Abstract
Claudins are tight junction membrane proteins that regulate paracellular permeability of renal epithelia to small ions, solutes and water. Claudins interact within the cell membrane and between neighboring cells to form tight junction strands and constitute both the paracellular barrier and pore. The first extracellular domain of claudins is thought to be the pore-lining domain and contains the determinants of charge selectivity. Multiple claudins are expressed in different nephron segments and this likely determines the permeability properties of each segment. Recent evidence has identified claudin-2 as constituting the cation-reabsorptive pathway in the proximal tubule, claudin-14, -16 and -19 as forming a complex that regulates calcium transport in the thick ascending limb of the loop of Henle, and claudin-4, -7 and -8 as determinants of collecting duct choride permeability. Mutations in claudin-16 and -19 cause familial hypercalciuric hypomagnesemia. The roles of other claudins in kidney diseases remain to be fully elucidated.
Keywords: Tight junction, paracellular, permeability, ion channel, renal tubule, sodium, calcium
INTRODUCTION
Epithelial cells in the glomerulus and renal tubule of the kidney play critical roles in determining their permeability to solutes and water and hence in the homeostasis of extracellular fluid composition. Transepithelial transport can occur via the transcellular or paracellular route. Paracellular transport, which refers to transport in between cells, is restricted by the tight junction, which is the most apical structure of the intercellular junctional complex. The tight junction is composed of a large number of different proteins, which include membrane proteins, cytoplasmic scaffolding proteins, and signalling proteins. Of these, the membrane proteins are likely to play the major role in determining paracellular permeability because their extracellular domains protrude into the paracellular space and are therefore ideally situated to influence paracellular solute movement.
The claudins are members of a large family of tight junction membrane proteins that were first identified in 1998 by Furuse et al. (1). In 1999, Simon et al. identified a new member of this family, claudin-16 or paracellin, that was mutated in familial hypercalciuric hypomagnesemia with nephrocalcinosis (FHHNC) (2). Because FHHNC appeared to be due to defective thick ascending limb paracellular Ca and Mg reaborption, this was the first indication that perhaps claudin-16, and by inference claudins in general, might play an important role in paracellular solute permeability in the kidney. We now know from overexpression, knockdown/knockout and mutagenesis experiments that claudins are the major determinants of paracellular permeability to small solutes and act both as the barrier and pore.
CLAUDIN TAXONOMY AND STRUCTURE
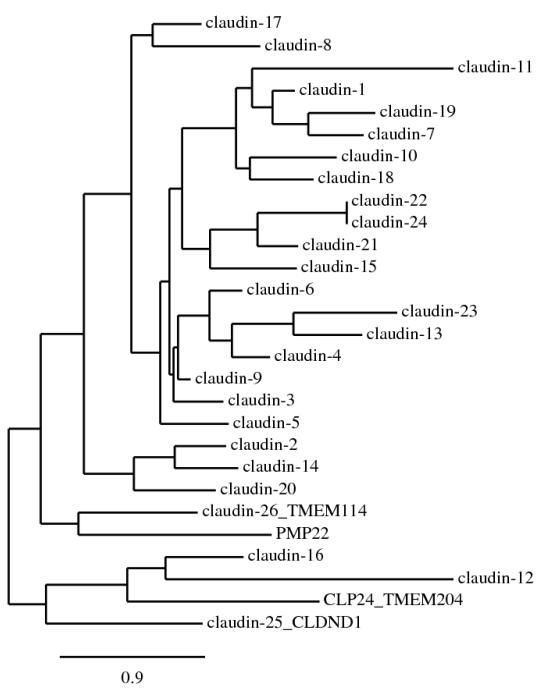
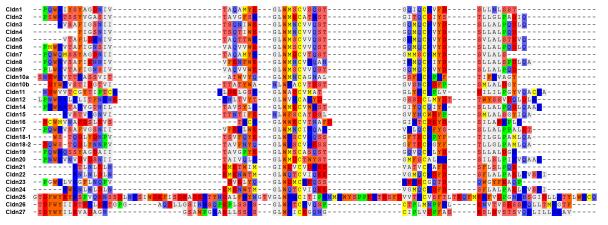
Claudins are tetraspan proteins consisting of a family of at least 26 members in mammals (3-5). They are characterized by a common motif (GLWCC; PROSITE ID: PS01346) in the first extracellular loop (ECL1) (6). Phylogenetic analyses show that the claudin proteins (1-24, except 12 and 16) cluster closely to each other compared with the outgroup protein, PMP22 (peripheral myelin protein 22) (Figure 1). Claudins 1-10, 14, 15, 17 and 19 share sequence homology and functional similarity, and are often known as the classic claudins (7). Claudin-12 and -16 are closely related to the CLP24 (claudin-like protein-24; also known as TMEM204) molecule that is localized in the adherens junction but not in the TJ (8). Two novel claudins (25 and 26, also known as CLDND1 [claudin domain containing 1] and TMEM114 [transmembrane protein 114] respectively) (4) are positioned at the outer portion of the clade of previously identified claudin family members. A recent database search has identified a potentially novel claudin, designated as claudin-27 (also known as LOC283999) which, however, is not localized in the TJ and thus is excluded from this review (4).
Figure 1.
Phylogenetic tree of the mouse claudin family. The tree was constructed by the neighbor-joining method. The scale bar indicates the rate of amino acid substitutions per site.
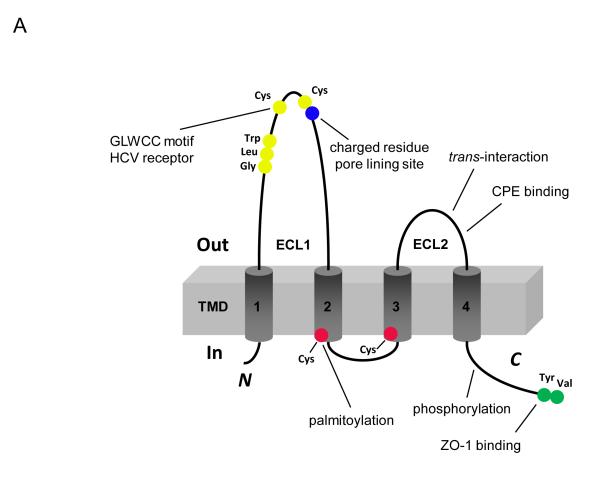
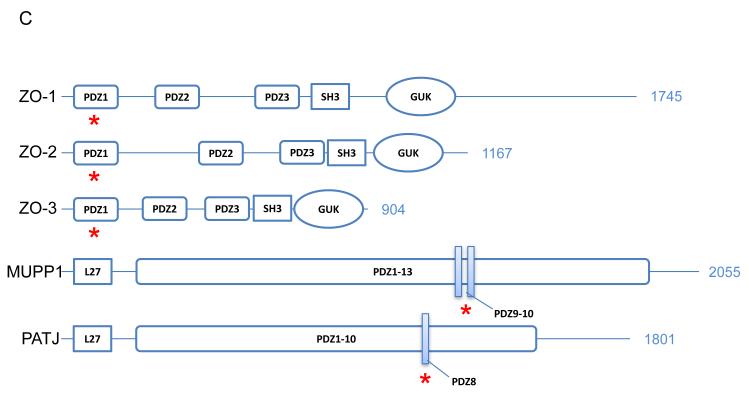
Claudins are 21–28 kD proteins and consist of four transmembrane domains, two extracellular loops, amino- and carboxy-terminal cytoplasmic domains, and a short cytoplasmic turn (Figure 2A). The ECL1 of claudin consists of ~50 amino acids with the common GLWCC motif (7) and negatively (9, 10) and positively (6, 11) charged residues that contribute to paracellular ion selectivity. The GLWCC motif in claudin-1 is critical for hepatitis C virus entry (12). The charges in ECL1 regulate paracellular ion selectivity through electrostatic effects. The second extracellular loop (ECL2) consists of ~25 amino acids with a predicted helix–turn–helix motif (13) that mediate trans-claudin interactions and claudin interactions with the Clostridium perfringens enterotoxin (CPE) (14). The carboxy-terminal domain of claudin contains a PDZ (postsynaptic density 95/discs large/zonula occludens-1) binding motif (YV) that is critical for interaction with the submembrane scaffold proteins ZO-1, -2 and -3, and MUPP-1 (15, 16). However, this motif is not required for the correct localization of claudins in the TJ (17-20) and its function is unknown. The YV motif is conserved among classic claudins, whereas non-classic claudins show greater variations (H/S/Y/D/E/R–V/L) in their PDZ binding domain and have different affinities for ZO-1 (21). Post-translational modifications such as palmitoylation and phosphorylation occur in the cytoplasmic domain of claudin. Claudins have membrane-proximal cysteines in the CxxC motif near the ends of the second and fourth transmembrane domains. Mutation in any of these cysteines in claudin-14 prevented its palmitoylation and reduced the efficiency of TJ localization (22). Claudin phosphorylation contributes to its ion selectivity. WNK4, a gene mutated in pseudohypoaldosteronism type II (PHAII), has been suggested to phosphorylate the carboxy terminus of claudin-4 and alter paracellular Cl− permeability in cultured MDCK cells (23). Claudin-16 is phosphorylated at the S217 residue of carboxy terminus by PKA; phosphorylation is required for claudin-16 mediated Mg2+ transport (24).
