Abstract
Negative urgency (i.e., the tendency to engage in rash action in response to negative affect) has emerged as a critical personality trait contributing to individual differences in binge eating. However, studies investigating the extent to which genetic and/or environmental influences underlie the effects of negative urgency on binge eating are lacking. Moreover, it remains unclear whether negative urgency-binge eating associations are simply due to the well-established role of negative affect in the development/maintenance of binge eating. The current study addresses these gaps by examining phenotypic and etiologic associations between negative urgency, negative affect, and dysregulated eating (i.e., binge eating, emotional eating) in a sample of 222 same-sex female twin pairs from the Michigan State Twin Registry. Negative urgency was significantly associated with both dysregulated eating symptoms, even after controlling for the effects of negative affect. Genetic factors accounted for the majority (62–77%) of this phenotypic association, although a significant proportion of this genetic covariation was due to genetic influences in common with negative affect. Non-shared environmental factors accounted for a relatively smaller (23–38%) proportion of the association, but these non-shared environmental effects were independent of negative affect. Findings suggest that the presence of emotion-based rash action, combined with high levels of negative affect, may significantly increase genetic risk for dysregulated eating.
Keywords: binge eating, emotional eating, impulsivity, negative urgency, negative affect, twin study
Personality traits are critical etiologic factors for eating disorders (Lilenfeld, Wonderlich, Riso, Crosby, & Mitchell, 2006), helping to explain why some individuals develop eating disorders and others do not. Impulsivity is perhaps the most important trait to consider for binge eating and associated eating disorders. Although most individuals with eating disorders are high on negative emotionality/neuroticism, an impulsive temperament tends to differentiate patients with binge/purge behaviors from those with restrictive eating disorders (Claes, Vandereycken, & Vertommen, 2005). Impulsivity and binge eating symptoms are positively associated in community samples (Fischer, Smith, & Anderson, 2003; Racine, Culbert, Larson, & Klump, 2009) and, perhaps most importantly, impulsivity appears to prospectively increase risk for the development of bulimic symptoms (Bodell, Joiner, & Ialongo, 2012; Wonderlich, Connolly, & Stice, 2004).
Unfortunately, research on the role of impulsivity in binge eating is limited by the fact that impulsivity is a broad umbrella term encompassing multiple constructs (e.g., lack of planning, sensation seeking, lack of perseverance, affect-driven impulsivity; Whiteside & Lynam, 2001). Pinpointing the impulsive personality trait(s) that confer greatest risk for specific phenotypes is important when considering the context and function that the impulsive behavior may serve. For example, determining whether individuals binge eat due to a need for stimulation, to distract from negative affect, or because they simply do not consider the long-term consequences of their behavior, can be important for the development of etiologic models and treatment approaches for binge eating.
Research using self-report measures that assess distinct impulsivity constructs has begun to accumulate. These studies convincingly suggest that negative urgency (i.e., the tendency to act rashly in response to negative affect) is the most relevant form of impulsivity for binge eating. When examined in concert with other specific impulsive traits (e.g., lack of planning, lack of perseverance, sensation seeking), negative urgency has consistently emerged as the best predictor of binge eating (Anestis, Smith, Fink, & Joiner, 2009; Claes et al., 2005; Fischer & Smith, 2008). Moreover, a meta-analysis that classified studies investigating impulsivity-bulimic symptom associations based on the type of impulsive trait examined found that negative urgency was most important for binge eating (i.e., effect size for negative urgency = .38; effect sizes for other impulsive traits = .08–.16; Fischer, Smith, & Cyders, 2008). Finally, recent longitudinal research has specified negative urgency as a prospective risk factor for binge eating in both middle school and college-aged samples (Fischer, Peterson, & McCarthy, in press; Pearson, Combs, Zapolski, & Smith, 2012) Thus, individuals who tend to respond to negative affect with rash action may be at increased risk for binge eating because they may use binge eating as an attempt to regulate negative emotions (Fischer et al., 2008).
Importantly, studies thus far have only focused on phenotypic associations between negative urgency and binge eating; thus, very little is known regarding etiologic factors that underlie negative urgency-binge eating relationships. At the level of broad mechanisms, common genetic/biological factors and/or common environmental contexts might explain the robust phenotypic association between negative urgency and binge eating. For example, it may be that the genes that predispose someone to have higher levels of negative urgency also lead to binge eating. Alternatively, certain environmental experiences (e.g., child abuse/trauma; Brodsky et al., 2001) may influence the development of an impulsive temperament, which could subsequently increase risk for binge eating. Findings such as these could help advance etiologic models of binge eating development and ultimately inform targeted prevention and intervention programs that explicitly aim to avert risk processes.
Twin studies are especially useful for providing an initial indication of the relative contribution of genetic and environmental factors to the relationship between two variables, as they decompose the covariance into genetic and environmental components. Notably, no study to date has investigated genetic and environmental covariance between negative urgency and binge eating, and in fact, studies have not yet identified whether genetic and/or environmental factors underlie relationships between any impulsive trait and binge eating. Twin studies have, however, examined etiologic associations with other relevant personality traits, and findings suggest that genetic and non-shared environmental influences contribute approximately equally to phenotypic relationships between binge eating and the traits of negative emotionality (Klump, McGue, & Iacono, 2002) and emotional dysregulation (Livesley, Jang, & Thordarson, 2004). Given these findings, we might expect that negative urgency-binge eating associations are similarly influenced by both genetic and non-shared environmental factors.
One important consideration for both phenotypic and etiologic studies examining the relationship between negative urgency and binge eating is the potential role of negative affect. As a reminder, negative urgency integrates the experience of negative affect with the tendency to engage in rash action, and negative affect is a strong, proximal trigger for binge eating (Haedt-Matt & Keel, 2011). Thus, individuals high on negative urgency may be prone to binge eating simply because they frequently experience high levels of negative affect. Similarly, any significant genetic/environmental overlap between urgency and binge eating may be completely accounted for by etiologic influences on trait levels of negative affect. To our knowledge, only one study has examined the independent predictive power of negative urgency and negative affect for binge eating; this study reported that negative urgency significantly predicted binge eating over and above the effects of negative affect (Anestis et al., 2009). We are not aware of any twin studies examining etiologic overlap between negative affect and binge eating; however, we might expect significant genetic/environmental associations for these constructs based on twin study findings for binge eating and the personality trait of negative emotionality (from the Multidimensional Personality Questionnaire (MPQ)) (see above)1. In sum, research at both the phenotypic and etiologic levels is needed to determine whether negative urgency is uniquely associated with binge eating, distinct from general elevations on negative affect. Findings can help shed light on the nature of the personality trait of negative urgency, more generally, as well as its specific contribution to binge eating risk.
Given the above, the aim of the current study was to investigate phenotypic and etiologic associations among negative urgency, negative affect, and dysregulated eating (i.e., binge eating, emotional eating) in a sample of same-sex female twins. We sought to replicate associations between negative urgency and dysregulated eating as well as to extend previous findings by demonstrating that relationships are present over and above the effects of negative affect. Next, we used a twin design to investigate the extent to which negative urgency-dysregulated eating relationships were due to common genetic and/or environmental factors and to determine the proportion of etiologic overlap that was accounted for by genetic/environmental influences in common with negative affect.
