Abstract
In rats, opioids produce analgesia in large part by their effects on two cell populations in the medullary raphe magnus (RM). To extend our mechanistic understanding of opioid analgesia to the genetically tractable mouse, we characterized behavioral reactions and RM neural responses to opioid administration. DAMGO, a mu-opioid receptor agonist, microinjected into the murine RM produced cardiorespiratory depression and reduced slow wave EEG activity as well as increased the noxious heat-evoked withdrawal latencies. As in rat, RM cell types that were excited and inhibited by noxious stimuli, termed on and off cells respectively, were observed in mice. However, in contrast to findings in rat, opioid doses that suppressed withdrawals did not alter the background discharge rate of murine on and off cells, suggesting that the cellular mechanisms by which the murine RM generates opioid analgesia are substantially different from those in rats. Murine on cell discharge did not predict the latency or magnitude of an ensuing withdrawal but did correlate to the magnitude and latency of concurrent withdrawals. Although opioids failed to alter the background discharge of on and off cells, they reduced the responses of RM neurons to noxious stimulation, further evidence that RM modulates on-going withdrawals. In characterizing the role of RM in respiratory modulation, we found that on cells burst and off cells paused during tachypneic events. The effects of opioids in the murine RM on homeostasis and the association of on and off cell discharge with tachypnea corroborate roles for opioid signaling in RM beyond analgesia.
Introduction
Morphine analgesia, the mainstay of clinical pain management, depends upon the medullary raphe magnus (RM) (Azami et al. 1982; Gilbert and Franklin 2002; Mitchell et al. 1998; Young et al. 1984). Morphine acts on two physiological classes of RM neurons that were originally defined by their physiological responses to noxious stimulation and morphine. On cells in RM are excited by noxious cutaneous stimulation and inhibited by opioids administered either centrally or peripherally (Barbaro et al. 1986; Fields et al. 1983a). In contrast, RM off cells are inhibited by noxious cutaneous stimulation and excited by analgesic doses of central or peripheral opioids (Barbaro et al. 1986; Fields et al. 1983a, b). Because the background firing rates of on and off cells correlate, inversely and proportionately, respectively, with the level of analgesia across a wide range of conditions, on cells are thought to facilitate nociception and off cells to suppress nociception (Fields et al. 1991). This physiological model has proved of great heuristic value, spawning a multitude of experiments whence new insights into the mechanisms of both analgesia and persistent pain have emerged (Hurley and Hammond 2000; 2001; Meng et al. 1998; Porreca et al. 2002). Yet, this model of medullary pain modulation is founded entirely on electrophysiological and microinjection studies in rats. To enable the use of mouse genetics to further our understanding of RM’s role in pain modulation, we have tested whether three fundamental findings observed in rats also occur in mice: 1) opioid microinjection into RM produces antinociception (Fang et al. 1989; Hurley and Hammond 2000; Jones and Gebhart 1988); 2) the response of RM cells to noxious stimulation predicts the response to opioid analgesic administration; and 3) the discharge of murine RM cells predicts nociceptive responsiveness (Foo and Mason 2003b; Heinricher et al. 1989; Jinks et al. 2004).
In addition to participating in pain modulation, studies in rat demonstrate that RM contributes to normal physiological functioning (for review see Mason 2001; Nakamura et al. 2005; Richerson 2004). In the rat, RM neurons project oligosynaptically to all sympathetic and parasympathetic targets tested to date as well as to somatic targets involved in micturition, breathing, and escape (Cao et al. 2006; Kerman et al. 2003; Mason 2001; Nadelhaft and Vera 1996, 2001). While some have argued that RM cells mediate specific physiological functions such as cold defense or fever (Blessing and Nalivaiko 2000; Morrison et al. 1999; Nakamura et al. 2004; Rathner et al. 2001; Tanaka et al. 2006), the multitude of pathways from RM neurons to widespread targets as well as pathways from some individual RM neurons to multiple targets (Jansen et al. 1995; Nadelhaft and Vera 2001) make unlikely that RM is involved in only a single, specific physiological function (for review see Mason 2005). Consistent with the idea that RM modulates many physiological functions, opioid microinjections into the rat RM alter cold defense, micturition and locomotion as well as nociceptive behavior (da Silva and Menescal-de-Oliveira 2007; Morgan and Whitney 2000; Nason and Mason 2006; York et al. 2005). Further, RM lesions in the rat block intestinal paralysis evoked by opioid administration into the midbrain periaqueductal gray (Parolaro et al. 1985) as well as cardiovascular responses evoked by systemic morphine (Randich et al. 1992). To examine the role of the murine RM in physiological homeostasis (Craig 2003), we tested: 1) whether opioid microinjection into the murine RM alters cardiorespiratory activity; and 2) whether the discharge of murine RM cells predicts spontaneous changes in cardiorespiratory function. Finally, complementary experiments in the rat permitted a direct comparison of RM function and physiology in rat and mouse.
Methods
Mouse surgery
Male C57BL/6 (n=70, 20–28 g; Charles River, Portage, MI) and DBA (n=5, 20–25 g) mice were anesthetized with 5% isoflurane. Anesthesia was maintained by 1.2–1.6% isoflurane throughout the duration of the surgery. Core temperature was maintained with a water-perfused heating pad. In experiments with i.c.v. injections, an additional craniotomy was made over the right lateral ventricle. Needle electrodes were placed into the thorax bilaterally to record the electrocardiogram (EKG) and into the biceps femoris to record the electromyographic activity (EMG) of the hindlimb withdrawal muscles. In some animals, screw electrodes were inserted into the skull over the parietal region to record electroencephalographic activity (EEG). A 1 mm diameter craniotomy was made 1–2 mm caudal from lambda to allow for access to RM. After surgery, animals were given one hour to allow the animal to equilibrate to 1.0% isoflurane (Eger 1984).
Drugs
Morphine sulfate (Malinckrodt, St. Louis MO) and d-Ala2, N-Me-Phe4-Gly5ol-enkephalin (DAMGO; Multiple Peptide Systems, San Diego, CA) were dissolved in phosphate buffered saline (PBS). Naloxone hydrochloride was purchased already dissolved in saline (0.4 mg in 1 ml, Abbott Laboratories) and administered i.m. in rats, but s.c. in mice because of the large volume.
Electrophysiological methods
Tungsten metal electrodes (5 MΩ, A-M Systems, Pullman, WA) were lowered at a 0–10° angle (2.0–3.0 mm posterior from lambda, 0.0–0.5 mm lateral, 5.0–6.0 mm ventral from cerebellar surface) into the region of the RM. The unit waveform was acquired at 40 kHz by a CED Micro1401 interface (CED, Cambridge, UK) and spikes were sorted offline using template matching. Locations of all recorded neurons were marked with lesions by passing 20 µA anodal current for 4 mins.
Neuronal characterization
Cells were characterized by their responses to at least 3 trials of noxious paw heat applied using a peltier device (Yale Instrumentation, New Haven, CT). The heat stimulus consisted of a 1.9 s ramp from 32°C to 51°C with a 4.5 s plateau at the peak temperature. The peltier platform then ramped back down to 32°C over the course of 1.8 s. The footpad and toes of the hindpaw were affixed, using Velcro straps, to the peltier platform (2 cm square) so that they were exposed to the full-duration stimulus. Between stimuli, the peltier platform was maintained at 32°C. Although evoked withdrawals in mice and rats were similar, an inter-stimulus interval of 500 s was necessary to prevent withdrawal fatigue in the mouse whereas an interval of 300 s was sufficient in the rat.
Neurons that were successfully isolated and demonstrated either an excitatory or inhibitory response to noxious stimulation were investigated further, thus biasing the sample to on and off cells. Since our initial electrophysiological experiments resulted in only 7 off cells, we recorded from an additional 8 mice with the specific intention of increasing the sample of off cells. In these additional experiments, we tried to reduce the surgical time and anesthetic exposure, both of which appear to suppress off cell firing. To this end, we omitted putting in an i.c.v. cannula and were thus able to record an off cell in each additional experiment.
Morphine administration and cell recording
After characterization of a cell, as described above, morphine was administered after which paw heat trials continued at 500 s intervals. After at least 3 trials of paw heat were recorded post-morphine administration, additional dosages of morphine or naloxone hydrochloride were injected and followed by more trials of heat stimulation.
DAMGO microinjections into RM
Mice were prepared with a guide cannula (Plastics One, Roanoke, VA) into RM as well as with EMG electrodes as above. After equilibration at 1.0% isoflurane, 3 paw heat trials were applied at 500 s intervals. DAMGO was then injected into RM or dorsally as a placement control. Paw heat trials continued to be applied at 500 s intervals after microinjections. After at least 3 post-injection trials had been collected, naloxone (0.4 mg/1 ml saline, s.c.) was administered and again paw heat trials were applied at 500 s intervals. In 4 mice, saline (500 nl) was injected prior to DAMGO injection and another 4 mice received saline followed by naloxone. The injection cannula was left in place until the conclusion of the experiment when it was withdrawn and filled with either India ink or Pontamine Sky blue. The dye-filled injector was placed back into the guide cannula and an injection of 500 nl was made to mark the microinjection site.
Histology
Animals were overdosed with 5% isoflurane and 10 mg pentobarbital, and perfused with a fixative containing 4% paraformaldehyde and 7% sucrose in 0.1 M phosphate-buffered saline. The brainstem was removed, post-fixed for 24 hrs, and then immersed in 30% sucrose in 0.1 M PBS. Coronal sections (50 µm) were cut on a freezing microtome. Sections were mounted on gelatin-coated slides, and then stained with cresyl violet. Microinjection and lesion sites were identified and drawn under 50x magnification. Sites were assigned an anterior-posterior location and then plotted on standard sections adapted from Figure 2 of VanderHorst and Ulfhake (2005). In the mouse, RM included a region 600 µm wide centered on the midline to a point 1000 µm dorsal to the base of the brain. The nucleus reticularis magnocellularis (NRMC), adjacent to RM, which is termed nucleus reticularis gigantocellularis pars alpha by some, was considered to encompass regions located 300 to 600 µm on either side of the midline and from the ventral surface to a point 1000 µm dorsal to the base of the brain. The anterior and posterior borders of RM and NRMC were the first section caudal to the nucleus of the trapezoid body and the first section rostral to nucleus ambiguous, respectively. Sites superficial to RM and NRMC that were located between the lateral edges of the pyramids were considered to be in nucleus reticularis gigantocellularis (NRGC). Neurons outside of RM and NRMC are not included in this report. DAMGO injections outside of RM and NRMC are considered as placement controls (see below).
