Abstract
Macrophage migration inhibitory factor (MIF) is an immunoregulatory cytokine that is produced by different inflammatory and immune cell types. MIF exerts activating responses on many cellular targets, and it is expressed within the rheumatoid synovium as well as in experimental models of disease. Immunoneutralization of MIF decreases joint destruction in the collagen-induced, rat adjuvant-induced, and antigen-induced models of arthritis.
MIF deficient mice were used to induce arthritis by serum transfer from K/BxN mice. Macrophage transfers were done to investigate the specific role of macrophage derived MIF. Arthritis was evaluated by histological analysis, ankle swelling and clinical index and cytokine measurements. The present study was undertaken to investigate the immunopathologic role of MIF in the K/BxN serum-induced model of arthritis, which is critically dependent on innate pathways of joint destruction. We show first that MIF is essential for disease development as both joint inflammation and cartilage destruction are significantly reduced in MIF-deficient (MIF−/−) versus wild-type mice. The adoptive transfer of wild-type macrophages into MIF−/− mice restored the sensitivity of MIF−/− mice to arthritis development, and this affect was associated with a restoration in serum IL-1β and IL-6 production. These results indicate that MIF plays a critical role in inflammation and joint destruction in K/BxN serum-induced arthritis and that the systemic expression of MIF by a subpopulation of macrophages is necessary and sufficient for the full development of arthritis.
Keywords: Macrophage, Fibroblast-like synoviocytes (FLS), Cytokine, Macrophage migration inhibitory factor, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic and debilitating autoimmune disease characterized by inflammation and destruction of synovial joints (1). The affected joints display inflammatory infiltration by neutrophils, macrophages, T cells and dendritic cells, persistent pro-inflammatory cytokine production and complement deposition, the formation of a hyperplastic and invasive pannus, and the erosion and remodeling of cartilage and bone (2). It has been difficult to study the initial stages of disease because individuals are diagnosed only after joint inflammation becomes established. Animal models of arthritis in which disease can be induced in a synchronized fashion thus allow for the investigation of the role of specific cell types and effector molecules to the pathologic steps leading to disease.
The cytokine known as macrophage migration inhibitory factor (MIF) is becoming increasingly recognized for its upstream role in regulating the immune and inflammatory response. While the original description of this mediator focused on its ability to arrest macrophage migration, MIF is now known to be released by diverse cell types including macrophages and to up-regulate activation responses by specific signal transduction and antiapoptotic pathways (3–5). Within the RA synovium, MIF is expressed by macrophages, CD4+ T cells and fibroblast-like synoviocytes (FLS). FLS-derived MIF up-regulates the release of monocyte tumor necrosis factor (TNF-α), suggesting that MIF acts in a paracrine “feed-forward” manner to sustain the pro-inflammatory cytokine network within rheumatoid synovium (6). MIF also activates FLS expression of key pro-inflammatory mediators, such as prostaglandin E2 via induction of phospholipase A2 and cyclooxygenase-2 expression (7), the cytokines IL-1, IL-6 and IL-8, and the matrix metalloproteases involved in cartilage destruction (8–10). Synovial leucocyte trafficking also has been demonstrated to be impaired in the absence of MIF (11).
Mikulowska et al. (12) first explored the role of MIF in joint inflammation by studying murine collagen-induced arthritis: treatment with a neutralizing anti-MIF antibody before immunization with type II collagen led to delayed onset and lowered frequency of arthritis. Leech et al. (13) subsequently reported that anti-MIF monoclonal antibody administration produced a dramatic reduction in clinical and histological parameters of disease in the rat adjuvant arthritis. The progression of antigen-induced arthritis also was profoundly inhibited by immunoneutralization or genetic deficiency of MIF (14). Of note, recent genetic studies in RA patients have shown a significant relationship between high-expression MIF alleles and more severe, erosive disease (15). MIF plays a role in chemotactic neutrophil recruitment (16) and T cell activation regulating ERK MAP kinase phosphorylation (17) in arthritis. Also, MIF has been recently shown to be critical in Ross River virus-induced arthritis (18).
In this study, we analyzed the impact of genetic MIF deficiency in the K/BxN serum transfer model of arthritis (19), in which joint damage is strictly dependent on macrophages and ensues in the absence of T or B cell-mediated pathways (20,21). We also studied the role of macrophage production of MIF in reconstitution studies employing wild-type macrophages. MIF−/− mice were protected from serum-induced arthritis and showed a significant decrease in serum interleukin-1β (IL-1 β) and IL-6 levels; however, the adoptive transfer of wild-type, MIF-producing macrophages to MIF−/− mice produced an arthritis that was indistinguishable from that which developed in wild-type mice.
Methods
Experimental animals
KRN TCR-transgenic mice on the C57BL/6 background (19) were a kind gift from Drs. D. Mathis and C. Benoist (Strasbourg, France) and were crossed to NOD/Lt (N) mice to generate K/BxN mice. MIF-deficient mice maintained on a pure C57BL/6 background have been described previously (22). C57BL/6 mice were obtained from Charles River Laboratories, Sulzfeld, Germany. All mice were maintained under pathogen-free conditions and bred at the animal facility of the University of Applied Sciences, Rheinbach, Germany. All mice were sex-and age-matched for experiments and were between 8–10 weeks of age. Animal experimentation was performed in accordance with local guidelines and with permission of the ethical committee.
Preparation of K/BxN arthritogenic serum
The K/BxN progenies bearing the Vβ6-transgenic TCR were identified by flow cytometry of peripheral blood lymphocytes using anti-CD4-PE-an d anti-Vβ6 -FITC-labeled mAb (BD PharMingen, Heidelberg, Germany). Serum was separated from the blood obtained from tail sampling of arthritic mice; these were pooled and frozen at −20° C until use. Arthritis was induced in mice by intraperitoneal (i.p.) injection of 200 µl equivalent of serum from K/BxN or control mice.
Clinical and histological assessment of arthritis
Ankle thickness (mm) was measured with a caliper and clinical scores were determined on alternate days upon evidence of redness and swelling using a scale ranging from 0–4 as described (14). Arthritis was assessed visually as the clinical index score; where 1 = 1 ankle affected, 2 = 2 ankles affected, 3 = 3 ankles affected, and 4 = all 4 ankles affected. Samples for histological analysis were prepared by fixing the paws with ankle joints for 24–48 h in 4% phosphate-buffered formaldehyde (Sigma Aldrich, Deisenhofen, Germany). Fixed joints were decalcified by treatment with 14% EDTA solution (Merck KGgA, Darmstadt, Germany) for 2 weeks with gentle rocking and daily replacement of the solution. Samples then were washed with PBS, dehydrated with a series of ethanol washes (50% ethanol, followed by 70%, 95% and 100% ethanol) and embedded in paraffin. Sections of tissue 4 µm thick were stained with hematoxylin/eosin and histological evaluation of inflammation and joint destruction was performed in a blinded fashion. The extent of joint inflammation was defined by synovial hyperplasia and degree of infiltration of the synovial tissue with polymorphonuclear and mononuclear leukocytes as follows: 0 = no changes; 1 = mild hyperplasia and infiltration; 2 = moderate changes; 3 = severe changes. The extent of damage of the cartilage surface and bone structures was evaluated on a scale of 0–3, where 0 = no damage, 1 = mild destruction, 2 = moderate destruction, and 3 = severe destruction of cartilage and bone (extensive area of cartilage destruction, deep invasive bone erosions.