We focused on two dimensional measures of dysregulated eating behaviors given that the prevalence of binge episodes would be expected to be too low in our community sample for formal twin analyses. Specifically, we examined: 1) thoughts and behaviors related to binge eating using the Minnesota Eating Behaviors Survey (MEBS) Binge Eating subscale, and 2) the tendency to eat in response to negative emotions using the Dutch Eating Behaviors Questionnaire (DEBQ) Emotional Eating scale. Several previous studies investigating negative urgency-binge eating associations have used the Eating Disorders Inventory Bulimia Scale, which is very similar to MEBS Binge Eating. Thus, we were able to replicate results and investigate etiologic associations using a binge eating measure previously examined in the literature. In addition, we are the first to investigate associations between negative urgency and emotional eating, a symptom that is defined by a tendency to act in response to negative emotions. Taken together, findings may help to more broadly understand the role of negative urgency in dysregulated eating behaviors.
Methods
Participants
Participants included 222 same-sex female twin pairs (444 twins; 246 monozygotic (MZ) twins; 198 dizygotic (DZ) twins) between the ages of 16 and 25 years (M= 18.45 years, SD = 2.18) from the Michigan State University Twin Registry (MSUTR; Burt & Klump, in press; Klump & Burt, 2006). MSUTR twins are recruited using birth record methods previously described (Klump & Burt, 2006). Data from previous studies (Culbert, Breedlove, Burt, & Klump, 2008) and the current study indicate that MSUTR participants are demographically representative of the recruitment region (81.1% Caucasian; 15.8% African American; 1.8% Asian/Pacific Islander; 1.4% Native American; http://www.michigan.gov).
Data were drawn from the Twin Study of Hormones and Behavior across the Menstrual Cycle (response rate = 56%; Klump et al., in press). The parent study consists of daily data collection across 45 days as well as three in-person assessments at the beginning, middle, and end of the 45-day period. With regards to measures for the current study, the MEBS Binge Eating Scale and the negative urgency measure were administered on one occasion only (i.e., intake assessment), whereas DEBQ Emotional Eating and negative affect were assessed daily for 45 days. Given that the main variable of interest (i.e., negative urgency) was only measured once, we averaged levels of emotional eating and negative affect over the 45 days. This allowed us to examine both dysregulated eating symptoms over a similar time frame and to approximate trait levels of negative affect (r = .53 for the correlation between trait and aggregated daily negative affect scores; Watson & Clark, 1999). Importantly, a previous study by our group describes the longitudinal association between negative affect and emotional eating in this sample (Haedt-Matt et al., submitted).
Because the parent study focused on hormones, a number of inclusion criteria were necessary to capture natural hormonal variation: 1) menstruation every 22–32 days for past 6 months; 2) no psychotropic or steroid medications in past 4 weeks; 3) no pregnancy or lactation in past 6 months; and 4) no history of genetic/medical conditions known to influence hormone functioning or appetite/weight. The majority of our sample (87%) was also required to be free from hormonal contraceptive use over the past 3 months, although a smaller subset (13%) were participants from a related pilot study that specifically recruited for current hormonal contraceptive use. Importantly, comparisons between our participants and those from previous MSUTR studies without these restrictions indicated very small differences on measures of negative affect, general impulsivity, and binge eating (average d = .11, range = .01–.20), suggesting that our participants are representative of the larger population of twins on these constructs.
Measures
Zygosity determination
Similar to other large-scale twin registries (e.g., Kendler, Heath, Neale, Kessler, & Eaves, 1992), a physical similarity questionnaire was used as the primary determinant of zygosity. This questionnaire has previously demonstrated over 95% accuracy when compared to genotyping (Lykken, Bouchard, McGue, & Tellegen, 1990). Twins, twins’ guardians (for 16–17 year-old twins), and research assistants completed this questionnaire, yielding up to 9 independent ratings of physical similarity. Discrepancies were resolved by having the principal investigator (KLK) review questionnaire responses and examine twin photographs. In addition, DNA was available for 82% of the sample and was used to ensure that twins classified as MZ had identical genotyping results across polymorphisms. Only 2.7% of MZ twins had their zygosity changed based on DNA information.
Dysregulated eating
The MEBS Binge Eating scale (von Ranson, Klump, Iacono, & McGue, 2005)2 measures binge eating risk, including contemplating binge eating (e.g., “I think a lot about overeating (eating a really large amount of food)”) and engaging in binge eating behaviors (e.g. “Sometimes I eat lots and lots of food and feel like I can’t stop”), via seven items. Internal consistency for this subscale is adequate in the current sample (α = .71) and previous young adult samples (von Ranson et al., 2005). In addition, women with bulimia nervosa have higher MEBS Binge Eating scores than unaffected control women (von Ranson et al., 2005).
The DEBQ Emotional Eating scale (van Strien, Frijters, Bergers, & Defares, 1986) consists of thirteen items that assess the tendency to eat in response to negative affective cues (e.g., “Did you have a desire to eat when you were discouraged?”). Internal consistency for the DEBQ Emotional Eating scale is excellent in previous research (van Strien et al., 1986) and in the current study (α = .90). This scale correlates with established measures of binge eating (e.g., r’s = .55–.69) (Racine et al., 2009; van Strien, 2000) as well as with palatable food intake (i.e., ice cream) in the laboratory (van Strien, 2000). Moreover, DEBQ emotional eating score distinguish between individuals with bulimia nervosa/binge eating, overweight individuals, and college students (Wardle, 1987).
In order to validate the use of these two measures for assessing dysregulated eating symptoms often present in binge eating individuals, we compared scores in women who endorsed current objective binge episodes (OBEs; eating a large amount of food in a short period of time accompanied by a sense of loss of control (LOC)) (N=13) to women with no history of OBEs (N=371–375), as assessed via a structured eating disorder interview. As expected, current binge eaters had substantially higher mean scores on MEBS Binge Eating (M (SD) = 3.92 (2.36)) and DEBQ Emotional Eating (M (SD) = 0.63 (.50)) compared to women free of binge eating (MEBS M(SD) = .90 (1.19), p = .001, Cohen’s d = 1.62; DEBQ M(SD) = 0.28 (.38), p = .001, d = .79). We further examined the criterion validity of these scales by comparing women with current OBEs to those who reported currently eating large amounts of food without LOC (N=5) and those who reported LOC without consuming large amounts of food (N = 16). Both comparisons revealed clinically significant group differences, with those reporting OBEs scoring highest on the MEBS Binge Eating Scale (p’s = .02–.06; d’s = .84–1.13). Although differences were less pronounced for emotional eating, they represented moderate effect sizes and clinically meaningful effects (p’s = .25–.40; d’s = .44–.46). Notably, findings were identical when including individuals with a lifetime history of OBEs, overeating, and LOC in analyses.