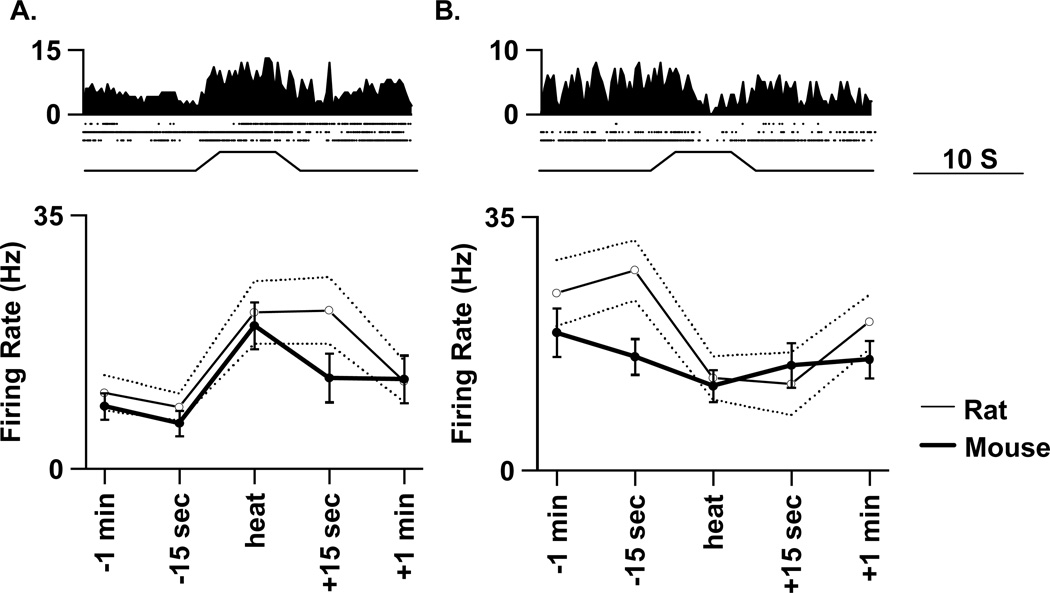
Figure 2.
Comparison of on (A) and off (B) cell responses to paw heat stimulation in mouse and rat. Average histograms (250 ms bins) and raster plots of 3 trials of heat stimulation (bottom trace) from a representative murine on cell (left) and a representative murine off cell (right) are shown at top. Below, the average activity a min prior to stimulation, 15 s prior to stimulation, during stimulation (“heat”), and 15 s and a min after the stimulation for all murine on (n= 32, left) or off (n=15, right) cells is indicated by the thick solid line and filled symbols. The thin solid line and open symbols represent the mean and the dashed lines the standard error from a cohort of rat on (n=35) and off (n=10) cells.
Cellular analysis
Although cells with a slow and regular pattern of discharge that are highly likely to be serotonergic (Li and Bayliss 1998; Mason 1997; Wang et al. 2001) were not studied, an algorithm that physiologically identifies serotonergic and non-serotonergic cells was employed to gain further confidence that studied cells were indeed non-serotonergic (Mason 1997).
A previously described statistical algorithm was used to classify cells as ON, off or NEUTRAL (Leung and Mason 1998). Briefly, the standard deviation of the change in discharge rate across sequential 10-s bins was calculated from a 500 s period without stimulation. Evoked changes were considered significant if they exceeded a threshold defined as two times this standard deviation. Using this criterion, responses were determined and then cells were classified as on or off if they were excited or inhibited, respectively, in a majority of stimulation trials and as NEUTRAL if they did not respond consistently.
Mean background discharge rate for each cell was calculated from the 60 s prior to each paw heat trial. Evoked discharge was defined as the mean discharge rate during (“stim”) or after (“post”) each paw heat trial less the background discharge rate. Then for each cell, values from multiple paw heat trials for each variable (background, evoked stim, evoked post) were averaged during baseline, post-DAMGO, -saline, and -naloxone periods. Population means, calculated for on and off cells during baseline and post-drug periods, were compared between groups using an ANOVA followed by post hoc comparisons using Newman-Keuls.
EEG analysis
EEG recordings were conducted on a subset of the mice receiving microinjections. The EEG was analyzed with a fast Fourier transform on a 64-s window (256 Hz, 214 points). Delta activity was the power summed within the frequency range of 1–4 Hz. In order to determine the effect of opioids on cortical activity, artifact-free periods of 500 s before and after DAMGO (excluding electrical artifact produced by movement of the microinjection cannula) and naloxone, during which no stimuli were applied, were analyzed as above for delta activity and compared between groups using an ANOVA.
Analysis of physiological reactions
Heart rate was calculated as the reciprocal of the interval between successive R waves in the EKG. The EKG signal was then high pass filtered to eliminate the slow QRS waves. The remaining fast EMG activity, reflective of intercostal muscle activity, was full-wave rectified and integrated. From this processed signal, respiratory rate was calculated as the reciprocal of the interval between successive peaks (each peak marking inspiration).
Pre-heat trial heart and respiratory rates were considered baseline variables. For each of these variables, the mean values for the 60 s prior to each paw heat trial were calculated. Then for each animal, the mean values before and after each drug were calculated. Finally, population means for each experimental group were calculated and compared between groups using an ANOVA.
The hindlimb EMG recording was full wave rectified and integrated. Evoked withdrawal magnitude was quantified as the sum of the EMG for 8.2 s (the full duration of the heat stimulus) after stimulus onset less the sum for 8.2 s prior to the stimulus. The onset of the evoked withdrawal was determined by the time when the amplitude of the EMG exceeded 2 standard deviations above the mean, pre-stimulus EMG activity. For each trial and each experimental group, mean withdrawal magnitude and latency were calculated and then compared between groups using an ANOVA.
Correlational analyses
For each cell, the mean withdrawal latency, peak magnitude, and integrated magnitude and the mean cellular response to each heat trial during the baseline period were calculated. All variables were normalized by a Z-score transformation. Briefly the Z-score is equal to (R–X)/SD where R is the variable from an individual trial, X is the mean variable from the animal (for withdrawal variables) or cell (for cell discharge), and SD is the standard deviation of that variable for all trials in the animal or cell. This transformation compensates for variability between animals and neurons. A correlation analysis was then conducted between cellular discharge, during different epochs (as described in Results), and all variables of the withdrawal reaction.
To determine the relationship between RM cell discharge and brief episodes of tachypnea, an automatic search program identified increases in respiratory rate that exceeded 5%. The search program computed the average respiration rate in rolling (100 ms advancing interval) 30-s time bins. Tachypneic episodes were then defined as events when the second bin’s average exceeded the first bin’s average by 5%. For every tachypneic event, the average cell activity, heart rate and respiration rate were calculated for both time bins with the first bin representing baseline and the second bin considered tachypnea.
Rat experiments
Most experiments on male Sprague–Dawley rats (n = 20, 250–500 g; Charles River, Portage, MI) were reported in Brink et al. (2006). The experimental protocol in these rat studies was similar to that in the mouse studies with the following exceptions: 1) rats were anesthetized with halothane; 2) an arterial catheter was placed in the femoral artery; 3) rats received intermittent colorectal distension stimulation; and 4) DAMGO (50 ng) rather than morphine was administered i.c.v. Additionally, previously unpublished experiments in rats (n = 12) examining the effect of 0.5 mg i.m. morphine on RM cellular activity are also included. Of note, in the former experiments, 2–4 electrodes were inserted together, resulting in the simultaneous recording of multiple single units in each animal.
Statistics
Each variable is expressed as a mean ± standard error. Statistical tests were performed using SigmaStat (SPSS Science, Chicago, IL). For multiple comparisons, repeated measures ANOVA with Bonferonni corrections were performed. Pearson correlations were calculated to test the relationship of firing rates with withdrawal parameters using Z-score normalization to account for variability between subjects. All p values greater than 0.001 are reported exactly and those less than 0.001 are noted as “p<0.001”. A p value less than 0.05 was considered significant.
Results
Microinjection of mu-opioid receptor agonists into the murine RM produces analgesia, bradycardia, bradypnea and alters EEG activity
DAMGO microinjection into RM (50 ng, 500 nl) significantly increased the latency (p=0.003) and reduced the magnitude (p=0.05) of withdrawals from noxious paw heat in lightly anesthetized mice (n=11, Fig. 1). Subsequent naloxone administration (0.4 mg, 1 ml, s.c.) significantly decreased the withdrawal latency of mice that had received DAMGO (p=0.03, n=11). Saline microinjection into RM (n=8) or DAGMO microinjection into the dorsally located NRGC (n=6) had no effect on withdrawal latency (saline p=0.72, NRGC p= 0.19) or magnitude (saline into RM: p=0.42; DAMGO into NRGC p=0.50).
Figure 1.
