Abstract
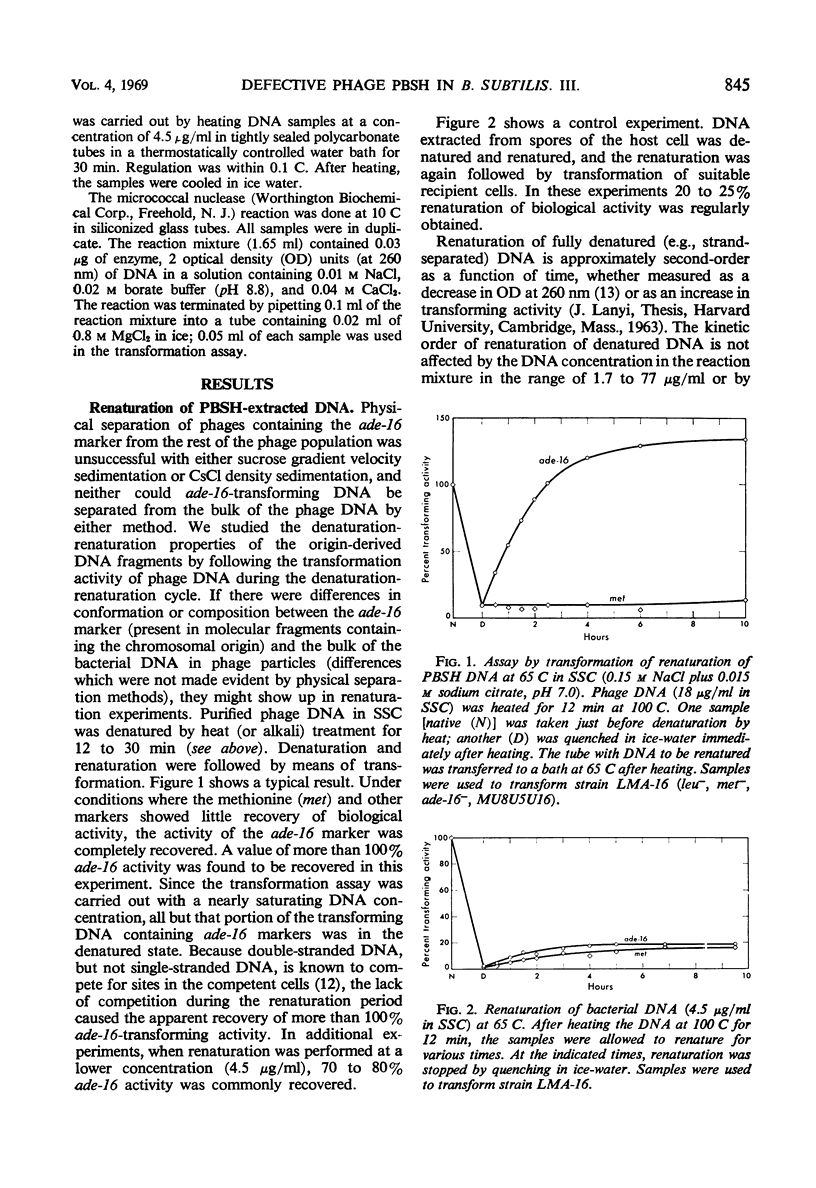
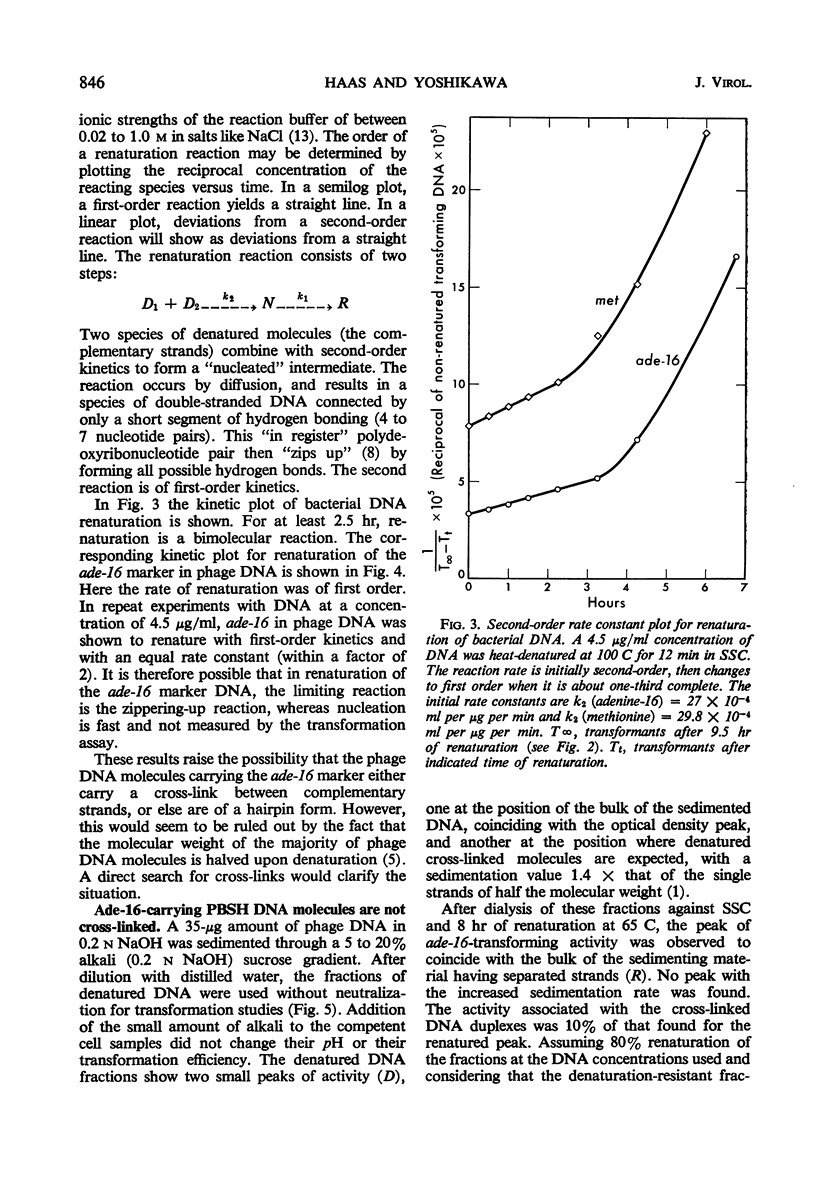
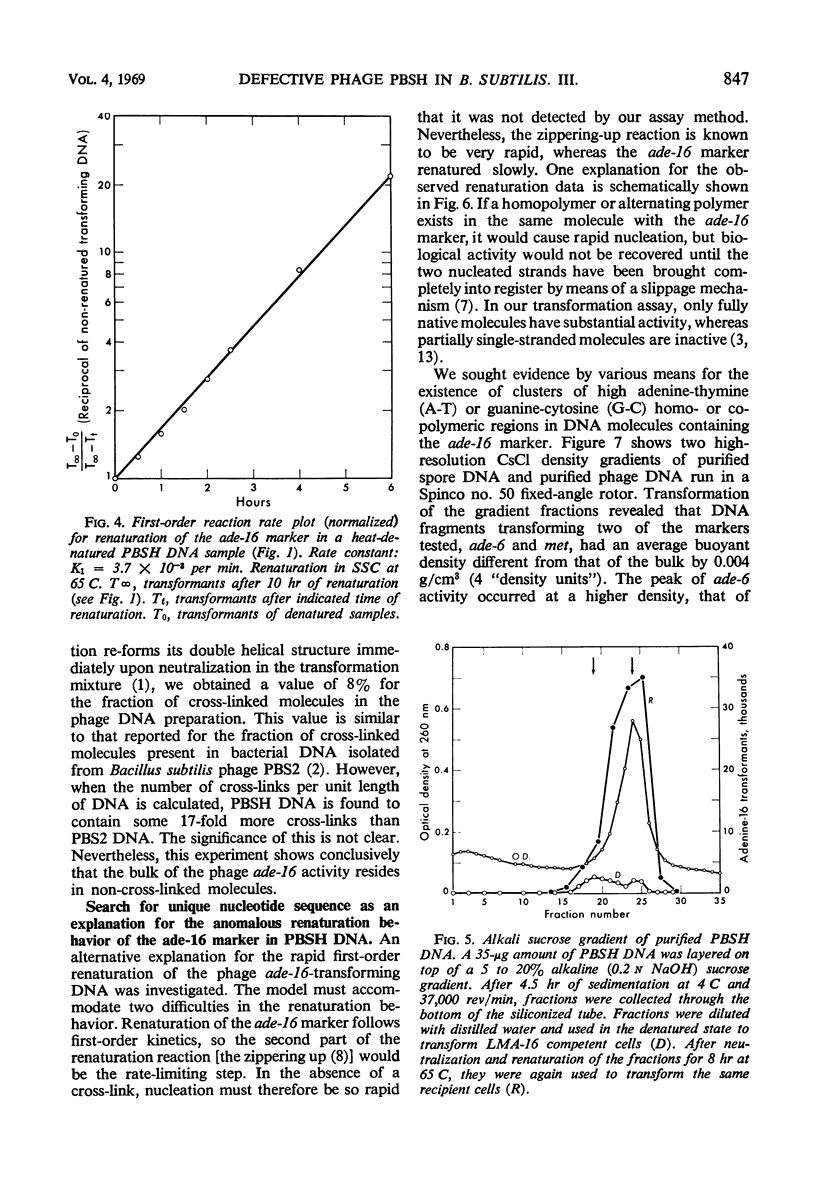
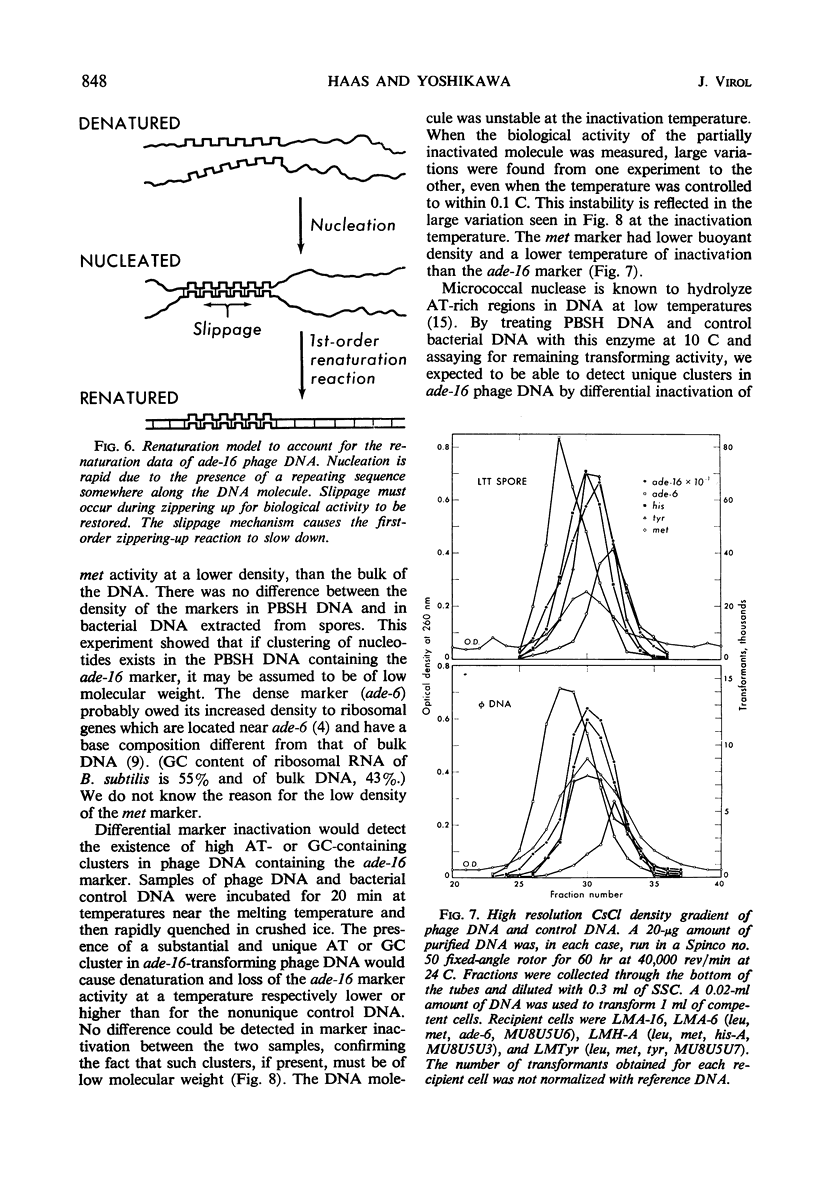
The adenine-16 (ade-16) marker (the marker nearest the chromosomal origin of Bacillus subtilis) in purified PBSH deoxyribonucleic acid (DNA) renatured more rapidly and to a greater extent than any other marker in the phage DNA, and more rapidly and to a greater extent than all markers, including ade-16, in bacterial DNA. The renaturation of the phage DNA ade-16 marker followed a first-order reaction, whereas renaturation of bacterial markers was initially a second-order reaction. No cross-linkages were detected in DNA molecules containing the ade-16 marker. Buoyant density measurements and inactivation by heat and micrococcal deoxyribonuclease of the ade-16 marker did not reveal large