monoamines. Brain Res. 1981;215:135–145. doi: 10.1016/0006-8993(81)90497-2. [DOI] [PubMed] [Google Scholar]
- Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system I. Demonstration of monoamine in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;232:1–36. [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Castiglioni P, Omboni S, Ferrari AU, Ramirez AJ, Pedotti A, Mancia G. Role of sinoaortic afferents in modulating BP and pulse-interval spectral characteristics in unanesthetized cats. Am J Physiol. 1991;261:H1811–H1818. doi: 10.1152/ajpheart.1991.261.6.H1811. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Specific responses of rat raphe neurones to skin temperature. J Physiol. 1977;273:227–293. doi: 10.1113/jphysiol.1977.sp012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke PC, Fownes D, Wade JG. Halothane depresses baroreflex control of heart rate in man. Anesthesiology. 1977;46:184–187. doi: 10.1097/00000542-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol. 1985;88:590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- Gang S, Mizuguchi A, Kobayashi N, Aoki M. Descending axonal projections from the medial parabrachial and Kolliker-Fuse nuclear complex to the nucleus raphe magnus in cats. Neurosci Lett. 1990;118:273–275. doi: 10.1016/0304-3940(90)90645-p. [DOI] [PubMed] [Google Scholar]
- Gao K, Lin Y, Guo X. Changes of 5-hydroxyindoleacetic acid in nucleus paragigantocellularis lateralis detected with in vivo voltammetry. Chin J Physiol Sci. 1991;7:27–33. [Google Scholar]
- Gao K, Mason P. The discharge of a subset of serotonergic raphe magnus cells is influenced by baroreceptor input. Brain Res. 2001a;900:306–313. doi: 10.1016/s0006-8993(01)02294-6. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Physiological and anatomic evidence for functional subclasses of serotonergic raphe magnus cells. J Comp Neurol. 2001b;439:426–439. doi: 10.1002/cne.1360. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–1725. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Somatodendritic morphology and axonal anatomy of intracellularly labeled serotonergic neurons in the rat medulla. J Comp Neurol. 1997;389:309–328. [PubMed] [Google Scholar]
- Gilbey MP, Coote JH, Macleod VH, Peterson DF. Inhibition of sympathetic activity by stimulating in the raphe nuclei and the role of 5-hydroxytryptamine in this effect. Brain Res. 1981;226:131–142. doi: 10.1016/0006-8993(81)91088-x. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Anatomical and functional connections of neurons of the rostral medullary raphe of the rabbit. Brain Res. 1988a;453:176–182. doi: 10.1016/0006-8993(88)90156-4. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Cardiovascular responses elicited by electrical and chemical stimulation of the rostral medullary raphe of the rabbit. Brain Res. 1988b;453:167–175. doi: 10.1016/0006-8993(88)90155-2. [DOI] [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical evidence for a strong ventral parabrachial projection to nucleus raphe magnus and adjacent tegmental field. Brain Res. 1988;447:154–158. doi: 10.1016/0006-8993(88)90977-8. [DOI] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, King KA. Effect of activation of 5-HT1A receptors at the ventral medulla on phrenic nerve activity. Eur J Pharmacol. 1994;253:307–310. doi: 10.1016/0014-2999(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Norman WP, Skirboll L, Dretchen KL, Cuello C, Visser TJ, Hokfelt T, Gillis RA. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984;4:1064–1071. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JRJ, Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Julien C. The enigma of Mayer waves: Facts and models. Cardiovasc Res. 2006;70:12–21. doi: 10.1016/j.cardiores.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. J Neurosci Res. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- Kadzielawa K. Antagonism of the excitatory effects of 5-hydroxytryptamine on sympathetic preganglionic neurones and neurones activated by visceral afferents. Neuropharmacology. 1983;22:19–27. doi: 10.1016/0028-3908(83)90256-3. [DOI] [PubMed] [Google Scholar]
- Key BJ, Wigfield CC. The influence of the ventrolateral medulla on thermoregulatory circulations in the rat. J Auton Nerv Sys. 1994;48:79–89. doi: 10.1016/0165-1838(94)90162-7. [DOI] [PubMed] [Google Scholar]