Antinociception, cardiorespiratory depression, and cortical arousal evoked by 50 ng DAMGO microinjection into RM and reversal by systemic naloxone. The panels on the left show, from top to bottom, noxious heat-evoked withdrawal latency (in s), heart rate (HR in Hz), respiratory rate (RR in Hz), EEG delta (1–4 Hz) power (in µV2) during baseline (left of D), after DAMGO (between D and N) and after naloxone (dotted line marked N) from one animal. An arrow in the graph of delta power indicates the electrical artifact produced by introducing the microinjection cannula. The line graphs on the right represent mean values (from 3–5 measurements as detailed in the Methods) of each of the labeled measures for individual mice receiving either DAMGO microinjection into the RM (left column), DAMGO into the dorsally located NRGC (right column - solid circles), or saline into the RM (right column - hollow circles). A baseline period (b) preceded all microinjections (d) which were followed by systemic naloxone administration (n).
Heart rate (n=11, p<0.001) and respiratory rate (n=10, p<0.001) decreased after DAMGO microinjection. In one mouse receiving a DAMGO microinjection it was not possible to effectively determine respiratory rate because of a defective electrode. In all mice receiving DAMGO microinjection (n=11), cardiorespiratory changes were sustained until naloxone was administered, when they were partially reversed (Fig. 1). Saline microinjection into RM (n=8) or DAGMO microinjection into NRGC (n=6) did not evoke a significant change in heart rate (saline into RM: p= 0.83; DAMGO into NRGC: p= 0.98) or respiratory rate (saline into RM: p=0.07; DAMGO into NRGC: p=0.33).
Hypothesizing that the observed cardiorespiratory depression reflected a change in arousal state, EEG recordings were performed on mice receiving DAMGO in RM (n=8), saline in RM (n=6), or DAMGO in NRGC (n=4). An overall “flattening” of the EEG after DAMGO administration into RM was visible to the experimenter even prior to FFT analysis. Indeed, delta activity, a measure of synchronized slow cortical activity, decreased after DAMGO administration into RM (Fig. 1; p=0.03). While a decrease in delta power implies a heightened level of arousal (Grahn and Heller 1989), mice were simultaneously less responsiveness to noxious stimuli. No significant effect on delta power was observed when saline was microinjected into RM (p=0.91) or when DAMGO was microinjected into NRGC (p=0.25).
Background discharge and heat-evoked responses of murine RM cells resemble those of rat RM cells
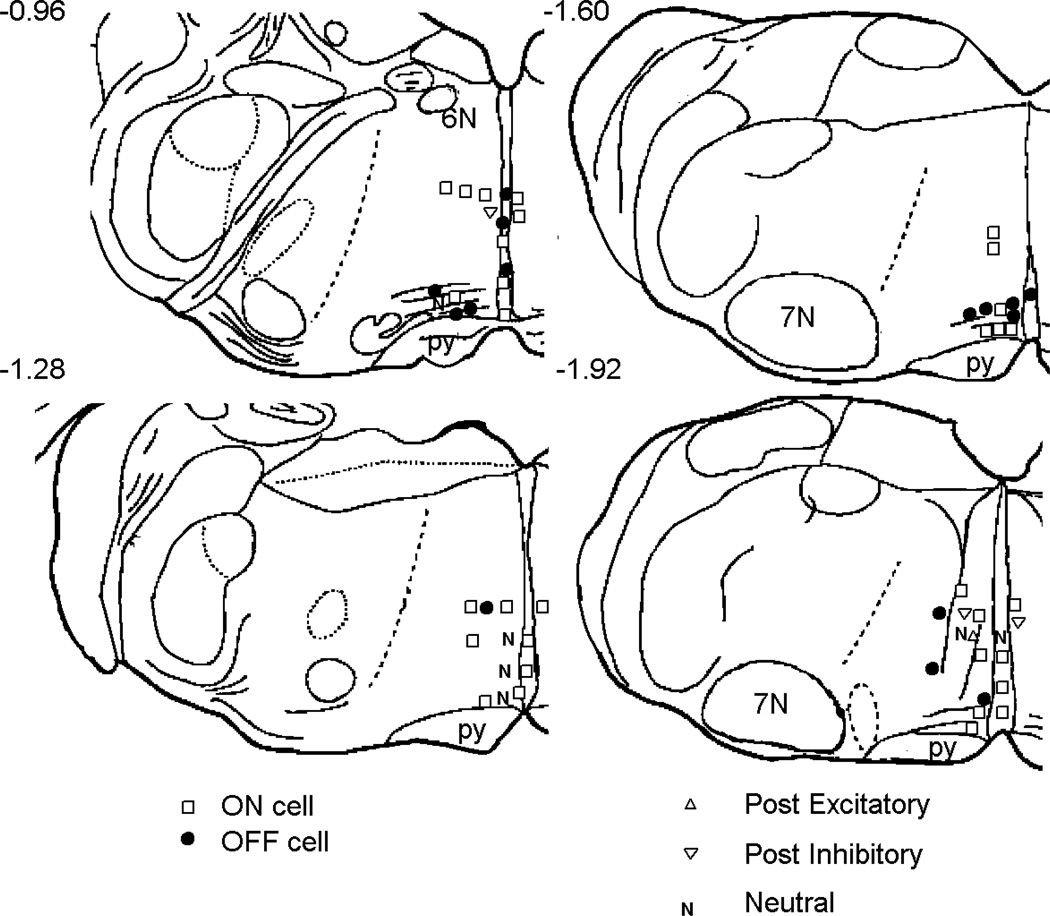
Each neuron was characterized by its resting discharge and response to noxious stimulation (Fig. 2). Only cells located in RM (n=42) or the adjacent NRMC (n=15) were studied (Fig. 3). Although we intended to study only cells that were excited or inhibited by a heat stimulus, post hoc analysis revealed that some cells did not respond consistently to heat stimuli and thus were classified as NEUTRAL (n=6). No neurons had the slow and steady discharge characteristic of serotonergic neurons in the rat (Mason 1997) or cat (Auerbach et al. 1985). In 49 out of 57 neurons recorded, the coefficient of variation of the interspike interval was greater than 1, indicating a discharge that includes bursts and frank pauses (cv=5.6 ± 0.8, firing rate=12.7 ± 2.6 Hz). The remaining 8 neurons with a coefficient of variation less than 1 were unlikely to be serotonergic because they all had firing rates that exceeded 10 Hz. As previously observed in rats, more cells were excited (ON cells, n=32, Fig. 2A) than inhibited (OFF cells, n=15, Fig. 2B) by noxious heat. We often (10–25% of all cell encounters) encountered cells that appeared to respond to noxious heat only after the stimulus had ended, but only characterized a few such neurons that were clearly inhibited (n=3 cells) or excited (n=1 cells) after the offset of the heat stimulus (data not shown); these cells are not included in the analysis below.
Figure 3.
Locations of recording sites. The relative position of different characterized cell types are plotted on a line drawing modified from Vanderhorst and Ulfhake (2006). Cell locations on the left have been transposed and marked on the right side of the brain, although recordings were made bilaterally. The numbers indicate the distance in mm to the interaural line as measured by Vanderhorst and Ulfhake 2006. Abbreviations: 6N, abducens nucleus. 7N, facial nucleus. py, pyramid.
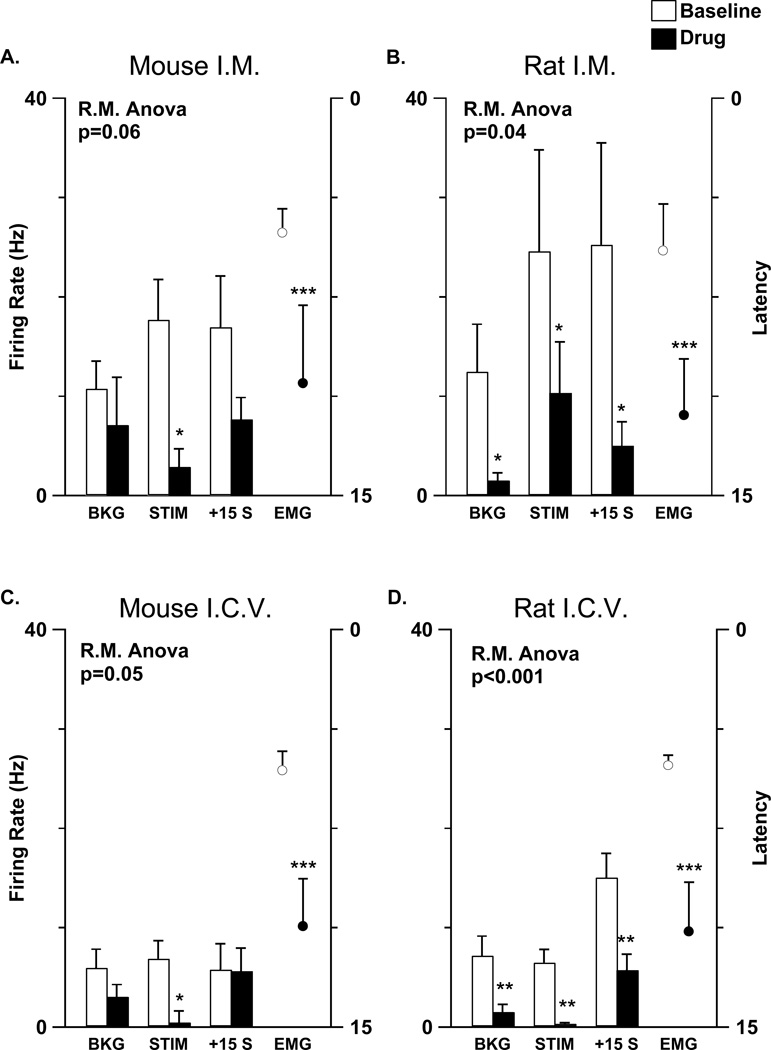
To compare the physiological features of RM cells from rat and mouse directly, a cohort of rat on (n=35) and off (n=10) cells was studied using a similar experimental setup to that employed in studying mice. In both rats and mice, a response threshold was calculated from the variability in the background discharge; this response threshold was proportional to discharge variability such that it was greater in cells that fired in bursts than in cells that fired steadily (see Leung and Mason 1998 and Methods for details). To compare the noxious heat-evoked responses of cells from rat and mouse, all responses were normalized to this response threshold so that, for example, a change in discharge that is 3-fold greater than the response threshold was assigned a value of 3.0. This analysis revealed two significant differences. First, the decrease in off cell discharge during heat stimulation was greater in rat (3.1 ± 0.8) than in mouse (1.4 ± 0.1; p=0.04). Second, one minute after stimulation, on cell discharge was still greater than threshold in rat (2.5 ± 0.4) but not in mouse (0.4 ± 0.3; p<0.001).