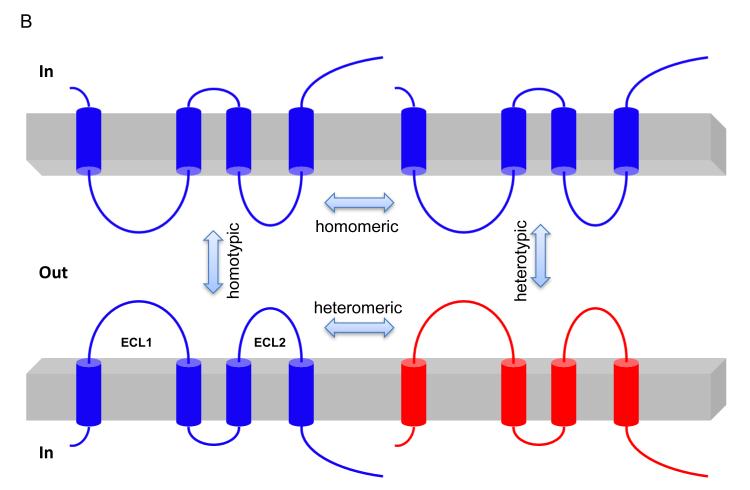
Figure 2.
Structure and function of claudin. (A) Schematic presentation of the topology of claudin monomer. The model depicts the conserved structural features of claudin and the known interactions and modifications. ECL1 and ECL2 denote the extracellular loops 1 and 2, respectively. The transmembrane domains 1 to 4 (TM1-TM4) and the regions important for hepatitis C virus (HCV) entry, paracellular ion selectivity, Clostridium perfringens enterotoxin (CPE) binding, palmitoylation, phosphorylation and ZO-1 binding are shown. Modified from Figure 1 in Hou, J. (121). (B) Schematic presentation of interaction possibilities between claudin molecules. The cis interaction includes homomeric or heteromeric interaction; the trans interaction includes homotypic or heterotypic interaction. Modified from Figure 3 in Hou, J. (121).
Claudins associate by cis interactions within the plasma membrane of the same cell into dimers, or higher oligomeric states (25, 26), and by trans interactions between claudins in adjacent cells. Additional cis interactions likely assemble claudin oligomers into TJ strands. The cis interaction can involve a single type of claudin (homomeric interaction) or different types of claudins (heteromeric interaction); the trans interaction can occur in homotypic or heterotypic mode (27)(Figure 2B). Claudin-16 and claudin-19, for example, interact in cis to generate a cation-selective channel (28). Although there are few data available demonstrating the critical loci for cis interaction within claudin molecule, the extracellular loops (ECL1 and ECL2) appear not to be involved (26). The heteromeric interaction between claudin-16 and claudin-19 is not affected by mutations in ECL1 of either claudin (26), and the homomeric interaction of claudin-5 is not affected by amino acid exchange in ECL2 (29). In contrast, trans claudin interactions do depend upon the ECL2 domain. While claudin-5 heterotypically interacts with claudin-3, but not with claudin-4, chimeras exchanging the ECL2 of claudin-4 with claudin-3 confer the ability to bind to claudin-5 (30). Mutagenesis has identified a locus of amino acids in ECL2 (F147, Y148, Y158) critical for homotypic claudin-5 interaction (13).
TJ strand formation requires both cis and trans claudin interactions. Genetic ablation of either claudin-16 or claudin-19 prevents the assembly of both claudins into TJ strands (31), ostensibly due to the loss of cis interaction. Preliminary biochemical studies of claudin-4 in insect Sf9 cells (25) and claudin-5 in human fibroblast NIH/3T3 cells (32) suggest that claudins preferentially form hexamers via cis interaction. By contrast, the loss of trans interaction between claudin-5 in neighboring cells reduced the TJ strand density even though cis interaction remained intact (13).
GENERAL PROPERTIES OF CLAUDINS
Barrier vs. pore claudins
Several studies have shown that claudins are responsible for the size- and charge-selective conductance properties of the paracellular pathway. Expressing exogenous claudins in cultured epithelial cells significantly changes their transepithelial resistance (TER) (1, 33, 34). Claudins are loosely classified as barrier or pore claudins based on whether their expression increases or decreases TER. For example, over-expression of claudin-4, in low resistance Madin Darby canine kidney II (MDCK II) epithelial cells tripled the transepithelial resistance; thus claudin-4 is classified as a barrier claudin (34). Conversely, exogenous expression of a pore claudin, claudin-2, in high resistance MDCK I cell sheets decreased TER by 20-fold (1). Several over-expression studies have categorized claudin-1, -4, -5, -6, -8, -9, -11, -15 and -19 as barrier claudins (34-40) while claudin-2, -10 and 16 are thought to be predominantly pore claudins (10, 41, 42), though this is also quite dependent on the background permeability of the cell line (see below). Furthermore, in the nephron, pore claudins have been observed to be associated with leakier tubule segments while barrier claudins are expressed in tighter tubule segments. For example, the pore claudin, claudin-2, is localized to the leakier proximal tubule (43) while a barrier claudin, claudin-8, is expressed in the tighter distal nephron (44).
Charge selectivity of claudins
Claudins can selectively increase the the paracellular permeability of cations over anions and vice versa. For example, over-expression of the barrier claudin, claudin-4, in low resistance MDCK II cells, selectively decreases the permeability of Na+ over Cl− (34). Conversely, over-expression of the pore claudin, claudin-2, in high resistance MDCK II or C7 cells preferentially increases the permeability of Na+ over Cl− (41, 45, 46). Table 1 summarizes current knowledge of the properties of claudin isoforms. Several claudins (e.g. 4, 8, 11, 15) behave differently depending on the background permeability and selectivity of the cell line in which they are expressed, but retain their overall charge selectivity. Other claudins (e.g. 7, 16, 19) show markedly different properties in different studies that are currently difficult to reconcile.
Table 1.