Macrophage preparation and reconstitution of MIF−/− mice
Peritoneal macrophages were isolated from 6–8 week old wild-type or MIF−/− mice (both in the C57BL/6 background). 5–8 ml of ice-cold PBS were injected intraperitoneally, the mice were euthanized by cervical dislocation, and the cells in the peritoneal fluid collected. After centrifugation of the peritoneal exudate, the cells were resuspended in PBS. Macrophages then were purified by adherence on 90 mm diameter culture dishes (Greiner, Frickenhausen, Germany) for 1 h in 5% CO2 at 37°C, and the non-adherent cells, which were mainly lymphocytes, were removed by washing two times with warm PBS. The adherent macrophages then were collected, re-suspended in PBS, and 1.5×107 cells (in a total volume of 0.2 ml) were injected intraperitoneally into recipient, MIF−/− mice.
Quantification of cytokines by Luminex methodology and ELISA
Mouse sera were diluted 1:3 with sera dilute (Bio-Rad) and cytokines (with the exception of MIF) were quantified using a bead-based multiplex assay on a Bio-Plex Luminex analyzer (Bio-Rad) according to manufacture’s protocol. Data were analyzed using Graphpad Prism version 4.00 for Windows (GraphPadSoftware, San Diego, CA, USA). For the measurement of MIF, sera were diluted 1:3 with 1× reaction buffer (0.1% BSA-1 mM EDTA-0.05% Tween 20-PBS) and subjected to a murine specific MIF ELISA assay as described previously (21).
For the quantification of synovial cytokine content, mouse ankle joints were frozen in liquid nitrogen and ground into the powder (60 secs) using Mikro Dismembrator (B. Braun Biotech International, Germany). The powder then was suspended in 400 µl of lysis buffer (10ml RIPA buffer, 1 tablet of protease inhibitor cocktail from Roche Diagnostics, Mannheim, Germany) and incubated on ice for 1hr. The homogenate was centrifuged at 1,870×g for 15 mins, and the resulting supernatant was centrifuged again at 13,23000D7; for 5 mins. Each supernatant then was adjusted to 40 mg protein/ml using the Bio-Rad protein assay reagent. 50 µl of supernatant then was utilized for cytokine determination by Luminex assay or in the case of MIF, by ELISA. Values were expressed relative to total tissue protein.
Statistical Analysis
Statistical significance was analyzed using the Graphpad Prism software. Data are expressed as mean ±SEM. Comparisons were performed using the unpaired Student’s t-test or the Mann-Whitney U-test and by analysis of variance. Differences were considered statistically significant when P < 0.05.
Results
MIF-deficient mice are protected from K/BxN serum-induced arthritis
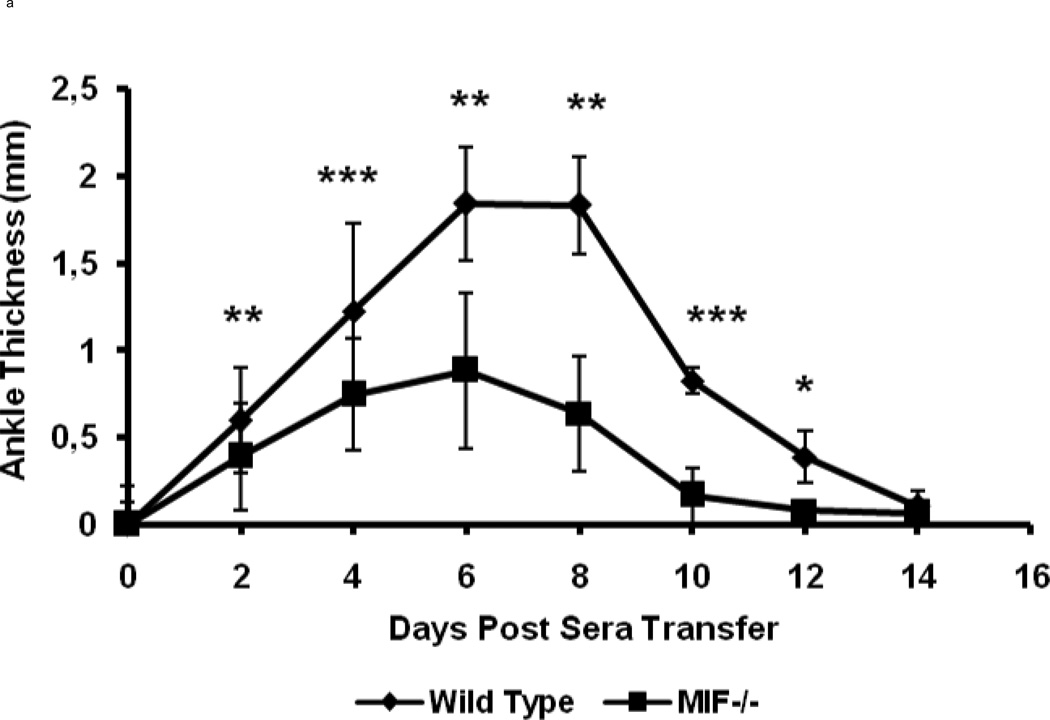
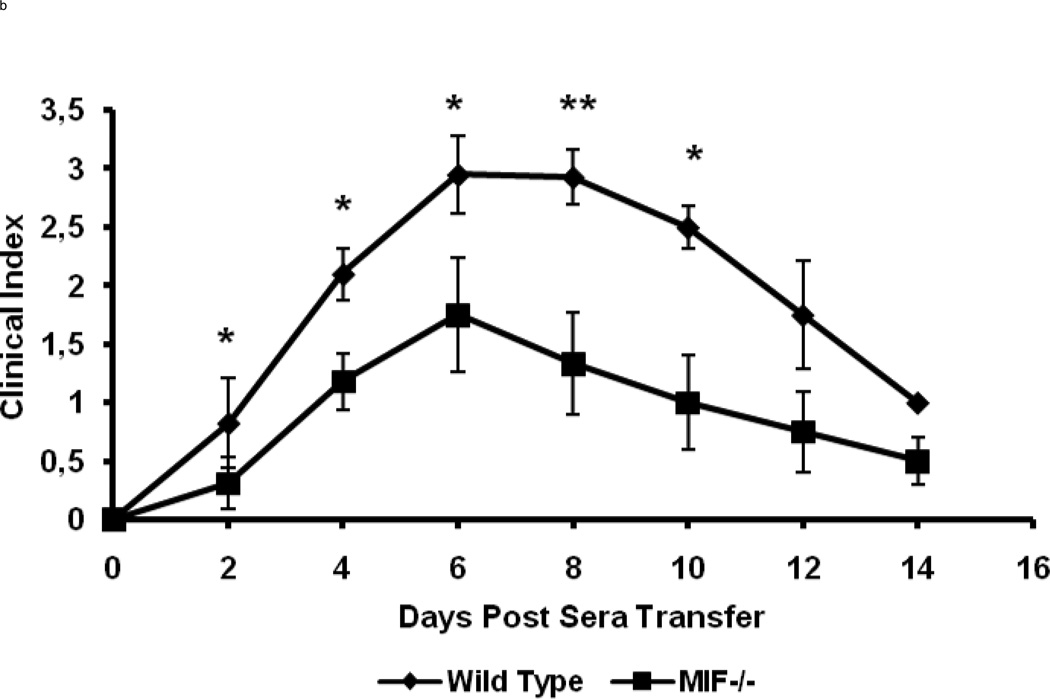
MIF−/− and wild-type control (C57BL/6) mice were injected i.p. with 200 µl of K/BxN sera at day 0. The wild-type controls showed the clinical signs and symptoms of arthritis that are typical of this model (Fig 1). Ankle swelling in the wild-type mice was evident beginning at day 2 after serum injection, with peak swelling measured at days 7 and 8, after which disease began to resolve. By contrast, MIF−/− mice were resistant to the development of arthritis and showed significantly decreased joint disease after serum injection, as assessed by ankle thickness and the clinical index score (Fig 1).
