Abstract
Despite the recent realization of Interleukin (IL)-35 in tumorigenesis, its exact impact on colorectal cancer (CRC) progression and prognosis, however, is yet to be elucidated clearly. We thus in the present report conducted comparative analysis of IL-35 levels between CRC patients and matched control subjects. IL-35 is highly expressed in all CRC tissues, which can be detected in vast majority of colorectal cancer cells. IL-35 levels in CRC lysates and serum samples are highly correlated to the severity of malignancy and the clinical stage of tumor. Particularly, a significant reduction for serum IL-35 was noted in patients after surgical resection, indicating that IL-35 promotes CRC progression associated with poor prognosis. Mechanistic study demonstrated a significant correlation between serum IL-35 levels and the number of peripheral regulatory T (Treg) cells in CRC patients, suggesting that IL-35 implicates in CRC pathogenesis probably by inducing Treg cells, while cancer cell-derived IL-35 may also recruit Treg cells into the tumor microenvironment in favor of tumor growth. Together, our data support that IL-35 could be a valuable biomarker for assessing CRC progression and prognosis in clinical settings.
Keywords: Interleukin-35, colorectal cancer, regulatory T cell, EBI3, IL-12p35
Introduction
Interleukin-35 (IL-35) is a heterodimeric cytokine composed of the p35 subunit of IL-12 and the Epstein-Barr Virus (EBV)-induced gene 3 (EBI3) subunit identified in B lymphocytes based on its induction following EBV infection [1-4]. Previous studies demonstrated that IL-35 is expressed in non-stimulated murine regulatory T cells (Tregs) [4] and in stimulated human Tregs [1,4,5]. Therefore, IL-35 is considered to be a characteristic factor for Treg cells [1,4,6-9], although its expression in non-stimulated human Tregs has yet to be detected [10]. Indeed, there is evidence supporting that IL-35 is effective to suppress the functionality of effector T cells such as Th1, Th2 and Th17 cells, and by which it attenuates the progression of inflammatory diseases and autoimmune diseases [1,4,11-14].
More recently, gene-expression analysis revealed that IL-35 may have a broader tissue distribution other than that observed in Tregs [15]. It was noted that EBI3 and IL-12p35 were upregulated in the placental trophoblasts [16], and EBI3 associates with p35 in the extracts of the trophoblastic components of human full-term normal placenta [2]. EBI3 has also been found expressed in Hodgkin lymphoma cells [17] and acute myeloid leukemia cells [18]. Immunohistochemical analysis further revealed that EBI3 and IL-12p35 are highly expressed in tumor tissues originated from lung cancer, colon cancer, esophageal carcinoma, hepatocellular carcinoma and cervical carcinoma [19]. Indeed, IL-35 can be induced in human cancer cell lines following TNF-α and IFN-γ stimulation [19]. Together, these data support a role for IL-35 in tumor development and metastasis.
In the tumor microenvironment, Foxp3+ Tregs and other Tregs are considered to be the primary IL-35 producer [20,21]. Treg-derived IL-35 has been found to potently inhibit antitumor T cell responses [13]. In line with this notion, knockdown of EBI3 inhibited lung cancer cell proliferation, while upregulation of EBI3 promoted lung cancer cell growth [22]. Therefore, EBI3 expression is considered to be correlated to poor prognosis for human lung cancer [22]. Other than Treg cells, tumor-infiltrating dendritic cells (DCs) are also found to express EBI3 [17,23], and tumor cells can produce EBI3 and IL-12p35 as well. Indeed, tumor-derived IL-35 induces CD11b+Gr1+ myeloid cell accumulation in the tumor microenvironment, and thereby, promotes tumor angiogenesis [24]. While these discoveries are important and exciting, the exact impact of IL-35 on tumorigenesis, particularly in human colorectal cancer development and metastasis, is yet to be fully addressed. We herein, in the present report, conducted studies in colorectal cancer (CRC) patients to address this question. Our data revealed that IL-35 is highly expressed in all CRC tissues and is detected in vast majority of tumor cells. Particularly, much higher levels of p35 and EBI3 expressions are detected in poorly differentiated CRC patients than that of moderately and well differentiated CRC patients. Moreover, serum IL-35 levels are positively correlated to Treg numbers in the peripheral blood of CRC patients, and a significant reduction of serum IL-35 was noted in patients after tumor resection. Together, our data support that IL-35 could be a valuable biomarker for assessing CRC progression and prognosis in clinical settings.
