Abstract
Epithelial-to-mesenchymal transition (EMT) is a process for fully differentiated epithelial cells to undergo a phenotypic change to fibroblasts via diverse intracellular signaling pathways. While the pivotal role of fibroblasts in renal fibrosis is widely accepted, their origin remains undefined. In addition, although a large number of studies have provided evidence of EMT in human kidney diseases, specific signaling pathways leading to EMT have not yet been discovered in humans. To evaluate the origin of interstitial fibroblasts and signaling pathways involved in the EMT process, we analyzed the differential expression of EMT-related molecules in paraffin-fixed sections from 19 human fibrotic kidneys and 4 control kidneys. In human fibrotic kidneys, tubular epithelial cells (TECs) with intact tubular basement membrane (TBM) showed loss or down-regulation of an epithelial marker (E-cadherin), de novo expression of mesenchymal markers (vimentin and fibronectin), and significant up-regulation of inducers and mediators controlling the EMT process (transforming growth factor-β1 (TGF-β1), p-Smad2/3, β1-integrin, p38 mitogen-activated protein kinase (MAPK), WNT5B and β-catenin) in the areas of interstitial inflammation and fibrosis, compared with their expression in control kidneys. In conclusion, the type II EMT process in humans is thought to be an adaptive response of TECs to chronic injury and is regulated by interconnections of TGF-β/Smad, integrin/integrin-linked kinase (ILK) and wnt/β-catenin signaling pathways.
Keywords: EMT, TGF-beta/Smad signaling, integrin, wnt signaling, renal fibrosis, immunohistochemistry
Introduction
Progression of chronic kidney disease (CKD), characterized by deposition of extracellular matrix (ECM), is an irreversible process which eventually leads to tubulointerstitial fibrosis and progressive loss of kidney function [1]. While the key role of matrix-producing fibroblasts in renal fibrogenesis is widely accepted, their origin leading to fibrosis is still controversial. Strutz et al. demonstrated that TECs can co-express fibroblast markers in disease states, postulating for the first time the possibility of EMT [2]. A challenging study by Iwano et al. showed that the cell lineage-tracing technique using reporter genes demonstrates interstitial fibroblasts derived from renal proximal tubules [3]. However, in human kidneys, there is little evidence regarding the origin of interstitial fibroblasts except two studies previous conducted using renal biopsies by Jinde et al. and Rastaldi et al. [4,5], which have been cited in several review papers [6,7].
EMT is defined as a phenotypic change of fully differentiated epithelial cells to matrix-producing fibroblasts [8]. It is characterized by the loss of epithelial characteristics (E-cadherin) and the acquisition of a mesenchymal phenotype (vimentin and fibronectin) [8,9]. According to the functional consequences and biological context, EMT is divided into three subtypes [9-11]; type I EMT occurs during embryogenesis, in which it produces motile cells but does not lead to ECM deposition or intravascular invasion. Type II EMT induces a morphogenetic change during organ fibrosis or wound healing, which is associated with ECM production and muscle-like characteristics. Type III EMT is involved in carcinoma-metastatic transition.
EMT is a complex biological process through the TGF-β/Smad [6,8,12,13], TGF-β/non-Smad [6,14-16], integrin/ILK [6,8,17] or wnt/β-catenin [6,18-20] signaling pathway, which is induced by a wide variety of stimuli such as TGF-β1, wnt protein, and ECM. Figure 1 shows the major intracellular signaling pathways regulating the EMT process with reference to the simplified figure in Liu’s papers [6,8], which are intricately interconnected and converged by activation of β-catenin, a good controller of the EMT process [6,18]. Despite a large number of papers on the evidence of EMT and a great deal of intense studies on signaling pathways leading to EMT, we still do not know which signaling pathway is involved in human renal fibrogenesis.
Figure 1.
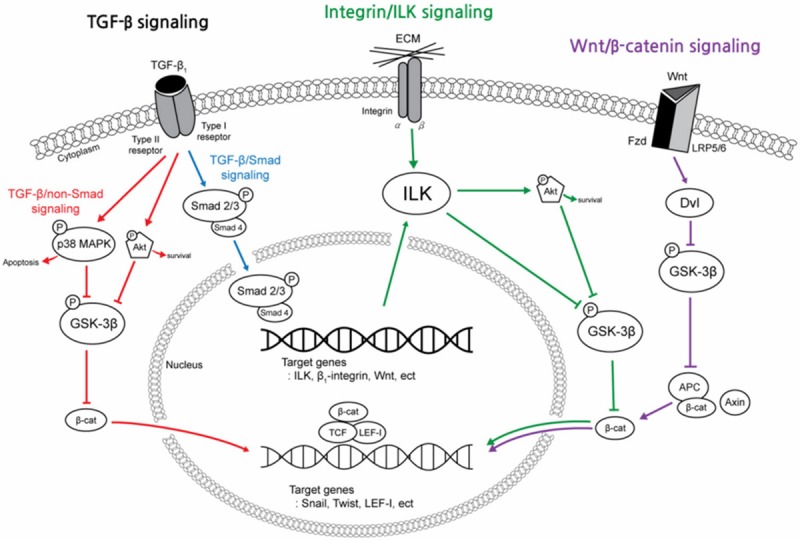
A simplified schematic shows the major intracellular signal transduction pathways that regulate the epithelial-to-mesenchymal transition (EMT) process in fibrotic kidneys. EMT is a complex biological process through the TGF-β1, integrin/ILK or wnt/β-catenin signaling pathway. These pathways are intricately interconnected and converged by activation of β-catenin. TGF-β1 is a chief inducer of the EMT process, and p-Smad2/3, p38 MAPK and ILK function as mediators of the TGF-β1 signaling pathway. (TGF-β1, transforming growth factor-β1; p-Smad2/3, phosphorylated-Smad2/3; MAPK, mitogen-activated protein kinase; GSK-3β, glycogen synthase kinase-3β; ILK, integrin-linked kinase; Fzd, frizzled receptors; LRP 5/6, low density lipoprotein receptor-related protein 5/6; Dvl, Disheveled; APC, adenomatosis polyposis coli; TCF/LEF-1 complex, T cell factor/lymphoid enhancer-binding factor-1 complex).