Ion permeability and selectivity of claudinisoformsa
| Cation-selective claudins | |
|---|---|
| Predominantly cation pore-forming | |
| Claudin-2 | (41, 45, 46) |
| Claudin-10b | (72) |
| Claudin-16b | (10, 83, 114)(42) |
|
| |
| Predominantly anion barrier-forming | |
|
| |
| Claudin-7b | (11, 101) |
| Claudin-19b | (28) |
|
| |
| Potential to form cation pore or anion barrier | |
|
| |
| Claudin-15 | (47, 115-117) |
|
| |
| Anion-selective claudins | |
|
| |
| Predominantly anion pore-forming | |
|
| |
| Claudin-7b | (67) |
| Claudin-10a | (72) |
| Claudin-17 | (74) |
|
| |
| Predominantly cation barrier-forming | |
|
| |
| Claudin-1 | (33, 35) |
| Claudin-3 | (118) |
| Claudin-5 | (36) |
| Claudin-6 | (38) |
| Claudin-9 | (38) |
| Claudin-14 | (119) |
| Claudin-18-2 | (120) |
| Claudin-19b | (40) |
|
| |
| Potential to form anion pore or cation barrier | |
|
| |
| Claudin-4 | (34, 67, 102) |
| Claudin-8 | (40, 44) |
| Claudin-11 | (119) |
Based on in vitro overexpression or knockdown studies in cultured cell lines. We assume that permeability and selectivity are properties intrinsic to individual claudin isoforms, and ignore the possible confounding effect of heteromeric interactions between isoforms. Pore-forming claudins refer to those that predominantly decrease TER or increase solute permeability, while barrier-forming claudins refer to those that predominantly increase TER or decrease solute permeability. The distinction is somewhat arbitrary since most claudins probably have some finite permeability to most solutes, and the observable phenotype is highly dependent on the properties of the host cell line ((40, 119)). References are listed in the right column.
The properties of claudin-7, -16 and -19 are somewhat controversial. Published studies disagree on their permeability and selectivity in ways that are not easily reconciled.
The molecular basis for paracellular charge selectivity is now known to be encoded in the ECL1 of claudins. This was shown in 2003 by Colegio et al. in a series of elegant molecular chimera studies, in which the ECL1 of claudins 2 and 4 were swapped (9). Furthermore, Van Itallie et al. found a correlation between the net charge of all residues in the second half of the ECL1 and the charge selectivity of the claudin (39). Claudins with a more negative ECL1 (claudin 2, claudin 15, claudin 10a) seem to be cation-selective while claudins with a net positively charged ECL1 (claudin 10b, claudin 17) are anion-selective (Figure 3) (7).
Figure 3.
Alignment of the first extracellular loop of claudins. The predicted amino acid residues that comprise the first extracellular loop of human claudins 1-27 were obtained using the TMHMM2 program. These sequences were then aligned using a pairwise alignment algorithm (Clustalw2). Residues are color coded by their side-chain composition and properties as follows. Acidic, red; basic, dark blue; amido, light blue; hydroxyl, pink; hydrophobic, grey; aromatic, orange; sulfur, yellow; proline, green.
Consistent with the idea that electrostatic potential in the ECL1 determines charge selectivity of ion permeation, substituting certain charged residues in the ECL1 with oppositely charged residues can reverse the charge selectivity of the claudin (47). For example, substituting a negative amino acid for the positive amino acid at position 65 in claudin-4 increased its permeability to Na+ (47). Conversely, mutating an aspartic acid at the homologous position in claudin-2 to neutralize its charge markedly reduced its cation selectivity (46). These studies indicate that the charge selectivity of claudins is regulated by the electrostatic interaction between partially dehydrated permeating ions and discrete charged pore-lining residues.
Angelow et al. (48) have gone on to identify additional pore-lining residues by mutating ECL1 amino acid residues to cysteines and then testing the accessibility of these mutated residues to sulfhydryl-reactive reagents, like methanethiosulfonates (MTS). Sites that were accessible to covalent modification by MTS and consequent block of the paracellular pore were then categorized as being pore-lining residues (48).
Size selectivity of claudins
An early study by van Os et al. in 1974 (49) showed that rabbit gall bladder epithelium has a large paracellular permeability to small electrolytes and hydrophilic molecules up to 8 Å in diameter (the size of glycerol). In addition, they also observed a smaller but significant flux of very large uncharged solutes, such as dextran and inulin. Since then, paracellular pathways have been shown to also allow passage of polyethylene glycol tracers (PEGs) of varying diameters up to 15 Å (50) (51). These studies suggest that there are two distinct paracellular pathways: (a) a high capacity, size-selective pore pathway that allows passage of small uncharged solutes (up to 8-9 Å in diameter), and (b) a non-selective leak pathway that allows a small flux of large solutes. Claudins mediate the size-selective pore pathway. Van Itallie et al. (51) measured the paracellular flux of PEGs (50) and showed that changes in the expression of claudin-2 altered the permeability of a size-selective pore with an apparent diameter of 8 Å but did not have any effect on the flux of larger molecules (51) (Figure 4). Similarly, Yu et al. measured permeability to a series of organic cation probes and showed that claudin-2 functioned as a size-selective cation pore of about 6.5-7 Å in diameter (46).
Figure 4.
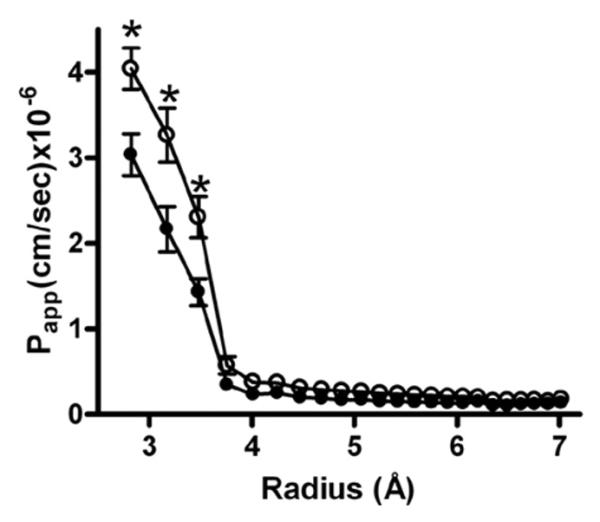
Size selectivity of claudins. Claudin-2 was inducibly overexpressed in MDCK-II cells and the relative permeability to polyethylene glycols of different diameters in uninduced (solid circles) and induced ( open circles) cell monolayers was compared. Overexpression of claudin-2 in MDCK-II cells increased paracellular flux of smaller PEGs ( radius < 3.5 Å) but not of larger PEGs. This suggests that claudins form a size-selective barrier in epithelial monolayers. Modified with permission from Van Itallie et al. (51).
Role of background in phenotype of claudin
The study of claudin function and phenotype has primarily been carried out by over-expression studies where exogenous claudins are expressed in cell lines or by RNA interference (RNAi) studies in which endogenously expressed claudins are knocked down. In such studies, the apparent phenotype of the claudins has been found to be dependent on the background of the cell lines in which they are expressed (39). For example, when pore-forming claudin-2 was over-expressed in low-resistance MDCK-II cells (9), there was a small increase in TER, but when it was over-expressed in high resistance MDCK-I cells it caused a large decrease in TER (41). This has also been observed with charge selectivity of claudins. In the anion-selective LLC-PK1 cells, over-expression of the anion-selective claudin-4 had no effect on paracellular permeability while claudin-2 (cation-selective claudin) over-expression reversed the charge selectivity, making the paracellular barrier more cation-selective. Conversely, over-expression of claudin-4 in the cation-permeable MDCK-II cell line significantly decreased PNa and made it less cation-selective (9).
LOCALIZATION, FUNCTION AND REGULATION OF RENAL CLAUDINS
A variety of claudins are expressed in the kidney. Here we focus on those that are expressed along the nephron (listed in Table 2).
Table 2.
Localization of claudins in the mammalian nephron
| Nephron segment | Claudin(s) | References |
|---|---|---|
| Glomerular parietal epithelium | 1 | (53, 54) |
| Podocytes | 5, 6 | (60)(61) |
| Proximal tubule | 2, 10a, 17 | (53)(43)(42, 74) |
| Thin descending limb of Henle’s loop |
2, 7, 8 | (43, 44) |
| Thin ascending limb of Henle’s loop |
3, 4, 10, 16, 17, 18, 19 |
(6, 40, 53, 74) |
| Thick ascending limb of Henle’s loop |
3, 10, 14, 16, 18, 19 | (2, 6, 31, 40, 53, 82) |
| Distal convoluted tubule | 3, 7, 8 | (44, 53, 101) |
| Collecting duct | 3, 4, 7, 8, 18 | (31, 44, 53, 101, 102) |
a. Glomerulus
The parietal epithelium that lines Bowman’s capsule acts as a barrier to macromolecules (52). In glomerulonephritis, this barrier is disrupted and macromolecules (and presumably albumin) that gain access to Bowman’s space are able to leak into the space between the parietal epithelium and the basement membrane as well as into the extraglomerular space. Claudin-1 is expressed uniquely at the tight junction of parietal epithelial cells (53, 54). In vitro, claudin-1 functions as a barrier to ion conductance and to permeation of 4-40 kDa dextran (33, 35), suggesting that it may be responsible for the barrier function of Bowman’s capsule.
