Abstract
Arsenic, a human skin carcinogen, suppresses differentiation of cultured keratinocytes. Exploring the mechanism of this suppression revealed that BMP-6 greatly increased levels of mRNA for keratins 1 and 10, two of the earliest differentiation markers expressed, a process prevented by co-treatment with arsenite. BMP also stimulated, and arsenite suppressed, mRNA for FOXN1, an important transcription factor driving early keratinocyte differentiation. Keratin mRNAs increased slowly after BMP-6 addition, suggesting they are indirect transcriptional targets. Inhibition of Notch1 activation blocked BMP induction of keratins 1 and 10, while FOXN1 induction was largely unaffected. Supporting a requirement for Notch1 signaling in keratin induction, BMP increased levels of activated Notch1, which was blocked by arsenite. BMP also greatly decreased active ERK, while co-treatment with arsenite maintained active ERK. Inhibition of ERK signaling mimicked BMP by inducing keratin and FOXN1 mRNAs and by increasing active Notch1, effects blocked by arsenite. Of 6 dual-specificity phosphatases (DUSPs) targeting ERK, two were induced by BMP unless prevented by simultaneous exposure to arsenite and EGF. Knockdown of DUSP2 or DUSP14 using shRNAs greatly reduced FOXN1, and keratins 1 and 10 mRNA levels and their induction by BMP. Knockdown also decreased activated Notch1, keratin 1 and keratin 10 protein levels, both in the presence and absence of BMP. Thus, one of the earliest effects of BMP is induction of DUSPs which increase FOXN1 transcription factor and activate Notch1, both required for keratin gene expression. Arsenite prevents this cascade by maintaining ERK signaling, at least in part by suppressing DUSP expression.
Keywords: Dual specificity phosphatases, EGF, ERK, FOXN1, Keratins 1 and 10, Notch1
Introduction
Chronic intake of inorganic arsenic leads to cancer in several tissue targets (National Research Council, 2001), including the skin. Elucidating the mechanism of arsenic-induced carcinogenesis may help manage the risk posed by natural contamination of drinking water for the millions of people exposed worldwide. To this end, identifying signaling pathways with which arsenic interferes may help in understanding the diversity of its pathophysiological actions. Such outcomes likely result from disruption of important cell signaling events in affected cell types that alter the balance between differentiation and proliferation. Indeed, in cell culture experiments, arsenite has been demonstrated to suppress differentiation and increase the proliferative potential of epidermal keratinocytes (Kachinskas et al., 1997; Patterson et al., 2005) by augmenting EGFR signaling (Patterson and Rice, 2007) and suppressing signaling by pro-differentiation participants such as Notch1 and PKCδ (Reznikova et al., 2010). An evident consequence is maintenance of keratinocytes with stem cell character. This phenomenon in the epidermis could result in a larger target pool for action of genotoxins, helping rationalize the arsenic-mediated increased tumorigenicity of ultraviolet light in mouse and human skin (Burns et al., 2004). Such action is consistent with arsenic transplacental carcinogenesis in mice (Waalkes et al., 2004) due at least in part to increasing the stem cell population (Tokar et al., 2011; Sun et al., 2012). Carcinogenic outcomes also likely are stimulated by arsenic targeting additional processes such as DNA repair (Qin et al., 2012).
In human keratinocyte cultures, two genes greatly suppressed by arsenic encode the superficial keratins, KRT1 and KRT10 (Reznikova et al., 2009). A major transcription factor affecting early steps of keratinocyte differentiation, including KRT1 and KRT10 expression, is FOXN1, mutation of which yields the nude phenotype in mice. Over-expression of this gene in mouse skin and in cultured human keratinocytes leads to increased KRT1 and KRT10 expression and decreased proliferative potential (Baxter and Brissette, 2002; Janes et al., 2004). FOXN1 is regulated negatively by the EGF receptor and ERK1, since knockdown of either of these increases FOXN1 expression (Mandinova et al., 2009). U1026, an inhibitor of the ERK kinase, MEK1/2, also increases FOXN1 levels in cultured mouse keratinocytes (Baxter and Brissette, 2002). Since arsenic maintains EGF receptor signaling, we investigated whether arsenic suppresses KRT1 and KRT10 by decreasing FOXN1.
In the hair follicle, FOXN1 is positively regulated by BMP (Kulessa et al., 2000; Andl et al., 2004; Cai et al., 2009), but this pathway has not yet been shown effective in interfollicular epidermis. Canonical BMP signaling involves binding of an extracellular ligand to a bipartite receptor consisting of members of the TGFβ superfamily. When activated by ligand binding, the receptor phosphorylates Smads 1, 5 and/or 8 on C terminal serine residues. This is followed by association with Smad4 and translocation to the nucleus, where the complex acts as a transcription factor (see Miyazono et al., 2010 for review). Interfollicular epidermis expresses BMP ligands and receptors in a differentiation dependent manner (reviewed in Botchkarev, 2003), and BMP6 is induced during differentiation initiated by cell suspension (Drozdoff et al., 1994). Furthermore, addition of BMP6 to the culture medium induces KRT1 (McDonnell et al., 2001) and KRT10 in keratinocytes (Gosselet et al., 2007). Since epidermal keratins depend upon FOXN1 expression, their induction by BMP may occur through increased FOXN1 in a pathway similar to that demonstrated in the hair follicle. Experiments described here utilize BMP6 because that form has been shown to affect differentiation in interfollicular epidermis. Other forms of BMP may have similar or distinct effects.
Finally, Notch1 signaling is critical for initiation of differentiation in suprabasal epidermis (Lowell et al., 2000; Rangarajan et al., 2001; Nickoloff et al., 2002). In the hair follicle, Notch1 is also required for proper differentiation and has recently been shown to function in a linear pathway from BMP to FOXN1 to Notch1 (Cai et al., 2009). Notch1 is a transmembrane protein that undergoes proteolytic cleavage after binding to a ligand on a neighboring cell. The cleaved Notch1 intracellular domain (NICD) then functions as a transcription factor after translocation to the nucleus and dimerization with a partner. Arsenite has been demonstrated to suppress NICD levels in cultured keratinocytes, while pharmacological inhibition of Notch1 processing has effects analogous to arsenite on differentiation marker expression and maintenance of proliferative potential (Reznikova et al., 2009). These findings suggested the possibility that arsenic suppresses KRT1 and KRT10 by interfering with BMP signaling, which has downstream effects on induction of FOXN1 and activation of Notch1.
Materials and methods
Cell Culture
Derived from foreskin, spontaneously immortalized human keratinocytes (SIK) (Rice et al., 1993), used in passages 20–30, were propagated in DMEM/F12 (2:1) medium supplemented with fetal bovine serum (5%), hydrocortisone (0.4 µg/ml), adenine (0.18 mM), insulin (5 µg/ml), transferrin (5 µg/ml) and triiodothyronine (20 pM) using a feeder layer of lethally irradiated 3T3 cells (Allen-Hoffmann and Rheinwald, 1984). Medium was further supplemented with cholera toxin (10 ng/ml) (EMD Biosciences, La Jolla, CA) at inoculation but not continued at subsequent medium changes. EGF (10 ng/ml) (Biomedical Technologies, Inc., Stoughton, MA) was added starting at the first medium change and continued except in certain experiments where it was omitted as the cells neared confluence. Cells were grown until just before confluence with medium changes at 3 to 4 day intervals, at which time they were treated with 2 µM sodium arsenite (Fisher Scientific, Houston, TX), 50 ng/ml recombinant human BMP6 (R&D Systems, Minneapolis, MN), 10 µM U1026, 10 µM SP600125, 10 µM SB203580 (LC Laboratories, Woburn, MA) or 10 µM DAPT (N-(N-(3, 5-difluorophenacetyl-L-alanyl)-S-phenylglycine t-butyl ester)) (EMD Biosciences, La Jolla, CA).
Lentiviral and retroviral Infection
Knockdowns of FOXN1, DUSP2 and DUSP14 in SIK cells were produced using Mission shRNAs (Sigma-Aldrich, St. Louis, MO). Viral particles were prepared in HEK293FT cells by co-transfection of the Mission shRNA with ViraPower Packaging Mix using Lipofectamine 2000 transfection reagent, both from Life Technologies (Carlsbad, CA). Medium containing virus was collected 48 h and 72 h after transfection, centrifuged to remove debris and stored at −80C. Viral titers were estimated by measuring lentiviral RNA in the collected medium after purification with QiaAmp Viral RNA purification kits (Qiagen, Valencia, CA). Contaminating plasmid was removed using Ambion Turbo DNA-free kits before synthesis of cDNA with a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). Viral cDNA was quantitated by PCR with Taqman Fast Universal PCR Master Mix and a Taqman Gene Expression assay detecting HIV LTR (Pa03453409_s1), both from Applied Biosystems (Foster City, CA). SIK cultures were infected with virus 1 day after plating on 6 well plates using 1 ml of virus stock (108 to 109 copies of virus RNA/ml) plus 1 ml of keratinocyte growth medium in the presence of 6 µg/ml hexadimethrine bromide (Sigma-Aldrich, St. Louis, MO). Medium was changed the next day and cells were grown to confluence before treating with BMP as indicated.