Therefore, the aims of this study were to (1) examine the phenotypic changes of TECs by evaluating the expression of epithelial and mesenchymal biomarkers and (2) identify the signaling pathway involved in tubular EMT in human fibrotic kidneys by evaluating the expression of EMT-related inducers and intracellular mediators.
Materials and methods
Patient and kidney tissue
Twenty three patients who underwent nephrectomy at Dague Catholic Medical Center from January 2006 to December 2011 were selected for the current study. Nineteen patients (male:female = 11:8, mean age: 62 years) underwent nephrectomy because of hydronephrosis, nephrolithiasis or renal atrophy along with chronic pyelonephritis. Four kidneys which were traumatically ruptured (males, mean age: 37.5 years) were used as controls. Tumor infiltration was excluded from this study. The demographic and clinical data of all patients are summarized in Table 1. Hematuria was defined as three or greater red blood cells (RBCs) per high power filed (HPF) on urinalysis with microscopy [21]. Pyuria was defined as the presence of more than 5-8 white blood cells (WBCs) per HPF of unspun, voided mid-stream urine [22].
Table 1.
Summary of demographic and clinical data of 23 patients
| Case No. | Sex/Age (yr) | Diagnosis | S-Cr (mg/dL) | U-albumin | U-RBC (per HPF) | U-WBC (per HPF) |
|---|---|---|---|---|---|---|
| 1* | M/25 | Rupture | 0.9 | NS | NS | NS |
| 2* | M/31 | Rupture | 0.9 | NS | NS | NS |
| 3* | M/46 | Rupture | 1.0 | NS | NS | NS |
| 4* | M/49 | Rupture | 1.2 | NS | NS | NS |
| 5 | M/74 | NL, CPN | 2.1 | - | 1-3 | 10-20 |
| 6 | F/52 | A (ESK) | 0.9 | - | 1-3 | > 30 |
| 7 | M/47 | HN, CPN | 1.9 | 2+ | 0-1 | 0-1 |
| 8 | M/55 | HN, CPN | 1.1 | 1+ | 1-3 | 1-3 |
| 9 | F/75 | HN (ESK) | 0.9 | Trace | 0-1 | 0-1 |
| 10 | M/48 | A (ESK) | 1.2 | - | 0-1 | > 30 |
| 11 | M/67 | HN, CPN | 1.1 | - | 3-5 | > 30 |
| 12 | F/51 | HN, NL, CPN | 0.7 | - | 1-3 | > 30 |
| 13 | M/77 | A (ESK), CPN | 1.7 | Trace | 0-1 | > 30 |
| 14 | M/71 | HN, CPN | 1.2 | - | 3-5 | 20-30 |
| 15 | F/74 | HN, CPN | 1.0 | Trace | 3-5 | 0-1 |
| 16 | M/70 | HN, CPN | 1.4 | 2+ | 0-1 | 0-1 |
| 17 | F/65 | NL, CPN | 0.8 | 2+ | 1-3 | > 30 |
| 18 | F/69 | HN, CPN | 0.8 | Trace | 0-1 | 0-1 |
| 19 | M/29 | HN, CPN | 1.0 | 1+ | 0-1 | 0-1 |
| 20 | M/80 | MCCs, CPN | 2.1 | 2+ | 20-30 | 20-30 |
| 21 | F/21 | HN, CPN | 3.3 | 2+ | 3-5 | 0-1 |
| 22 | M/57 | A (ESK), CPN | 1.3 | - | 0-1 | 0-1 |
| 23 | F/71 | HN, NL, CPN | 1.6 | 3+ | > 30 | > 30 |
No, number; yr, years; S, serum; Cr, creatinine (reference range: 0.6-1.5 mg/dL); U, urine; RBC, red blood cell (reference range: M: 0-1/HPF, F: 0-1/HPF); HPF, high power field; WBC, white blood cell (reference range: M: 0-1/HPF, F: 0-3/HPF);
control;
M, male; F, female; NS, not specific; NL, nephrolithiasis; CPN, chronic pyelonephritis; -, absent; +, present; A, atrophy; ESK, end stage kidney; HN, hydronephrosis; MCCs, multiple cortical cysts.