Patients and materials
Patient collection
A total of 50 CRC patients undergone large bowel resection were collected at the Department of Surgery of Affiliated Hospital of Guangdong Medical College in Zhanjiang and the Second Clinical Medical School of Guangdong Medical College in Dongguan. The collections are composed of 28 male patients and 22 female patients with a mean age of 51.1 yr old (21~78 yr old). Both cancer and normal tissues (> 5 cm away from cancer tissues) were obtained from operative specimens and stored in -80°C until use. For normal controls, 50 healthy volunteers (HV) were organized by the Affiliated Hospital of Guangdong Medical Examination Center, which included 27 males and 23 females with a mean age of 48.1 yr old (24~78 yr old). Blood samples were collected before and after surgery (> 7 days after surgery). Serum aliquots (200 μl for each) were rapidly frozen and stored in a -80°C freezer until use. PBMCs were isolated from peripheral blood samples and then subjected to flow cytometry analysis immediately. Patients with recent blood transfusion or immunotherapy were excluded from the study. Informed consent was obtained from all study subjects, and the studies were approved by the Internal Review Board of Human Assurance Committee at the Guangdong Medical College.
Immunofluorescence and immunohistochemistry
Tissue sections (4-μM) were prepared from frozen tissue blocks and then subjected to Hematoxylin & Eosin (HE) staining as reported [25]. The results were assessed by two pathologists to demonstrate the presence of tumor and the proportion of tumor cells in each section. For immunostaining, tissue sections were subjected to antigen retrieval with sodium citrate (pH 6.0), followed by staining with antibodies against IL-12 p35 (sc-7925, Santa Cruz Biotechnology, Inc.) or EBI3 (sc-32868, Santa Cruz Biotechnology, Inc.) overnight at 4°C, respectively. After washes, the sections were stained with a goat anti-rabbit IgG labeled with either PE (bs-0295G-PE, Beijing Biosynthesis Biotechnology Co., Ltd) or FITC (bs-0295G-FITC, Beijing Biosynthesis Biotechnology Co., Ltd) [26]. The sections were then assessed for p35 and EBI3 expression under a fluorescent microscope (Nikon Ti-U). Immunohistochemistry (IHC) was next employed for quantitative analysis of p35 and EBI3 expression levels, which included 50 cancer sections and 50 normal sections. A rabbit polyclonal antibody against IL-12p35 (sc-7925, Santa Cruz Biotechnology, Inc.) or EBI3 (sc-32868, Santa Cruz Biotechnology, Inc.) was applied for the analysis as previously reported [27]. In brief, the slides were incubated in H2O2 solution for 10 min at room temperature to block the endogenous peroxidase activity. Antigenic epitopes were next retrieved by heating for 5 min in 10 mmol/L citrate buffer (pH 6.0). The slides were then first incubated with the above indicated primary antibodies for 30 min at room temperature, followed by a secondary peroxidase anti-rabbit IgG at 1:500 dilutions.
Score of immunohistochemical sections
All sections were assessed by two pathologists in a blinded fashion to the clinical status of the patients. The immunoreactive area for EBI3 and p35 was scored as 1 (0~25%), 2 (25~50%), 3 (50~75%) or 4 (> 75%), as previously reported by Michalski and colleagues [28]. Based on the intensity of staining, 0 scored with absent of staining, 1 scored as weak staining, and 2 scored as strong staining. The final score was established by the average scores from two pathologists. Cases with high variations between two pathologists were evaluated again without the knowledge of previous results.
ELISA analysis
Frozen tissues (1 gm) were homogenized in a glass homogenizer in 200 μl PBS, supernatants were collected after centrifugation. A human IL-35 ELISA kit (BlueGene, Shanghai, China) was employed for analysis of IL-35 levels in the tissue supernatants and serum samples using the established techniques [29].