Immunoblotting
Cells were scraped directly into buffer containing 2% SDS, 62.5 mM Tris (pH 6.8), 10% glycerol, with 5 mM sodium pyrophosphate and 50 µM sodium vanadate added to inhibit phosphatases. Protein was measured with BCA Protein Assay (Smith et al., 1985) (Pierce, Rockford, IL) before addition of dithiothreitol to 20 mM and boiling for 3 min. Equal amounts of protein (from 10–50 µg, depending on the protein of interest) were loaded and separated by SDS polyacrylamide gel electrophoresis, transferred to Immobilon membranes (Millipore, Danvers, MA), blocked with 5% dry milk in Tris buffered saline, 0.05% Tween 20, incubated with the indicated antibodies and detected using ECL Plus chemiluminescence detection reagent (Pierce, Rockford, IL) and a Fuji LAS3000 imaging system (GE, Piscataway, NJ). Quantitation was performed using the associated Multi-Gauge imaging software. Antibodies were obtained from the following sources: β-Actin (mouse monoclonal, clone AC-74) from Sigma-Aldrich (St. Louis, MO); KRT1 (rabbit polyclonal PRB-149P) from Covance (Berkeley, CA), KRT10 (mouse monoclonal DE-K10) from Thermo Scientific and cleaved Notch1 (#2421), total Notch1 (#3608), phospho-ERK1/2 (#4376), total ERK1/2 (#4695), phospho-MEK1/2 (#9154), total MEK1/2(#9126), and phospho-Smad1/5 (#9516), all from Cell Signaling Technology (Danvers, MA).
Real time PCR
For analysis of mRNA amounts by quantitative real-time PCR, total RNA was isolated using Trizol reagent (Life Technologies, Carlsbad, CA), and cDNA synthesis was performed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). PCR was performed with TaqMan Fast Universal PCR Master Mix and TaqMan Gene Expression assays (Applied Biosystems). The assays were performed in an ABI 7500 Fast Sequence Detection System. mRNA expression, normalized to the housekeeping gene, GusB, is presented relative to untreated control cultures (set to 1.0).
Statistics
Results illustrate the means and standard deviations of 3 or 4 independent experiments except as noted. One way ANOVA statistical testing (with Bonferroni corrections) for significance (p<0.05) was performed using Stata/SE9.2 software for Windows. In certain cases, as noted, values from independent experiments were subjected to 2-tailed Student’s t-testing using Excel for calculation of p values.
Results
BMP6 induces early differentiation markers
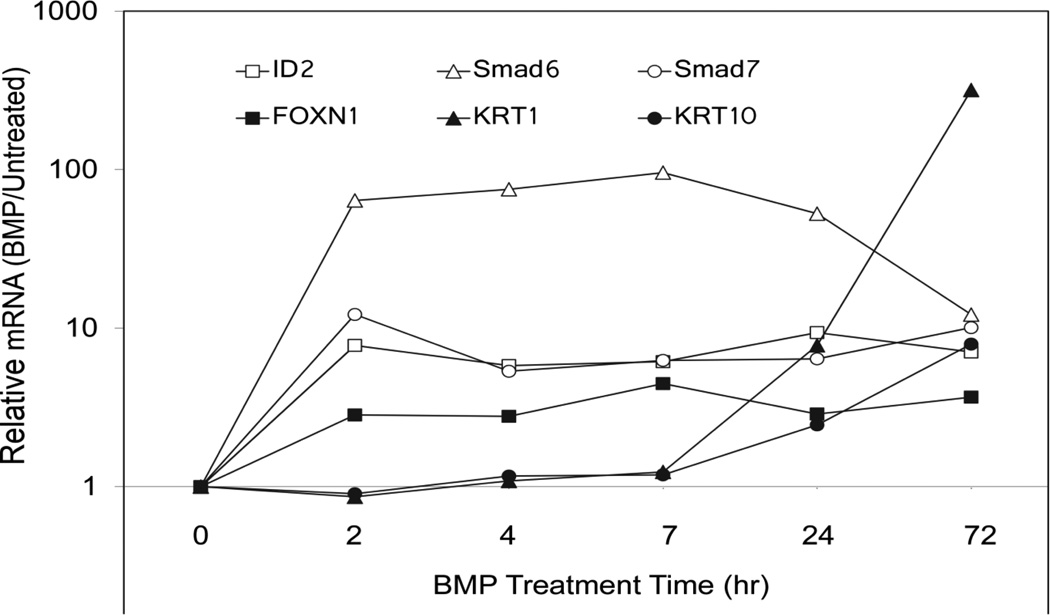
Cultured epidermal cells display dramatic transcriptional responses to BMP6. Smad6, Smad7, ID2 and FOXN1 are all stimulated 3–100 fold within 2 h of treatment with little increase over the next 72 h, as shown in Figure 1. KRT1 and 10 mRNAs, although stimulated 10–100 fold, exhibited much slower increases, beginning to rise at 24 h and increasing further by 72h. ID2 and the negative regulators, Smad6 and Smad7, have all been shown to be directly regulated by Smad transcription factors in other cell types with similar kinetics (see Miyazono et al., 2010 for review). Although the suprabasal keratins, KRT1 and KRT10, are two of the earliest genes to be induced at the onset of keratinocyte differentiation, the slower kinetics of their mRNA accumulation suggest indirect regulation by BMP.
Fig. 1.
Time course of mRNA induction by BMP6. Confluent SIK cultures were treated with BMP6, or left untreated, for the indicated times. Cells were harvested in Trizol reagent and quantitated by real time PCR. For each time point, the amount of mRNA in the BMP6-treated sample was calculated relative to the untreated sample. Illustrated are averages of duplicate measurements. Independent experiments gave similar results for induction times although the magnitude of the induction varied.
Arsenite blocks BMP6 stimulation of keratinocyte early differentiation markers
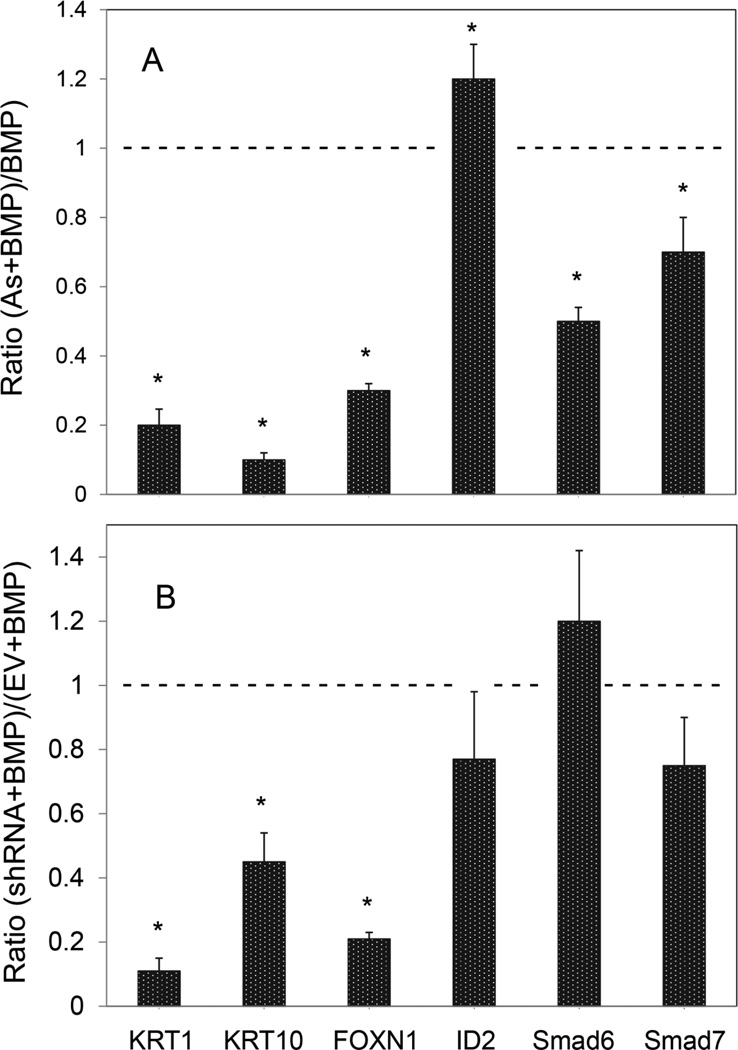
The large increases in KRT1 and KRT10 mRNAs in confluent SIK cultures upon exposure to BMP6 were largely prevented by simultaneous exposure to arsenite and, in parallel, arsenite suppressed BMP induction of FOXN1 mRNA (Figure 2A and Figure 1A, Appendix), a putative transcription factor that stimulates KRT1 expression (Baxter and Brissette, 2002). As shown, ID2, Smad6 and Smad7 induction were less affected by arsenite. Arsenite also suppressed basal levels of KRT1, KRT10 and FOXN1 but not ID2, Smad6 and Smad7 (Figure A1, Appendix). Like arsenite exposure, knockdown of FOXN1 with specific shRNAs suppressed BMP stimulation of KRT1, KRT10 and FOXN1 mRNA expression with little effect on ID2, Smad6 and Smad7 (Figure 2B). Arsenite and FOXN1 shRNA do not produce identical effects, likely because arsenite affects additional signaling pathways that are not impacted by knockdown of FOXN1.