Figure 1.
MIF−/− mice (n=8) and wild-type C57BL/6 mice (n=8) were treated with an intraperitoneal injection of 200µl of K/BxN sera and arthritis evaluated by measuring ankle thickness (a) and clinical index (b). The values represent the mean ± SEM of ankles/time points, which were compared with the student t-test (* P < 0.05, ** P < = 0.01,*** P < 0.001).
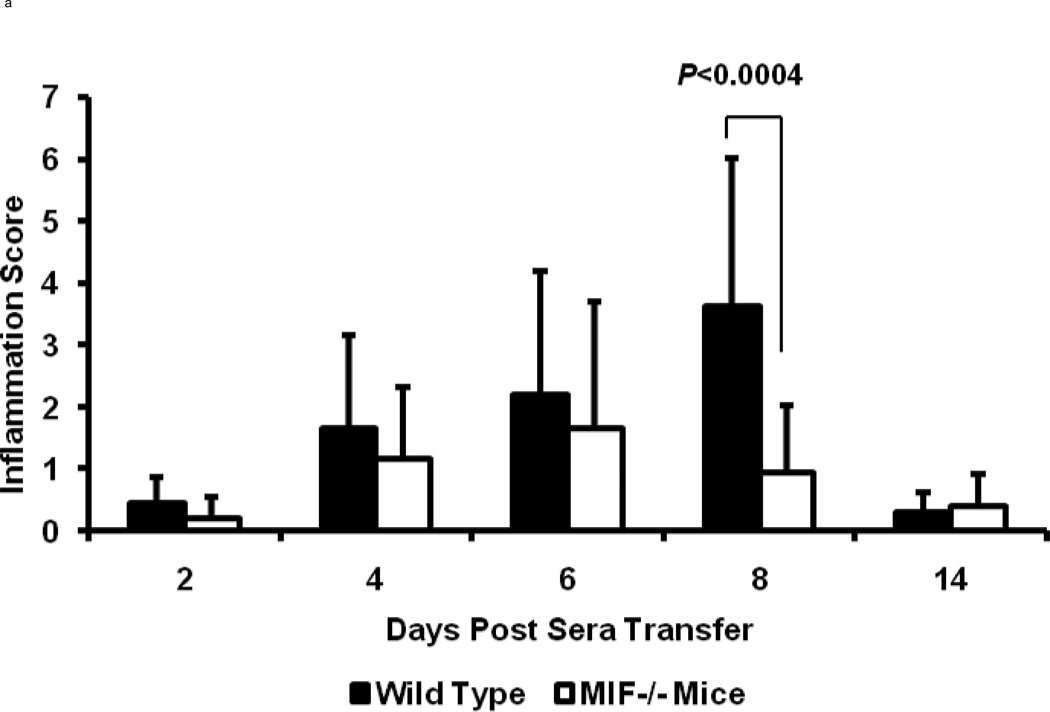
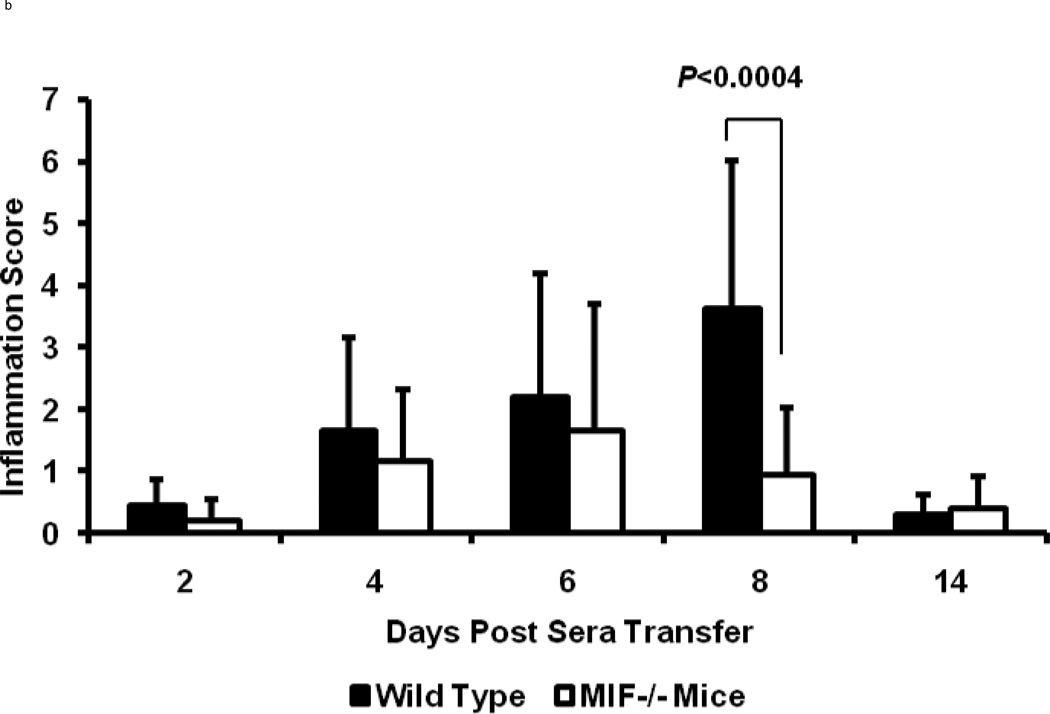
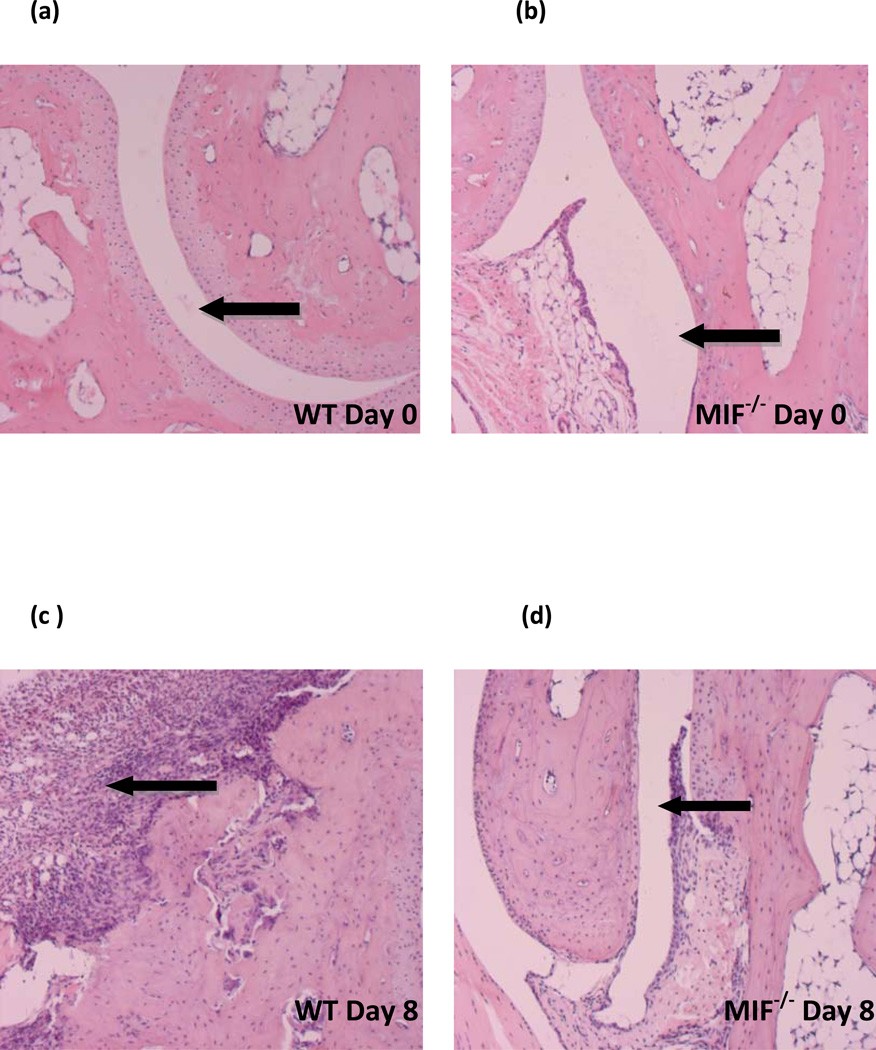
To more accurately determine the degree of inflammation and destruction of cartilage and bone, sections of ankle joints were examined and graded using a histopathologic scoring system. Joint swelling and the clinical severity of arthritis were higher in wild-type mice when compared to MIF−/− mice (day 8; inflammation score: 4.0±0.5 in wild-type mice, 1.0±0.5 in MIF−/− mice, P < 0.0004; destruction score: 0.9±0.5 in wild-type mice, 0.2±0.5 in MIF−/− mice, P < 0.0043) (Fig 2). Histologic evaluation of joints from the wild-type mice revealed the exudation of polymorphonuclear leukocytes into the joint space, hyperplasia of the synovial lining layer, infiltration of inflammatory cells into the synovial tissue, and expansion of synovial tissue (pannus) over the articular cartilage surface with degradation of the cartilage and erosion of the bone (Fig 3). By contrast, the joints of MIF−/− mice showed no visible evidence of cartilage or bone erosion, or cellular proliferation, and there was scant infiltration by inflammatory cells.