In the adult glomerulus, visceral epithelial cells (podocytes) form a specialized intercellular junction, the slit diaphragm. True tight junctions do form between immature podocytes in the fetal glomerulus and are of uncertain functional significance (55, 56). These disappear during development, but can reappear during nephrotic states, coincident with effacement of the foot processes and obliteration of the slit pore (57-59). Claudin-5 is expressed throughout the plasma membrane of podocytes (60), while claudin-6 is found at the basal membrane, and the base of the slit diaphragm (61). In experimental nephrosis induced by puromycin, claudin-6 is upregulated and concentrated, together with claudin-5, at the newly formed tight junctions between podocytes, but their functions are unknown.
b. Proximal tubule
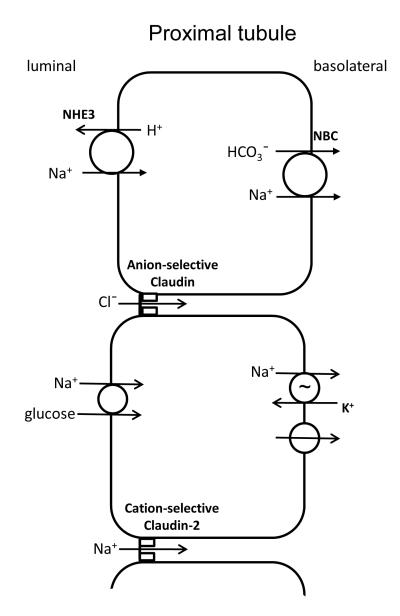
The adult proximal tubule is a leaky epithelium that is known to reabsorb up to two-thirds of the filtered Cl− load (62) as well as two-thirds of the volume of the ultrafiltrate (63). Almost half of the NaCl is reabsorbed through paracellular pathways (64). The early proximal tubule reabsorbs Na+ and HCO3− preferentially over Cl− (65). This makes the peritubular fluid delivered to the mid-late proximal tubule lower in bicarbonate and higher in Cl− concentration than the plasma (65). Here, the paracellular pathway is preferentially permeable to Cl− which is reabsorbed by passive diffusion down its concentration gradient, and in turn generates a lumen-positive potential (66). This electrical gradient then drives paracellular reabsorption of Na+ (Figure 5).
Figure 5.
Sodium, chloride, and fluid reabsorption in the proximal tubule. In the initial portion of the proximal tubule there is reabsorption of both Na+ and bicarbonate via the Na+/H+ exchanger on the apical membrane and a Na+-bicarbonate co-transporter on the basolateral membrane. This increases the Cl− concentration in late proximal luminal fluid, driving passive paracellular Cl− reabsorption down its chemical gradient, through as-yet unidentified anion-selective claudin(s). Paracellular Cl− reabsorption then creates a slightly lumen-positive voltage which then drives the claudin-2-mediated reabsorption of Na+. With the reabsorption of salt, amino acids and glucose occurring in the proximal tubule, there is a significant osmotic gratdient generated that drives reabsorption of water, mostly transcellularly through aquaporin-1, but also in part paracellularly via claudin-2.
Claudin-2, which has been shown to act as a paracellular cation pore (41, 45, 46) (67), is highly expressed in the proximal tubule as well as the upper segment of the thin descending limb of long loops of Henle, with the levels of expression showing an axial increase (43, 53). Claudin-2 has also been shown to be able to transport K+ and Ca2+ (46). The properties of claudin-2 make it an excellent candidate for the cation-reabsorbing paracellular pore in the proximal tubule. This was confirmed in 2010 by Muto et al (68). They generated a claudin-2 knockout mouse which had decreased cation permeability and reduced NaCl and water reabsorption when measured in isolated perfused proximal tubules. In whole animal balance studies, the fractional excretions of Na+ and Cl− were comparable to the values for wild-type mice under normal conditions. However, they were significantly elevated when mice were placed on a high salt diet (68). These mice did not have any overt change in K+ metabolism but they were hypercalciuric, raising the possibility that claudin-2 also mediates Ca2+ reabsorption.
Another important function of the proximal tubule is the reabsorption of water. The osmotic gradient established by the reabsorption of salt is thought to drive water transport, much of which occurs through aquaporin-1 (69). Rosenthal et al. (70) measured transepithelial water flux in cells over-expressing claudin-2 and found that claudin-2 increased water reabsorption driven either by an osmotic gradient or a NaCl concentration gradient (70). These data suggest that claudin-2 can mediate water transport and could account for that part of water reabsorption in the proximal tubules that is not mediated by aquaporin-1 (70, 71).
In addition to claudin-2, proximal tubules have also been shown to express claudins 10a (42, 72) (68), 12 (73) and 17 (74). Claudins 10a and 17 (74) can function as anion-selective pores, and could potentially be responsible for paracellular Cl-reabsorption in the proximal tubule. In intestinal epithelium, claudin-12 is upregulated by vitamin D and functions as a Ca2+-selective pore (75). It is interesting to speculate that it may play a similar role, perhaps in concert with claudin-2, in Ca2+ reabsorption in the proximal tubule.
c. Thick ascending limb of Henle
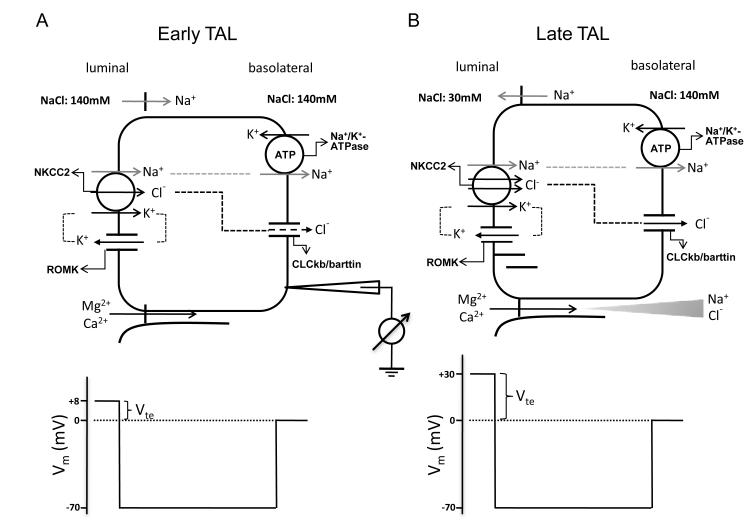
The thick ascending limb (TAL) of the loop of Henle is responsible for reabsorbing 25–40% of filtered Na+ (76), 50–60% of filtered Mg2+ (77) and 30–35% of filtered Ca2+ (78). The TAL actively transports Na+ and Cl− via the transcellular route, and provides a paracellular pathway for the selective absorption of Mg2+ and Ca2+ (79, 80). Na+, K+ and Cl− enter the cell through the Na+-K+-2Cl− cotransporter (NKCC2) in the luminal membrane. Na+ exits the cell through the Na+/K+-ATPase, in exchange for K+ entry. K+ is secreted into the lumen through the renal outer medullary potassium channel. Cl− leaves the cell through a basolateral Cl− channel. Mg2+ and Ca2+ are passively reabsorbed through the paracellular pathway, driven by a lumen-positive transepithelial voltage (Vte). The generation of this lumen-positive Vte can be attributed to two mechanisms: (I) the active transport Vte due to apical K+ secretion and basolateral Cl− exit; and (II) the diffusion Vte generated because of the transepithelial NaCl concentration gradient and the cation selectivity of the paracellular pathway of TAL (81). This second component is therefore dependent on the high permeability ratio between Na and Cl (PNa/PCl). Both components are present in parallel along the TAL (Figure 6); however, their contributions vary at different parts of TAL. At the beginning of the TAL segment, it is the first mechanism that provides a voltage around +8 mV. There is minimal contribution of diffusion potential at this stage since the concentration gradient has not yet been built up (Figure 6A). With continuous NaCl reabsorption along the axis of the TAL, the lumen fluid is diluted and a large NaCl gradient is generated at the end of the TAL. Because the paracellular permeability of the TAL is cation-selective, the diffusion Vte is superimposed onto the active transport Vte and becomes the major source of the lumen-positive Vte, which now increases substantially - estimated to be over +30 mV (Figure 6B).