On cells’ responses to noxious stimulation, but not background firing rates, were reduced by an analgesic dose of morphine
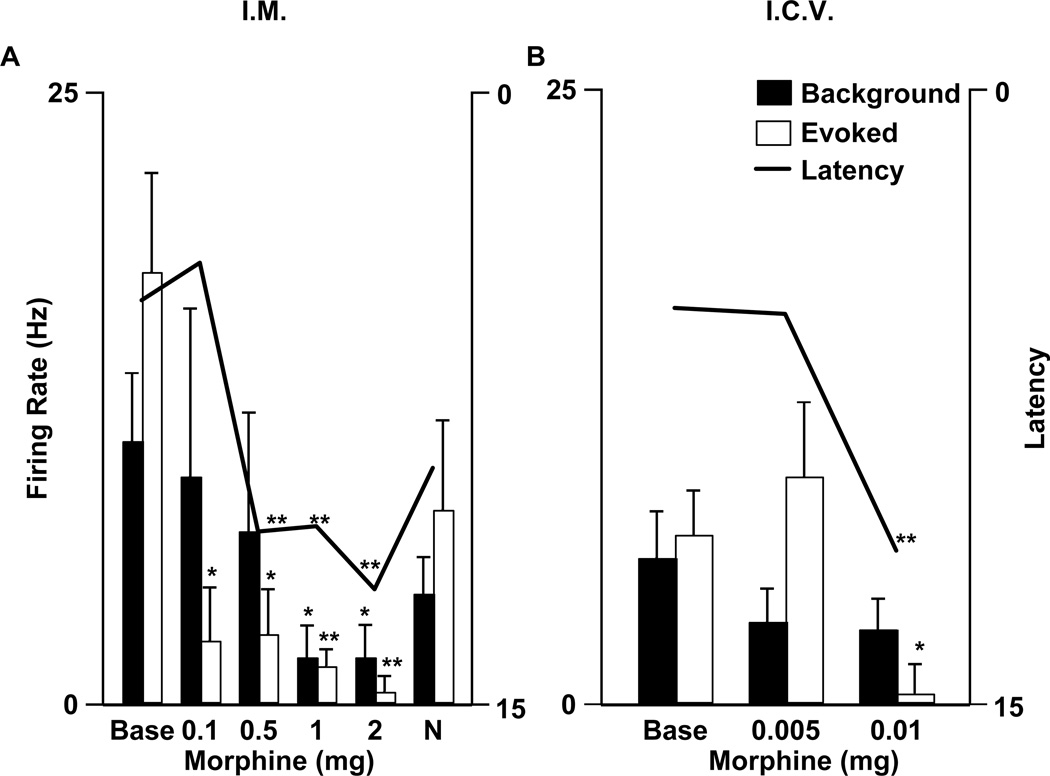
A comparison between the effects of identical opioid doses, in grams per body weight, on RM cells in mouse and rat would be inappropriate as the doses required to produce analgesia are 10 to 100 times greater in mouse than in rat (Mogil 1999; Mogil and Wilson 1997; Szekely et al. 1984). Therefore we decided to compare low doses that produce reliable analgesia. For rats, doses were chosen from the literature in order to avoid using animals to reconfirm well-established findings. The doses were 500 µg (~ 1.25 mg/kg based on the average weight of rats used, 400 g) of systemic morphine (Tyler and Advokat 1986) and 50 ng (~ 125 ng/kg) of i.c.v. DAMGO (Kepler et al. 1991). For mice, a series of doses were tested. As illustrated in Figure 4, 500 µg (n=20; ~ 20 mg/kg, based on the average weight of mice used, 25 g), but not 100 µg (n=5; ~ 4 mg/kg) of systemic morphine produced analgesia in mice; therefore the effect of a 500 µg dose of systemic morphine on murine RM cell firing was tested. When administered i.c.v., 10 µg (n=7; ~40 µg /kg) but not 5 µg (n=5; ~20 µg /kg) DAMGO significantly increased the paw withdrawal latency (Fig. 4B). The effect of 10 µg DAMGO (i.c.v.) was then tested on the discharge of RM cells in mice.
Figure 4.
Average background firing rates (filled bars, left ordinate) and noxious heat-evoked responses (solid bars, left ordinate) of murine on cells are compared to mean withdrawal latency (line, right ordinate) before (base) and after multiple dosages (as labeled) of systemic (A) and central (B) morphine. The same variables are also illustrated after systemic naloxone administration (N) in A. The number of cells tested with each dose were as follows in A: 100 µg, n=5; 500 µg, n=20; 1 mg, n=9; 2 mg, n=7; naloxone, n=18; and in B: 5 µg, n=5; 10 µg, n=7. Significant differences are marked by 1 (p<0.05) or 2 (p<0.01) asterices.
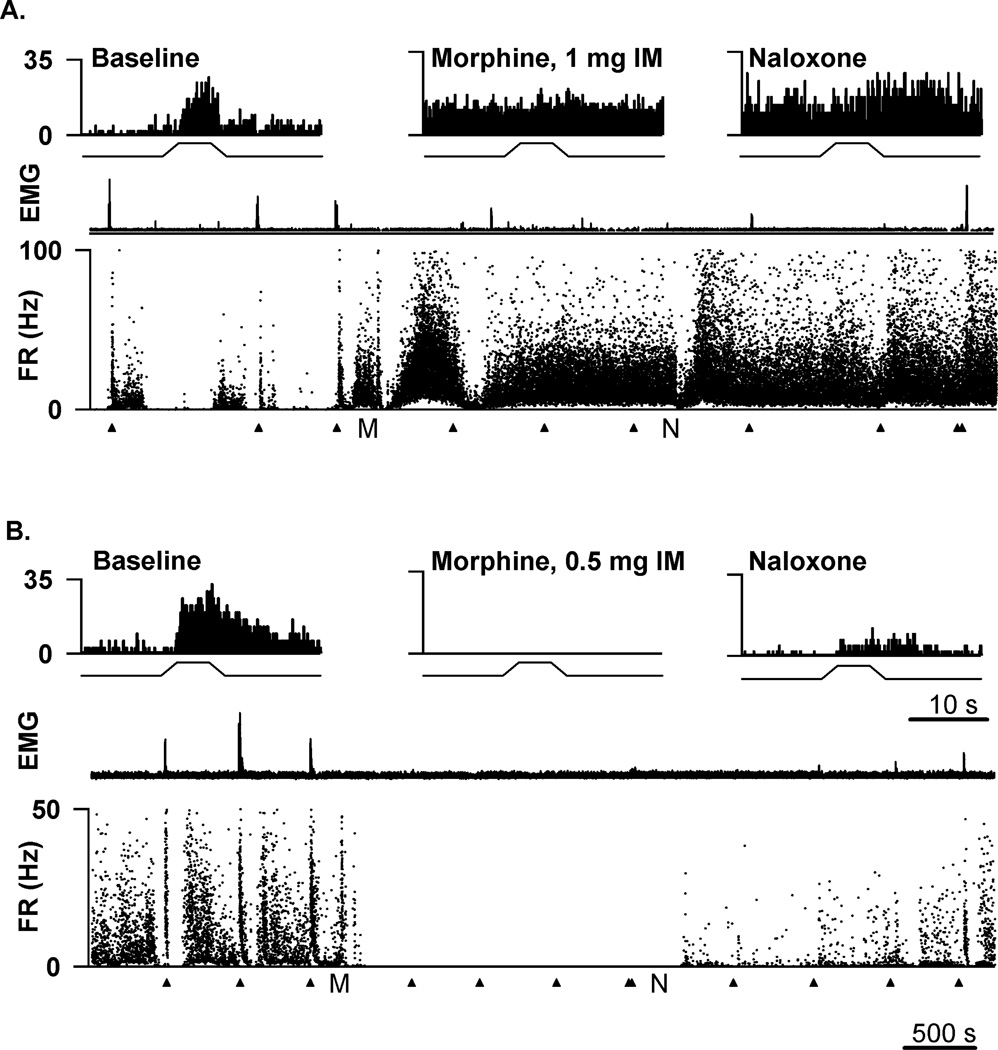
To examine the role of RM in opioid analgesia, the mean background firing rate and noxious heat-evoked responses of the on cell population were computed before and after an analgesic opioid dose. Morphine (500 µg, i.m.) significantly increased the withdrawal latency (p=0.004, n=20 animals) and reduced responses of on cells (p=0.003, n=20 cells) evoked by noxious paw heat. However, this same analgesic dose of morphine had no effect on the background firing of on cells (p=0.53, n=20 cells) (Fig. 4A). At a higher dose of systemic morphine (2 mg), declines in both background firing (p=0.03) and noxious heat-evoked responses (p=0.008) were observed in the population of 7 on cells tested (Fig. 4A). In a second analysis, the firing rates before and after morphine administration, in each cell, were compared. The background firing rate of most on cells tested (17/20) was not different or increased after systemic morphine (500 µg, i.m.) administration (Fig. 5A). In the case of only 3 on cells, morphine (500 µg, i.m.) elicited a decrease in background firing rate (Fig. 5B).
Figure 5.