Figure 2.
Arthritis scores of 8 week old wild-type C57BL/6 or MIF−/− mice after administration of K/BxN serum (n=3 per genotype per time point). (a) Inflammation (hyperplasia of the synovial lining layer, exudation of granulocytes, infiltration of the synovial membrane by polymorphonuclear and mononuclear leukocytes, pannus formation) and (b) joint destruction (damage of articular cartilage surfaces and bone erosion) in mice with K/BxN serum-induced arthritis. The histologic scores for synovial inflammation and joint destruction are significantly less for MIF−/− mice compared to the wild-type mice (P < 0.0004 and P = 0.004, respectively). Bars show the mean ± SEM. P values were determined by the Mann-Whitney U-test.
Figure 3.
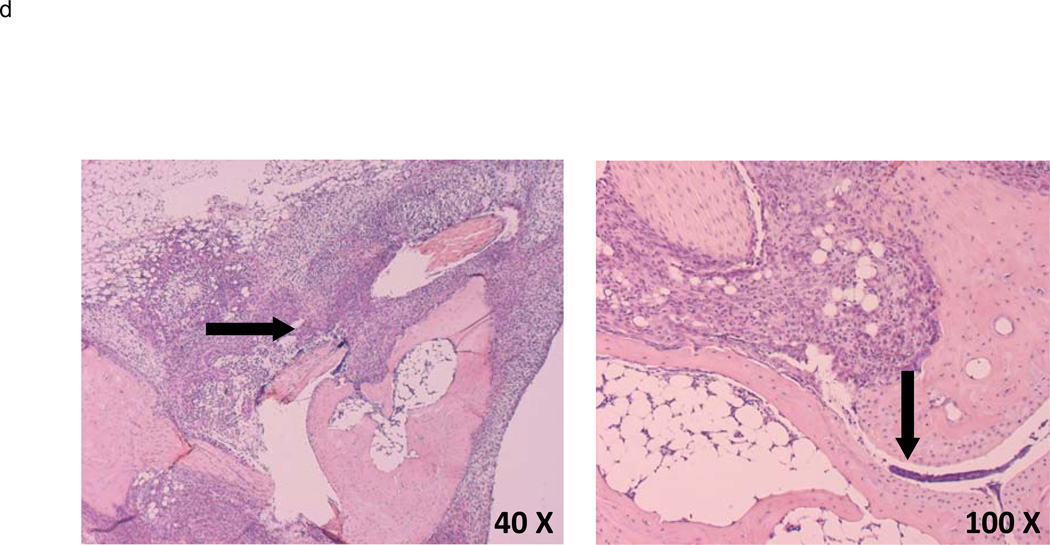
Representative histology of ankle joints from wild-type and MIF−/− mice at (a,b) day 0 (before K/BxN serum transfer) and (c,d) day 8 (post K/BxN serum transfer) (c) Wild-type joints display synovial inflammation, hyperplasia, pannus formation, and cartilage and bone destruction whereas (d) MIF−/− joints are free of inflammation and synovium retains its relatively acellular composition.