Fig. 2.
BMP stimulation of keratinocyte early differentiation and suppression by arsenite treatment or FOXN1 knockdown. (A) Confluent SIK cultures, treated with BMP6 alone or in combination with arsenite (As) for 3 days, were analyzed for specific mRNAs by real time PCR relative to the untreated control. Illustrated are the ratios of arsenite + BMP to BMP alone). A value of 1 (dashed line) indicates that arsenite had no effect on BMP stimulation, while ratios less than one indicate suppression. (B) Preconfluent SIK cultures were infected with a lentiviral vector expressing FOXN1 shRNA or with the empty vector (EV). At confluence, cultures were treated with BMP6 for 3 days before harvesting for quantitation of the specific mRNAs indicated. Results are shown as the amount of mRNA in FOXN1 shRNA expressing cells relative to cells infected with the empty vector (EV), normalized to 1 (dashed line). Values are the means ± SD of 2 independent experiments, each with 2 different shRNAs. Average knockdown of FOXN1 mRNA in the absence of BMP was to 40% of control levels. Asterisks indicate statistically significant differences between cultures treated with arsenite (A) or shRNA (B) and control cultures not so treated (dashed line).
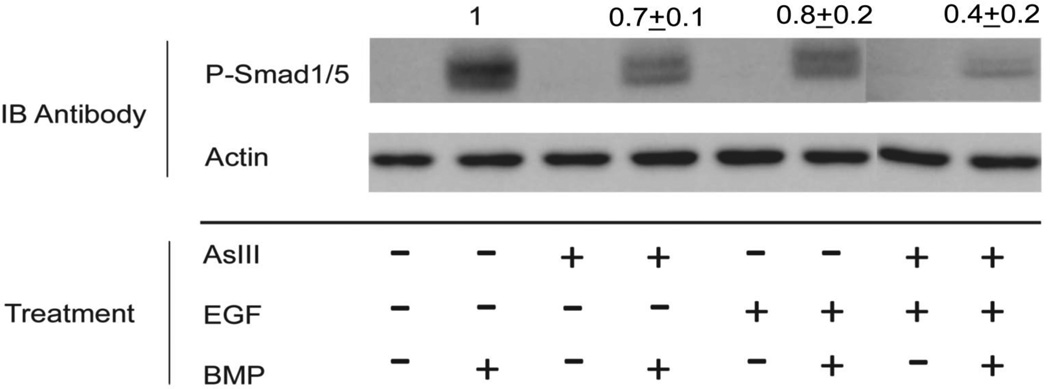
Western blotting with phospho-Smad1/5 antibodies showed that arsenite pre-treatment decreased signaling through Smad1/5 in response to BMP (Figure 3), which was further decreased in combination with EGF. Arsenite was not effective immediately upon addition, but required pre-treatment times of 1 day or more (data not shown). Since arsenite had only a small effect on the direct Smad targets, ID2, Smad6 and Smad7 (Figure 2A), suppression of BMP-mediated KRT1 and KRT10 transcription likely occurs by an additional or alternative mechanism.
Fig. 3.
Suppression of Smad phosphorylation by arsenite. SIK cultures were treated at confluence for 3 days with arsenite (AsIII) or EGF or left untreated as indicated. BMP6 was added, then cells were harvested 1 h later for immunoblotting (IB) with phospho-Smad1/5 antibody. A representative blot is shown. Membranes were also probed with β-actin antibody as a gel loading control. There was no detectable phospho-Smad in untreated cells. Numbers (mean ± SD of 5 experiments) above the P-Smad panel indicate relative amounts of phospho-Smad in BMP-treated samples (after normalization to β-actin) compared to untreated samples.
BMP induction of KRT1 and KRT10 requires Notch signaling
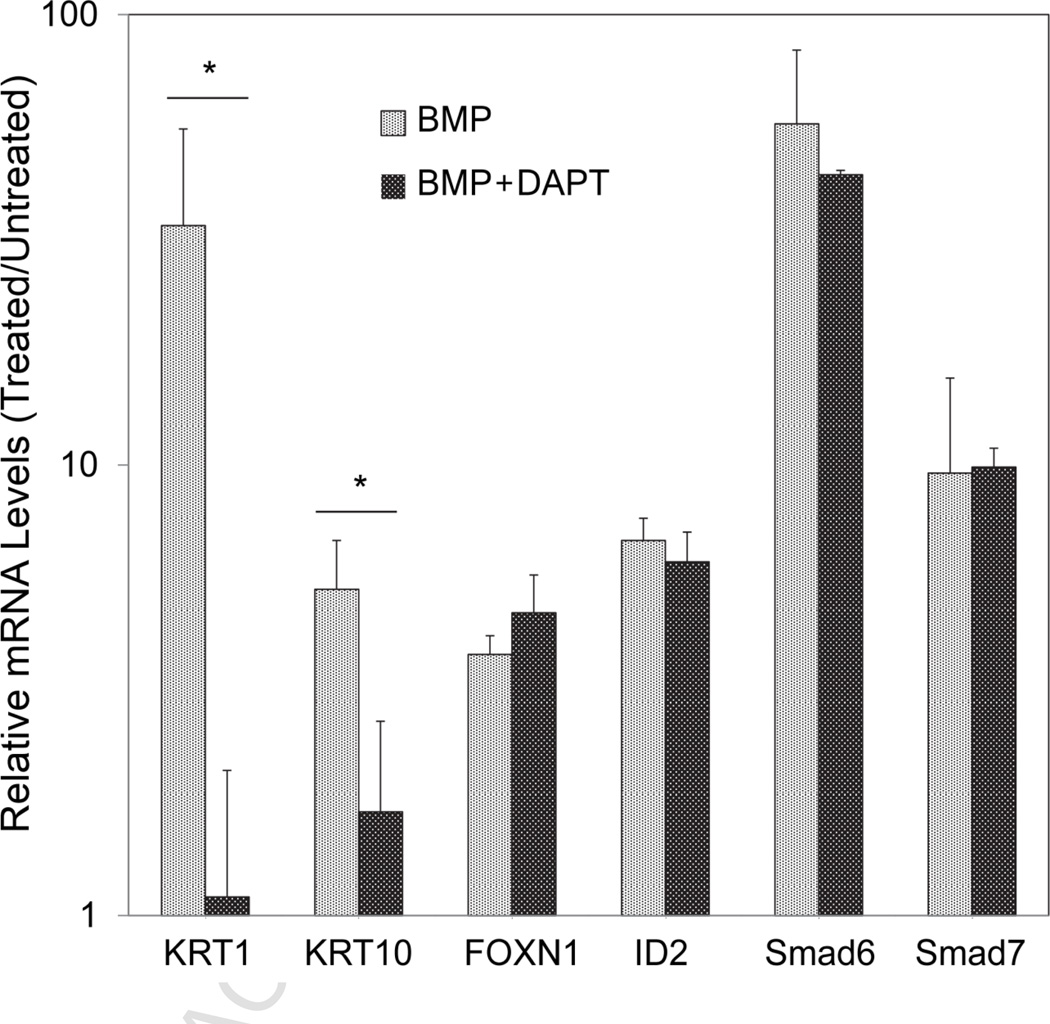
We (Reznikova et al., 2009) and others (Kolev et al., 2008) have demonstrated a role for Notch1 signaling in KRT1 and KRT10 gene expression. To investigate a potential role for Notch1 in BMP regulation of keratins, cells were treated with BMP in the presence and absence of DAPT, an inhibitor of Notch1 activation. KRT1 and KRT10 mRNAs were greatly increased by BMP treatment, an effect that was largely blocked by simultaneous exposure to DAPT (Figure 4). In BMP-treated cells, KRT1 and KRT10 mRNA levels were 28 and 3 times higher in the absence of DAPT, while FOXN1, ID2, Smad6 and Smad7 mRNA levels were similar in both conditions. These results suggest that Notch1 signaling is required for BMP induction of keratin expression. Supporting this view, Western blotting experiments showed that BMP increased generation of NICD (active Notch1) (Figure 5) with little change in total amounts of Notch1 (BMP/untreated control = 0.8 ± 0.5, mean ± SD of 3 experiments). As observed previously (Reznikova et al., 2009), arsenite alone suppressed generation of NICD and now is shown to prevent the increase by BMP (Fig. 5).