Negative Urgency
The (Negative) Urgency, (lack of) Premeditation, (lack of) Perseverance, Sensation Seeking-Positive Urgency (UPPS-P) Impulsive Behavior Scale (Lynam, Smith, Whiteside, & Cyders, 2006) was used to assess negative urgency. The Negative Urgency scale consists of 12 items, and internal consistency was high in the current study (α = .85) and previous work (Fischer & Smith, 2008). Convergent and discriminant validity have been established, as negative urgency measured via self-report and interview assessments are highly correlated, and negative urgency exhibits much lower correlations with other UPPS-P impulsivity scales measured using either self-report or interview (Smith et al., 2007). Moreover, test-retest reliability for negative urgency is good in studies of college students over 1 month (r = .73; Anestis et al., 2009) and middle school students over 6 months-1 year (r’s = .53–.66; Peterson et al., 2012).
Negative affect
The Negative Affect scale from the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) consists of 10 items that assess the full spectrum of daily negative emotions (e.g., fear, distress, irritability, nervousness). This scale exhibits good convergent and discriminant validity (Watson et al., 1988), and internal consistency was excellent in the current study (α = .85).
Statistical Analyses
Binge eating and negative affect scores were log-transformed prior to analyses to account for positive skew. The log transformation brought skewness values for these measures from 1.85 and 1.38 to .69 and .77, respectively. An arctan transformation was used for emotional eating given significant kurtosis (7.88) and more moderate skewness (2.32). After transformation, skewness and kurtosis were 1.27 and 1.41, respectively. Negative urgency was normally distributed and was not transformed.
Phenotypic analyses
Within-person, Pearson correlations were used to examine initial phenotypic associations among negative urgency, negative affect, and dysregulated eating. Hierarchical linear models (HLM; also known as mixed linear models) were then fit to examine relationships between negative urgency and dysregulated eating while controlling for negative affect and the dyadic nature of the twin data. Non-independence was accounted for by nesting a level 1 variable (individual twin) within a level 2 unit (twin pair). As recommended (Simmons, Nelson, & Simonsohn, 2011), two HLMs were conducted in order to directly examine the effects of negative affect on the relationship between negative urgency and dysregulated eating. Model 1 examined the “simple” main effect of negative urgency on dysregulated eating. Model 2 included both negative urgency and negative affect as predictors in order to determine whether negative urgency influences dysregulated eating over and above any effects of negative affect. Given that HLM provides unstandardized estimates of effects, we standardized all variables in order to compare effect sizes across models.
Etiologic analyses
Twin correlations and biometric model fitting were used to examine whether genetic and/or environmental influences contribute to the variance in negative urgency, negative affect, and dysregulated eating as well as the covariation among these phenotypes.
Twin correlations
Intraclass correlations were first calculated separately for negative urgency, negative affect, and dysregulated eating measures by zygosity (MZ vs. DZ) using the double entry method in order to provide an initial indication of the relative influence of genetic and environmental factors on each phenotype. Next, cross-twin cross-trait correlations (e.g., correlation between Twin 1’s level of negative urgency and Twin 2’s level of binge eating) were calculated to determine the extent to which phenotypic associations between negative urgency, negative affect, and dysregulated eating are accounted for by common genes and/or common environmental factors. For both sets of correlations, additive genetic factors (A; genetic influences that add across genes) are suggested if the MZ twin correlation is approximately twice the DZ twin correlation. Non-additive genetic effects (D; interaction of genetic effects at same locus) are implied if MZ correlations are more than double DZ correlations. Shared environmental effects (C; factors that make co-twins similar to one another) are inferred if MZ and DZ correlations are approximately equal. Finally, non-shared environmental effects (E; factors that make co-twins different from one another, including measurement error) are implied if the MZ correlation is less than 1.0 (for intraclass correlations) or less than the corresponding phenotypic correlation (for cross-twin cross-trait correlations). Specifically, MZ cross-twin cross-trait correlations equal to phenotypic correlations suggest that, for example, we can predict Twin 1’s level of binge eating from Twin 2’s level of negative urgency just as well as from Twin 1’s level of negative urgency. Alternatively, if MZ cross-twin cross-trait correlations are lower than corresponding phenotypic correlations, it suggests there are individual specific (i.e., non-shared) influences on the relationship between two traits.
Biometric model fitting
Trivariate, Cholesky decomposition models were used to examine the extent to which additive genetic, non-additive genetic, shared environmental, and/or non-shared environmental influences accounted for relationships among negative urgency, negative affect, and dysregulated eating. Although independent pathway and common pathway models are often fitted when modeling three variables, we only examined a Cholesky model given our a priori set of “directional” hypotheses (see below).
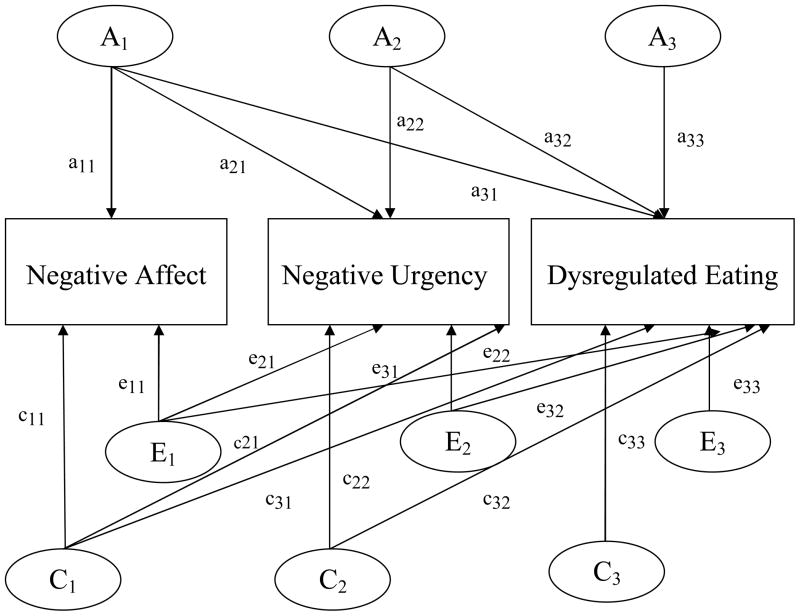
Figure 1 presents the trivariate Cholesky model with genetic, shared environmental, and non-shared environmental effects. Non-additive genetic effects are not presented, given that non-additive genetic and shared environmental effects cannot be estimated together when examining only MZ and DZ reared-together twins (Neale & Cardon, 1992). As shown in the figure, the trivariate model provides information regarding the magnitude of the genetic and environmental influences on each phenotype and the extent to which these influences contribute to the covariation among phenotypes. Although the ordering of the variables (i.e., first, second, third) does not affect how well the model fits the data, the ordering is critical for the parameter estimates produced. The current study aimed to determine whether genetic/environmental effects on negative urgency account for a significant proportion of the genetic/environmental influences on dysregulated eating. In addition, we wanted to determine the extent to which etiologic influences in common with negative affect account for genetic/environmental covariation between negative urgency and dysregulated eating. Therefore, we ordered the variables in the following way: 1) negative affect, 2) negative urgency, 3) dysregulated eating (see Figure 1). This ordering allowed for the variance in dysregulated eating to be decomposed into: 1) genetic/environmental effects attributable to negative affect (a31, c31, e31; see Figure 1); 2) genetic/environmental effects attributable to negative urgency but not shared with negative affect (a32, c32, e32); and 3) residual genetic/environmental effects specific to dysregulated eating (a33, c33, e33). The variance in negative urgency is decomposed into genetic/environmental influences overlapping with negative affect (a21, c21, e21) and those specific to negative urgency (a22, c22, e22), whereas there is no decomposition of genetic/environmental effects on negative affect (a11, c11, e11).