Immunohistochemical staining
Four μm-thick sections were prepared from representative 10% formalin-fixed, paraffin-embedded tissues for immunohistochemical studies. Immunohistochemical stains were performed mostly using an Autostainer (Bond-Max, Leica, USA). Primary antibodies used are summarized in Table 2. p-Smad2/3 staining was manually performed. Briefly, 4 μm-thick sections from each paraffin block were cut, deparaffinized with xylene and rehydrated with a graded series of ethanol solutions. After washing with 1% phosphate-buffered saline (PBS) three times for 5 min, the slides were immersed in 30% H2O2 in methanol at room temperature for 15 min to inhibit endogenous peroxidase. After washing as described above, the slides were treated with proteinase K (20 μg/ml) and incubated in a humidified chamber at 37°C for 15 min for antigen retrieval. The slides were boiled with 0.1 M citrate buffer (pH 6.0) in a microwave oven for 10 min, and then left at room temperature for 20 min to cool down. After washing with PBS, the primary antibody against p-Smad2/3 was applied to the tissue sections. The slides were incubated in a humidified chamber at 37°C for 1 hour or overnight at 4°C. After washing with PBS, labeling was done using the streptavidin-biotin immunoperoxidase method with a commercial kit (LSAB kit, DAKO, USA). Tissues were visualized using diaminobenzidine (DAB) as a chromogene to produce a brown color. Counterstaining was performed with Mayer’s hematoxylin. The slides were hydrated with ethanol solutions starting from 70% to 100%, and then mounted. Serial sections were stained with individual antibodies.
Table 2.
List of antibodies used for immunohistochemistry
| Antibody | Clone/Source | Supplier | Dilution |
|---|---|---|---|
| Epithelial biomarker | |||
| E-cadherin | Monoclonal rabbit | Cell signaling technology, # 3195 | 1:200 |
| Mesenchymal biomarker | |||
| Vimentin | Monoclonal mouse | BD Biosciences, 550513 | 1:200 |
| Fibronectin | Monoclonal mouse | Santa Cruz Biotechnology, sc-71113 | 1:100 |
| EMT-related molecules | |||
| TGF-β1 | Polyclonal rabbit | Santa Cruz Biotechnology, sc-146 | 1:100 |
| p-Smad2/3 | Polyclonal goat | Santa Cruz Biotechnology, sc-11769 | 1:50 |
| β1-integrin | Monoclonal rabbit | Epitomics, # 1798-1 | 1:100 |
| p38 MAPK | Monoclonal rabbit | Epitomics, # 1544-1 | 1:100 |
| WNT5B | Polyclonal rabbit | Epitomics, # T3288 | 1:100 |
| β-catenin | Polyclonal rabbit | Epitomics, ab6302 | 1:200 |
EMT, epithelial to mesenchymal transition; TGF-β1, transforming growth factor-β1; p-Smad2/3, phosphorylated-Smad 2/3; MAPK, mitogen-activated protein kinase.
Analysis of immunohistochemical staining
A negative/positive relative scale was used to grade the amount of E-cadherin immunostaining: negative (–), loss or reduced membrane expression of E-cadherin; and positive (+), preserved membrane expression of E-cadherin in TECs. A semiquantitative assessment of vimentin, fibronectin, TGF-β1, p-Smad2/3, β1 integrin, p38 MAPK and WNT5B staining in TECs was performed as follows: –, no immunostaining; +, less than 10% immunostaining; ++, 10% to 25% immunostaining; +++, 25% to 50% immunostaining, and ++++, greater than 50% immunostaining. For β-catenin, cytoplasmic and nuclear staining of TECs was assessed as described above. The results were analyzed independently by two pathologists, who were unaware of the patients’ clinicopathologic variables. Images were captured by a digital camera with OLYMPUS (Tokyo, Japan) BX51 microscopy.
Statistical analysis
Comparisons between the groups were asse-ssed by Fisher’s exact test. A value of P < 0.05 was considered statistically significant. All statistical analyses were performed by the software IBM SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA).
Results
Normal human kidneys
The expression of E-cadherin appeared in TECs of the distal tubules and collecting ducts rather than in TECs of the proximal tubules (Figures 2A and 3A). Periodic acid Schiff (PAS) stain demonstrated a characteristic brush border of the proximal tubule that was not stained by E-cadherin (Figure 2B). The expression of vim-entin was observed in smooth muscle cells of blood vessels and in a few scattered interstitial cells, but not in TECs (Figure 3B). The expression of fibronectin, TGF-β1, p-Smad2/3, p38 MAPK, and WNT5B was not identified in TECs (Figures 3C, 4A, 4B, 4D and 5A). On the other hand, β1 integrin was weakly expressed in TECs (Figure 4C). β-catenin was expressed in the cytoplasmic membranes of TECs (Figure 5B).
Figure 2.