Fig. 4.
Role of Notch1 in BMP regulation of keratinocyte differentiation. SIK cultures were treated at confluence for 3 days with BMP6 ± DAPT and harvested for quantitation of specific mRNAs. Shown are the levels relative to untreated controls set to a value of 1. Asterisks indicate significant differences between samples treated with BMP versus BMP+DAPT.
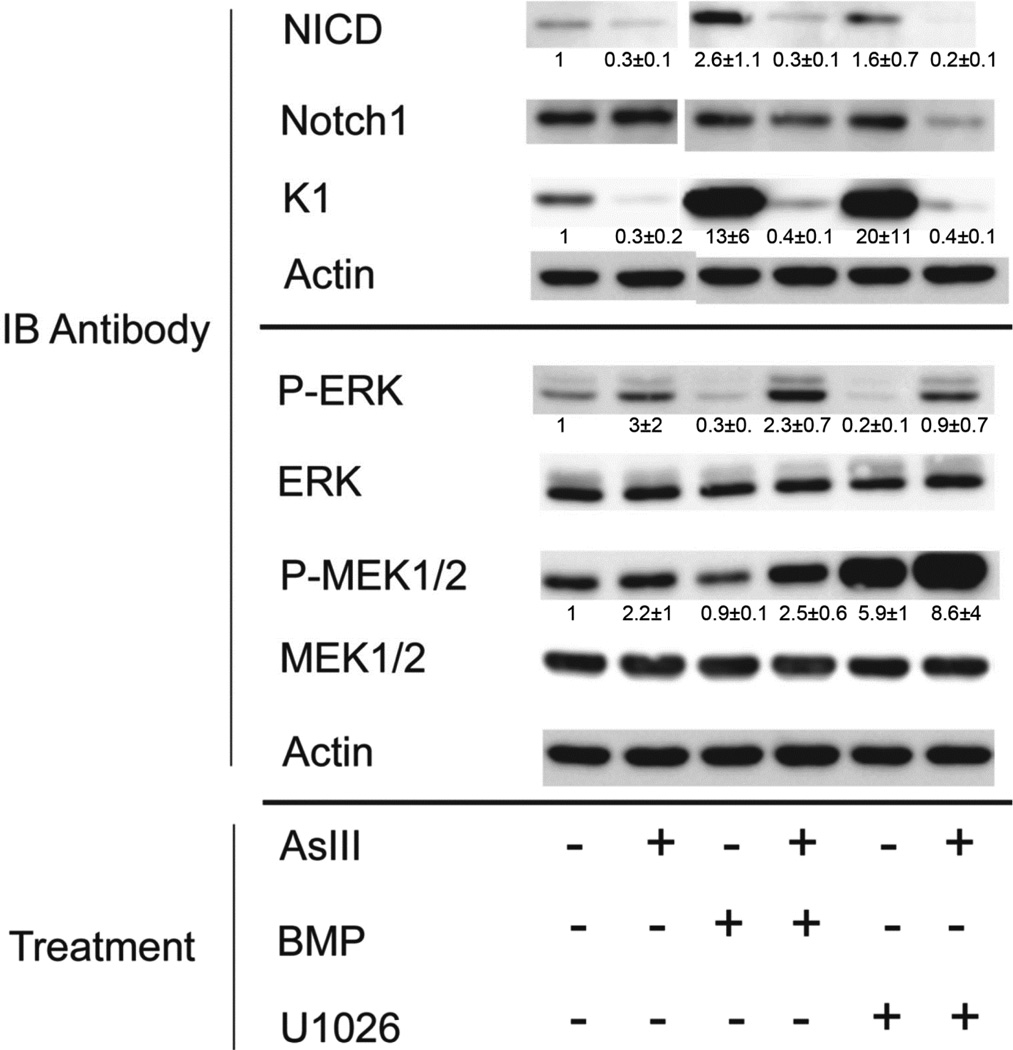
Fig. 5.
Effects of arsenite, BMP6 and U1026 (MEK1/2 inhibitor) on cell signaling pathways. SIK cultures were treated at confluence for 3 days (ERK and MEK antibodies) or 6 days (NICD, Notch1 and KRT1 antibodies) with arsenite or BMP6 or U1026 or combinations or left untreated as indicated, then harvested for immunoblotting (IB). The blot shown is representative of 3 experiments. Numbers beneath the lanes indicate average ± SD of 3 experiments relative to the untreated sample.
BMP decreases active ERK, which is prevented by co-treatment with arsenite
A striking finding was that BMP substantially decreased active ERK1/2, as assessed by Western blotting with phospho-ERK (Thr202/Tyr204) antibody (Figure 5). This effect was accompanied by a small decrease in active MEK1/2 (detected with phospho-MEK1/2 (Ser217/221) antibody), but with little change in total amounts of ERK1/2 or MEK1/2. Treatment of cells with arsenite alone slightly increased active ERK, and reversed the suppression by BMP when the two compounds were added together. With continued presence of BMP in the culture medium, suppression of phospho-ERK lasted up to at least 1 week after initial exposure (not shown).
MEK inhibition mimics BMP
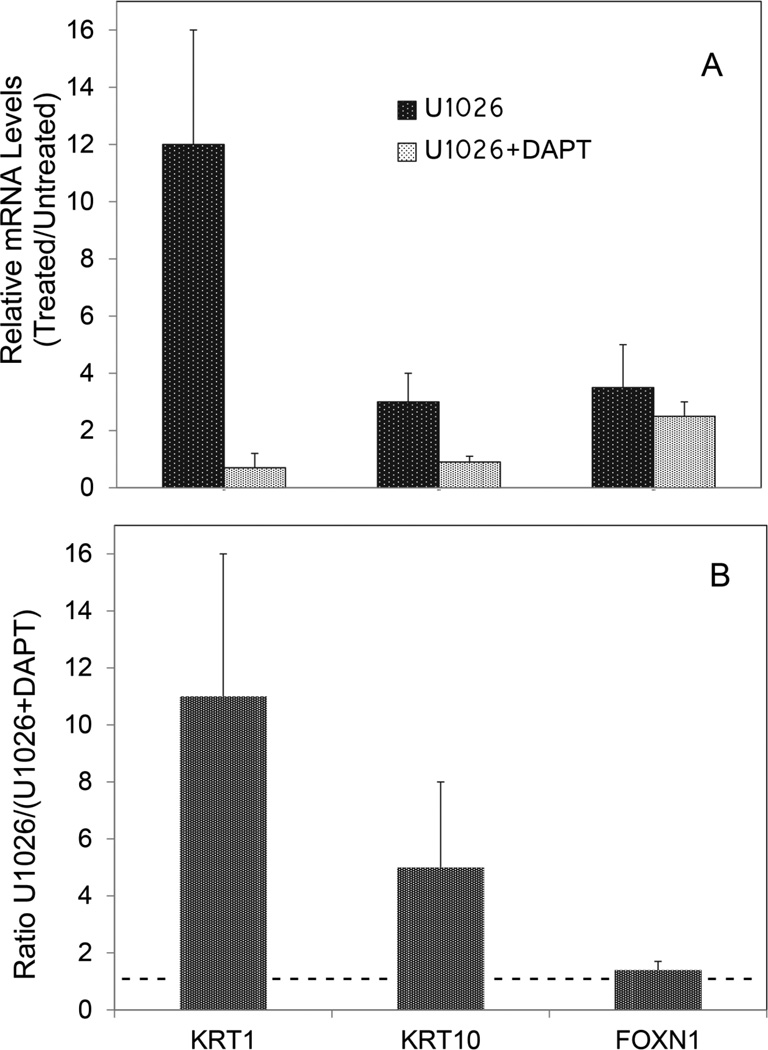
The relevance of ERK suppression by BMP to later effects on Notch1 activation and induction of suprabasal keratins was investigated by determining whether an inhibitor of the upstream ERK kinase, MEK1/2, could mimic BMP. Cultures were treated for three days with the MEK1/2 inhibitor, U1026, in the presence and absence of arsenite. Like BMP, U1026 increased active Notch1 (NICD) and KRT1, and both effects were prevented by simultaneous treatment with arsenite (Figure 5). In real time PCR experiments, U1026, like BMP, induced KRT1 and KRT10 mRNAs (Figure 6A, dark bars). Also like BMP, U1026 induction of KRT1 and KTR10 was diminished by inhibition of Notch1 signaling with DAPT (Figure 6A, light bars). As previously shown (Baxter and Brissette, 2002), U1026 induced FOXN1 (Figure A2, Appendix) but, unlike the keratins, this induction was unaffected by DAPT (not shown).