Figure 1. Path diagram for the trivariate Cholesky decomposition model.
The variance in liability to each disorder is assumed to be comprised of additive genetic effects (A1, A2, A3), shared environmental effects (C1, C2, C3), and non-shared environmental (E1, E2, E3). Pathways are represented by lowercase letters and two numbers, the first which represents the variable being influenced, and the second which reflects the latent factor.
Path estimates from the trivariate model can be used to produce two additional sets of indices that quantify the degree of covariation between the phenotypes: 1) genetic/environmental correlations, and 2) proportions of covariance accounted for by genetic/environmental factors. Path estimates can be standardized on their respective variances to produce genetic and environmental correlations that describe the degree to which, for example, the genetic/environmental influences on negative urgency are the same as those on binge eating. Correlations are often presented in multivariate twin studies, as they range from −1 to 1 and provide an easily interpretable estimate of etiologic overlap between two phenotypes. Because they are free from measurement error, it is possible to have genetic and shared environmental correlations of 1.0 (e.g., Kendler, Neale, Kessler, Heath, & Eaves, 1992). A genetic correlation of 1.0 would indicate that the genetic influences on two phenotypes are identical, and a genetic correlation of 0 would suggest that genetic influences on a set of phenotypes are completely distinct. Unlike the attributable genetic and environmental estimates described above, these correlations index the degree of genetic/environmental covariation between each pair of phenotypes without removing variance associated with the other phenotype(s) in the model. Therefore, we can evaluate genetic and environmental overlap between negative urgency and dysregulated eating without accounting for negative affect using these correlations.
The phenotypic correlations between negative affect, negative urgency, and dysregulated eating also can be decomposed into the proportion of the association that is due to genetic factors versus environmental factors. These estimates are different from genetic/environmental correlations in that they provide information about the relative importance of genetic and environmental factors to the relationship between two traits. For example, a genetic correlation could be very large, but if the heritability estimates for the two traits are low, shared genetic influences are unlikely to substantially contribute to the covariation between the traits.
Model fit and selection
Model fitting was conducted using full-information maximum likelihood raw data techniques in MX statistical software (Neale, Boker, Xie, & Maes, 2003). Raw data techniques treat missing data as missing-at-random (Little & Rubin, 1987) and allow for the retention of twin pairs in which one co-twin has missing data. Full ACE and ADE models were both examined, based on the pattern of twin correlations (see Results). This allowed us to determine whether shared environmental or non-additive genetic parameters were more important for inclusion in the models. Nested sub-models were also fit and were compared to these full models (i.e., AE and CE models compared to ACE model; AE model compared to ADE model).3
Model fit comparisons were made by taking the difference in minus twice the log-likelihood (−2lnL) between the full models and the nested sub-models. Under certain regularity conditions, this comparison results in a chi-square difference test, with the degrees of freedom (df) for this test representing the difference between the df for the full and nested models. Statistically significant chi-square values lead to the rejection of the nested model in favor of the full model. Aikake’s Information Criterion (AIC; χ2 – 2df) was also used as an index of model fit. AIC measures model fit relative to parsimony, and AIC is lowest/more negative in the best-fitting models.
Results
Phenotypic Analyses
Descriptive statistics and Pearson correlations are presented in Table 1. The correlation between binge eating and emotional eating was lower than expected based on previous research in community samples (r’s = .55–.69) (Racine et al., 2009; van Strien, 2000), but still in the moderate range (r = .34). Negative urgency was positively associated with both measures of dysregulated eating (r’s = .26–.46), as was negative affect (r’s = .24–.49). Finally, the correlation between negative affect and negative urgency was moderate (r = .34), indicating that these are overlapping, yet distinct, constructs.
Table 1.
Descriptive Statistics and Pearson Correlations
| Variables | Mean (S.D) | Range | Binge Eating | Emotional Eating | Neg. Urgency | Neg. Affect |
|---|---|---|---|---|---|---|
| Dysregulated eating | ||||||
| Binge Eating | 1.05 (1.43) | 0–7 | - | - | - | - |
| Emotional Eating | 0.30 (.38) | 0–3 | .34 (.24–.45) | - | - | - |
| Predictors | ||||||
| Neg. Urgency | 2.03 (.55) | 1–3.67 | .46 (.36–.55) | .26 (.18–.35) | - | - |
| Neg. Affect | 14.74 (3.56) | 10–30 | .24 (.14–.35) | .49 (.41, .59) | .34 (.23–.43) | - |
Note. 97.5% confidence intervals for correlations presented in parentheses.
HLM results are presented in Table 2. Negative urgency was significantly associated with both dysregulated eating symptoms when only negative urgency was included in the model. Importantly, negative urgency continued to significantly predict binge eating and emotional eating after controlling for negative affect in Model 2. Thus, negative urgency is related to dysregulated eating above and beyond the effects of negative affect. Notably, negative affect was not significantly associated with binge eating in Model 2, but negative affect was a stronger predictor of emotional eating than negative urgency.
Table 2.
Predictive Associations between Negative Urgency, Negative Affect, and Dysregulated Eating
| Model | Binge Eating | Emotional Eating | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| b (S.E) | t (df) | p | b (S.E) | t (df) | p | |
| Model 1 | ||||||
| Neg. Urgency | .45 (.04) | 10.58 (429.42) | <.001 | .23 (.04) | 5.13 (418.66) | <.001 |
| Model 2 | ||||||
| Neg. Urgency | .43 (.05) | 9.55 (419.98) | <.001 | .10 (.04) | 2.51 (420.67) | .01 |
| Neg. Affect | .07 (.05) | 1.44 (394.40) | .15 | .42 (.04) | 9.37 (406.89) | <.001 |
Etiologic Analyses
Twin correlations
Twin intraclass and cross-twin cross-trait correlations are presented in Table 3. Higher MZ than DZ correlations, and MZ correlations less than 1.0, indicate the presence of genetic and non-shared environmental influences, respectively, on all constructs. In addition, non-additive genetic effects may be important for negative urgency and binge eating, given that MZ twin correlations were more than double DZ twin correlations. Finally, shared environmental factors appear to be relevant for emotional eating, as the MZ correlation was less than double the DZ correlation.
Table 3.