Reconstitution of MIF-deficient mice with wild-type macrophages restores susceptibility to arthritis
While MIF is produced upon the inflammatory activation of a number of different cell types, early studies of lethal endotoxemia suggested that the macrophage is an important source of MIF in vivo (3). We tested the hypothesis that macrophage-derived MIF is important for the pathogenesis of K/BxN serum-induced arthritis by adoptively transferring 1.5 × 107 peritoneal-derived, wild-type macrophages into MIF−/− mice. Wild-type mice injected with PBS alone, and MIF−/− mice injected with MIF−/− macrophages also were studied in parallel for arthritis development.
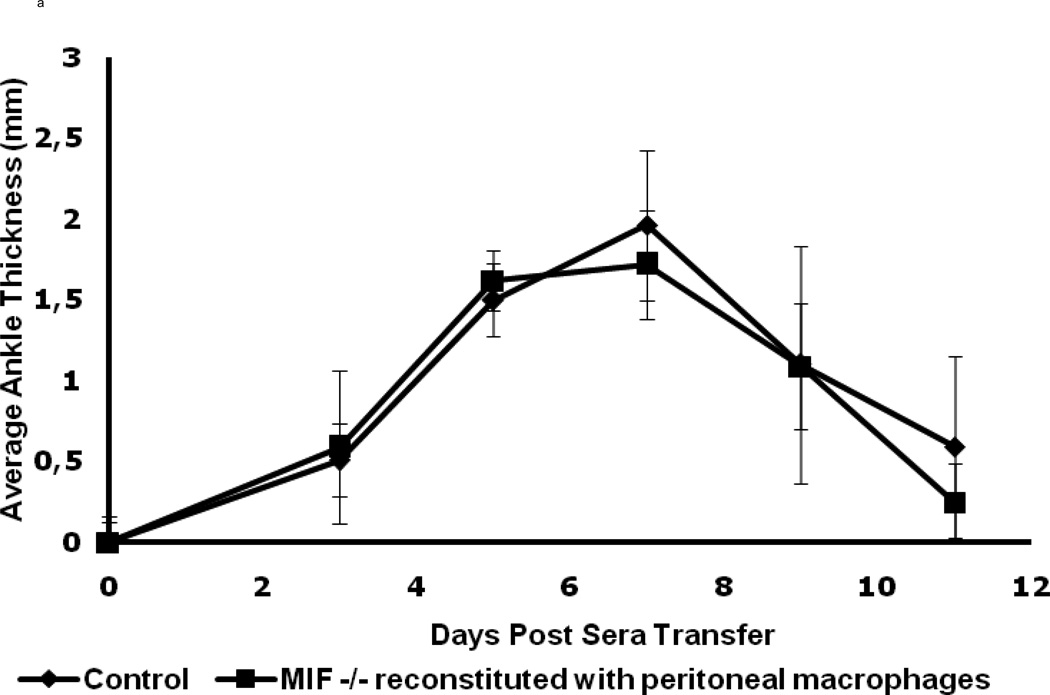
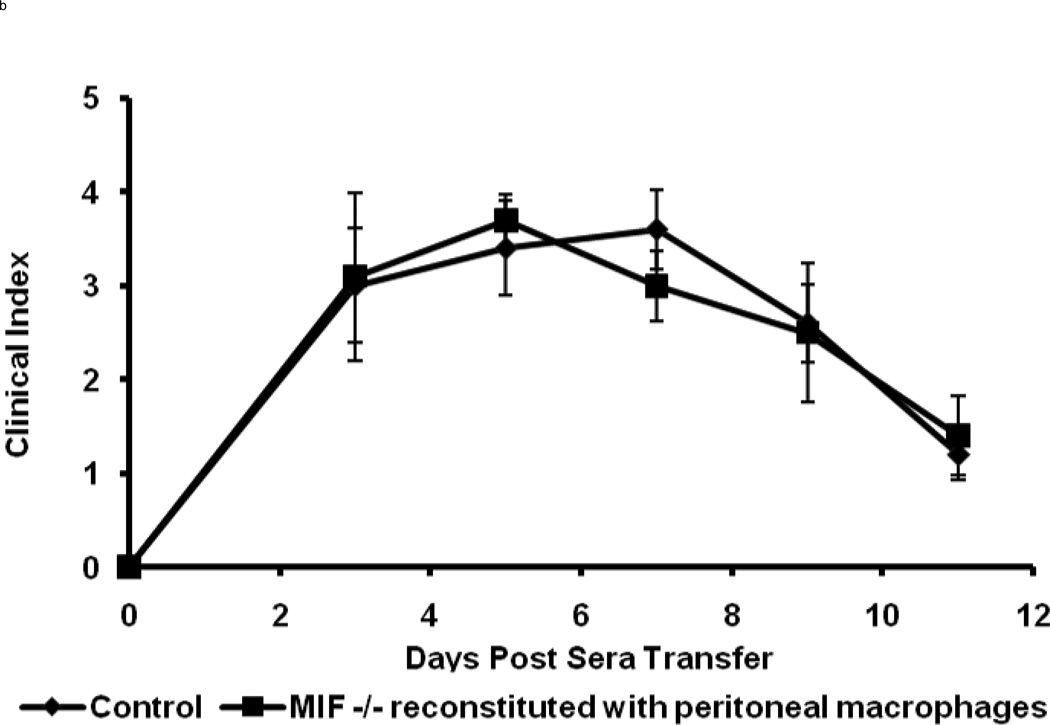
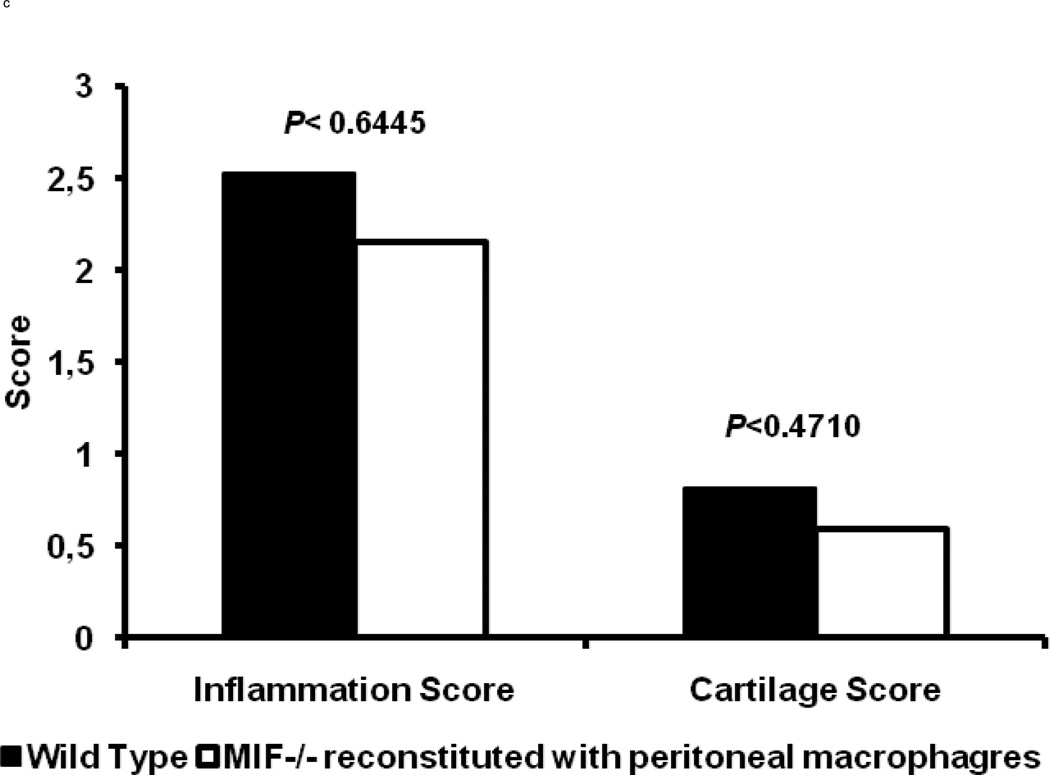
The wild-type macrophage reconstituted MIF−/− mice were observed to be fully susceptible to arthritis induction upon K/BxN serum transfer, as evident from the ankle thickness and clinical index scores (Fig. 4). The typical features of K/BxN serum-induced arthritis were manifested with a peak in joint swelling at days 5 – 7, which then decreased at days 9 – 11. No significant differences in the histological evaluation of inflammation (P < 0.64) or cartilage and bone destruction (P < 0.47) were evident upon comparison of wild-type animals with MIF−/− mice reconstituted with wild-type macrophages (Fig 4c). Microscopic examination of joints also confirmed that the wild-type macrophage-reconstituted MIF−/− mice showed the classic histological picture of inflammatory cell infiltration and pannus invasion into cartilage (Fig. 4d). No difference in arthritis was observed upon K/BxN serum administration to MIF−/− mice injected with MIF−/− macrophages when compared to MIF−/− mice alone (data not shown). The restoration of inflammatory joint pathology by the reconstitution of MIF−/− mice with wild-type peritoneal macrophages supports the conclusion that macrophage-derived MIF is necessary for the full development of K/BxN serum-induced arthritis.
Figure 4.
Macrophage-reconstituted MIF−/− mice are susceptible to arthritis. Wild-type peritoneal macrophages were injected intraperitoneally into MIF−/− mice, followed by 200 µl of arthritogenic K/BxN serum. The mice were assessed for arthritis development by ankle thickness (a) and clinical index (b) scores on day 0, 3, 5, 7, 9 and 11. MIF−/− mice reconstituted with wild-type macrophages showed arthritis identical to that of wild-type mice (control). Data are expressed as mean ± SEM; n=5. (c) Inflammation and joint destruction of wild-type mice and MIF−/− mice reconstituted with wild-type macrophages. (d) Representative ankle joint histology of MIF−/− mice reconstituted with wild-type macrophages and administered 200 µl of K/BxN serum.