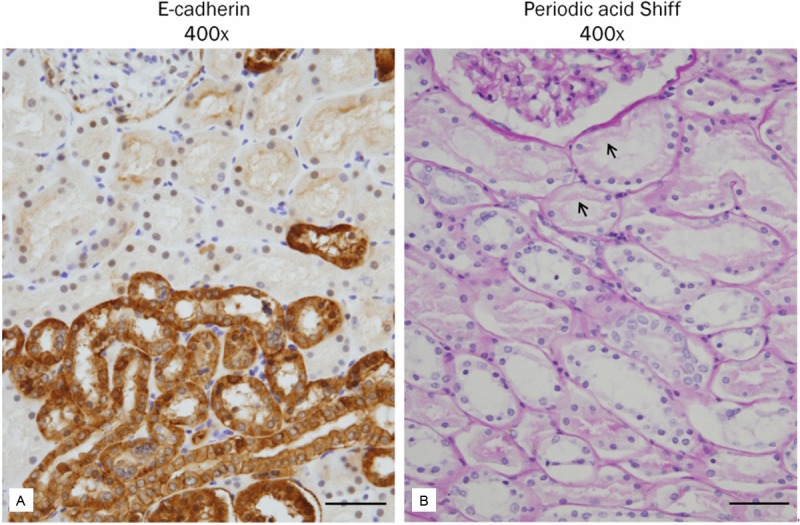
Differential expression of E-cadherin in the proximal and distal tubules of human kidney. A: E-cadherin is widely expressed in the distal tubules and collecting ducts, but not in the proximal tubules. B: The proximal tubule demonstrates a characteristic brush border (periodic acid Shiff). Scale bars: 50 μm.
Figure 3.
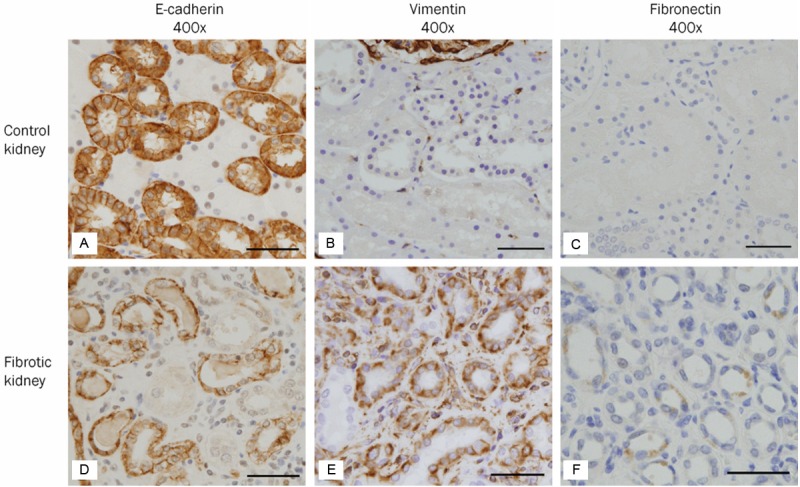
Expression of biomarkers suggesting phenotypic transition in human fibrotic kidneys. A-C: In the control kidney, E-cadherin (A), an epithelial biomarker, is strongly expressed in the cytoplasmic membrane of distal tubular and collecting duct epithelial cells. Vimentin (B) and fibronectin (C), mesenchymal biomarkers, are not expressed in tubular epithelial cells. A few scattered interstitial cells and smooth muscle cells of blood vessels express vimentin. D-F: In the fibrotic kidney, the expression of E-cadherin (D) is significantly decreased in cuboidal epithelial cells with intact tubular basement membrane surrounding interstitial inflammation and fibrosis. On the other hand, de novo expression of vimentin (E) and fibronectin (F) is observed in tubular epithelial cells in the areas of interstitial inflammation and fibrosis. Scale bars: 50 μm.
Figure 4.
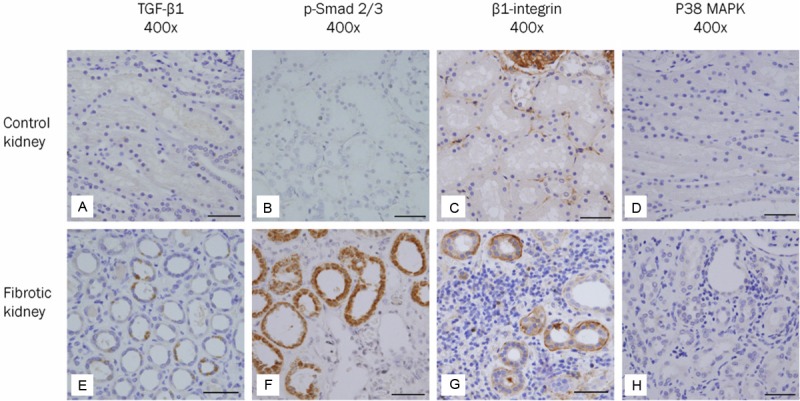
Expression of molecules involved in the TGF-β signaling and integrin/integrin-linked kinase (ILK) signaling pathways in human fibrotic kidneys. A-D: In the control kidney, tubular epithelial cells do not express TGF-β1 (A), p-Smad2/3 (B) and p38 MAPK (D). On the other hand, tubular epithelial cells weakly express β1-integrin (C). E-H: In the fibrotic kidney, expression of TGF-β1 (E), p-Smad2/3 (F) and β1-integrin (G) is observed in some cuboidal epithelial cells with intact tubular basement membrane surrounding interstitial inflammation and fibrosis. However, the expression of p38 MAPK (H) is not detected in tubular epithelial cells. Scale bars: 50 μm.