- King KA, Holtman JR., Jr Characterization of the effects of activation of ventral medullary serotonin receptor subtypes on cardiovascular activity and respiratory motor outflow to the diaphragm and larynx. J Pharmacol Exp Ther. 1990;252:665–674. [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological properties of medullary raphe neurons during sleep and waking. J Neurophysiol. 1999;81:584–595. doi: 10.1152/jn.1999.81.2.584. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Spectral analysis of arterial blood pressure and raphe magnus neuronal activity in anesthetized rats. Am J Physiol. 1996;271:R483–R489. doi: 10.1152/ajpregu.1996.271.2.R483. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The actions of 5-hydroxytryptamine on the membrane of putative sympatho-excitatory neurones in the rostral ventrolateral medulla of the adult rat in vitro. Brain Res. 1993;609:103–109. doi: 10.1016/0006-8993(93)90861-g. [DOI] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. The influence of 5-hydroxytryptamine agonists and antagonists on identified sympathetic preganglionic neurones in the rat, in vivo. Br J Pharmacol. 1990;99:667–672. doi: 10.1111/j.1476-5381.1990.tb12987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick TA. Cardiovascular responses to 5-HT in the ventrolateral medulla of the rat. J Auton Nerv Sys. 1989;28:35–41. doi: 10.1016/0165-1838(89)90005-2. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Zhong PY, Kellar KJ, Gillis RA. Ventrolateral medulla: an important site of action for the hypotensive effect of drugs that activate serotonin-1A receptors. Circ Res. 1990;15:S49–60. [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Ann Rev Neurosci. 2001;25:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- McCall RB. Evidence for a serotonergically mediated sympathoexcitatory response to stimulation of medullary raphe nuclei. Brain Res. 1984;311:131–139. doi: 10.1016/0006-8993(84)91405-7. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004 doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Ootsuka Y, Blessing WW. Activation of 5-HT1A receptors in the medullary raphe reduces cardiovascular changes elicited by acute psychological and inflammatory stresses in rabbits. Am J Physiol. 2005;289:R596–R604. doi: 10.1152/ajpregu.00845.2004. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol. 2004;92:510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol. 2001;90:1247–1257. doi: 10.1152/jappl.2001.90.4.1247. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Bourgoin S, Hery F, Besson JM, Hamon M. The topographical distribution of serotoninergic terminals in the spinal cord of the cat: biochemical mapping by the combined use of microdissection and microassay procedures. Brain Res. 1977;138:393–406. doi: 10.1016/0006-8993(77)90680-1. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol. 2005;288:R846–855. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- Persson PB, Ehmke H, Kohler WW, Kirchheim HR. Identification of major slow blood pressure oscillations in conscious dogs. Am J Physiol. 1990;259:H1050–H1055. doi: 10.1152/ajpheart.1990.259.4.H1050. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, Lipski J, Voss MD. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990;10:1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Bohmer G, Merkelbach S. Serotonergic control of phrenic motoneuronal activity at the level of the spinal cord of the rabbit. Neurosci Lett. 1990;116:204–209. doi: 10.1016/0304-3940(90)90411-2. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol. 2005;566:543–557. doi: 10.1113/jphysiol.2005.083873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Urbain N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner TA, Goodchild AK, Pilowsky PM. A mapping study of cardiorespiratory responses to chemical stimulation of the midline medulla oblongata in ventilated and freely breathing rats. Am J Physiol. 2004;287:R411–421. doi: 10.1152/ajpregu.00019.2004. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Descending projections of the posterior nucleus of the hypothalamus: Phaseolus vulgaris leucoagglutinin analysis in the rat. J Comp Neurol. 1996;374:607–631. doi: 10.1002/(SICI)1096-9861(19961028)374:4<607::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511 (Pt 2):433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Lovick TA. Excitatory 5-HT2-mediated effects on rostral ventrolateral medullary neurones in rats. Neurosci Lett. 1992a;141:89–92. doi: 10.1016/0304-3940(92)90341-4. [DOI] [PubMed] [Google Scholar]
- Wang WH, Lovick TA. Inhibitory serotonergic effects on rostral ventrolateral medullary neurons. Eur J Physiol. 1992b;422:93–97. doi: 10.1007/BF00370407. [DOI] [PubMed] [Google Scholar]
- Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neuroscience. 1993;55:849–867. doi: 10.1016/0306-4522(93)90446-m. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sykes KT, Buhler AV, Hammond DL. Electrophysiological heterogeneity of spinally-projecting serotonergic and non-serotonergic neurons in the rostral ventromedial medulla. J Neurophysiol. 2006;95:1853–1863. doi: 10.1152/jn.00883.2005. [DOI] [PubMed] [Google Scholar]