Serum inflammatory mediators IL-1β and IL-6 levels are reduced in MIF−/− mice but restored in MIF−/− mice reconstituted with wild-type peritoneal macrophages
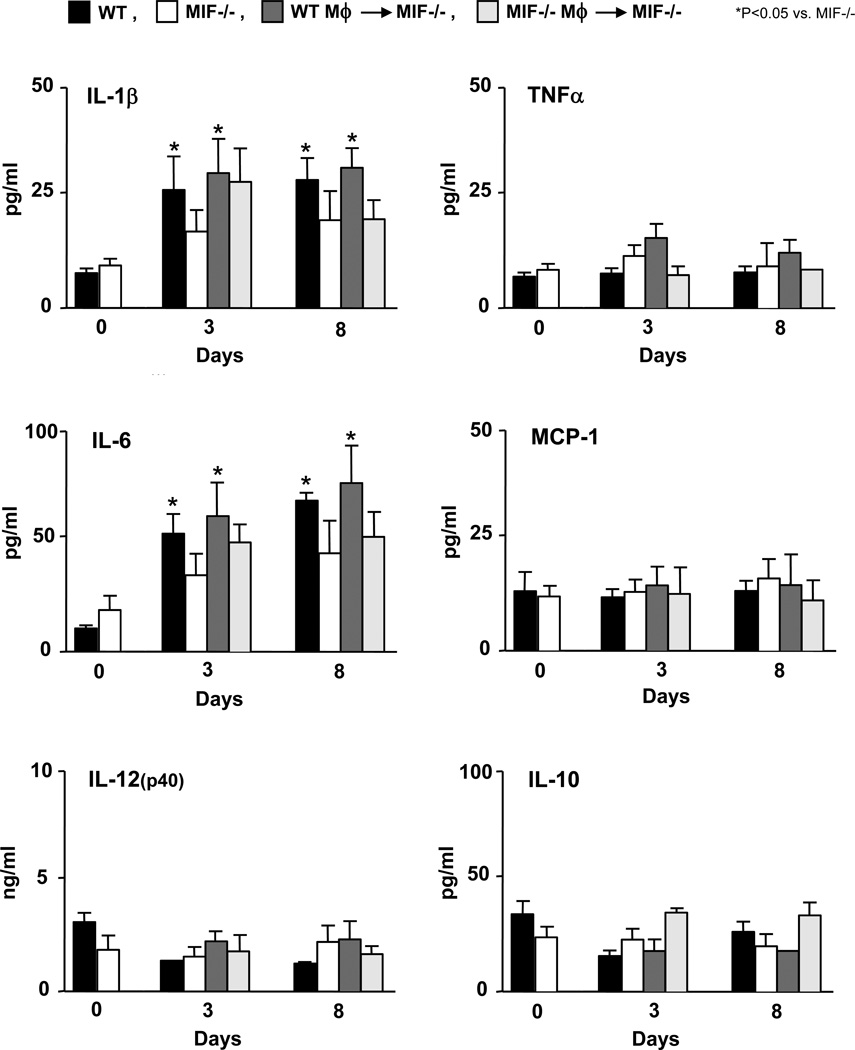
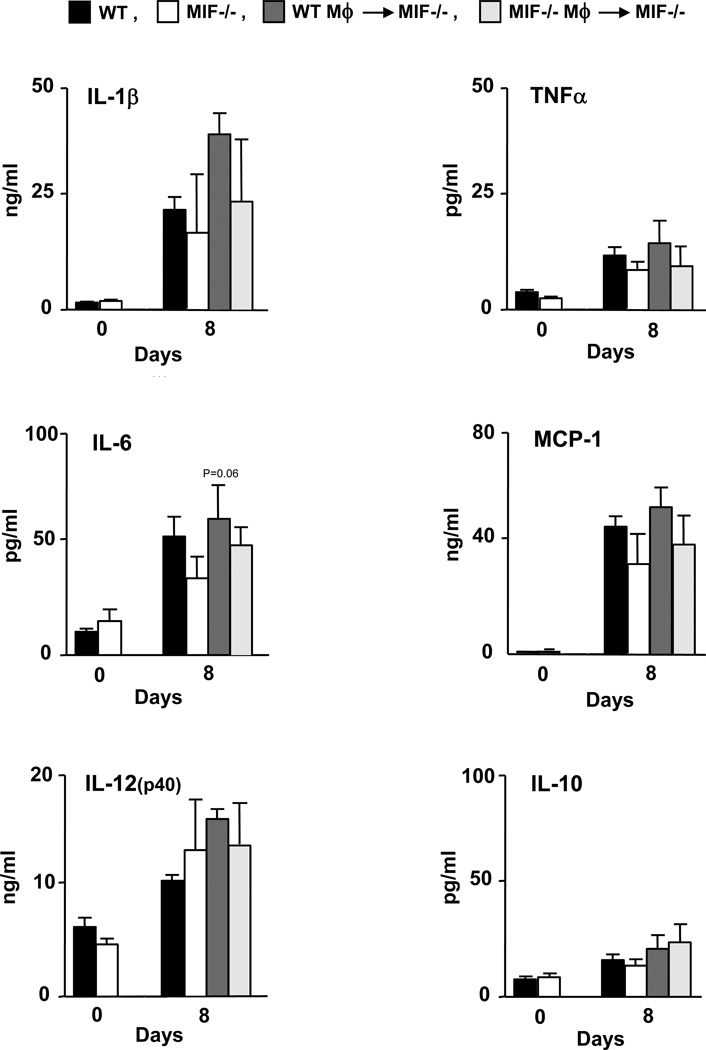
Serum levels of the cytokines IL-1β, TNF-α, IL-6, MCP-1, IL-12(p40) and IL-10 were measured following the administration of K/BxN serum to wild-type mice, MIF−/− mice, MIF−/− mice reconstituted with wild-type macrophages, and MIF−/− mice reconstituted with MIF−/− macrophages. Serum was isolated from mice on days 3 and 8 after arthritis induction (Fig. 5). Both wild-type mice and MIF−/− mice injected with wild-type macrophages showed significantly higher serum levels of IL-1β and IL-6 on days 3 and 8 post arthritis induction when compared with MIF−/− mice (or MIF−/− mice reconstituted with MIF−/− macrophages) (P < 0.05 versus MIF−/− mice). No changes in the circulating levels of TNF-α, MCP-1, IL-12(p40) or IL-10 were detected in the different experimental groups. The ELISA measurement of the intrasynovial concentration of these cytokines showed very low overall values, although a time-dependent increase in IL-1β, IL-6, IL-12, and MCP-1 could be detected (Fig. 6). There were no statistically significant differences in the synovial tissue content of cytokines between the different experimental groups, which likely reflects the low sensitivity of this analysis, although a trend for increased intrasynovial IL-6 in MIF−/− mice reconstituted with WT macrophage versus MIF−/− mice was detected (P < 0.06).
Figure 5.
Serum cytokine levels following the administration of K/BxN serum into wild-type mice (WT), MIF−/− mice, MIF−/− mice reconstituted with wild-type macrophages (WT Mφ), and MIF−/− mice reconstituted with MIF−/− macrophages (MIF−/− Mφ). Serum samples (untreated: n=5, day 3 and 8: n=3) were examined for IL-1β, IL-6, TNF-α, MCP-1, IL-12(p40) and IL-10 levels using a Luminex-based assay. Bar show the mean and SEM. P values were determined by Student’s t-test (* P < 0.05).
Figure 6.