Fig. 6.
Stimulation of KRT1 and KRT10 mRNA by ERK inhibition and requirement for Notch1 activation. Cultures were treated at confluence for 3 d with U1026 ± DAPT and specific mRNA levels were quantitated. mRNA levels are illustrated relative to untreated control cultures set to 1. Asterisks indicate values significantly different from 1.
Keratin and FOXN1 induction by mitogen-activated kinase inhibitors was specific for the ERK pathway, since JNK and p38 kinase inhibitors exhibited opposite effects from U1026 and actually decreased keratin mRNA (Figure A2, Appendix). Both of these inhibitors also suppressed the induction of keratin mRNAs by BMP in co-treatment experiments (Figure A2B, Appendix).
BMP induces ERK phosphatases
Decreased ERK phosphorylation in the presence of BMP could occur by induction of an ERK phosphatase. This possibility was explored using Western blotting to measure the rate of decline of phospho-ERK after addition of U1026 to arrest ERK phosphorylation. At 37C phospho-ERK levels declined rapidly after addition of U1026 with an estimated t½ of about 1.5 min in untreated cultures (not shown). The rate of decline was increased in cultures treated for 3 days with BMP (estimated t½ of less than 1 min, not shown). These results supported the hypothesis that BMP increased the amount or activity of one or more ERK phosphatases and provided impetus for their identification.
The DUSPs (dual-specificity phosphatases) comprise a major family of MAP kinase phosphatases. These enzymes dephosphorylate both the tyrosine and threonine residues in the activation loop of MAP kinases that are phosphorylated by the MAP kinase kinases. Within this family, DUSPs 1, 2, 4, 5, 6, 7, 9, 14 and 26 show high activity toward ERK (Jeffrey et al., 2007; Patterson et al., 2009) and, of these, DUSPs 1, 2, 5, 6, 7, and 14 are expressed in SIK cultures as determined by PCR (Figure A3, Appendix). BMP induced mRNAs encoding both nuclear DUSP 2 and cytoplasmic DUSP 14 as determined in real time PCR experiments (Figure A4, Appendix). (Levels of other DUSP mRNAs were largely unaffected or reduced.) Induction of each by BMP was diminished by simultaneous treatment with either arsenite or EGF (not shown) and more robustly by the combination (Figure 7).
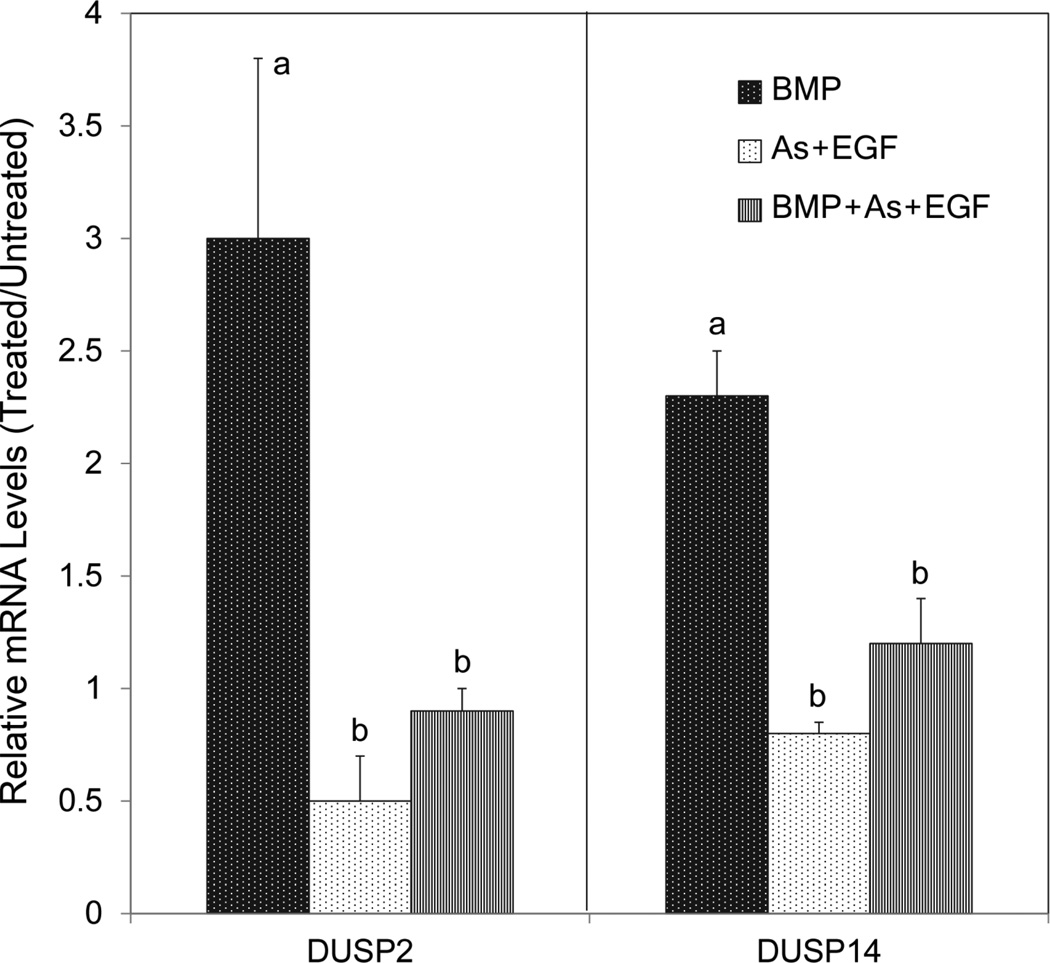
Fig. 7.
Arsenite suppression of DUSP gene induction by BMP. Confluent SIK cultures were treated for 3 days with BMP6, with arsenite (As) plus EGF or with all three agents, then harvested and the mRNAs quantitated. Resulting mRNA levels are shown relative to the untreated control, set at 1. The mRNA levels of samples treated with As + EGF with or without BMP6 (b) were significantly less than those treated with BMP6 alone (a).