Twin Correlations for Negative Urgency, Negative Affect, and Dysregulated Eating
| Variables | MZ twins (N = 237–244) | DZ twins (N = 186–196) | Z | p |
|---|---|---|---|---|
| Intraclass correlations | ||||
| Neg. Urgency | .39 (.29, .48) | −.03 (−.17, .12) | 4.48 | <.001 |
| Neg. Affect | .55 (.46, .64) | .24 (.09, .37) | 3.84 | <.001 |
| Binge Eating | .42 (.29, .52) | .02 (−.12, .17) | 4.28 | <.001 |
| Emotional Eating | .41 (.29, .53) | .28 (.13, .42) | 1.50 | .07 |
| Cross-twin cross-trait correlations | ||||
| Neg. Urgency-Neg. Affect | .25 (.13, .38) | .07 (−.08, .21) | 1.89 | .03 |
| Neg. Urgency-Binge Eating | .27 (.15, .39) | .02 (−.11, .15) | 2.64 | .004 |
| Neg. Urgency-Emotional Eating | .17 (.06, .28) | .08 (−.05, .22) | 0.93 | .18 |
| Neg. Affect-Binge Eating | .21 (.07, .34) | .14 (.01, .27) | 0.73 | .23 |
| Neg. Affect-Emotional Eating | .35 (.20, .48) | .29 (.16, .42) | 0.67 | .25 |
Note. MZ = monozygotic; DZ = dizygotic. Z = Fisher r-to-z transformation test of equality. p value for one-tailed test examining whether the MZ correlation is larger than the DZ correlation. 95% confidence intervals for correlations presented in parentheses.
Regarding cross-twin cross-trait correlations, higher MZ than DZ correlations indicate that genetic factors likely contribute to the covariation among negative affect, negative urgency, and dysregulated eating measures. This pattern was particularly pronounced for the association between negative urgency and binge eating (see Table 3), whereas differences between MZ and DZ twin correlations were more modest for negative affect-dysregulated eating symptom relationships. Finally, non-shared environmental effects are implicated in the covariation of all pairs of phenotypes, given MZ cross-twin cross-trait correlations less than the corresponding phenotypic correlations (see Tables 1 and 3).
Biometric model fitting
Trivariate model fit statistics and parameter estimates for full and nested models (i.e., ACE, ADE, AE, CE) are presented in Table 4. Parameter estimates from the full models suggested that, in general, additive genetic effects and non-shared environmental effects are most important for the phenotypes examined. These sources of variance made significant contributions to negative urgency, negative affect, and both dysregulated eating symptoms, whereas shared environmental effects and non-additive genetic parameters were non-significant across models. Confirming these impressions, model-fit comparisons indicated that the best-fitting model for all phenotypes was the AE model. The AE models did not fit significantly worse than the ACE or ADE models, according to the chi-square difference tests, and they also produced the lowest AIC values (see Table 4).
Table 4.
Parameter Estimates and Test Statistics for the Comparison of Trivariate Cholesky Decomposition Models
| Model | Standardized Parameter Estimates | Test Statistics | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| a2 | c2/d2 | e2 | −2lnL (df) | χ2 (df) | p | AICa | |
| Binge Eating | |||||||
| ACE | 3478.83 (1290) | - | - | −7.05 | |||
| Neg. Affect | .43 (.13, .64) | .11 (0, .37) | .46 (.35, .59) | ||||
| Neg. Urgency | .34 (.15, .48) | .01 (0, .16) | .65 (.51, .79) | ||||
| Binge Eating | .37 (.15, .52) | .02 (0, .18) | .61 (.47, .77) | ||||
| CE | 3499.32 (1296) | 20.49 (6) | .002 | 1.44 | |||
| Neg. Affect | - | .42 (.31, .53) | .58 (.47, .69) | ||||
| Neg. Urgency | - | .21 (.08, .33) | .79 (.67, .92) | ||||
| Binge Eating | - | .23 (.10, .35) | .77 (.65, .90) | ||||
| AE | 3039.82 (1296) | .78 (6) | .99 | −18.32 | |||
| Neg. Affect | .55 (.42, .65) | - | .45 (.35, .58) | ||||
| Neg. Urgency | .35 (.21, .49) | - | .65 (.51, .79) | ||||
| Binge Eating | .39 (.23, .53) | - | .61 (.47, .77) | ||||
| ADE | 3473.42 (1290) | - | - | −12.46 | |||
| Neg. Affect | .37 (0, .63) | .19 (0, .62) | .45 (.35, .57) | ||||
| Neg. Urgency | .01 (0, .38) | .38 (0, .52) | .61 (.49, .75) | ||||
| Binge Eating | .07 (0, .41) | .37 (0, .55) | .56 (.44, .72) | ||||
| AE | 3479.56 (1296) | 6.14 (6) | .41 | −18.32 | |||
| Neg. Affect | .55 (.42, .65) | - | .45 (.35, .58) | ||||
| Neg. Urgency | .35 (.21, .49) | - | .65 (.51, .79) | ||||
| Binge Eating | .39 (.23, .53) | - | .61 (.47, .77) | ||||
|
| |||||||
| Emotional Eating | |||||||
| ACE | |||||||
| Neg. Affect | .42 (.12, .63) | .12 (0, .38) | .46 (.36, .59) | 3445.74 (1287) | - | - | −23.21 |
| Neg. Urgency | .34 (.12, .47) | 0 (0, .17) | .66 (.53, .81) | ||||
| Emotional Eating | .26 (.01, .53) | .17 (0, .40) | .58 (.45, .72) | ||||
| CE | 3461.15 (1293) | 15.41 (6) | .02 | −19.90 | |||
| Neg. Affect | - | .42 (.30, .52) | .58 (.48, .69) | ||||
| Neg. Urgency | - | .21 (.09, .34) | .79 (.66, .91) | ||||
| Emotional Eating | - | .35 (.23, .46) | .65 (.54, .77) | ||||
| AE | 3447.25 (1293) | 1.51 (6) | .96 | −33.80 | |||
| Neg. Affect | .55 (.42, .65) | - | .46 (.35, .58) | ||||
| Neg. Urgency | .34 (.19, .47) | - | .66 (.53, .81) | ||||
| Emotional Eating | .44 (.30, .56) | - | 56 (.44, .70) | ||||
| ADE | 3443.25 (1287) | - | - | −25.80 | |||
| Neg. Affect | .37 (.01, .62) | .19 (0, .57) | .44 (.35, .57) | ||||
| Neg. Urgency | .02 (0, .41) | .35 (0, .49) | .63 (.50, .78) | ||||
| Emotional Eating | .38 (.02, .55) | .07 (0, .45) | .55 (.43, .69) | ||||
| AE | 3447.25 (1293) | 4.00 (6) | .68 | −33.80 | |||
| Neg. Affect | .55 (.42, .65) | - | .46 (.35, .58) | ||||
| Neg. Urgency | .34 (.19, .47) | - | .66 (.53, .81) | ||||
| Emotional Eating | .44 (.30, .56) | - | 56 (.44, .70) | ||||
Note. −2lnL = −2 times log likelihood; df = degrees of freedom; AIC = Aikake Information Criteria. Best-fitting model is indicated by bold type. 95% confidence intervals for variance estimates are presented in parentheses.