segments of clusters of the individual bases in these molecules. Alternative mechanisms for the unique renaturation behavior of the ade-16 marker are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M. Characterization of a naturally occurring, cross-linked fraction of DNA. II. Origin of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):405–421. doi: 10.1016/0022-2836(68)90018-1. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bernardi G. Residual transforming activity of denatured Haemophilus influenzae DNA. J Mol Biol. 1968 Mar 14;32(2):437–451. doi: 10.1016/0022-2836(68)90020-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. I. Induction, purification, and physical properties of the bacteriophage and its deoxyribonucleic acid. J Virol. 1969 Feb;3(2):233–247. doi: 10.1128/jvi.3.2.233-247.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Yoshikawa H. Defective bacteriophage PBSH in Bacillus subtilis. II. Intracellular development of the induced prophage. J Virol. 1969 Feb;3(2):248–260. doi: 10.1128/jvi.3.2.248-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S. The reversible reaction of Hg (II) and double-stranded polynucleotides. A step-function theory and its significance. Biochim Biophys Acta. 1963 Feb 26;68:240–253. doi: 10.1016/0006-3002(63)90139-2. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Spears C. L., Doty P. Inter-strand crosslinking of DNA by nitrogen mustard. J Mol Biol. 1966 Aug;19(2):266–288. doi: 10.1016/s0022-2836(66)80004-9. [DOI] [PubMed] [Google Scholar]
- Oishi M. The transcribing strands of bacillus subtilis DNA for ribosomal and transfer RNA. Proc Natl Acad Sci U S A. 1969 Jan;62(1):256–262. doi: 10.1073/pnas.62.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Marmur J. Conversion of Bacillus subtilis DNA to phage DNA following mitomycin C induction. J Mol Biol. 1968 Jun 28;34(3):429–437. doi: 10.1016/0022-2836(68)90170-8. [DOI] [PubMed] [Google Scholar]
- Rownd R., Green D. M., Sternglanz R., Doty P. Origin of the residual transforming activity of denatured Bacillus subtilis DNA. J Mol Biol. 1968 Mar 14;32(2):369–377. doi: 10.1016/0022-2836(68)90016-8. [DOI] [PubMed] [Google Scholar]
- Subirana J. A., Doty P. Kinetics of Renaturation of Denatured DNA. I. Spectrophotometric results. Biopolymers. 1966;4(2):171–187. doi: 10.1002/bip.1966.360040204. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wingert L., Von Hippel P. H. The conformation dependent hydrolysis of DNA by micrococcal nuclease. Biochim Biophys Acta. 1968 Mar 18;157(1):114–126. doi: 10.1016/0005-2787(68)90270-0. [DOI] [PubMed] [Google Scholar]