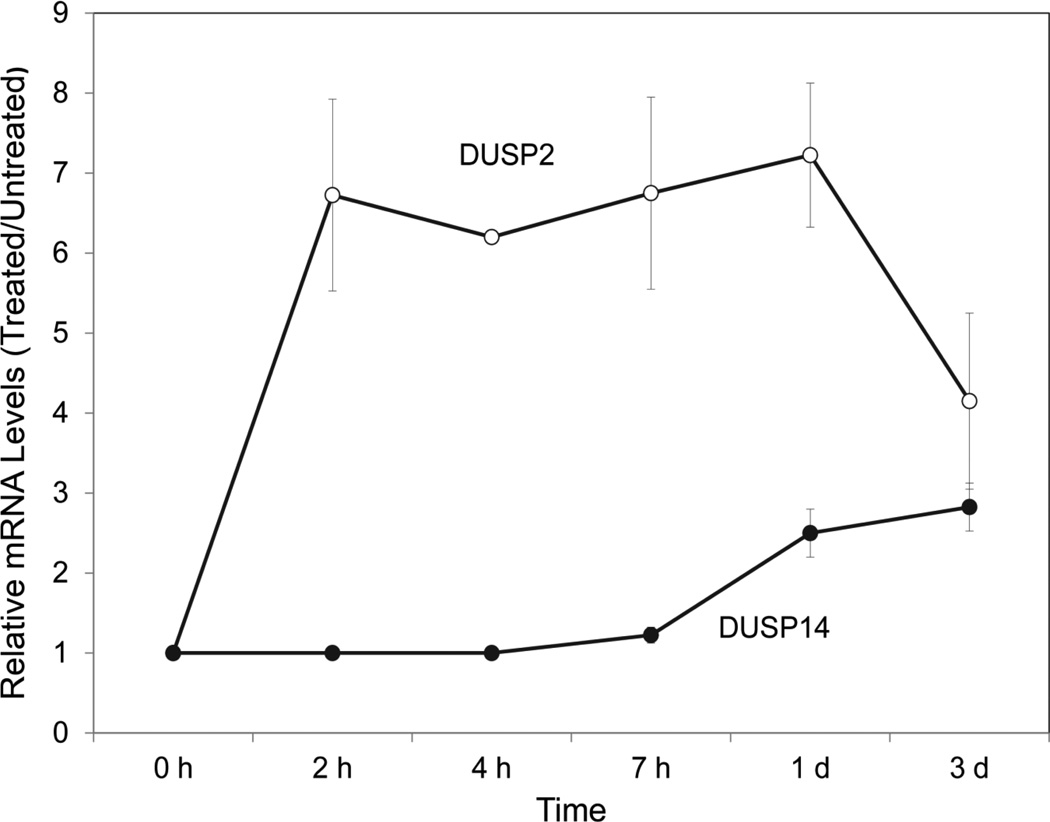
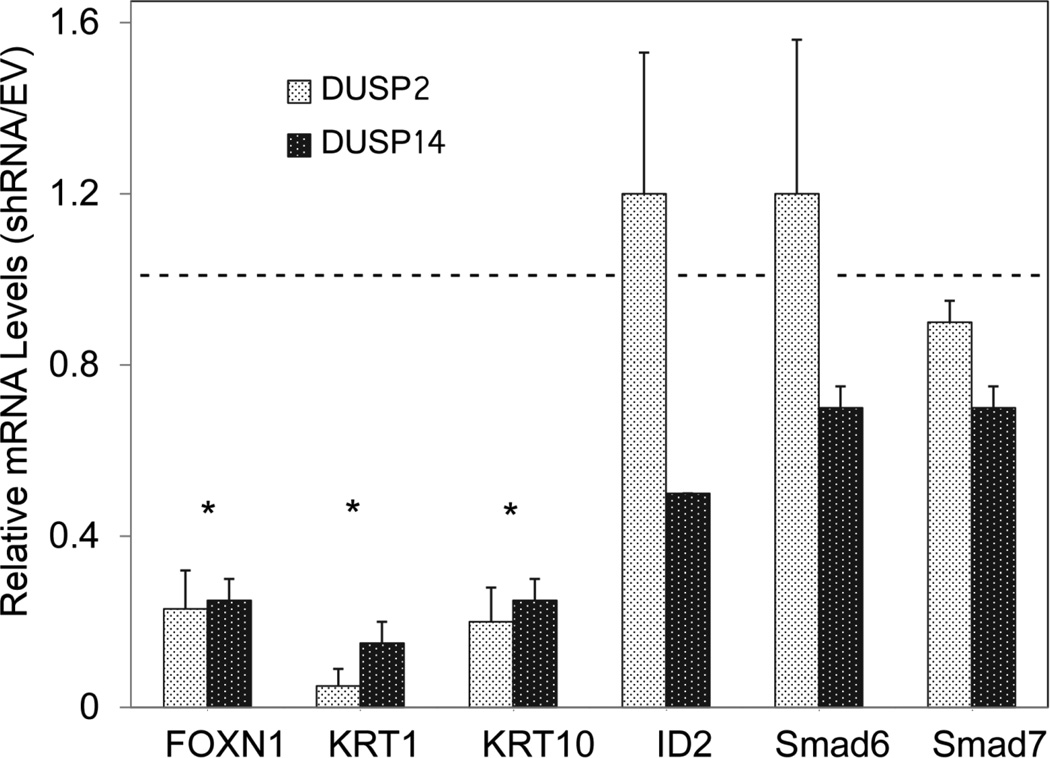
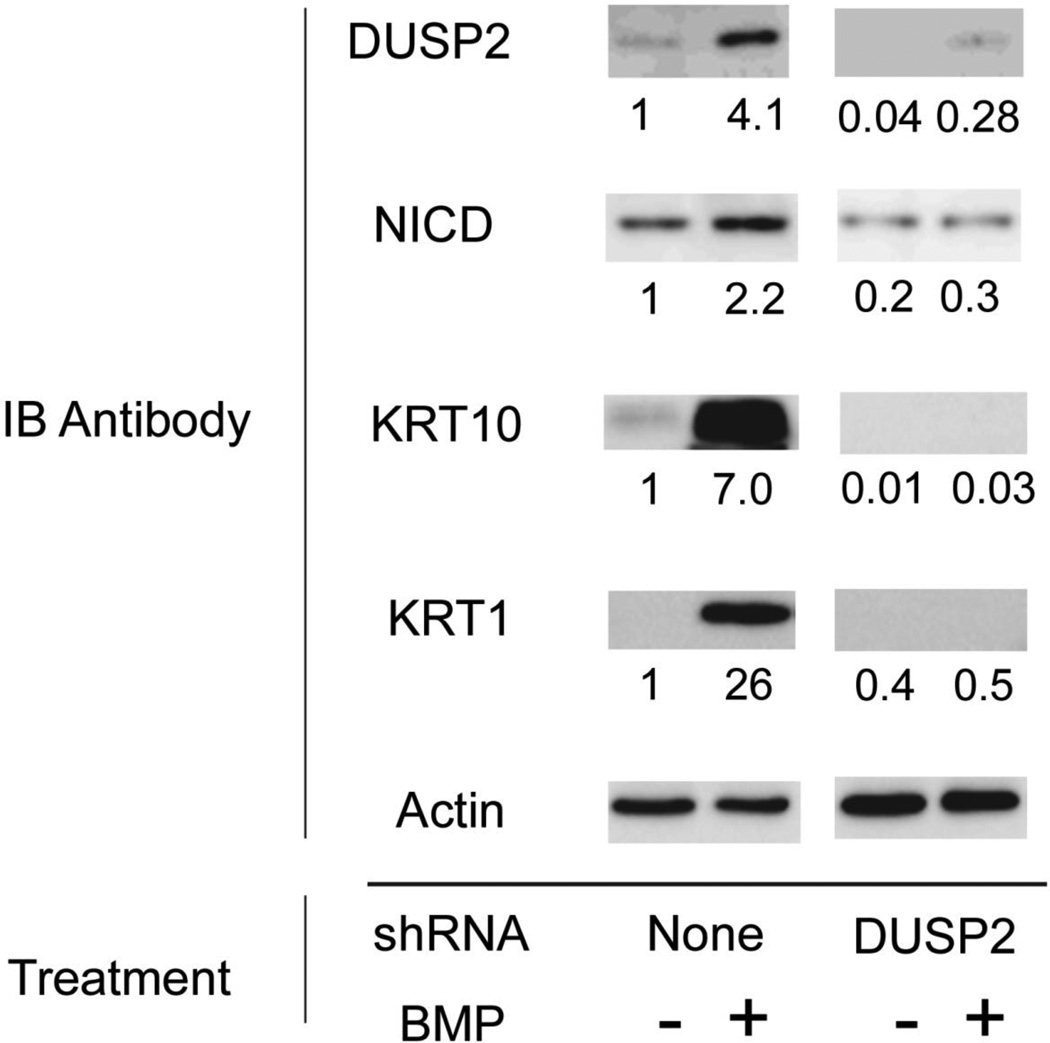
DUSP 2 mRNA levels increased rapidly after BMP addition and reached a plateau by 2 h (Figure 8). DUSP 14 mRNA increased more slowly with maximal levels being achieved only after 1 day of treatment. Whether new protein synthesis was required for this induction by BMP was not testable using cycloheximide, since the latter agent alone induced both DUSPs (Figure A5, Appendix). Increased levels of both DUSP mRNAs were maintained for at least 3 days after addition of BMP. Unlike induction of KRT1 and KRT10 mRNAs by BMP, induction of DUSPs 2 and 14 by BMP did not require Notch1 signaling since it was not blocked by DAPT (Figure A6, Appendix). Knockdown of DUSP2 or DUSP14 by expression of shRNA greatly reduced induction of FOXN1, K1 and K10 mRNAs by BMP (Figure 9), while basal levels of the mRNAs were reduced similarly in the absence of BMP (Figure A7, Appendix). In the presence of either shRNA, BMP induction of ID2, Smad6 or Smad7 was less affected. That the DUSP2 shRNA was effective in reducing DUSP2 protein was shown by Western blotting (Figure 10). Although BMP treatment still induced DUSP2, the amounts were much reduced. Knockdown also reduced amounts of activated Notch1 (NICD), KRT1 and KRT10, both in the presence and absence of BMP, placing DUSP2 upstream of Notch activation and keratin expression.