Figure 6.
Transepithelial ion transport in the thick ascending limb. (A) When similar salt concentrations are present at the luminal and basolateral sides, the luminal spontaneous potential Vte of +8mV is generated by the concerted action of luminal K+ channels, basolateral Cl− channels, the Na+2Cl−K+ cotransporter, and the Na+,K+-ATPase. Vte drives Na+ absorption through the paracellular pathway. (B) When a dilute luminal fluid is present after NaCl absorption along the water-tight TAL, the luminal potential Vte of +30mV is now generated as a diffusion voltage by the ‘backleak’ of Na+. The diffusion voltage depends on the permselectivity of the tight junction. The membrane voltage (Vm) trace depicts the virtual measurement by an electrode that is pushed from the basolateral side through the cell to the luminal side. Modified from Figure 1 in Hou and Goodenough (2009).
The molecular basis for TAL divalent cation reabsorption remained unknown until the discovery of mutations in claudin-16 and -19 as the cause of the rare autosomal recessive renal disorder, FHHNC, either without ocular involvement (OMIM#248250) (2) or with ocular involvement (OMIM#248190) (82), respectively. FHHNC is characterized by renal wasting of Mg2+ and Ca2+, and is thought to be due to a defect in the TALH. Thus, the identification of claudin-16 and 19 as the culprit genes implicated them in the mechanism for paracellular divalent cation transport in this nephron segment.
It was initially hypothesized that claudin-16 might form the selective paracellular Mg2+ and Ca2+ channel itself, and this was tested in a number of in vitro studies. Ikari et al (24) transfected the low-resistance MDCK II cells with claudin-16 and reported increased Ca2+ permeability that appeared to be unidirectional. Kausalya et al. (83) transfected the high-resistance MDCK-C7 cells but only found a modest increase in transepithelial Mg2+ flux. However, Hou et al (10) transfected a model cell line, LLC-PK1, that has low endogenous paracellular cation permeability and found that claudin-16 profoundly increased transepithelial Na+ permeability (PNa) accompanied by an only moderately enhanced Mg2+ permeability (PMg). PNa was greatly reduced or completely disappeared in all FHHNC disease mutants of claudin-16, suggesting that changes in Na+ permeability, rather than Ca2+ or Mg2+ permeability, might be pathogenic for this disease. Claudin-16 deficient knockdown (KD) mice showed significantly reduced plasma Mg2+ levels and increased urinary excretion of Mg2+ and Ca2+, and nephrocalcinosis (84). This phenotype was very similar to FHHNC, suggesting that FHHNC might be due to loss of claudin-16 function. Consistent with the hypothesis that claudin-16 acts primarily as a Na+ permeability pathway, when TAL segments were isolated and perfused ex vivo, the paracellular ion selectivity (PNa/PCl) of the TAL was significantly reduced in claudin-16 KD mice (84). Since the component of the lumen-positive Vte due to the NaCl diffusion potential (mechanism II) is dependent on a high PNa/PCl, this suggests that claudin-16 facilitates TAL Ca2+ transport by increasing PNa and hence increasing the electrical driving force for paracellular divalent cation reabsorption.
Some of the mutations in claudin-16 cause FHHNC due to protein trafficking defects (83, 85). This is important because pharmacological chaperones could rescue some of these trafficking defects in claudin-16 (83), suggesting a potential therapeutic approach to this disease.
The other main cause of FHHNC is mutations in claudin-19. Hou et al (28) found that claudin-19 profoundly decreased the Cl− permeability (PCl) and functioned as a Cl− barrier when expressed in LLC-PK1 cells. The FHHNC mutations from human patients either partially or completely abolished the claudin-19 effects on PCl. Coexpression of claudin-16 and claudin-19 in LLC-PK1 cells resulted in the simultaneous upregulation of PNa and downregulation of PCl, and hence a large increase in PNa/PCl, generating a highly cation-selective paracellular pathway (28). Thus, it was hypothesized that claudin-16 and -19 work together in the TAL to facilitate the generation of the NaCl diffusion potential.
Claudin-19 KD animals phenocopied claudin-16 KD and develop the FHHNC manifestations of reduced plasma Mg++ levels and excessive renal wasting of Mg2+ and Ca2+ (31). The phenotypic similarities of claudin-19 KD with claudin-16 KD can be explained by the cis heteromeric interaction between the two claudins (28). Several FHHNC mutations in claudin-16 and claudin-19 were shown to disrupt this interaction, and also abolished their cation selectivity when coexpressed in LLC-PK1 cells, suggesting a role for claudin interaction in the development of FHHNC (28).
High extracellular Ca2+ is known to inhibit Ca2+ and Mg2+ reabsorption in the TAL, thus providing a homeostatic mechanism to excrete excess Ca2+. This is mediated by a Ca2+-sensing receptor (CaSR) in the TAL (86). CaSR activation works in part via the transcellular pathway to inhibit the apical NKCC2 transporter (87, 88) and the ROMK channel (89), but there is also evidence that activation of the CaSR can directly inhibit paracellular Ca2+ permeability in the TAL (90, 91). Interestingly, Motoyama noted that activation of the CaSR reduced the NaCl diffusion potential in TAL perfused with an asymmetric salt solution, suggesting that the driving force for Ca2+ reabsorption was also reduced (91), although the molecular basis was not known at the time.
A recent genome-wide association study identified claudin-14 as a major risk gene for hypercalciuric nephrolithiasis, making it another candidate protein involved in TAL divalent cation transport (92). The role of claudin-14 has now been investigated by Gong et al. (93). The renal localization of claudin-14 was found predominantly in the TAL (93), and to a lesser extent in the proximal tubules (94). Claudin-14 proteins were observed to interact with claudin-16 but not claudin-19. In transfected LLC-PK1 cells, claudin-14 diminished the cation permeability of the claudin-16 channel (93). This suggested that claudin-14 might physiologically bind to the claudin-16/19 complex to act as a negative regulator of divalent cation reabsorption in the TAL.
Consistent with this, claudin-14 knockout mice developed hypermagnesemia, hypomagnesiuria and hypocalciuria on a high Ca2+ diet (93), exactly the opposite phenotype to that of claudin-16 KD mice (84). The mechanism by which Ca2+ regulates claudin-14 was found to be through a microRNA-based suppression of claudin-14 gene expression. The microRNA molecules, miR-9 and miR-374, recognize partially complementary binding sites located in 3′-UTRs of claudin-14 transcript, suppress its protein translation and induce its mRNA decay. Extracellular Ca2+, via activation of the CaSR, reduced expression of these microRNAs, thereby activating claudin-14 and inhibiting claudin-16/19-facilitated divalent cation reabsorption.
d. Collecting duct
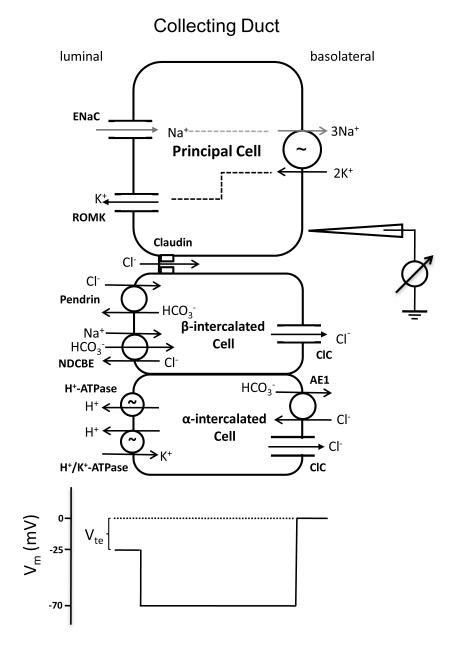
The aldosterone-sensitive distal nephron (ASDN) is responsible for the reabsorption of 2-3% filtered NaCl, and plays a vital role in the regulation of extracellular fluid volume and blood pressure control (95, 96). Na+ reabsorption in the collecting duct is driven by basolateral efflux through the Na+/K+-ATPase and apical entry through the epithelial sodium channel (ENaC) in the principal cell (97) and is responsible for generating the lumen-negative transepithelial potential. This creates the electrical driving force for reabsorption Cl−, which can be transported by both paracellular and transcellular pathways. Collecting ducts are known to have a significant passive Cl− conductance that is presumed to be paracellular (98-100). Defects in paracellular Cl– shunt would be expected to increase the magnitude of Vte, depolarize the luminal membrane, and consequently inhibit ENaC.