Figure 5.
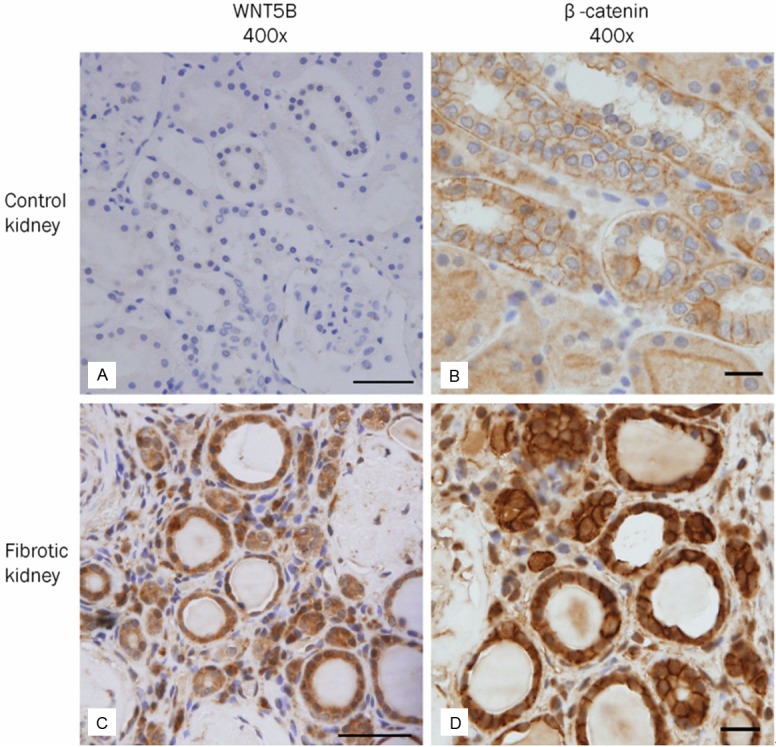
Expression of molecules involved in the wnt/β-catenin signaling pathway in human fibrotic kidneys. A, B: In the control kidney, tubular epithelial cells do not express WNT5B (A). Membranous expression of β-catenin (B) in tubular epithelial cells is characteristic. C, D: In the fibrotic kidney, a large number of tubular epithelial cells with intact basement membrane show up-regulation of WNT5B (C) and β-catenin (D) accompanied by positive nuclear staining. Several interstitial cells in the areas of interstitial inflammation and fibrosis are also positive for WNT5B and β-catenin. Scale bars: A, C = 50 μm, B, D = 20 μm.
Expression of biomarkers suggesting phenotypic transition in human fibrotic kidneys
Phenotypic change of TECs was examined on the basis of loss or decrease of the epithelial marker, E-cadherin, and de novo expression of mesenchymal markers, vimentin and fibronectin. The expression of E-cadherin was shown to disappear or decrease in some TECs of each case (Figure 3D). On the other hand, vimentin was expressed in a large number of cuboidal or flattened epithelial cells with intact or damaged TBM in all cases (Figure 3E). The expression of fibronectin was detected in a small number of TECs (Figure 3F). The differential expression of these EMT-related biomarkers was marked in the areas of interstitial inflammation and fibrosis.
Expression of molecules involved in the TGF-β signaling and integrin-linked kinase (ILK) signaling pathways in the type II EMT process
We analyzed the tubular expression of molecules involved in each pathway of TGF-β1/Smad, TGF-β1/non-Smad and integrin/ILK signaling. The expression of TGF-β1, p-Smad2/3 and β1 integrin was regulated significantly upward in some TECs with intact TBM in the areas of interstitial inflammation and fibrosis (Figure 4E-G). However, the expression of p38 MAPK, an intracellular mediator of TGF-β1/non-Smad pathway, was not detected in TECs (Figure 4H).
Expression of molecules involved in the wnt/β-catenin signaling pathway in the type II EMT process
In human fibrotic kidneys, a large number of TECs with intact TBM showed up-regulation of WNT5B and β-catenin, accompanied by nuclear staining (Figure 5C and 5D). A few interstitial cells in the areas of inflammation and fibrosis were also stained for WNT5B and β-catenin. The differential expression of type II EMT-related molecules and possible signaling pathways in human renal fibrogenesis are summarized in Table 3.
Table 3.