Fig. 8.
Time course of BMP induction of DUSP2 and DUSP14 mRNAs. Cultures were treated with BMP, or left untreated, for the indicated times, and the mRNAs were quantitated. Results are expressed relative to amounts of DUSP mRNA in untreated cells, set at 1.
Fig. 9.
Suppression of KRT1, KRT10 and FOXN1 expression by DUSP knockdown. Confluent SIK cultures expressing DUSP2 or DUSP14 shRNAs or the control empty vector (EV) were treated for 3 days with BMP6. The indicated mRNAs, measured by real time PCR, are shown normalized to cells treated with the empty vector, set at 1 (dashed line). Results are the means and SD of 3 independent experiments with a single DUSP2 shRNA or one experiment using 2 different DUSP14 shRNAs. Amounts of DUSP mRNA in shRNA expressing cells were 40 ± 16 % (DUSP2) and 55 ± 5 % (DUSP14) of control. Asterisks indicate values significantly different from 1.
Fig. 10.
Effect of DUSP2 knockdown on activated Notch1 (NICD), KRT1 and KRT10. Parallel cultures were infected with lentivral vector expressing DUSP2 shRNA or left uninfected. At confluence, cultures were treated for 6 days with BMP or left untreated, as indicated. Western blots were probed with the indicated antibodies, imaged and quantitated. Protein amounts were normalized to actin. Numbers beneath the lanes show amounts relative to the uninfected, untreated samples, set at 1.
Discussion
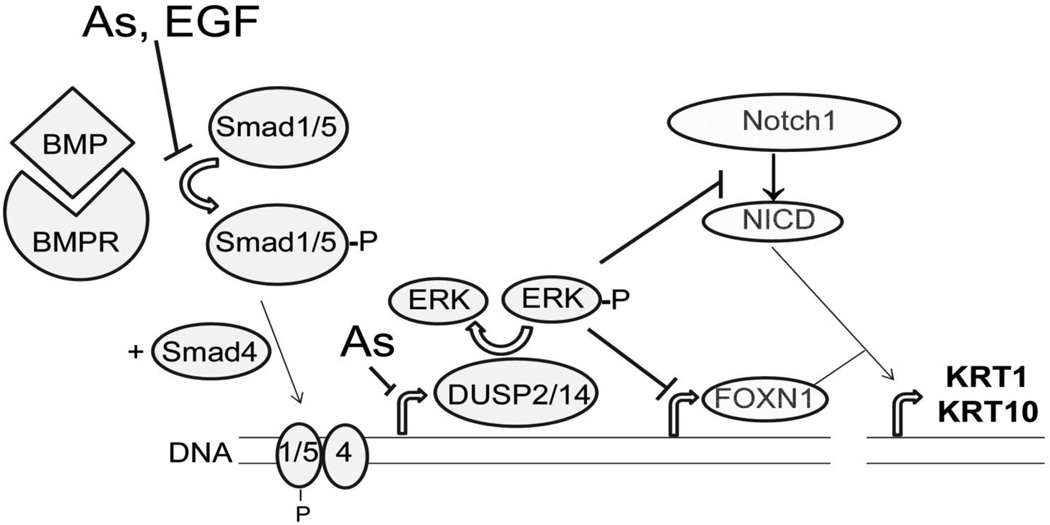
This investigation is the first to show that FOXN1 induction is suppressed by arsenite. Rationalizing this action, arsenite decreased phosphorylation of Smad1/5 transcription factors, classic targets of the BMP receptor kinase. This action was more powerful in the presence of EGF, reminiscent of arsenite augmentation of EGF signaling (Patterson and Rice, 2007) and consistent with observations that expression of KRT1 and KRT10 is negatively regulated by EGF (Rea et al., 1998; Tran et al., 2012). However, this alone was unlikely to result in the large suppression of BMP effects on FOXN1 and keratins since arsenite had little effect on BMP induction of three genes directly regulated by Smad transcription factors (ID2, Smad6 and Smad7). Figure 11 illustrates this and another site of arsenic intervention affecting ERK signaling through DUSP induction.
Fig 11.
Signaling pathway and proposed sites of arsenite perturbation. Illustrated is the phosphorylation of Smad1/5 by the liganded BMP receptor, a phenomenon partially suppressed by arsenite (As) and EGF. The resulting induction of DUSP2 and DUSP14 (the latter possibly indirect) by Smad transcription factors is also shown as a site of arsenite intervention. Arsenite inhibition of DUSP dephosphorylation of ERK is possible but was not tested. Active ERK (phosphorylated) suppresses FOXN1 mRNA levels and Notch1 activation. The latter may occur at least in part by reduced FOXN1 binding to response elements reported in the upstream promoter region of Notch1 (Cai et al., 2009), not shown for simplicity. It is not known whether FOXN1 and activated Notch1 (NICD) bind directly to keratin gene promoters or instead regulate the expression of other factors affecting keratin gene transcription.
EGF receptor and ERK signaling have been shown to antagonize BMP signaling in keratinocytes (McDonnell et al., 2001; Gosselet et al., 2007) and other cell types (Kretzschmar et al., 1997; Sapkota et al., 2007) and, conversely, BMP is a negative regulator of ERK (Li et al., 2012). Since arsenite augments EGF receptor signaling by maintaining receptor levels (Patterson and Rice, 2007), effects on the EGF receptor-ERK pathway may contribute to arsenite suppression of FOXN1 and keratin induction by BMP. This view is supported by the demonstration that U1026, an inhibitor of the ERK kinase, MEK1/2, increases FOXN1, KRT1 and KRT10, just as BMP does, and arsenite blocks these effects while maintaining levels of active ERK. Conversely, in preliminary experiments, over-expression of a constitutively active ERK, like arsenite, blocked BMP induction of FOXN1 and keratin mRNAs (unpublished). In addition, siRNAs targeting EGF receptor or ERK1 or the AP-1 transcription factors c-Jun or c-Fos have been shown to increase FOXN1 mRNA in cultured human keratinocytes, while over-expression of c-Jun suppressed the activity of the FOXN1 promoter (Mandinova et al., 2009). Although we have not investigated the roles of c-Fos and c-Jun in regulation of FOXN1 by BMP and arsenite, such a mechanism is plausible. In any case, rapid BMP induction of FOXN1 may be a direct effect of Smad transcription factor binding to the FOXN1 promoter.
In embryonic stem cells, BMP4 suppresses ERK signaling by induction of the ERK phosphatase, DUSP9 (Li et al., 2012). Although DUSP9 is confined to embryonic cells, it is plausible that BMP6 acts similarly in keratinocytes and decreases phospho-ERK by induction of a different DUSP. Several DUSPs are expressed in keratinocytes and, of these, DUSP2 and DUSP14 were demonstrated to be induced by BMP. DUSP2 increased rapidly after addition of BMP and was maximal by 2 h, while DUSP14 took a day to be maximally induced. These results suggest that DUSP2 may be a direct target of BMP signaling while DUSP14 induction is likely to be a secondary effect.
The ability of arsenite to increase ERK phosphorylation in various cell types at relatively high concentration has been known for some time (Samet et al., 1998). In more recent experiments in human keratinocyte cultures, acute exposure to arsenite at low micromolar concentrations substantially increased ERK phosphorylation with highest levels of induction after hours of treatment (Trouba and Germolec, 2004). In those experiments, MEK1/2 phosphorylation increased in parallel with ERK phosphorylation, suggesting that the target of arsenite was upstream of ERK. In present work, examining the effects of chronic treatment with arsenite revealed its main effect was to prevent decreased ERK phosphorylation by BMP, while arsenite alone had only small effects. The longer exposure time likely allows secondary effects of arsenite treatment to occur, such as suppression of DUSP induction, and would miss a transient increase in ERK phosphorylation. In addition, arsenite may plausibly directly inhibit an ERK phosphatase since it maintains high levels of phospho-ERK even in the presence of a MEK1/2 inhibitor.
Chronic treatment of epidermal cultures with arsenite has been shown to result in higher EGF receptor levels than are present in parallel untreated cultures (Patterson and Rice, 2007). Although not investigated in keratinocytes, the mechanism could reflect altered receptor trafficking and degradation as observed in lung carcinoma cell cultures treated with oxidants such as H2O2 (Khan et al., 2006). The delayed response may be due to a requirement for altered gene expression.