Responses of two murine on cells to systemic morphine. (A) An on cell in RM was excited by 1 mg morphine. (B) An on cell in RM was inhibited by 0.5 mg morphine. Histograms at the top of each panel show average responses (250 ms bins) to 3 trials of heat stimulation during the baseline period (left), after morphine administration (middle), and after naloxone (right). Beneath the histograms are the full-length recordings from each cell with the rectified integrated EMG (top) and the instantaneous firing rate (FR, bottom) for the entire duration of the experiment (~1.5 hr). Tail heat stimulation was applied regularly throughout the recordings at the times marked by the arrowheads. The time points at which morphine and naloxone were injected are indicated by M and N, respectively. The scale bar at the bottom of B applies to the long records in A and B. The scale bar beneath the right histogram in B applies to all histograms.
To test whether the background discharge of murine RM on cells is inhibited by centrally administered morphine, even in the absence of such a response to systemic morphine, the effect of morphine (10 µg) administered i.c.v. on RM cell discharge was tested. Morphine (10 µg, i.c.v.) increased paw withdrawal latency (p=0.003, n=7 animals) and also reduced RM on cell responses to noxious paw heat in the mouse (p=0.04, n=7 cells). However, the background discharge of murine on cells was unaffected by central morphine (p=0.61, n=7 cells) (Fig. 4B). At higher doses of i.c.v. morphine (30–50 µg), the background discharge of two on cells was unaffected and that of one was inhibited (data not shown).
Morphine’s failure to reduce the background discharge of on cells in the murine RM is in sharp contrast to the significant reduction of on cell background discharge by opioid receptor agonists in rats (Brink et al. 2006; Fields et al. 1983; Fields et al. 1983). To ensure that there was nothing in the experimental setup preventing us from seeing a more typical (for the rat) on cell response to opioids, morphine’s reduction of rat on cell discharge was confirmed here in a set of experiments. The background, heat-evoked, and post-stimulus discharge rates of rat on cells decreased after systemic morphine (n=8; p values <0.05; Fig. 6B) or i.c.v. DAMGO (n=27; p values <0.01; Fig. 6D). An analysis of individual cell responses showed that the discharge rate of all rat on cells tested (n=8) decreased after systemic morphine and nearly all on cells in the rat (25/27) were similarly inhibited by i.c.v. DAMGO.
Figure 6.
A comparison of mouse (A, C) and rat (B, D) on cell responses to systemic (A, B) and central (C, D) opioid administration. The bar graphs represent background (BKG), heat-evoked (STIM) and post-stimulus (+15 s) firing rates (left axis) before (open bars) and after (filled bars) opioid administration at doses that caused analgesia. The open and filled markers indicate withdrawal latency (right axis) before and after opioid administration, respectively. A: Morphine (500 µg, i.m.) delayed withdrawal latencies in mice and reduced heat-evoked responses but had no significant effects on background on cell (n=20) firing. B: Morphine (500 µg, i.m.) delayed withdrawal latencies in rats while significantly reducing the background activity and noxious stimulus-evoked responses of on cells (n=8). C: Morphine (10 µg, i.c.v.) delayed withdrawal latencies and significantly reduced the stimulus-evoked responses of on cells (n=7) in mice. D: DAMGO (50 ng, i.c.v.) delayed withdrawal latencies and significantly reduced background and evoked activity in rat on cells (n=27). The significance of each repeated measures ANOVA for all cell parameters shown is in the upper left of each panel. Significant differences are marked by 1 (p<0.05), 2 (p<0.01), or 3 (p<0.001) asterices.
Opioids did not excite murine off cells
The activity of 12 murine off cells was recorded in response to morphine. In 10 mice receiving systemic injections, there was no consistent effect of 0.5 mg morphine on off cell background firing rate (p=0.96) despite consistent increases in withdrawal latency (p<0.01) (Fig. 7). Although 2 individual off cells were significantly excited by morphine, another 2 off cells were inhibited. Even when an additional dosage of 1 mg morphine was injected in 4 cases, there was no observable excitation of off cells. In the case of 2 off cells, morphine i.c.v. significantly decreased background firing rate.
Figure 7.
Systemic administration of 0.5 mg morphine reduced withdrawal amplitude (traces marked EMG) but did not excite this murine off cell. A–C: Average responses (250 ms bins, top traces) to repeated trials of heat stimulation (bottom traces) during the baseline period (A, n=3 trials), after 0.5 mg morphine administration (B, n=3), and after naloxone (0.4 mg; C, n=2). D: The full-length recording from this cell with the rectified integrated EMG (top) and the instantaneous discharge (bottom) for the entire duration of the experiment (~1 hr). Tail heat stimulation was applied regularly throughout the recording at the times marked by the arrowheads. The time points at which morphine and naloxone were injected are indicated by M and N, respectively. The scale bar in C applies to the records in A–C. The scale bar beneath D applies to D only.
In the rat cohort, off cell background activity was increased by at least 2 standard deviations by i.c.v. DAMGO (6/9 cells) and i.m. morphine (4/4 cells). Even in the 3 rat off cells whose discharge was not significantly increased by 2 standard deviations, there were nonetheless long-lasting increases in firing rate.
Prestimulus on cell activity does not correlate with the magnitude of withdrawal in mouse
Since reduction of background on cell activity is a primary effect of opioid drug administration in rats but not mice, we anticipated that there may be an additional difference between rats and mice in the relationship of on cell activity to withdrawal magnitude even in the absence of morphine. To test whether on cells modulate future noxious stimulus-evoked withdrawals, on cell activity was correlated to the magnitude of withdrawals in the baseline condition (all variables were transformed to Z-scores as described in the Methods). The data set contained recordings of neurons paired with withdrawals in mice (32 neurons, 137 withdrawals) and rats (31 neurons, 125 withdrawals).
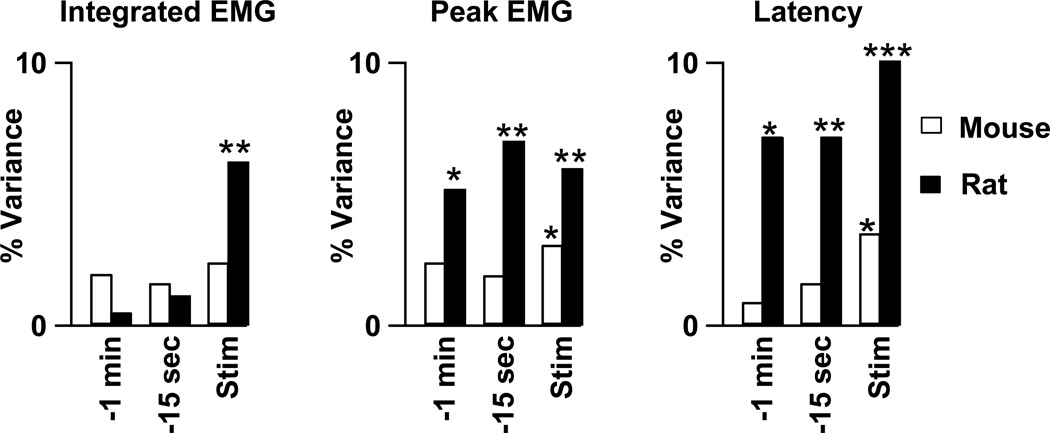
In mice, there were no significant correlations between firing rate prior to withdrawal and the parameters describing the ensuing withdrawal (Fig. 8). In rats, firing rates during the 15 s and 1 minute periods prior to withdrawal correlated with peak EMG amplitude (− min: r2=0.05, p=0.01; −15 s: r2=0.07, p=0.003) and withdrawal latency (−1 min: r2=0.04, p=0.02; −15 s: r2=0.07, p=0.002) but not with integrated EMG amplitude (−1 min: r2=0.01, p=0.43; −15 s: r2=0.01, p=0.43). We also examined the relationship between firing rate and the concurrent withdrawal. In mice the firing rate during noxious stimulation was significantly correlated with two measures of withdrawal strength: peak EMG amplitude (r2=0.03, p=0.04) and withdrawal latency (r2=0.03, p=0.02). In rats, there were correlations between evoked firing rate and all 3 parameters describing withdrawals (r2 values >0.06, p values <0.005). In sum, the background discharge of rat on cells significantly predicted ensuing withdrawal strength but the background discharge of murine on cells did not. Further, the noxious heat-evoked discharge of both murine and rat on cells correlated significantly with the simultaneously-occurring withdrawal reaction.
Figure 8.
A linear regression analysis was performed between on cell firing rate and withdrawal strength in mice (open bars) and rats (solid bars). Please note that off cell firing rate was not included in this analysis. Three measures of on cell firing rate (categories along the abscissa) were regressed upon three measures of withdrawal strength (three columns) and the r2 values plotted. All cellular and motor measures were normalized as fully detailed in the Methods. The measures of on cell firing rate were the mean firing rate during the minute prior to heat stimulation (−1 min), the 15 s prior to heat stimulation (−15 sec) and the 15 s during heat stimulation (Stim). The withdrawal measures were integrated EMG magnitude (left), peak EMG value (middle), and withdrawal latency (right). Only trials prior to opioid administration were included. Asterices denote significant correlations between a withdrawal parameter and the associated firing rate. Significant differences are marked by 1 (p<0.05), 2 (p<0.01), or 3 (p<0.001) asterices.