Cytokine production in ankle joint tissue following administration of K/BxN serum into wild-type mice (WT), MIF−/− mice, MIF−/− mice reconstituted with wild-type macrophages (WT Mφ), and MIF−/− mice reconstituted with MIF−/− macrophages (MIF−/− Mφ). Mice were euthanized prior to the induction of arthritis (day 0) or 8 days after serum transfer. Ankle joint tissue from mice (n=3 per assayed group) were isolated, snap frozen in the liquid nitrogen and ground into fine powder, lyzed and examined by Luminex-based assay (untreated n=5, day 8 n=3) for IL-1β, IL-6, TNF-α, MCP-1, IL-12(p40) and IL-10. Bar show the mean and SEM. P values were determined by Student’s t-test. Values are expressed as cytokine content relative to 1 mg of total synovial protein.
We also measured the levels of MIF on day 8 after arthritis induction. While MIF was detectable in the serum (1.8 ng/ml) and the joint tissue (1.9 ng/mg tissue) of wild-type mice with arthritis, MIF levels remained undetectable in the serum or joint tissue of MIF−/− mice or MIF−/− mice reconstituted with wild-type macrophages.
Discussion
The cytokine profile of RA synovium is an area of intense research interest, both with respect to the particular cytokines that initiate joint inflammation and those that mediate joint destruction. Pro-inflammatory cytokines such as TNF-α and IL-1β have been shown to induce in vitro many of the mediators of inflammation and joint injury that are expressed in RA. A closer understanding of the role of these cytokines in the immunopathology of RA also has led to the development of effective therapeutic strategies. Yet, even with pharmacologic inhibition of TNF, as many as 40% of RA patients show an inadequate clinical response (23). Furthermore, current anti-cytokine strategies have not proved curative and the search for better therapeutic targets in RA continues.
Many aspects of the function of MIF suggest that it is an important cytokine in RA. MIF sustains the activation of monocytes/macrophages by inhibiting activation-induced apoptosis (24), thereby promoting the production of TNF, IL-1β, nitric oxide, and PGE2. MIF also up-regulates the expression of MMPs and cyclooxygenase-2 by rheumatoid synovial fibroblasts (13,25). Elevated MIF levels have been reported in RA serum, synovial fluid, and synovial tissue, with the later correlating with disease activity (6,26). The cellular sources of MIF in RA include monocytes/macrophages, fibroblast-like synoviocytes (FLS) (6), endothelial cells, and, to a lesser extent, T cells (4).