Overview of the differential expression of EMT-related molecules and possible signaling pathways in human renal fibrogenesis
| Case No. | Epithelial biomarker | Mesenchymal biomarkers | Signaling molecules (Inducers and mediators) | Pathway involved in type II EMT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| E-cadherin | Vimentin | Fibronectin | TGF-β1 | p-Smad2/3 | β1-integrin | p38 MAPK | WNT5B | β-catenin | ||
| 1* | + | - | - | - | - | - | - | - | - | - |
| 2* | + | - | - | - | - | - | - | - | - | - |
| 3* | + | - | - | - | - | - | - | - | - | - |
| 4* | + | - | - | - | - | - | - | - | - | - |
| 5 | - | +++ | + | ++ | ++ | + | - | ++ | ++ | 1, 2, 3 |
| 6 | - | ++ | - | - | - | - | - | +++ | ++ | 3 |
| 7 | - | ++ | + | + | + | + | - | - | + | 1, 2 |
| 8 | - | +++ | - | + | + | + | - | + | + | 1, 2, 3 |
| 9 | - | ++ | - | - | - | - | - | ++ | + | 3 |
| 10 | - | ++ | - | + | + | + | - | +++ | ++ | 1, 2, 3 |
| 11 | - | ++ | - | + | + | ++ | - | + | + | 1, 2, 3 |
| 12 | - | +++ | - | + | ++ | ++ | - | + | + | 1, 2, 3 |
| 13 | - | ++ | - | + | + | + | - | + | + | 1, 2, 3 |
| 14 | - | ++ | - | + | + | + | - | + | + | 1, 2, 3 |
| 15 | - | +++ | ++ | ++ | ++ | ++ | - | - | + | 1, 2 |
| 16 | - | +++ | - | + | + | + | - | + | + | 1, 2, 3 |
| 17 | - | ++ | - | - | - | - | - | + | + | 3 |
| 18 | - | ++ | - | + | + | + | - | + | + | 1, 2, 3 |
| 19 | - | +++ | - | - | - | - | - | + | + | 3 |
| 20 | - | ++ | - | + | + | + | - | ++ | + | 1, 2, 3 |
| 21 | - | + | - | - | - | - | - | + | + | 3 |
| 22 | - | ++ | - | - | - | - | - | ++ | + | 3 |
| 23 | - | ++ | - | + | + | + | - | + | + | 1, 2, 3 |
No, number;
control;
TGF-β1, transforming growth factor-β1; p-Smad2/3, phosphorylated-Smad2/3; MAPK, mitogen-activated protein kinase; EMT, epithelial to mesenchymal transition. In cases of E-cadherin stain, positive (+), preserved membrane expression of E-cadherin; negative (–), loss or reduced membrane expression of E-cadherin in tubular epithelial cells. In cases of vimentin, fibronectin, TGF-β1, p-Smad2/3, β1 integrin, p38 MAPK, and WNT5B stains, -, no immunostaining; +, less than 10% immunostaining; ++, 10% to 25% immunostaining; +++, 25% to 50% immunostaining, and ++++, greater than 50% immunostaining. For β-catenin, the same scale for nuclear staining was applied. In cases of involved pathway, 1, TGF-β/Smad; 2, integrin/ILK; 3, wnt/β-catenin signaling pathway.
Relationship between clinical data and EMT-related signaling pathways
We compared the clinical parameters reflecting renal function by each of the EMT-related signaling pathways. Associations between serum creatinine, proteinuria, hematuria or pyuria and EMT-related signaling pathways were not statistically significant (Table 4).
Table 4.
Relationship between clinical data and EMT-related signaling pathways
| Variable | EMT-related signaling pathways | No. of involved signaling pathways | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| TGF-β/Smad (n = 13) | Integrin/ILK (n = 13) | Wnt/β-catenin (n = 17) | 1 (n = 6) | 2 (n=2) | 3 (n = 11) | |
| S-Cr > 1.5 mg/dL | ||||||
| - | 8 (61.5%) | 8 (61.5%) | 12 (70.6%) | 5 (26.3%) | 1 (5.3%) | 7 (36.8%) |
| + | 5 (38.5%) | 5 (38.5%) | 5 (29.4%) | 1 (5.3%) | 1 (5.3%) | 4 (21.1%) |
| p-value† | 0.605 | 0.605 | 1.000 | 0.635 | ||
| Proteinuria | ||||||
| - | 5 (38.5%) | 5 (38.5%) | 7 (41.2%) | 2 (10.5%) | 0 (0%) | 5 (26.3%) |
| + | 8 (61.5%) | 8 (61.5%) | 10 (58.8%) | 4 (21.1%) | 2 (10.5%) | 6 (31.6%) |
| p-value† | 1.000 | 1.000 | 0.509 | 0.804 | ||
| Hematuria | ||||||
| - | 8 (61.5%) | 8 (61.5%) | 12 (70.6%) | 5 (26.3%) | 1 (5.3%) | 7 (36.8%) |
| + | 5 (38.5%) | 5 (38.5%) | 5 (29.4%) | 1 (5.3%) | 1 (5.3%) | 4 (21.1%) |
| p-value† | 0.605 | 0.605 | 1.000 | 0.635 | ||
| Pyuria | ||||||
| - | 5 (38.5%) | 5 (38.5%) | 7 (41.2%) | 4 (21.1%) | 2 (10.5%) | 3 (15.8%) |
| + | 8 (61.5%) | 8 (61.5%) | 10 (58.8%) | 2 (10.5%) | 0 (0%) | 8 (42.1%) |
| p-value† | 0.350 | 0.350 | 0.211 | 0.095 | ||
EMT, epithelial to mesenchymal transition; No, number; TGF-β, transforming growth factor-β; ILK, integrin-linked kinase; S, serum; Cr, creatinine; -, absent; +, present;
Calculated by Fisher’s exact tests for categorical variables;
Hematuria, defined as ≥ 3 red blood cells per high power field on urinalysis with microscopy; Pyuria, defined as > 5-8 white blood cell per high power field of unspun, mid-stream urine.