Several claudins are expressed in the collecting duct, including claudin-3, -4, -7 and -8 (53) (44, 101). The first collecting duct claudin to be investigated functionally was claudin-4, which was transfected into MDCK II cells and decreased PNa (34). Because MDCK II cells are quite leaky and express a high level of claudin-2, they may have been a poor model for the collecting duct. Hou et al (102) used two collecting duct cell lines, M-1 and mIMCD3, to study claudin-4 function. They showed that knockdown of claudin-4 significantly decreased paracellular anion permeabilities, including that of Cl−, in the collecting duct cells. Furthermore, aldosterone was shown to phosphorylate claudin-4 and increase paracellular Cl− conductance in cultured rat cortical collecting duct cells (103). Thus, claudin-4 provides a potential molecular mechanism for coupling Cl− transport to Na+ reabsorption in the collecting duct both at baseline and in response to aldosterone stimulation.
Claudin-8 also plays a potentially important role in collecting duct Cl− permeability. In both M-1 and mIMCD3 cells, claudin-8 KD was found to decrease the Cl− permeability (PCl) to a similar level as in claudin-4 KD (104). The observed claudin-8 effects depended upon its cis interaction with claudin-4. Claudin-8 was shown to interact with claudin-4 in cell membranes by several criteria: (i) a positive yeast two-hybrid assay; (ii) coimmunoprecipitation in epithelial cells; (iii) co-trafficking and co-localization in epithelial cells; and (iv) claudin-8 recruitment of claudin-4 to TJs (104). Without claudin-8, claudin-4 was confined to the endoplasmic reticulum and the Golgi apparatus, suggesting that their interaction is required for claudin-4 protein to pass the endoplasmic reticulum quality control checkpoint (104).
One of the unanswered questions regarding claudin-8 function in collecting duct function is the nature of the paracellular channel formed by claudin-8. Removal of claudin-8 depleted both claudin-4 and claudin-8 at the level of the TJ, but the reduction in PCl was no greater than with deletion of claudin-4 alone (104). This suggested that claudin-8 does not form an additional Cl− channel by itself. Consistent with this, when expressed alone in MDCK cells, claudin-8 reduced PNa without affecting PCl (105). Aldosterone was shown to upregulate the transcription of claudin-8 in the distal colon (106). If claudin-8 gene expression is regulated similarly in ASDN as in the distal colon, aldosterone would be expected to upregulate claudin-8 and increase paracellular Cl− conductance.
To determine the role of claudin-3 and claudin-7, Hou et al (104) knocked down their expression in the collecting duct M-1 and mIMCD3 cells. While claudin-3 KD showed no significant effect on PNa or PCl, claudin-7 KD resulted in a 30% decrease in TER with no significant change in ion selectivity. This suggested that claudin-7 acts as a non-selective ion barrier in the collecting duct. Consistent with this, claudin-7 knockout mice exhibited severe renal wasting of Na+, K+, Cl−, and water, accompanied by a surge of aldosterone synthesis, and died within 12 days after birth. The loss of Na+ and Cl− is consistent with the loss of a non-selective paracellular ion barrier leading to backleak of reabsorbed NaCl in the collecting duct. The consequent volume loss would be expected to lead to secondary hyperaldosteronism, which might be the cause of the K+ wasting. The TJ localization of claudin-4 or claudin-8 was not affected in claudin-7 knockout mice, ruling out the possibility that claudin-7 is part of the paracellular Cl− channel complex.
A potential role for a paracellular Cl− channel in the distal nephron in blood pressure regulation has been implicated by several studies. WNK1 and WNK4, two genes associated with the inherited hypertensive disorder, PHAII, have been suggested to phosphorylate claudins 1-4 and/or 7 and to induce a concomitant increase of PCl in cultured epithelial cells (23, 107-109). Mutations that cause PHAII appeared to augment PCl and so would be predicted to increase collecting duct NaCl reabsorption and reduce Vte, leading to hypertension and hyperkalemia. However, another study did not find any effect of WNK4 on claudin-4 phosphorylation (110). Furthermore, transgenic mice harboring the PHAII mutations in WNK4 revealed no difference in the paracellular Cl− permeability of the collecting duct (111). Thus, the validity of the hypothesis that WNK kinases regulate paracellular Cl- permeability is currently uncertain.
CLAUDINS AND KIDNEY DISEASE
Claudins play potentially important pathogenic roles in kidney diseases. As described above, mutations in claudin-16 and -19 cause FHHNC (2, 82), while polymorphisms in claudin-14 have been strongly associated with the development of kidney stones and low bone mineral density, suggesting that they are associated with hypercalciuria (82). Regulation of claudin-mediated Cl- permeability in the ASDN may contribute to the pathogenesis of PHAII (23, 107-109). In polycystic kidney disease, cyst growth is due in part to secretion of fluid into cyst lumens, driven by transcellular Cl− secretion (112). It is predicted that the tight junction in cyst epithelia will be relatively impermeable to Cl− and highly permeable to Na+. Claudin-7 has been found to be disproportionately expressed in autosomal dominant polycystic kidney disease cyst epithelia (113). The role of claudins in other kidney disorders remains largely unexplored.
SUMMARY POINTS.
Claudins constitute a family of at least 26 genes that encode tight junction membrane proteins with four transmembrane domains.
Claudins interact with each other both in the same cell (in cis) and across adjacent cells (in trans) to form tight junction strands.
The first extracellular domains of claudins regulate paracellular permeability to small ions of less than ~8 Å diameter, forming both barriers and pores in the tight junction.
Claudins are expressed in a nephron segment-specific manner along the renal tubule, with each cell expressing multiple different claudins.
The complement of claudins in each nephron segment determines its paracellular permeability and hence ability to reabsorb or secrete solutes and water.
Mutations, polymorphisms and acquired abnormalities in renal claudins contribute to the pathogenesis of familial hypercalciuric hypomagnesemia, kidney stones and polycystic kidney disease.
FUTURE ISSUES.
The tertiary and quaternary structure of claudins remains unknown. Elucidating this will be important to truly understand at the molecular level how claudin molecules interact in cis and in trans, and how pore and barrier structures are formed.
The single channel activity of a paracellular pore has never been visualized. Whether it gates at all, and what the unitary conductance of a claudin might be, is unknown. The ability to patch clamp the paracellular pathway in the future would constitute a major technical advance.
The C-terminal cytoplasmic tail of most claudins contains multiple phosphorylation sites. It remains to be determined what is the physiological function of most of these sites.
There is likely much to be learned about functions of claudins other than regulation of paracellular permeability. For example, many claudins are found along the lateral membrane and likely play roles in cell-matrix interactions.
The role of the more distantly related claudins (21 and above) remain undefined and it is uncertain whether they also regulate paracellular permeability. Likewise, it is unclear if there are other members of the PMP-22/EMP/MP20 superfamily that have claudin-like functions.