Macrophages are present in high numbers in inflamed synovium and at the cartilage-pannus interface in RA, and they have been shown to play a crucial role in the K/BxN serum-induced model of arthritis (20). The data presented here demonstrate an important role for MIF in the development of K/BxN serum-induced arthritis. Within 2 days after the administration of arthritic K/BxN serum, mice genetically deficient in MIF showed resistance to arthritis development. By days 6 – 8 there was a significant reduction in ankle thickness and clinical arthritis index in these mice when compared to wild-type controls.
The observation that an arthritis-susceptible phenotype is restored in MIF−/− mice by the adoptive transfer of wild-type macrophages indicates an important role for macrophage-derived MIF in the pathogenesis of joint inflammation. This finding also suggests that a systemic, macrophage MIF release response is sufficient to initiate the cytokine cascade responsible for the inflammatory infiltration of joints, the formation of invasive pannus, and cartilage and bony destruction. Serum IL-1β and IL-6 levels were significantly decreased in the arthritis-resistant MIF−/− mice, whereas the levels of these cytokines were restored in MIF−/− mice reconstituted with wild-type macrophages. TNF-α levels in serum and joint tissue were not found to differ between wild type and MIF−/− mice (or MIF−/− mice reconstituted with wild-type macrophages), despite marked differences joint pathology. That TNF-α levels were not influenced by the reconstitution of MIF−/− mice with wild-type macrophages also supports the conclusion that the inflammatory cascade induced by arthritogenic K/BxN serum proceeds independently of TNF-α hese data are in accord with those studies that have shown that TNF−/− mice display a variable response to K/BxN serum-induced arthritis and that the presence of TNF receptor types I and II are not essential for immunopathology (27). IL-1 β is required for the development of K/BxN serum transfer arthritis however (28), and our data suggest that serum IL-1β (and IL-6) expression not only depends on the presence of MIF, but that a systemic MIF response provided by adoptively transferred macrophages is sufficient to restore IL-1β and IL-1β–dependent effector responses. We did not detect significant differences in the synovial content of cytokines, which likely reflects the low sensitivity of the joint tissue ELISA measurements. These data nevertheless point to the dominant influence of adoptively transferred, macrophage-derived MIF on systemic cytokine expression, which was more readily detectable and may contribute importantly to arthritis development.
In conclusion, we have shown that a deficiency in the MIF gene inhibits joint inflammation and destruction induced by arthritogenic K/BxN serum. We also show for the first time that macrophage-derived MIF, as provided by a systemically administered macrophage subpopulation, is necessary to drive the full arthritis development. These results support the hypothesis that MIF is an important, upstream mediator leading to inflammatory joint disease and further suggest that the pharmacologic targeting of MIF may offer unique therapeutic utility in those RA patients unresponsive to TNF inhibitors.
Acknowledgments
Funding: This work was supported by AUTOROME European Community grant no. LSHMCT-2004-0052 to HI and the German Research Foundation (Br 1372/9) and Interdisciplinary Center for Clinical Research (IZKF) Jena to RBr. LL, JF, and RBu were supported by NIH AR049610 and AR050498.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7849. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proceedings of the National Academy of Sciences of the United States of America. 1966;56:72. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, Weedon H, Holdsworth SR, Bucala R, Morand EF. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis and rheumatism. 1999;42:1601. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Sampey AV, Hall PH, Mitchell RA, Metz CN, Morand EF. Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis and rheumatism. 2001;44:1273. doi: 10.1002/1529-0131(200106)44:6<1273::AID-ART219>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31:268. [PubMed] [Google Scholar]
- 9.Onodera S, Nishihira J, Koyama Y, Majima T, Aoki Y, Ichiyama H, Ishibashi T, Minami A. Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis and rheumatism. 2004;50:1437. doi: 10.1002/art.20190. [DOI] [PubMed] [Google Scholar]
- 10.Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000;275:444. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 11.Gregory JL, Leech MT, David JR, Yang YH, Dacumos A, Hickey MJ. Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis and rheumatism. 2004;50:3023. doi: 10.1002/art.20470. [DOI] [PubMed] [Google Scholar]
- 12.Mikulowska A, Metz CN, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514. [PubMed] [Google Scholar]
- 13.Leech M, Metz C, Santos L, Peng T, Holdsworth SR, Bucala R, Morand EF. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis and rheumatism. 1998;41:910. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Santos L, Hall P, Metz C, Bucala R, Morand EF. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clinical and experimental immunology. 2001;123:309. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radstake TR, Sweep FC, Welsing P, Franke B, Vermeulen SH, Geurts-Moespot A, Calandra T, Donn R, van Riel PL. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis and rheumatism. 2005;52:3020. doi: 10.1002/art.21285. [DOI] [PubMed] [Google Scholar]
- 16.Santos LL, Fan H, Hall P, Ngo D, Mackay CR, Fingerle-Rowson G, Bucala R, Hickey MJ, Morand EF. Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis and rheumatism. 2011;63:960. doi: 10.1002/art.30203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos LL, Dacumos A, Yamana J, Sharma L, Morand EF. Reduced arthritis in MIF deficient mice is associated with reduced T cell activation: down-regulation of ERK MAP kinase phosphorylation. Clinical and experimental immunology. 2008;152:372. doi: 10.1111/j.1365-2249.2008.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrero LJ, Nelson M, Srikiatkhachorn A, Gu R, Anantapreecha S, Fingerle-Rowson G, Bucala R, Morand E, Santos LL, Mahalingam S. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12048. doi: 10.1073/pnas.1101089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 20.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur J Immunol. 2005;35:3064. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 21.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 22.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Muller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9354. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maini RN, Brennan FM, Williams R, Chu CQ, Cope AP, Gibbons D, Elliott M, Feldmann M. TNF-alpha in rheumatoid arthritis and prospects of anti-TNF therapy. Clin Exp Rheumatol. 1993;11(Suppl 8):S173. [PubMed] [Google Scholar]
- 24.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:345. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurigt U, Pfirschke C, Irmler IM, Huckel M, Gajda M, Janik T, Baumgrass R, Bernhagen J, Brauer R. Interactions of T helper cells with fibroblast-like synoviocytes: up-regulation of matrix metalloproteinases by macrophage migration inhibitory factor from both Th1 and Th2 cells. Arthritis and rheumatism. 2008;58:3030. doi: 10.1002/art.23904. [DOI] [PubMed] [Google Scholar]
- 26.Morand EF, Leech M, Weedon H, Metz C, Bucala R, Smith MD. Macrophage migration inhibitory factor in rheumatoid arthritis: clinical correlations. Rheumatology (Oxford) 2002;41:558. doi: 10.1093/rheumatology/41.5.558. [DOI] [PubMed] [Google Scholar]
- 27.Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196:77. doi: 10.1084/jem.20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197:537. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]