Discussion
Accumulation of interstitial fibroblasts is associated with progressive renal fibrosis, resulting in end-stage renal failure [1]. In renal fibrogenesis, several cell types are known to undergo phenotypic transition to matrix-producing interstitial fibroblasts after injury, participating in type II EMT; TECs [2,3,23], resident interstitial fibroblasts [24], circulating fibrocytes [23,24], vascular pericytes [25], endothelial cells [26] and glomerular podocytes [27]. Tubular EMT is a highly regulated process consisting of four steps [28]; (1) loss of epithelial cell adhesion, (2) de novo expression of mesenchymal markers such as α-SMA and reorganization of actin cytoskeleton, (3) disruption of TBM, and (4) enhancement of cell migration and invasion. The current study demonstrated a phenotypic transition of a large number of TECs that lost their epithelial phenotype and acquired mesenchymal features, by observing the loss or decreased expression of E-cadherin and de novo expression of vimentin and fibronectin in the areas of interstitial inflammation and fibrosis. These findings suggest additional evidence for the possibility of tubular EMT during renal fibrosis in humans. An additional finding in this study is that E-cadherin expression was abundant in the distal tubules and collecting ducts, but not in the proximal tubules of normal kidneys. This finding is similar to the result of Chea and coworker which showed high expression of E-cadherin in human distal tubules [29].
In fact, in human tissues, it is impossible to identify a serial process showing a change from TECs to fibroblasts, followed by disruption of the integrity of TBM and then migration into the interstitium. Only a histologic ‘snapshot’ exhibiting the coexistence of epithelial and mesenchymal markers can be used to identify transitioning TECs in the midst of the EMT process, like in previous studies using human renal biopsies [4,5]. Moreover, it is difficult to differentiate whether the expression of EMT-related molecules in damaged tubular cells means transitioning tubular cells or entered interstitial fibroblasts into the tubules through the damaged TBM. However, if TECs with intact TBM express EMT-related molecules, it clearly indicates TECs in the midst of the EMT process. In the present study, this phenotypic change was observed in TECs with intact TBM, and the majority of TECs still preserved their distinctive cuboidal appearance.
Renal fibrosis is generally considered a failure of tissue injury/repair response, which is closely associated with chronic interstitial inflammation [1,2,6]. In response to various environmental or injurious stimuli, TECs produce various chemokines and cytokines, and attract various inflammatory cells to the tubulointerstitial space [1,2,6]. Infiltrating cells in turn produce proinflammatory cytokine IL-1, profibrotic cytokine TGF-β1, and other cytokines, establishing a fibrogenic environment in the interstitial space that drives phenotypic transition of TECs [6]. On the other hand, Akt [16,30] and Snail [31] are known to play an important role in modulating cell survival during the EMT process (Figure 1). In the current study, apoptosis of TECs was rarely observed even in the areas of severe interstitial inflammation. This finding suggests that tubular EMT could be an adaptive response of TECs to chronic injury for the sake of escaping apoptosis, as mentioned by Liu [6].
EMT is a complex biological process, involving the TGF-β/Smad, TGF-β/non-Smad, Integrin/ILK, or wnt/β-catenin signaling pathway. In the TGF-β/Smad pathway, TGF-β1-mediated activation of Smad2 and Smad3 leads to forming complexes with Smad4, which then enter the nucleus where they control the transcription of various TGF-β1 responsive EMT-related genes, such as ILK, β1-integrin, Wnt, Snail, and so on (Figure 1) [6,8,12,13]. In the current study, cuboidal or flattened tubular cells showed significant up-regulation of p-Smad2/3 expression in the areas of interstitial inflammation and fibrosis. The up-regulation of p-Smad2/3 expression corresponded to the increase of TGF-β1 expression. Moreover, there was a highly significant correlation between the expression of TGF-β1 and p-Smad2/3 and the expression of TGF-β1 responsive EMT-related genes including β1-integrin and WNT5B in TECs (case No. 5, 7, 8, 10-16, 18, 20 and 23). TGF-β1-induced, Smad-dependent expression of β1-integrin or WNT5B in TECs suggests that the TGF-β/Smad signaling pathway is involved in the tubular EMT process in human fibrotic kidneys. Despite the well-known crucial role of TGF-β1 in the EMT process, the reason why TGF-β1 was not widely expressed in TECs may be that TGF-β1 is involved as an inducer in the early step of the tubular EMT process [28].