It is likely that as reagents for studying claudins (antibodies, genetically modified mouse models) become widely available, claudins will be found to be important in the pathogenesis of a wide variety of kidney and particularly renal tubular diseases.
Finally, no drugs have yet been developed that modulate claudin function and whether claudin proteins are even “druggable” is unknown. This may open up new avenues for treatment of disorders of the renal tubule and of extracellular fluid volume and composition.
WNK (With No Lysine) kinases are a family of proteins that seem to play major roles in orchestrating electrolyte transport in the renal tubule and other epithelia. WNK1 and WNK4 are, along with Kelch-like 3 and cullin-3, mutated in pseudohypoaldosteronism type II or Gordon’s syndrome, an autosomal dominant disorder characterized by hypertension and hyperkalemia and thought to be due to excessive Cl− reabsorption in the distal nephron. A major role for WNK1, 3 and 4 is to form a signaling network that regulates the thiazide-sensitive NaCl cotransporter, NCC. However, WNK3 and WNK4 are specifically localized to the tight junction suggesting the intriguing possibility that they may also regulate paracellular Cl− permeability, either directly, by phosphorylating claudins, or by indirect effects on the tight junction.
Figure 7.
Ion transport mechanism in the collecting duct. The membrane voltage (Vm) trace depicts the virtual measurement by an electrode that is pushed from the basolateral side through the cell to the luminal side. In this example, the basolateral membrane voltage is −70 mV and the luminal membrane voltage is −45 mV, resulting in a transepithelial Vte of −25 mV with respect to the basolateral side. Vte drives Cl− transport through the paracellular channel. Modified from Figure 1 in Hou, J. (93).
Acknowledgements
This work was supported by National Institutes of Health grants R01 DK062283 and U01 GM094627 (A.Y.), and RO1 DK084059 and P30 DK079333 (J.H.).
ACRONYMS AND DEFINITIONS
- FHHNC
Familial hypercalciuric hypomagnesemia with nephrocalcinosis, an autosomal recessive disorder characterized by renal Ca2+ and Mg2+ wasting.
- ECL1
The first extracellular loop of claudins which is thought to be the paracellular pore-lining domain.
- ECL2
The second extracellular loop of claudins, which participates in trans interactions and can potentially bind C. perfringens enterotoxin.
- PDZ binding motif
Motif that binds to PDZ domains, named after the first three proteins (PSD95, Dlg1 and ZO-1) that were discovered to share this domain.
- TER
Transepithelial electrical resistance, a measure of the permeability of an epithelium to small ions.
- MDCK
Madin-Darby Canine Kidney, a renal tubule epithelial cell line commonly used for cell biology and transport studies. Several strains exist with different properties.
- Diffusion potential
Equilibrium voltage generated across an epithelium by bathing in solutions of different ionic composition on apical and basolateral sides.
- TAL
Thick ascending limb of the loop of Henle.
- CaSR
Ca2+ sensing receptor, a G-protein-coupled plasma membrane receptor found in parathyroid gland and kidney.
- ASDN
Aldosterone-sensitive distal nephron, which encompasses the distal convoluted tubule, connecting tubule, and collecting duct.
- PHAII
Pseudohypoaldosteronism type II, also known as Gordon’s syndrome, a disorder characterized by hypertension and hyperkalemia.
Literature Cited
- 1.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin- 1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–6. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 3.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–12. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 6.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 7.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Kearsey J, Petit S, De Oliveira C, Schweighoffer F. A novel four transmembrane spanning protein, CLP24. A hypoxically regulated cell junction protein. Eur J Biochem. 2004;271:2584–92. doi: 10.1111/j.1432-1033.2004.04186.x. [DOI] [PubMed] [Google Scholar]
- 9.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346–54. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–18. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 11.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 12.Cukierman L, Meertens L, Bertaux C, Kajumo F, Dragic T. Residues in a highly conserved claudin-1 motif are required for hepatitis C virus entry and mediate the formation of cell-cell contacts. J Virol. 2009;83:5477–84. doi: 10.1128/JVI.02262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. Faseb J. 2008;22:146–58. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 14.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–61. doi: 10.1016/s0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 15.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–61. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 16.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–63. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi J, Inai T, Shibata Y. Formation of tight junction strands by expression of claudin-1 mutants in their ZO-1 binding site in MDCK cells. Histochem Cell Biol. 2002;117:29–39. doi: 10.1007/s00418-001-0359-x. [DOI] [PubMed] [Google Scholar]
- 18.Van Itallie CM, Anderson JM. The molecular physiology of tight junction pores. Physiology (Bethesda) 2004;19:331–8. doi: 10.1152/physiol.00027.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol. 2004;83:135–44. doi: 10.1078/0171-9335-00366. [DOI] [PubMed] [Google Scholar]
- 20.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol. 2004;199:29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Yeh S, Appleton BA, Held HA, Kausalya PJ, Phua DC, Wong WL, Lasky LA, Wiesmann C, Hunziker W, Sidhu SS. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J Biol Chem. 2006;281:22299–311. doi: 10.1074/jbc.M602902200. [DOI] [PubMed] [Google Scholar]
- 22.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci. 2005;118:1427–36. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci U S A. 2004;101:4690–4. doi: 10.1073/pnas.0306924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci. 2006;119:1781–9. doi: 10.1242/jcs.02901. [DOI] [PubMed] [Google Scholar]
- 25.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;12:218–27. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem. 2011;286:3442–50. doi: 10.1074/jbc.M110.195578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–28. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63:505–14. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem. 2007;282:30005–13. doi: 10.1074/jbc.M703547200. [DOI] [PubMed] [Google Scholar]
- 31.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–5. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–78. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- 33.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol. 1999;78:849–55. doi: 10.1016/S0171-9335(99)80086-7. [DOI] [PubMed] [Google Scholar]
- 34.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–27. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci. 2000;113:3387–98. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 36.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol. 2004;24:8408–17. doi: 10.1128/MCB.24.19.8408-8417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–9. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 38.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1713–9. doi: 10.1152/ajpregu.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 40.Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol. 2007;293:F166–77. doi: 10.1152/ajprenal.00087.2007. [DOI] [PubMed] [Google Scholar]
- 41.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of Zonulae Occludentes from Tight to Leaky Strand Type by Introducing Claudin-2 into Madin-Darby Canine Kidney I Cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci. 2009;122:1507–17. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- 43.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281:F966–74. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- 44.Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol. 2004;286:F1063–71. doi: 10.1152/ajprenal.00384.2003. [DOI] [PubMed] [Google Scholar]
- 45.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 46.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–27. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–7. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- 48.Angelow S, Yu AS. Cysteine mutagenesis to study the structure of claudin-2 paracellular pores. Ann N Y Acad Sci. 2009;1165:143–7. doi: 10.1111/j.1749-6632.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- 49.van Os CH, de Jong MD, Slegers JF. Dimensions of polar pathways through rabbit gallbladder epithelium. The effect of phloretin on nonelectrolyte permeability. J Membr Biol. 1974;15:363–82. doi: 10.1007/BF01870095. [DOI] [PubMed] [Google Scholar]
- 50.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol. 2001;281:C388–97. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- 51.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 52.Ohse T, Chang AM, Pippin JW, Jarad G, Hudkins KL, Alpers CE, Miner JH, Shankland SJ. A new function for parietal epithelial cells: a second glomerular barrier. Am J Physiol Renal Physiol. 2009;297:F1566–74. doi: 10.1152/ajprenal.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–86. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 54.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol. 2008;19:1879–90. doi: 10.1681/ASN.2007101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humbert F, Montesano R, Perrelet A, Orci L. Junctions in developing human and rat kidney: a freeze-fracture study. J Ultrastruct Res. 1976;56:202–14. doi: 10.1016/s0022-5320(76)80166-9. [DOI] [PubMed] [Google Scholar]