All AICs were calculated by taking the difference in −2lnL values between a baseline, unrestricted model (i.e., a model that freely estimates variances, covariances, and means) and all other models. −2lnL(df) for baseline trivariate MEBS Binge Eating model: 3419.88 (1257); −2lnL(df) for baseline trivariate DEBQ Emotional Eating model: 3403.05 (1254)
Genetic/environmental correlations and the proportions of variance accounted for by genetic/environmental factors are presented in Table 5. Genetic correlations between negative urgency and dysregulated eating symptoms were large and significant for both binge eating (rg = .77 (CIs: .54, .99)) and emotional eating (rg = .52 (CIs: .25, .79)). The non-shared environmental correlation was significant between negative urgency and binge eating (re = .29 (CIs: .13, .43)) but not between negative urgency and emotional eating (re = .11 (CIs: −.05, .26)). The majority of the phenotypic covariation between negative urgency and dysregulated eating measures was accounted for by genetic influences (62–77%, see Table 5), with non-shared environmental factors contributing relatively less to these relationships (23–38%). Taken together, genetic factors impacting negative urgency and dysregulated eating are relatively similar, and genetic influences primarily underlie phenotypic relationships between negative urgency and dysregulated eating.
Table 5.
Genetic and Environmental Correlations and Proportions of Covariance Accounted for by Genetic and Environmental Factors
| Variables | Correlations | Proportion of covariance accounted for by: | ||
|---|---|---|---|---|
|
| ||||
| A | E | A | E | |
| Neg. Affect-Neg. Urgency | 63 (.41, .85) | .12 (−.04., .28) | .81 | .19 |
| Neg. Affect-Binge Eating | .55 (.31, .81) | −.03 (−.19, .14) | 1.00 | 0 |
| Neg. Urgency-Binge Eating | .77 (.54, .99) | .29 (.13, .43) | .62 | .38 |
| Neg. Affect-Emotional Eating | .82 (.66, .99) | .19 (.03, .34) | .82 | .18 |
| Neg. Urgency-Emotional Eating | .52 (.25, .79) | .11 (−.05, .26) | .77 | .23 |
Note. A = additive genetic effects; E = non-shared environmental effects. 95% confidence intervals for correlations presented in parentheses.
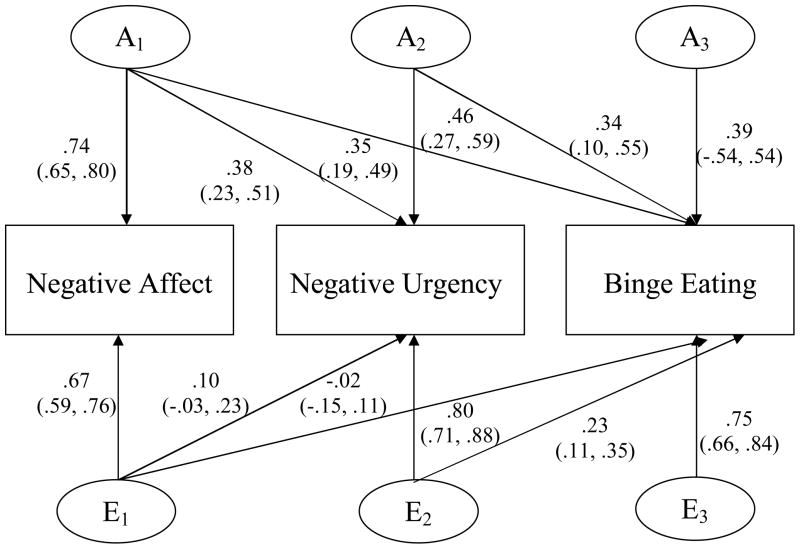
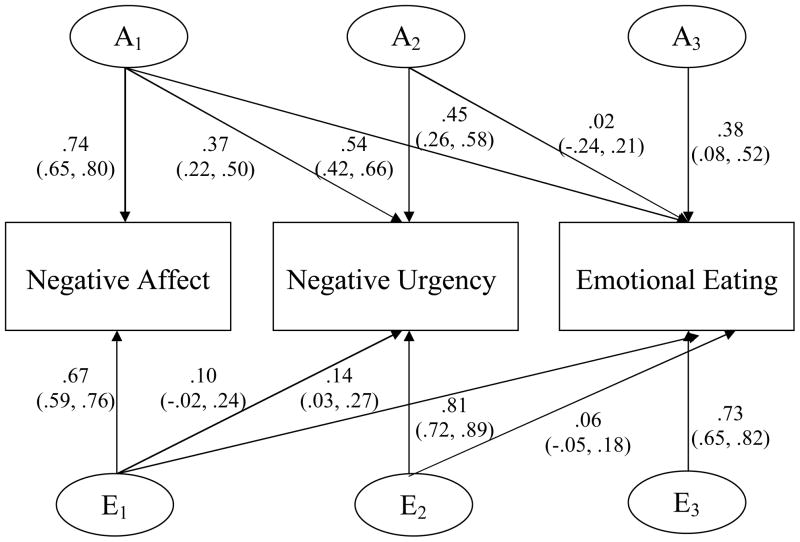
As previously stated, genetic and non-shared environmental correlations do not take into account whether etiologic overlap is independent of negative affect. For this question, we refer to the standardized path estimates (presented in Figures 2 and 3) which are squared to obtain estimates of attributable and unique variance (discussed in the text). As shown in Figures 2 and 3, genetic overlap with negative affect is important to consider for negative urgency-dysregulated eating associations given that genetic influences in common with negative affect significantly contribute to the variance in negative urgency (i.e., 14% of 35% total heritability), binge eating (i.e., 12% of 39% total heritability), and emotional eating (i.e., 31% of 44% total heritability). This etiologic overlap is likely to decrease the contribution of genetic/environmental influences unique to negative urgency to the variance in dysregulated eating.
Figure 2. Standardized path estimates for the additive genetic (A) and non-shared environmental contributions (E) to the variance within and covariance among negative affect, negative urgency, and binge eating.
95% confidence intervals presented in parentheses. Path estimates are squared to obtain variance components, which are discussed in the text.
Figure 3. Standardized path estimates for the additive genetic (A) and non-shared environmental contributiones (E) to the variance within and covariance among negative affect, negative urgency, and emotional eating.
95% confidence intervals presented in parentheses. Path estimates are squared to obtain variance components, which are discussed in the text.
Indeed, path estimates from negative urgency to binge eating (see Figures 2 and 3) suggest that the genetic covariance between negative urgency and dysregulayed eating is reduced after controlling for genetic influences in common with negative affect. Although genetic influences unique to negative urgency made a moderate and significant contribution to the total variance in binge eating (i.e., 12% of 39% total heritability), there was virtually no unique contribution of negative urgency to emotional eating. Non-shared environmental covariance between negative urgency and binge eating was completely independent of non-shared environmental factors on negative affect, but these non-shared environmental influences only contributed 5% to the total variance in binge eating.
Despite significant etiologic overlap among negative affect, negative urgency, and binge eating, residual genetic/environmental variance on dysregulated eating measures (i.e., that which is not accounted for by negative affect and negative urgency) was notable. Between 30% and 40% of the genetic variance on binge eating (i.e., 15% of 39% total heritability) and emotional eating (i.e., 14% of 44% total heritability) was unique, and greater than 90% (i.e., 56% of 61% for binge eating; 54% of 56% for emotional eating) of non-shared environmental influences were specific to binge eating.4
Discussion
This study was the first to go beyond investigating phenotypic associations between negative urgency and binge eating/dysregulated eating by examining potential etiologic factors that may underlie this relationship. Negative urgency was significantly associated with two measures of dysregulated eating (i.e., binge eating, emotional eating), and twin model results indicated that genetic and, to a lesser extent, non-shared environmental factors account for these phenotypic relationships. Moreover, the genetic factors that influence negative urgency are highly correlated with the genetic factors that influence dysregulated eating. Taken together, findings from the current study suggest that negative urgency likely increases risk for the development of binge eating and emotional eating through primarily genetic mechanisms.