The TGF-β1 signaling pathway is mainly mediated by Smad proteins. However, via a Smad-independent pathway, TGF-β1 can also activate p38 MAPK, leading to stabilization of β-catenin by escaping from ubiquitin-mediated degradation. Nuclear translocation of β-catenin in turn increases the transcription of the TGF-β1 responsive EMT-related gene (Figure 1) [6,14-16]. In the present study, TECs did not express p38 MAPK at all. Thus, the TGF-β1/non-Smad signaling pathway may not be involved in the tubular EMT process in human fibrotic kidneys.
ILK expression in TECs can be induced by TGF-β1 or ECM [6,8]. TGF-β1-induced, Smad-dependent ILK activation phosphorylates and activates Akt, leading to suppression of apoptosis and inhibition of GSK-3β [6,8,17,30]. This signal transduction participates in the EMT process through action of β-catenin [6,8,17]. ECM-induced ILK expression also inhibits GSK-3β, and then stabilizes β-catenin, resulting in EMT (Figure 1) [6,8]. In the current study, 13 cases presented increased expression of TGF-β1, p-Smad2/3, TGF-β1 responsive EMT-related genes (β1-integrin or WNT5B) and β-catenin without expressing p38 MAPK in TECs (case No. 5, 7, 8, 10-16, 18, 20 and 23). Thus, TGF-β1-induced, Smad-dependent ILK expression in TECs indicates the possibility of tubular EMT via the Integrin/ILK signaling pathway. However, the possibility of co-existence of the TGF-β1/Smad and wnt/β-catenin signaling pathways cannot be completely excluded.
Wnt proteins, a highly conserved family of secreted growth factors, interact with frizzled (Fzd) receptors and co-receptors of low density lipoprotein receptor-related protein (LRP) 5/6, inducing a serial of downstream signaling events involving Disheveled (Dvl), axin, adenomatosis polyposis coli (APC) and GSK-3β. This signal transduction leads to stabilization and nuclear translocation of β-catenin. β-catenin then binds to the T cell factor/ lymphoid enhancer-binding factor-1 (TCF/LEF-1) complex, stimulating transcription of β-catenin target genes such as Snail, Twist, LEF-1, and so on (Figure 1) [6,18-20]. Although WNT5B is not up-regulated in the renal fibrosis of UUO mouse models [20,32], the current study showed that WNT5B expression is indeed significantly up-regulated. The expression of WNT5B, accompanied by up-regulation of only β-catenin, suggests the occurrence of tubular EMT via the wnt/β-catenin signaling pathway in human fibrotic kidneys (case No. 6, 9, 17, 19, 21 and 22).
The expression of proteins involved in specific signaling pathways leading to EMT was definitely observed in TECs, consistent with the results of in vitro or in vivo studies that showed transdifferentiation of TECs to fibroblasts via each signaling pathway [12,14,17,18]. Therefore, the current study provides the possibility of tubular EMT through complex interconnections of the TGF-β/Smad, integrin/ILK and wnt/β-catenin signaling pathways rather than through one signaling pathway during renal fibrosis in humans. Although we superficially examined the signaling pathways leading to EMT through a diverse immunohistochemical staining procedures, we hope that this study can contribute to the understanding of the mechanism leading to renal fibrosis in humans.
In conclusion, TECs in the areas of interstitial inflammation and fibrosis showed decreased expression of epithelial biomarkers and de novo expression of mesenchymal biomarkers, showing significant correlation with increased expression of EMT-related inducers and mediators. In human fibrotic kidneys, the type II EMT process is thought to be an adaptive response of TECs to chronic injury and is regulated by interconnections of the TGF-β/Smad, integrin/ILK and wnt/β-catenin signaling pathways.
Acknowledgements
The authors greatly appreciate Byung-Ho Ko of Department of Digital Contents, Keimyung College University for the graphic assistance. This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu, Republic of Korea (2012).
Disclosure of conflict of interest
None.
References
- 1.Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- 2.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, Lan HY. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001;38:761–769. doi: 10.1053/ajkd.2001.27693. [DOI] [PubMed] [Google Scholar]
- 5.Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D'Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 2011;80:41–50. doi: 10.1038/ki.2011.77. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J. 2006;393:601–607. doi: 10.1042/BJ20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38 MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 15.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docherty NG, O'Sullivan OE, Healy DA, Murphy M, O'Neill AJ, Fitzpatrick JM, Watson RW. TGF-beta1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Renal Physiol. 2006;290:F1202–1212. doi: 10.1152/ajprenal.00406.2005. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang I, Seo EY, Ha H. Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease. Arch Pharm Res. 2009;32:1653–1662. doi: 10.1007/s12272-009-2200-3. [DOI] [PubMed] [Google Scholar]
- 21.Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, Miller SD, Peterson AC, Turk TM, Weitzel W. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473–2481. doi: 10.1016/j.juro.2012.09.078. [DOI] [PubMed] [Google Scholar]
- 22.Dieter RS. Sterile pyuria: a differential diagnosis. Compr Ther. 2000;26:150–152. doi: 10.1007/s12019-000-0001-1. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 25.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 26.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chea SW, Lee KB. TGF-beta mediated epithelial-mesenchymal transition in autosomal dominant polycystic kidney disease. Yonsei Med J. 2009;50:105–111. doi: 10.3349/ymj.2009.50.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172–2178. [PubMed] [Google Scholar]
- 31.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]