- 56.Reeves W, Caulfield JP, Farquhar MG. Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest. 1978;39:90–100. [PubMed] [Google Scholar]
- 57.Caulfield JP, Reid JJ, Farquhar MG. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976;34:43–59. [PubMed] [Google Scholar]
- 58.Ryan GB, Leventhal M, Karnovsky MJ. A freeze-fracture study of the junctions between glomerular epithelial cells in aminonucleoside nephrosis. Lab Invest. 1975;32:397–403. [PubMed] [Google Scholar]
- 59.Pricam C, Humbert F, Perrelet A, Amherdt M, Orci L. Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest. 1975;33:209–18. [PubMed] [Google Scholar]
- 60.Koda R, Zhao L, Yaoita E, Yoshida Y, Tsukita S, Tamura A, Nameta M, Zhang Y, Fujinaka H, Magdeldin S, Xu B, Narita I, Yamamoto T. Novel expression of claudin-5 in glomerular podocytes. Cell Tissue Res. 2011;343:637–48. doi: 10.1007/s00441-010-1117-y. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T. Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00862.2007. [DOI] [PubMed] [Google Scholar]
- 62.Aronson PS, Giebisch G. Mechanisms of chloride transport in the proximal tubule. Am J Physiol. 1997;273:F179–92. doi: 10.1152/ajprenal.1997.273.2.F179. [DOI] [PubMed] [Google Scholar]
- 63.Rector FC., Jr. Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–71. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 64.Alpern RJ, Howlin KJ, Preisig PA. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985;76:1360–6. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu FY, Cogan MG. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol. 1984;247:F816–21. doi: 10.1152/ajprenal.1984.247.5.F816. [DOI] [PubMed] [Google Scholar]
- 66.Green R, Giebisch G. Reflection coefficients and water permeability in rat proximal tubule. Am J Physiol. 1989;257:F658–68. doi: 10.1152/ajprenal.1989.257.4.F658. [DOI] [PubMed] [Google Scholar]
- 67.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–23. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- 68.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci U S A. 2010;107:8011–6. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallon V, Verkman AS, Schnermann J. Luminal hypotonicity in proximal tubules of aquaporin-1-knockout mice. Am J Physiol Renal Physiol. 2000;278:F1030–3. doi: 10.1152/ajprenal.2000.278.6.F1030. [DOI] [PubMed] [Google Scholar]
- 70.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–21. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 71.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci U S A. 1998;95:9660–4. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol. 2006;291:F1288–99. doi: 10.1152/ajprenal.00138.2006. [DOI] [PubMed] [Google Scholar]
- 73.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol. 2006;291:F1132–41. doi: 10.1152/ajprenal.00063.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-0949-x. First analysis of claudin-17 suggests it may be the key paracellular anion channel in the proximal tubule.
- 75.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 2008;19:1912–21. doi: 10.1091/mbc.E07-09-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giebisch G, Klose RM, Windhager EE. MICROPUNCTURE STUDY OF HYPERTONIC SODIUM CHLORIDE LOADING IN THE RAT. Am J Physiol. 1964;206:687–93. doi: 10.1152/ajplegacy.1964.206.4.687. [DOI] [PubMed] [Google Scholar]
- 77.Cole DE, Quamme GA. Inherited disorders of renal magnesium handling. J Am Soc Nephrol. 2000;11:1937–47. doi: 10.1681/ASN.V11101937. [DOI] [PubMed] [Google Scholar]
- 78.Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int. 2004;(Suppl):S28–33. doi: 10.1111/j.1523-1755.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 79.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol. 1981;241:F412–31. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 80.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol. 1981;241:F432–42. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- 81.Greger R. Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev. 1985;65:760–97. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 82.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the Tight-Junction Gene Claudin 19 (CLDN19) Are Associated with Renal Magnesium Wasting, Renal Failure, and Severe Ocular Involvement. Am J Hum Genet. 2006;79:949–57. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–91. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–22. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 85.Muller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol. 2010;298:F485–99. doi: 10.1152/ajprenal.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Jesus Ferreira MC, Bailly C. Extracellular Ca2+ decreases chloride reabsorption in rat CTAL by inhibiting cAMP pathway. Am J Physiol. 1998;275:F198–203. doi: 10.1152/ajprenal.1998.275.2.F198. [DOI] [PubMed] [Google Scholar]
- 88.Gamba G, Friedman PA. Thick ascending limb: the Na(+):K (+):2Cl (−) cotransporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch. 2009;458:61–76. doi: 10.1007/s00424-008-0607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W, Lu M, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol. 1997;273:F421–9. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 90.Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, Di Stefano A. Calcium-sensing receptor: regulation of electrolyte transport in the thick ascending limb of Henle’s loop. Kidney Blood Press Res. 1998;21:401–12. doi: 10.1159/000025892. [DOI] [PubMed] [Google Scholar]
- 91.Motoyama HI, Friedman PA. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol. 2002;283:F399–406. doi: 10.1152/ajprenal.00346.2001. [DOI] [PubMed] [Google Scholar]
- 92.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41:926–30. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 93.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca(++) transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. Demonstrated feedback mechanism by which Ca2+ overload signals Ca excretion via regulation of claudin-14.
- 94.Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–38. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252:1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 96.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 97.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–7. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 98.O’Neil RG, Boulpaep EL. Ionic conductive properties and electrophysiology of the rabbit cortical collecting tubule. Am J Physiol. 1982;243:F81–95. doi: 10.1152/ajprenal.1982.243.1.F81. [DOI] [PubMed] [Google Scholar]
- 99.O’Neil RG, Sansom SC. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol. 1984;82:281–95. doi: 10.1007/BF01871637. [DOI] [PubMed] [Google Scholar]
- 100.Sansom SC, Weinman EJ, O’Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984;247:F291–302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- 101.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–93. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 102.Hou J, Goodenough DA. Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens. 2010;19:483–8. doi: 10.1097/MNH.0b013e32833b7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289:C1513–21. doi: 10.1152/ajpcell.00314.2005. [DOI] [PubMed] [Google Scholar]
- 104.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–5. doi: 10.1073/pnas.1009399107. Delineates role for claudin-4 and -8 as chloride channels in collecting duct.
- 105.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in MDCK cells augments the paracellular barrier to cation permeation. J Biol Chem. 2003;278:17350–9. doi: 10.1074/jbc.M213286200. [DOI] [PubMed] [Google Scholar]
- 106.Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun. 2009;378:45–50. doi: 10.1016/j.bbrc.2008.10.164. [DOI] [PubMed] [Google Scholar]
- 107.Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci U S A. 2004;101:14877–82. doi: 10.1073/pnas.0406172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohta A, Yang SS, Rai T, Chiga M, Sasaki S, Uchida S. Overexpression of human WNK1 increases paracellular chloride permeability and phosphorylation of claudin-4 in MDCKII cells. Biochem Biophys Res Commun. 2006;349:804–8. doi: 10.1016/j.bbrc.2006.08.101. [DOI] [PubMed] [Google Scholar]
- 109.Tatum R, Zhang Y, Salleng K, Lu Z, Lin JJ, Lu Q, Jeansonne BG, Ding L, Chen YH. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24–34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–44. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiological reviews. 1998;78:1165–91. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- 113.Lanaspa MA, Andres-Hernando A, Rivard CJ, Dai Y, Berl T. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc Natl Acad Sci U S A. 2008;105:15797–802. doi: 10.1073/pnas.0805761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem. 2004;279:54826–32. doi: 10.1074/jbc.M406331200. [DOI] [PubMed] [Google Scholar]
- 115.Van Itallie C, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion selective epithelial lines by expression of different claudins. Am J Physiol Cell Physiol. 2003;286:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 116.Inai T, Kamimura T, Hirose E, Iida H, Shibata Y. The protoplasmic or exoplasmic face association of tight junction particles cannot predict paracellular permeability or heterotypic claudin compatibility. European journal of cell biology. 2010;89:547–56. doi: 10.1016/j.ejcb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–23. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 119.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–61. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 120.Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 121.Hou J. Lecture: New light on the role of claudins in the kidney. Organogenesis. 2012;8 doi: 10.4161/org.19808. [DOI] [PMC free article] [PubMed] [Google Scholar]