Notch1 signaling has been shown to be an important pathway for keratinocyte differentiation. Overexpression or addition of Notch1 ligands promotes differentiation (Lowell et al., 2000; Nickoloff et al., 2002). Conversely suppression of Notch1 signaling is associated with elevated incidence of squamous cell carcinoma, and interference with Notch1 signaling by expression of a dominant negative transcriptional co-activator produces squamous cell carcinomas in the mouse (Proweller et al., 2006). Previous work from our laboratory demonstrated suppression of Notch1 activation by arsenite (Reznikova et al., 2009), although the mechanism was unclear. More recent work demonstrated that EGF receptor signaling decreased Notch1 signaling by decreasing Notch1 transcription (Kolev et al., 2008). We found that arsenite reduced Notch1 mRNA to 60% of levels in untreated cells, but BMP also reduced Notch1 mRNA to a similar extent even though it increased active Notch1. These results suggest that these agents affect Notch1 signaling at an additional step.
γ-Secretase is the final enzyme in the cascade that cleaves and activates Notch1. Intriguingly, ERK has been shown to be a negative regulator of γ-secretase activity in mouse neuronal cells, probably through phosphorylation of nicastrin, one of the components of the γ-secretase complex (Kim et al., 2006). If such a mechanism were operative in keratinocytes, it would explain the stimulation of Notch1 activation by BMP due to induction of DUSPs and consequent decreased ERK signaling, as well as prevention of Notch1 activation by arsenite due to maintenance of active ERK. The possibility that arsenite alters Notch1 activity through perturbation of its posttranslational modification (Peng et al., 2012) cannot be ruled out.
Supplementary Material
Highlights.
BMP induces FOXN1 transcription.
BMP induces DUSP2 and DUSP14, suppressing ERK activation.
Arsenite suppresses levels of phosphorylated Smad1/5 and FOXN1 and DUSP mRNA.
These actions rationalize arsenite suppression of keratinocyte differentiation.
Acknowledgments
This work was supported by the USA National Institute of Environmental Health Sciences (grant 2P42 ES04699), the National Natural Science Foundation of China (grants 20921063 and 21177150) and the Chinese Academy of Sciences Key Program of Knowledge Innovation (KZCX2-EW-411). The funding agencies had no role in the study design, data collection and analysis, writing or decision to publish the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci USA. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Baxter RM, Brissette JL. Role of the nude gene in epithelial terminal differentiation. J Invest Dermatol. 2002;118:303–309. doi: 10.1046/j.0022-202x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study. Environ Hlth Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Lee J, Kopan R, Ma L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev Biol. 2009;326:420–430. doi: 10.1016/j.ydbio.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdoff V, Wall NA, Pledger WJ. Expression and growth inhibitory effect of decapentaplegic Vg-related protein 6: evidence for a regulatory role in keratinocyte differentiation. Proc Natl Acad Sci U S A. 1994;91:5528–5532. doi: 10.1073/pnas.91.12.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselet FP, Magnaldo T, Culerrier RM, Sarasin A, Ehrhart JC. BMP2 and BMP6 control p57(Kip2) expression and cell growth arrest/terminal differentiation in normal primary human epidermal keratinocytes. Cell Signal. 2007;19:731–739. doi: 10.1016/j.cellsig.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Janes SM, Ofstad TA, Campbell DH, Watt FM, Prowse DM. Transient activation of FOXN1 in keratinocytes induces a transcriptional programme that promotes terminal differentiation: contrasting roles of FOXN1 and Akt. J Cell Sci. 2004;117:4157–4168. doi: 10.1242/jcs.01302. [DOI] [PubMed] [Google Scholar]
- Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- Kachinskas DJ, Qin Q, Phillips MA, Rice RH. Arsenate suppression of human keratinocyte programming. Mutation Res. 1997;386:253–261. doi: 10.1016/s1383-5742(97)00015-x. [DOI] [PubMed] [Google Scholar]
- Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J Biol Chem. 2006;281:14486–14493. doi: 10.1074/jbc.M509332200. [DOI] [PubMed] [Google Scholar]
- Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW, Mook-Jung I. ERK1/2 is an endogenous negative regulator of the γ-secretase activity. FASEB J. 2006;20:157–159. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, Neel V, Dummer R, Wagner EF, Dotto GP. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–911. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, Zhang H, Han JD, Chen YG. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones PH, LeRoux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by Delta-Notch signaling at the boundaries of stem cell clusters. Curr Biol. 2000;10:491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Mandinova A, Kolev V, Neel V, Hu B, Stonely W, Lieb J, Wu X, Colli C, Han R, Pazin MJ, Ostano P, Dummer R, Brissette JL, Dotto GP. A positive FGFR3/FOXN1 feedback loop underlies benign skin keratosis versus squamous cell carcinoma formation in humans. J Clin Invest. 2009;119:3127–3137. doi: 10.1172/JCI38543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MA, Law BK, Serra R, Moses HL. Antagonistic effects of TGFβ1 and BMP-6 on skin keratinocyte differentiation. Exp Cell Res. 2001;263:265–273. doi: 10.1006/excr.2000.5117. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- National Research Council. Arsenic in Drinking Water. National Academy Press: Washington; 2001. [Google Scholar]
- Nickoloff BJ, Qin J-Z, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kB and PPARγ. Cell Death Differen. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Reznikova TV, Phillips MA, Rice RH. Arsenite maintains germinative state in cultured human epidermal cells. Toxicol Appl Pharmacol. 2005;207:69–77. doi: 10.1016/j.taap.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Patterson TJ, Rice RH. Arsenite and insulin exhibit opposing effects on epidermal growth factor receptor and keratinocyte proliferative potential. Toxicol Appl Pharmacol. 2007;221:119–128. doi: 10.1016/j.taap.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Kaplan N, Hamanaka RB, Katsnelson J, Blatt H, Yang W, Hao L, Bryar PJ, Johnson RS, Getsios S, Chandel NS, Lavker RM. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci USA. 2012;109:14030–14034. doi: 10.1073/pnas.1111292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS. Impaired notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res. 2006;66:7438–7444. doi: 10.1158/0008-5472.CAN-06-0793. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Liu W, Li YN, Sun X, Hai CX, Hudson LG, Liu KJ. Poly(ADP-ribose) polymerase-1 inhibition by arsenite promotes the survival of cells with unrepaired DNA lesions induced by UV exposure. Toxicol Sci. 2012;127:120–129. doi: 10.1093/toxsci/kfs099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster LC, Krishna S, Metzger D, Chambon P, Miele L, Aguet M, Radtke F, Dotto GP. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Phillips MA, deGraffenried LA, Qin Q, Rice RH. Modulation of human epidermal cell response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by epidermal growth factor. Carcinogenesis. 1998;19:479–483. doi: 10.1093/carcin/19.3.479. [DOI] [PubMed] [Google Scholar]
- Reznikova TV, Phillips MA, Patterson TJ, Rice RH. Opposing actions of insulin and arsenite converge on PKCδ to alter keratinocyte proliferative potential and differentiation. Molec Carcinogen. 2010;49:398–409. doi: 10.1002/mc.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikova TV, Phillips MA, Rice RH. Arsenite suppresses Notch1 signaling in human keratinocytes. J Invest Dermatol. 2009;129:155–161. doi: 10.1038/jid.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RH, Steinmann KE, deGraffenried LA, Qin Q, Taylor N, Schlegel R. Elevation of cell cycle control proteins during spontaneous immortalization of human keratinocytes. Molec Biol Cell. 1993;4:185–194. doi: 10.1091/mbc.4.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am J Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- Sapkota G, Alarcón C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP signaling through integrated inputs into the Smad1 linker. Molec Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analyt Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tokar EJ, Waalkes MP. Overabundance of putative cancer stem cells in human skin keratinocyte cells malignantly transformed by arsenic. Toxicol Sci. 2012;125:20–29. doi: 10.1093/toxsci/kfr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Qu W, Waalkes MP. Arsenic, stem cells, and the developmental basis of adult cancer. Toxicol Sci. 2011;120:S192–S203. doi: 10.1093/toxsci/kfq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran QT, Kennedy LH, Leon Carrion S, Bodreddigari S, Goodwin SB, Sutter CH, Sutter TR. EGFR regulation of epidermal barrier function. Physiol Genomics. 2012;44:455–469. doi: 10.1152/physiolgenomics.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouba KJ, Germolec DR. Micromolar concentrations of sodium arsenite induce cyclooxygenase-2 expression and stimulate p42/44 mitogen-activated protein kinase phosphorylation in normal human epidermal keratinocytes. Toxicol Sci. 2004;79:248–257. doi: 10.1093/toxsci/kfh132. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: Inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.