Increases in respiration are coincident with increases in on cell firing and decreases in off cell firing
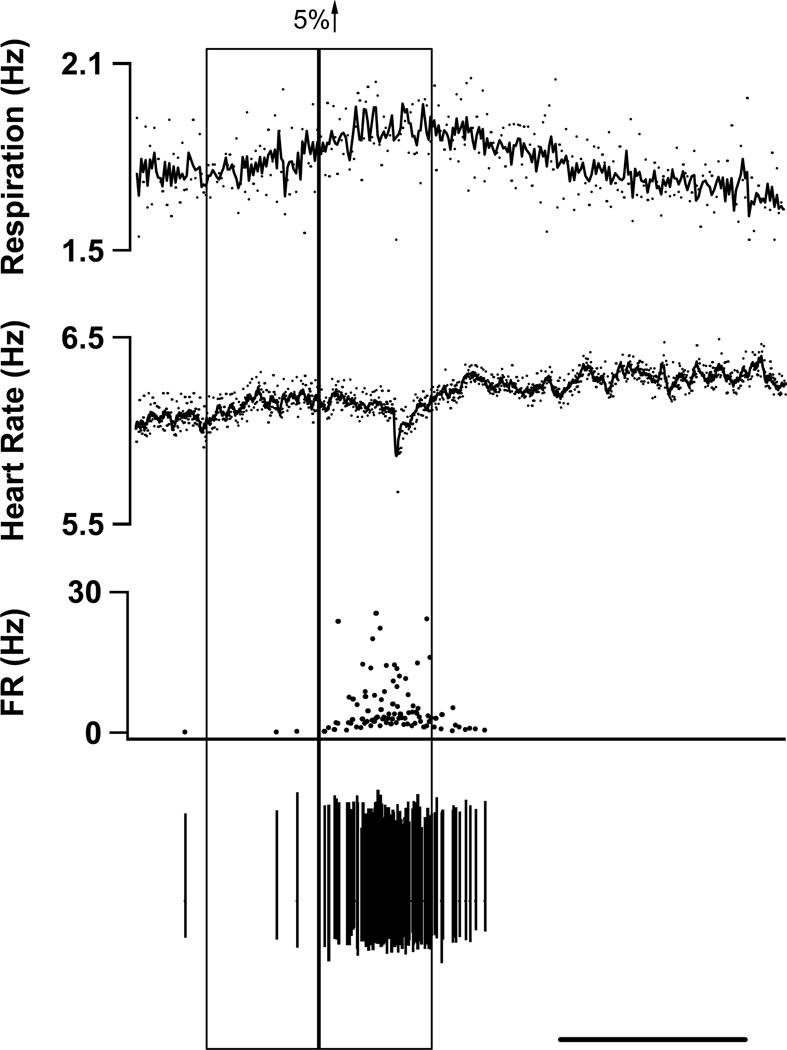
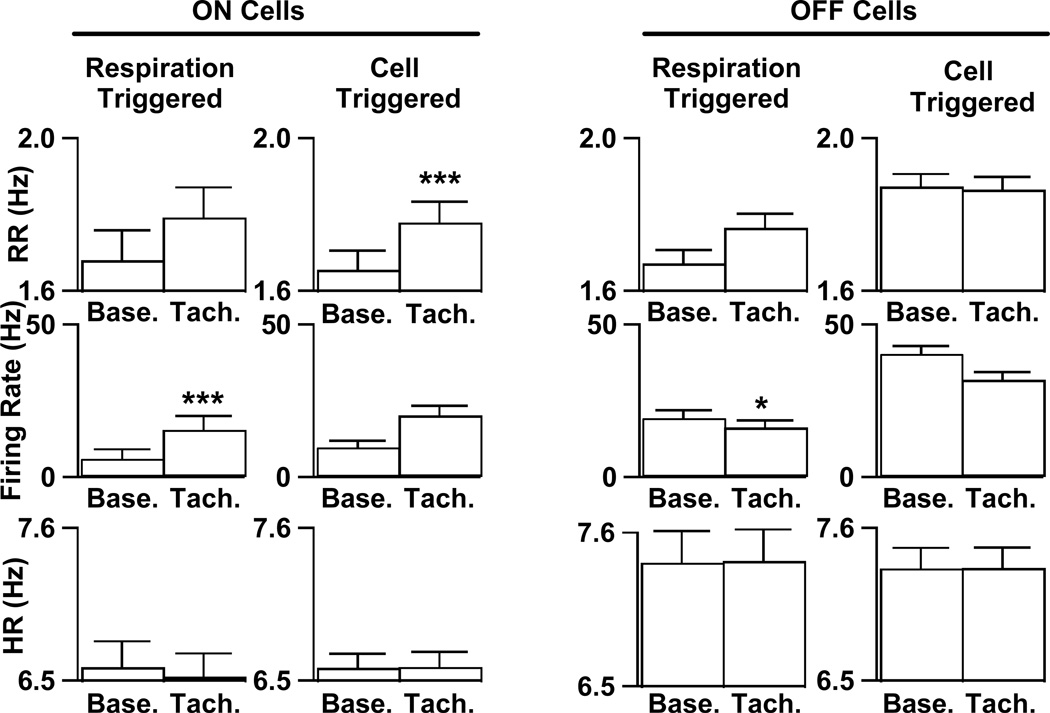
Given the poor correlation of background activity in mice to withdrawal magnitude, we visually examined the records to find other factors that might correlate with on cell firing. We observed that transient increases in respiration rate often occurred around the time of bursts of on cell activity (Fig. 9). To examine the consistency of this relationship, we identified all transient increases (≥5%) in respiration rate that were not evoked by a noxious stimulus (see Methods) and then analyzed on cell firing rates during these events. on cell firing rates increased by 9.0 ± 1.9 Hz (p <0.001, n=20) during transient episodes of tachypnea (Fig. 10). During these periods there were no changes in heart rate (p=0.39) or EMG tone (p=0.57). We also performed the reverse analysis to see if periods of increased on cell activity were related to increases in respiration rate. Periods of unstimulated, but increased on cell activity (n=30) were associated with a 7.5% increase in respiration (p<0.001), but not with alterations in heart rate (p=0.87) or EMG tone (p=0.31).
Figure 9.
A representative tachnypeic event with the associated on cell burst. All records were scanned for events where the respiratory rate increased by at least 5% from one bin to the next (see Methods). The vertical boxes show sequential 30-s bins across which respiratory rate (top trace) increased by at least 5%. In the respiratory and heart rate traces, the dots represent instantaneous event frequency and the line is a moving average. During the tachypneic event illustrated, heart rate (2nd trace) was unchanged while on cell activity (3rd trace) increased. Raw spikes are shown in the bottom trace. Scale bar represents 50 s.
Figure 10.
Alterations in firing rates were observed during tachypneic events and increased on cell firing rate predicted tachypnea. Bar graphs represent averages of respiration rate (top), on cell firing rate (middle) and heart rate (bottom) before (“Base.”) and during (“Tach.”) spontaneous tachypneic events. Events were either detected by an increase of ≥5% in respiration rate (columns labeled Respiration Triggered) or by a ≥5% change in cell firing (columns labeled Cell Triggered). For more details, please see text. Significant differences are marked by 1 (p<0.05) or 3 (p<0.001) asterices.
We also examined off cell discharge during spontaneous tachypneic events. Using the same analysis described above, we found that off cell firing rate decreased by 2.8 ± 1.3 Hz (p = 0.04) during episodes of tachypnea (n=97). Further examination of individual cell records revealed that while 10 off cells were consistently inhibited during tachypnea, 1 off cell was excited during tachypneic events (5.8 ± 1.8 Hz, n=19). Excluding this cell, the average decrease in off cell firing rate during tachypneic events (n=78) was 5.6 Hz ± 1.6 (p=0.001). In contrast to on cell bursts’ predicting tachypnea, decreased off cell firing (n=133) was not associated with any significant alteration in respiration rate (p=0.39).
Discussion
The murine RM differs from the rat RM in how it participates in nociceptive modulation but, like the rat RM, modulates physiological functions beyond nociception
The first goal of this study was to test whether findings basic to RM’s role in nociceptive modulation are observed in mouse as they are in rat in order that the pain modulation field could, with good reason, employ the mouse as a close analog of the rat. The unfortunate answer appears to be that the participation of RM in nociceptive modulation in the mouse differs in basic ways from that in the rat. Most importantly, the response of RM murine cells to noxious cutaneous stimulation does not predict the response to opioids as is true in the rat. Since this is the fundamental basis of the heuristic model developed by Fields, prudent researchers will not simply extend the rat on - off cell model to mice.
The second aim of this study was to determine whether the murine RM participates in modulation of physiological processes beyond nociception. The clear answer is yes. However, since we only tested RM’s modulation of heart rate, respiration rate and cortical synchrony, more studies are needed to determine the full range of physiological functions that RM modulates.
At first glance, the differences between male Sprague-Dawley rats and male C57BL/6 mice would appear to be due to a distinction between rats and mice. However, it should be noted that Sprague-Dawley is the strain used in all of the physiological studies of RM known to the authors. While results from the few DBA mice that we studied were not notably different than those obtained from C57BL/6 mice, a preliminary study has reported differences in the effects of DAMGO on the noxious stimulus-evoked responses of RM neurons in CBA/J and A/J mice (Sugino et al. 2006). Thus the differences observed may reflect either a true species difference or alternatively, a difference between strains that also happen to come from different species.
The murine RM contributes to opioid analgesia but not through a change in the background activity of on and off cells
DAMGO microinjection into the murine RM increases the latency and reduces the magnitude of noxious heat-evoked withdrawals, evidence that the RM plays a role in nociceptive modulation. However, in contrast to the case in the rat, the background activity of murine on cells – RM cells excited by noxious cutaneous stimulation - was not inhibited by opioids and that of murine off cells – RM cells inhibited by noxious cutaneous stimulation – was not excited by analgesic doses of opioids. Thus murine RM on and off cells mediate opioid analgesia differently than do the much more widely-studied rat RM cells.
The lack of a correspondence between murine RM cells’ responses to noxious heat and opioids was not due to any peculiarity in our experimental setup as we confirmed just such a correspondence in recordings from a cohort of rats. It is possible that we did not record from a sufficient number of cells or from cells in the right location in the mouse. However, this is unlikely as recordings from rat clearly confirmed that on cells are inhibited and off cells excited by opioids. Beyond the significance of the rat on and off cell populations’ responses to opioids and the lack of significance in the murine populations’ responses to opioids, 30 of 35 rat neurons responded to an analgesic opioid dose in the canonical way whereas only 5 of 39 murine cells responded in this manner. Further, the murine neurons recorded were made in the same region where opioid microinjection produced antinociception. Thus it is unlikely that on cell inhibition and off cell excitation play the same integral role in opioid analgesia in mouse as they do in rat.