We were also interested in investigating the role of negative affect, a well-established risk factor for binge eating, in explaining phenotypic and etiologic relationships between negative urgency and dysregulated eating. Negative urgency predicted both dysregulated eating symptoms over and above the effects of negative affect, indicating that phenotypic relationships between negative urgency and dysregulated eating cannot be entirely accounted for by negative affect. Genetic influences on negative affect significantly contributed to the variance in negative urgency, binge eating, and emotional eating and, after controlling these common genetic factors, genetic influences unique to negative urgency accounted for 0–12% of the total variance in dysregulated eating. Therefore, genetic influences shared with negative affect appear to significantly contribute to the common variance between negative urgency and dysregulated eating.
Notably this pattern of findings does not negate the importance of the construct of negative urgency for the etiology of dysregulated eating. Because the rash action of individuals high on negative urgency is conditional on momentary increases in negative affect, it might be expected that, after accounting for trait levels of negative affect, the remaining genetic variance in dysregulated eating attributable to negative urgency is small. Importantly, however, by including both negative affect and negative urgency in the same model, we provided a very strong test of our hypothesis regarding the specific role of negative urgency in dysregulated eating. Thus, it is impressive that genetic influences unique to negative urgency significantly contributed to the variance in binge eating. Moreover, negative affect and negative urgency together accounted for a substantial proportion (i.e., 60%) of the genetic variance in binge eating. Specifically, of the genetic influences on binge eating (i.e., 39%), 12% were due to genetic influences in common with negative affect and 12% were due to genetic influences unique to negative urgency. This percent of explained genetic variance is as high or higher than what is accounted for by other risk factors for binge eating such as negative emotionality, alcohol use, and weight/shape concerns (Klump et al., 2002; Munn et al., 2010; Slane, Burt, & Klump, 2012). Results point to negative urgency as a significant correlate for the genetic diathesis of binge eating and suggest that individuals most at risk may be those who experience high levels of negative affect and who have a tendency towards emotion-based rash action.
Although negative affect and negative urgency accounted for a significant proportion of the genetic variance in dysregulated eating, residual genetic variance was notable. Moreover, the majority of non-shared environmental influences were specific to dysregulated eating. In addition to personality traits and negative affect, other psychological (e.g., dietary restraint, alcohol use; Racine, Burt, Iacono, McGue, & Klump, 2011; Slane et al., 2012), psychosocial (e.g., peer/family influences), and biological (e.g., ovarian hormones; Klump et al., in press) factors appear to influence the development of dysregulated eating. Although this study and others by our group (Klump et al., in press; Slane et al., 2012) have focused on the main effects of these risk factors, it is likely that interactions between risk factors are relevant for the development of dysregulated eating and may explain a larger percentage of variance than main effects alone. For example, it may be that individuals high on negative urgency are more likely to develop binge eating (versus another kind of impulsive behavior) if they are exposed to a specific trigger for eating pathology, such as disorder relevant expectancies (i.e., eating will help alleviate negative emotions; Fischer, Settles, Collins, Gunn, & Smith, 2012), attempts to restrict food intake for weight loss (Racine et al., 2011), or a vulnerable hormonal milieu (Klump et al., in press). Additional research is needed to elucidate these types of complex interactions and develop a more in-depth understanding of binge eating and its risk factors.
Notably, although results were generally similar for binge eating and emotional eating, some differences emerged. In both phenotypic and etiologic analyses, negative urgency was more strongly related to binge eating, and negative affect was a stronger predictor of emotional eating. Differences in the constructs represented by these dysregulated eating measures could be responsible for these discrepant results. Emotional eating directly assesses eating in response to negative affective cues, whereas MEBS Binge Eating items focus mainly on behavioral indicators of impulsive, binge eating tendencies (e.g., eating a large amount of food at once, loss of control over eating). Alternatively, differential associations may be due to measurement issues since negative urgency and binge eating were both assessed one time during study intake, whereas negative affect and emotional eating were assessed daily (and then averaged). Thus, stronger associations between negative urgency and binge eating, and negative affect and emotional eating, may reflect similarities in the measurement window rather than true differential associations. To indirectly examine this possibility, we conducted post-hoc analyses investigating associations between another study variable that was assessed daily and during the intake session (i.e., MEBS Weight Preoccupation) and both negative urgency and negative affect. Results indicated modest-to-no differences in the magnitude of phenotypic associations (i.e., negative urgency and weight preoccupation scores at intake: r = .30 vs. negative urgency and daily weight preoccupation scores: r = .25; negative affect and weight preoccupation scores at intake and daily: r = .27). These findings suggest that different measurement windows are unlikely to account entirely for our differing phenotypic and etiologic associations. Nonetheless, future studies should replicate our results using measures administered across the same time frame in order to understand similarities/differences in phenotypic and etiologic associations between negative urgency, negative affect, and various dysregulated eating symptoms.
Although results from the current study enhance our understanding of negative urgency-dysregulated eating relationships, several additional limitations must be noted. First, our sample size was relatively small for a multivariate twin study, resulting in broad confidence intervals for some parameters and lower power to detect non-additive genetic and shared environmental effects. However, our findings regarding significant etiologic overlap among negative affect, negative urgency, and dysregulated eating are likely robust, as findings were replicated across two related measures. Even so, additional research in larger twin samples is needed to confirm our results.
Second, we examined dysregulated eating in a non-clinical sample of women rather than binge eating in a clinical sample of eating disorder patients. However, our findings are likely relevant for pathological binge eating given that our data support the criterion validity of both continuous measures for assessing binge eating risk and symptoms frequently present in binge eating populations. Further, research suggests that heritability estimates for binge eating are very similar across clinical and community samples and that there is substantial genetic overlap for binge eating and bulimia nervosa (Bulik, Sullivan, & Kendler, 1998; Wade, Bulik, Sullivan, Neale, & Kendler, 2000). Although it is likely that our findings would generalize to individuals with eating disorders who report regular OBEs, future studies should directly investigate this possibility.
Third, participants in our sample (ages 16–25 years) were not through the peak period of risk for binge eating, which may extend up until age 30. However, given our examination of dysregulated eating, our sample likely includes a number of “at risk” individuals who currently display dysregulated eating symptoms. Still, findings should be replicated in older samples using assessments of lifetime dysregulated eating and binge eating. Fourth, our research questions were examined using two self-report measures of dysregulated eating. Self-report measures have been criticized for overestimating the frequency of binge eating compared to interviews (Fairburn & Beglin, 1994). However, interview-based measures have also been shown to underestimate the heritability of various forms of psychopathology (Burt, 2009). Thus, there appear to be advantages of using both interview and self-report measures of dysregulated eating when conducting twin studies, but further research is needed to directly compare results.