Opioids’ failure to alter the background firing rates of RM cells in the anesthetized mouse resembles findings obtained in awake rats, where background OM cell firing rates are not affected by morphine (Martin et al. 1992). Thus although studies in the anesthetized rat have focused on the effect of opioids on spontaneous RM on cell activity rather than on evoked activity (Anderson et al. 1977; Azami et al. 1979; Barbaro et al. 1986; Chiang and Gao 1986; Chiang and Pan 1985; Deakin et al. 1977; Fields et al. 1983; Fields et al. 1983; Heinricher and Rosenfeld 1985; Satoh et al. 1979; Schnell et al. 2002; Toda 1982), it is clear that alterations in the spontaneous activity of RM on cells are not essential for opioid analgesia in awake rats or anesthetized mice.
The critical function of RM may be to modulate the size of on-going, rather than future, protective motor reactions
Opioids did not alter the background firing but did suppress the noxious stimulus-evoked responses of murine RM on and off cells. Similarly, a recent preliminary study reported that DAMGO suppressed the noxious stimulus-evoked responses of RM neurons from two different strains of mice (Sugino et al. 2006). This profile of opioid responses in the anesthetized mouse resembles that described in the unanesthetized rat. In the only study to test the opioid responses of cells with known responses to noxious stimulation in the awake rat, background on cell firing rates were not affected by morphine but on cell responses to noxious stimulation were reduced (Martin et al. 1992).
Here we found that the relative latency and magnitude of withdrawals were not predicted by the rate at which on cells fire prior to the noxious stimulus in the mouse. As a positive control, we were able to confirm previous studies (Foo and Mason 2003; Heinricher et al. 1989) that this predictive relationship exists in the rat. The amount of murine on cell discharge during noxious stimulation did correlate with the magnitude and latency of the withdrawals evoked concurrently, suggesting that the murine RM modulates withdrawals as they are occurring rather than withdrawals that are about to occur. Findings that RM neurons respond after the onset of the disynaptic jaw-opening reflex and after the onset of laser stimulus-evoked withdrawals further support this conclusion (Foo and Mason 2003; Mason et al. 1986).
While initially difficult to reconcile with a literal interpretation of Fields’ original model of RM on and off cell function, our current findings fit with the more extensive literature arising from experiments in anesthetized rats. First, our finding that DAMGO microinjection into the murine RM suppresses nociceptive withdrawals shows that the RM has the capacity to modulate nociceptive transmission in mouse as well as in rat and cat (Oliveras et al. 1975; Sessle and Hu 1981). Second, our finding that on cell responses to noxious stimulation correlate with the withdrawals evoked concurrently fits with a role for RM in modulating the magnitude of protective reactions to visceral and somatic stimuli. Just such a role has been documented in the somatomotor reaction to colorectal distension (Brink and Mason 2004) and the withdrawals evoked by cutaneous stimulation in the territory of an injured nerve (Burgess et al. 2002; Porreca et al. 2001). If this proves a consistent finding in rats and mice, anesthetized and unanesthetized, then modulating the size of on-going protective motor reactions may be a fundamental function of RM.
RM is important in regulation of physiological processes beyond nociception
Opioid microinjection into the murine RM evoked bradycardia and bradypnea. Widespread oligosynaptic pathways from RM neurons to most viscera and glands including heart (Ter Horst et al. 1996), cutaneous arteries (Smith et al. 1998), lungs, and trachea (Hadziefendic and Haxhiu 1999; Haxhiu et al. 1996) as well as to breathing muscles including the diaphragm (Billig et al. 2000; Yates et al. 1999) provide an anatomical substrate for the influence of RM upon heart rate and respiration rate.
DAMGO microinjection into RM caused the murine EEG to change from a state resembling a deep sleep stage IV to a state akin to the lighter sleep stage I/II (Rechtschaffen and Kales 1968) at the same time as responses to noxious stimuli were delayed. This coupling of cortical arousal and reduced responsiveness to noxious stimulation is consistent with morphine’s strong sedative (lowered responsiveness to external stimulation) but weak hypnotic (sleep-producing) effects (Kay 1975; Kay et al. 1979; Shaw et al. 2005). Previous studies have demonstrated that chemical microinjection into RM can modify cortical state, with bicuculline reducing the time spent awake after an innocuous stimulus (Foo and Mason 2000; 2003) and lidocaine producing arousal (Berner et al. 1999). Consistent with RM’s role in cortical state modulation, RM cells in the rat change their activity at transition points between EEG states in both anesthetized (Grahn and Heller 1989) and unanesthetized animals (Leung and Mason 1999). Finally, RM cells have been implicated in the suppression of somatic inputs during sleep (Foo and Mason 2003; Leung and Mason 1999; Mason et al. 2001). An increase in arousal during times of reduced sensitivity to external stimulation is adaptive as it provides a method for cognitive protection during a period of vulnerability.
The specific physiologic variables measured in this study were chosen primarily because they could be recorded with minimally invasive methods. Yet it is unlikely that RM’s modulatory targets are restricted to those measured. Instead, the measured changes in cardiorespiratory function and cortical activity are likely emblematic of a widespread modulatory influence exerted by RM upon homeostatic physiological functions. Certainly, oligosynaptic pathways exist, at least in the rat, from RM to every autonomic and respiratory target tested (see above). Thus, while our data are direct evidence that the murine RM has the capacity to simultaneously modulate nociception, cardiovascular function, breathing, and behavioral state, the physiological processes targeted by RM are unlikely to be restricted to the studied functions.
RM may modulate respiration and mediate morphine-induced respiratory depression
The respiratory depression produced by DAMGO microinjection into the RM and the power of RM cell firing to predict tachypneic events implies a specific role for the murine RM in respiration. Studies in the rat have noted that electrical stimulation of or glutamate microinjection into RM produces apnea (Aoki and Nakazono 1992; Cao et al. 2006a; Lalley 1986; Wang et al. 1988)), while bicuculline microinjection increases respiration rate (Nason and Mason 2004). RM neurons project to the phrenic motor nucleus (Cao et al. 2006b). Our results complement these findings by showing that on cell bursts predict spontaneous episodes of tachypnea. Further, the association of tachypnea with on cell bursts and off cell pauses suggests that RM cells contribute to generating tachypnea. The possibility also exists that on cell quiescence and off cell firing contributes to opioid respiratory depression. In mice, the highest doses of systemic morphine (≥1 mg) decreased background on cell activity and respiratory rate. In sum, the reduction in respiratory rate caused by DAMGO microinjection into RM and the predictive relationship of RM cell discharge upon respiratory activity imply that the RM participates in respiratory depression induced by high doses of morphine as well as in on-going respiratory modulation.
Similarities between RM physiology in mouse and rat provide insight into the core functions of the medullary raphe
The function of the murine RM resembles the rat RM in two key respects. First, RM cells modulate protective on-going withdrawals. Focusing modulation upon on-going rather than future withdrawals allows the initial reflex withdrawal to occur while also providing flexibility in regulating the duration and magnitude of the complete reaction. Second, RM modulates a number of physiological functions beyond nociception. These two features, present in both rat and mouse, are core to RM’s function.
Acknowledgments
This research was supported by NIH (R01 NS-043329). KMH was supported by a grant from the American Sleep Medicine Foundation, a foundation of the American Academy of Sleep Medicine. TSB was supported by a NIH Training Grant (T32 GM-07839). The authors appreciate valuable comments from Scott Mendelson, assistance from Ana Mrejeru and Daniel Rojas, and the kindness of Veronique VanderHorst in allowing us to adapt her drawings.
Bibliography
- Anderson SD, Basbaum AI, Fields HL. Response of medullary raphe neurons to peripheral stimulation and to systemic opiates. Brain Res. 1977;123:363–368. doi: 10.1016/0006-8993(77)90487-5. [DOI] [PubMed] [Google Scholar]
- Aoki M, Nakazono Y. Control of Breathing and its Modeling Perspective. New York: Plenum Press; 1992. Raphe magnus-induced inhibition of medullary and spinary respiratory activities in the cat; pp. 15–23. [Google Scholar]
- Auerbach S, Fornal C, Jacobs BL. Response of serotonin-containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. doi: 10.1016/0014-4886(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Azami J, Llewelyn MB, Roberts MH. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982;12:229–246. doi: 10.1016/0304-3959(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Azami J, Roberts MH, Wright DM. Effects of ionophoretic application of morphine and naloxone on responses of nucleus reticularis paragigantocellularis neurones to noxious stimulation in the rat. J Physiol. 1979;293:63P–64P. [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Grahn DA, Heller HC. 8-OH-DPAT-sensitive neurons in the nucleus raphe magnus modulate thermoregulatory output in rats. Brain Res. 1999;831:155–164. doi: 10.1016/s0006-8993(99)01426-2. [DOI] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- Brink TS, Mason P. Role for raphe magnus neuronal responses in the behavioral reactions to colorectal distension. J Neurophysiol. 2004;92:2302–2311. doi: 10.1152/jn.00374.2004. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Fujito Y, Matsuyama K, Aoki M. Effects of electrical stimulation of the medullary raphe nuclei on respiratory movement in rats. J Comp Physiol. 2006a;192:497–505. doi: 10.1007/s00359-005-0087-0. [DOI] [PubMed] [Google Scholar]
- Cao Y, Matsuyama K, Fujito Y, Aoki M. Involvement of medullary GABAergic and serotonergic raphe neurons in respiratory control: electrophysiological and immunohistochemical studies in rats. Neurosci Res. 2006b;56:322–331. doi: 10.1016/j.neures.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Gao B. The modification by systemic morphine of the responses of serotonergic and non-serotonergic neurons in nucleus raphe magnus to heating the tail. Pain. 1986;26:245–257. doi: 10.1016/0304-3959(86)90079-5. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Pan ZZ. Differential responses of serotonergic and non-serotonergic neurons in nucleus raphe magnus to systemic morphine in rats. Brain Res. 1985;337:146–150. doi: 10.1016/0006-8993(85)91620-8. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- da Silva LF, Menescal-de-Oliveira L. Role of opioidergic and GABAergic neurotransmission of the nucleus raphe magnus in the modulation of tonic immobility in guinea pigs. Brain Res Bull. 2007;72:25–31. doi: 10.1016/j.brainresbull.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Dickenson AH, Dostrovsky JO. Morphine effects on rat raphe magnus neurones [proceedings] J Physiol. 1977;267:43P–45P. [PubMed] [Google Scholar]
- Eger EI., 2nd The pharmacology of isoflurane. Br J Anaesth. 1984;56(Suppl 1):71S–99S. [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983a;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983b;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Bicuculline microinjection into the raphe magnus suppresses air puff-evoked awakenings in behaving rats. Soc Neurosci Abstract. 2000 [Google Scholar]
- Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Med Rev. 2003a;7:145–154. doi: 10.1053/smrv.2002.0224. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Discharge of raphe magnus ON and OFF cells is predictive of the motor facilitation evoked by repeated laser stimulation. J Neurosci. 2003b;23:1933–1940. doi: 10.1523/JNEUROSCI.23-05-01933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AK, Franklin KB. The role of descending fibers from the rostral ventromedial medulla in opioid analgesia in rats. Eur J Pharmacol. 2002;449:75–84. doi: 10.1016/s0014-2999(02)01974-x. [DOI] [PubMed] [Google Scholar]
- Grahn DA, Heller HC. Activity of most rostral ventromedial medulla neurons reflect EEG/EMG pattern changes. Am J Physiol. 1989;257:R1496–R1505. doi: 10.1152/ajpregu.1989.257.6.R1496. [DOI] [PubMed] [Google Scholar]
- Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Erokwu BO, Cherniack NS. The brainstem network involved in coordination of inspiratory activity and cholinergic outflow to the airways. J Auton Nerv Syst. 1996;61:155–161. doi: 10.1016/s0165-1838(96)00076-8. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Rosenfeld JP. Microinjection of morphine into nucleus reticularis paragigantocellularis of the rat: suppression of noxious-evoked activity of nucleus raphe magnus neurons. Brain Res. 1985;359:388–391. doi: 10.1016/0006-8993(85)91458-1. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Kay DC. Human sleep during chronic morphine intoxication. Psychopharmacologia. 1975;44:117–124. doi: 10.1007/BF00420997. [DOI] [PubMed] [Google Scholar]
- Kay DC, Pickworth WB, Neidert GL, Falcone D, Fishman PM, Othmer E. Opioid effects on computer-derived sleep and EEG parameters in nondependent human addicts. Sleep. 1979;2:175–191. doi: 10.1093/sleep/2.2.175. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Standifer KM, Paul D, Kest B, Pasternak GW, Bodnar RJ. Gender effects and central opioid analgesia. Pain. 1991;45:87–94. doi: 10.1016/0304-3959(91)90168-W. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol. 1986;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CG, Mason P. A physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol. 1998;80:1630–1646. doi: 10.1152/jn.1998.80.4.1630. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological properties of raphe magnus neurons during sleep and waking. J Neurophysiol. 1999;81:584–595. doi: 10.1152/jn.1999.81.2.584. [DOI] [PubMed] [Google Scholar]
- Li YW, Bayliss DA. Electrophysical properties, synaptic transmission and neuromodulation in serotonergic caudal raphe neurons. Clin Exp Pharmacol Physiol. 1998;25:468–473. doi: 10.1111/j.1440-1681.1998.tb02237.x. [DOI] [PubMed] [Google Scholar]
- Martin G, Montagne-Clavel J, Oliveras JL. Involvement of ventromedial medulla “multimodal, multireceptive” neurons in opiate spinal descending control system: a single-unit study of the effect of morphine in the awake, freely moving rat. J Neurosci. 1992;12:1511–1522. doi: 10.1523/JNEUROSCI.12-04-01511.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Mason P. Ventromedial medulla: pain modulation and beyond. J Comp Neurol. 2005;493:2–8. doi: 10.1002/cne.20751. [DOI] [PubMed] [Google Scholar]
- Mason P, Escobedo I, Burgin C, Bergan J, Lee JH, Last EJ, Holub AL. Nociceptive responsiveness during slow-wave sleep and waking in the rat. Sleep. 2001;24:32–38. doi: 10.1093/sleep/24.1.32. [DOI] [PubMed] [Google Scholar]
- Mason P, Strassman A, Maciewicz R. Intracellular responses of raphe magnus neurons during the jaw-opening reflex evoked by tooth pulp stimulation. Brain Res. 1986;379:232–241. doi: 10.1016/0006-8993(86)90776-6. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Lowe D, Fields HL. The contribution of the rostral ventromedial medulla to the antinociceptive effects of systemic morphine in restrained and unrestrained rats. Neuroscience. 1998;87:123–133. doi: 10.1016/s0306-4522(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG. Nociceptive and morphine antinociceptive sensitivity of 129 and C57BL/6 inbred mouse strains: implications for transgenic knock-out studies. Eur J Pain. 1997;1:293–297. doi: 10.1016/s1090-3801(97)90038-0. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK. Immobility accompanies the antinociception mediated by the rostral ventromedial medulla of the rat. Brain Res. 2000;872:276–281. doi: 10.1016/s0006-8993(00)02502-6. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–517. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Separate urinary bladder and external urethral sphincter neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. Brain Res. 2001;903:33–44. doi: 10.1016/s0006-8993(01)02349-6. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005;51:1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol. 2004;92:510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Medullary raphe neurons facilitate brown adipose tissue activation. J Neurosci. 2006;26:1190–1198. doi: 10.1523/JNEUROSCI.4707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveras JL, Redjemi F, Guilbaud G, Besson JM. Analgesia induced by electrical stimulation of the inferior centralis nucleus of the raphe in the cat. Pain. 1975;1:139–145. doi: 10.1016/0304-3959(75)90098-6. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Sala M, Crema G, Giagnoni G, Gori E. Cerebral sites of central action of dermorphin on intestinal motility in the rat. Peptides. 1985;6(Suppl 3):149–153. doi: 10.1016/0196-9781(85)90366-3. [DOI] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Randich A, Thurston CL, Ludwig PS, Robertson JD, Rasmussen C. Intravenous morphine-induced activation of vagal afferents: peripheral, spinal, and CNS substrates mediating inhibition of spinal nociception and cardiovascular responses. J Neurophysiol. 1992;68:1027–1045. doi: 10.1152/jn.1992.68.4.1027. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Owens NC, McAllen RM. Cold-activated raphe-spinal neurons in rats. J Physiol. 2001;535:841–854. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington D.C.: US Government Printing Office; 1968. [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Satoh M, Akaike A, Takagi H. Excitation by morphine and enkephalin of single neurons of nucleus reticularis paragigantocellularis in the rat: a probable mechanism of analgesic action of opioids. Brain Res. 1979;169:406–410. doi: 10.1016/0006-8993(79)91043-6. [DOI] [PubMed] [Google Scholar]
- Schnell C, Ulucan C, Ellrich J. Atypical on-, off- and neutral cells in the rostral ventromedial medulla oblongata in rat. Exp Brain Res. 2002;145:64–75. doi: 10.1007/s00221-002-1093-x. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Hu JW. Raphe-induced suppression of the jaw-opening reflex and single neurons in trigeminal subnucleus oralis, and influence of naloxone and subnucleus caudalis. Pain. 1981;10:19–36. doi: 10.1016/0304-3959(81)90042-7. [DOI] [PubMed] [Google Scholar]
- Shaw IR, Lavigne G, Mayer P, Choiniere M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28:677–682. doi: 10.1093/sleep/28.6.677. [DOI] [PubMed] [Google Scholar]
- Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- Sugino S, Kawamata M, Yamauchi M, Narimatsu E, Namiki A. Genetic differences in responses of rostral ventromedial medulla neurons induced by DAMGO, α μ-opioid receptor agonist in mouse inbred strains. Soc Neurosci Abstract. 2006 doi: 10.1016/j.neulet.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Szekely JI, Miglecz E, Bajusz S. Species differences in the relative analgesic potencies of some classical opiates and opioid peptides. Psychopharmacology. 1984;82:400–402. doi: 10.1007/BF00427694. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphe. J Physiol. 2006;572:569–583. doi: 10.1113/jphysiol.2005.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst GJ, Hautvast RW, De Jongste MJ, Korf J. Neuroanatomy of cardiac activity-regulating circuitry: a transneuronal retrograde viral labelling study in the rat. Eur J Neurosci. 1996;8:2029–2041. doi: 10.1111/j.1460-9568.1996.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Toda K. Responses of raphe magnus neurons to systemic morphine in rats. Brain Res Bull. 1982;8:101–103. doi: 10.1016/0361-9230(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Tyler CB, Advokat C. Investigation of “cross-tolerance” between systemic and intrathecal morphine in rats. Physiol Behav. 1986;37:27–32. doi: 10.1016/0031-9384(86)90379-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Wang YT, Wang N, Liu L. Studies on respiratory phase-switching effects of nucleus raphe magnus. Chi Sci Bull. 1988;33:1043–1048. [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- York EA, Baez MA, Mason P. Microinjection of DAMGO, a mu-opioid receptor agonist, into the ventromedial medulla delays volume-evoked micturition in anesthetized rats. Soc Neurosci Abstract. 2005 [Google Scholar]
- Young EG, Watkins LR, Mayer DJ. Comparison of the effects of ventral medullary lesions on systemic and microinjection morphine analgesia. Brain Res. 1984;290:119–129. doi: 10.1016/0006-8993(84)90741-8. [DOI] [PubMed] [Google Scholar]