Finally, data from our study cannot speak to causal associations and the direction of phenotypic, genetic, and environmental relationships between negative urgency and dysregulated eating. Recent data suggest that negative urgency increases risk for the later development of binge eating (Fischer et al., in press; Pearson et al., 2012), but additional twin research is needed to confirm that negative urgency is a prospective genetic and/or environmental risk factor for binge eating and emotional eating.
Acknowledgments
The research was supported by grants from the National Institute of Mental Health (NIMH) (1 R01 MH0820-54) (KLK, PKK, SAB, CLS, MN, SB) and the Canadian Institutes of Health Research (MDR-96630) (SER). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
Negative emotionality from the MPQ measures one’s disposition towards negative affect, negative interpersonal interactions, and withdrawal behaviors (Patrick, Curtin, & Tellegen, 2002), whereas negative affect is typically thought of as one’s state level of negative emotions and maps on to the affective component of negative emotionality. Correlations between negative affect, aggregated over time, and negative emotionality are modest (~ .50), suggesting that these are distinct yet overlapping constructs.
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory (M-EDI)) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, & Polivy (1983) Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
DE models are infrequently examined in behavior genetics studies given that the presence of non-additive genetic effects in the absence of additive genetic effects is theoretically unlikely (McGue & Christensen, 1997). Thus, DE models were not run in the current study.
Phenotypic and etiologic results were largely unchanged after including body mass index (BMI) as a covariate, which is not surprising given that BMI was not significantly associated with negative affect or negative urgency.
Parts of this manuscript were presented at the International Conference on Eating Disorders, Miami, Florida, April 27–30, 2011 and the Society for Research in Psychopathology Meeting, Ann Arbor, Michigan, October 4–7, 2012.
References
- Anestis MD, Smith AR, Fink EL, Joiner TE. Dysregulated eating and distress: Examining the specific role of negative urgency in a clinical sample. Cognitive Therapy and Research. 2009;33:390–397. doi: 10.1007/s10608-008-9201-2. [DOI] [Google Scholar]
- Bodell LP, Joiner TE, Ialongo NS. Longitudinal association between childhood impulsivity and bulimic symptoms in African American adolescent girls. Journal of Consulting and Clinical Psychology. 2012;80:313–316. doi: 10.1037/a0027093. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, Haas GL, Malone KM, Mann JJ. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158:1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/S0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychological Bulletin. 2009;135:608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry: An update. Twin Research and Human Genetics. doi: 10.1017/thg.2012.87. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes L, Vandereycken W, Vertommen H. Impulsivity-related traits in eating disorder patients. Personality and Individual Differences. 2005;39:739–749. doi: 10.1016/j.paid.2005.02.022. [DOI] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. doi: 10.1002/1098-108X(199412)16:4<363::AID-EAT2260160405>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Fischer S, Peterson CM, McCarthy D. A prospective test of the influence of negative urgency and expectancies on binge eating and purging. Psychology and Addictive Behaviors. doi: 10.1037/a0029323. in press. [DOI] [PubMed] [Google Scholar]
- Fischer S, Settles RE, Collins B, Gunn RL, Smith GT. The role of negative urgency and expectancies in problem drinking and disordered eating: Testing a model of comorbidity in pathological and at-risk samples. Psychology of Addictive Behaviors. 2012;26:112–123. doi: 10.1037/a0023460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Smith GT. Binge eating, problem drinking, and pathological gambling: Linking behavior to shared traits and social learning. Personality and Individual Differences. 2008;44:789–800. doi: 10.1016/j.paid.2007.10.008. [DOI] [Google Scholar]
- Fischer S, Smith GT, Anderson KG. Clarifying the role of impulsivity in bulimia nervosa. International Journal of Eating Disorders. 2003;33:406–411. doi: 10.1002/eat.10165. [DOI] [PubMed] [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: A meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review. 2008;28:1413–1425. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychological Bulletin. 2011;137:660–681. doi: 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK, Racine SE, Burt SA, Hu JY, Boker S, et al. Does emotional eating regulate affect? Concurrent and prospective associations and implications for risk models of binge eating. doi: 10.1002/eat.22247. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA: The Journal of the American Medical Association. 1992;268:1877–1882. doi: 10.1001/jama.1992.03490140085040. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder: Same genes,(partly) different environments? Archives of General Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk C, et al. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. doi: 10.1037/a0029524. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Genetic relationships between personality and eating attitudes and behaviors. Journal of Abnormal Psychology. 2002;111:380–389. doi: 10.1037/0021-843X.111.2.380. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LRR, Wonderlich S, Riso LP, Crosby R, Mitchell J. Eating disorders and personality: A methodological and empirical review. Clinical Psychology Review. 2006;26:299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Little RT, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Livesley WJ, Jang KL, Thordarson DS. Etiological relationships between eating disorder symptoms and dimensions of personality disorder. Eating Disorders. 2004;13:23–35. doi: 10.1080/10640260590893610. [DOI] [PubMed] [Google Scholar]
- Lykken D, Bouchard T, McGue M, Tellegen A. The Minnesota Twin Family Registry. Acta Geneticae Medicae et Gemellologiae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University; 2006. [Google Scholar]
- Munn MA, Stallings MC, Hyun Rhee S, Sobik LE, Corley RP, Rhea SA, et al. Bivariate analysis of disordered eating characteristics in adolescence and young adulthood. International Journal of Eating Disorders. 2010;43:751–761. doi: 10.1002/eat.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M, Boker S, Xie G, Maes H. Mx: Statistical Modeling. 6. Richmond, VA: Virginia Commonwealth University; 2003. [Google Scholar]
- Neale M, Cardon LR. Methdology for the genetic studies of twins and families. Norwell, MA: Kluwer; 1992. [Google Scholar]
- Pearson CM, Combs JL, Zapolski TCB, Smith GT. A longitudinal transactional risk model for early eating disorder onset. Journal of Abnormal Psychology. 2012;121:707–718. doi: 10.1037/a0027567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Burt SA, Iacono WG, McGue M, Klump KL. Dietary restraint moderates genetic risk for binge eating. Journal of Abnormal Psychology. 2011;120:119–128. doi: 10.1037/a0020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influence of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: Undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL. Bulimic behaviors and alcohol use: Shared genetic influences. Behavior Genetics. 2012;42:603–613. doi: 10.1007/s10519-012-9525-2. [DOI] [PubMed] [Google Scholar]
- Smith G, Fischer S, Cyders M, Annus A, Spillane N, McCarthy D. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- van Strien T. Ice-cream consumption, tendency toward overeating, and personality. International Journal of Eating Disorders. 2000;28:460–464. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. doi: 10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T. [DOI] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6:373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wade TD, Bulik CM, Sullivan PF, Neale MC, Kendler KS. The relation between risk factors for binge eating and bulimia nervosa: A population-based female twin study. Health Psychology. 2000;19:115–123. doi: 10.1037/0278-6133.19.2.115. [DOI] [PubMed] [Google Scholar]
- Wardle J. Eating style: A validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-expanded form. Iowa City, IA: University of Iowa; 1999. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Wonderlich SA, Connolly KM, Stice E. Impulsivity as a risk factor for eating disorder behavior: Assessment implications with adolescents. International Journal of Eating Disorders. 2004;36:172–182. doi: 10.1002/eat.20033. [DOI] [PubMed] [Google Scholar]