Flow cytometry analysis
Isolated PBMCs were resuspended in FACS buffer (PBS with 1% BSA and 0.1% sodium azide), and then subjected to staining with FITC labeled mouse anti-human CD4 (BD Phar-mingen, San Jose, CA), APC labeled mouse anti-human CD25 (BD Pharmingen, San Jose, CA), and PE labeled mouse anti-human Foxp3 (BD Pharmingen, San Jose, CA). After washes, the stained cells (2 x 106) were resuspended in 400 μL FACS buffer containing 2% paraformaldehyde and then analyzed via three-color flow cytometry (Epics Altre II, Beckman Coulter) as reported [30]. A mouse IgG isotype was served as negative controls. All data were analyzed using the Flowjo.7.6.1 software (Treestar, Ashland, OR, USA).
Statistical analysis
The normality test was first performed to determine whether our dataset was well-modeled by a normal distribution. Student’s t-test was employed for analysis of two-sample and two-tailed comparisons. Pearson correlation was used to measure the degree of dependency between variables by GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA). In all cases, p < 0.05 was considered with statistical significance.
Results
Demographics and clinical data
According to the WHO grading system, tumors in 14 patients (28%) were defined as well differentiated, 14 patients (28%) fell into the category of moderately differentiated, 10 patients (20%) were poorly differentiated, and 12 patients (24%) were diagnosed as mucinous adenocarcinoma. Based on the standard for tumor-node-metastasis (TNM) stage, 16 patients (32%) were in stage I, 13 patients (26%) were found in stage II, 14 patients (28%) were characterized in stage III, and 7 patients (14%) were defined in stage IV. Among all patients, 18 patients (36%) were noted with tumor size > 5 cm. The demographic and clinical characteristics of the selected subjects were summarized in Table 1. There was no significant difference in age and gender between CRC patients and control subjects.
Table 1.
Demographic and clinical characteristics for the enrolled subjects
| CRC patients | Healthy Volunteers | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| NO. | Gender | Age | Tumor size | Histological grade | TNM (stage) | NO. | Gender | Age |
| 1 | F | 27 | < 5 cm | Poorly | I | 1 | F | 32 |
| 2 | F | 64 | > 5 cm | Poorly | IV | 2 | M | 46 |
| 3 | F | 50 | < 5 cm | Well | III | 3 | F | 48 |
| 4 | M | 64 | < 5 cm | Moderately | I | 4 | M | 51 |
| 5 | F | 43 | > 5 cm | Poorly | III | 5 | F | 34 |
| 6 | M | 29 | < 5 cm | Adenocarcinoma | III | 6 | M | 38 |
| 7 | M | 47 | > 5 cm | Moderately | III | 7 | F | 46 |
| 8 | M | 49 | < 5 cm | Moderately | IV | 8 | F | 42 |
| 9 | F | 61 | < 5 cm | Well | III | 9 | F | 51 |
| 10 | M | 56 | > 5 cm | Well | III | 10 | M | 29 |
| 11 | M | 53 | > 5 cm | Moderately | II | 11 | M | 31 |
| 12 | F | 12 | > 5 cm | Adenocarcinoma | IV | 12 | M | 35 |
| 13 | M | 51 | < 5 cm | Moderately | IV | 13 | M | 64 |
| 14 | M | 65 | < 5 cm | Well | III | 14 | F | 62 |
| 15 | F | 56 | < 5 cm | Moderately | II | 15 | M | 45 |
| 16 | F | 78 | < 5 cm | Moderately | III | 16 | F | 58 |
| 17 | M | 54 | < 5 cm | Well | I | 17 | M | 67 |
| 18 | F | 55 | < 5 cm | Well | I | 18 | M | 38 |
| 19 | F | 57 | < 5 cm | Moderately | II | 19 | M | 42 |
| 20 | M | 50 | < 5 cm | Poorly | IV | 20 | F | 46 |
| 21 | M | 58 | > 5 cm | Adenocarcinoma | III | 21 | M | 49 |
| 22 | M | 46 | < 5 cm | Adenocarcinoma | I | 22 | F | 38 |
| 23 | M | 42 | > 5 cm | Adenocarcinoma | II | 23 | F | 65 |
| 24 | M | 34 | < 5 cm | Well | II | 24 | M | 64 |
| 25 | M | 71 | < 5 cm | Adenocarcinoma | III | 25 | F | 63 |
| 26 | M | 67 | < 5 cm | Well | I | 26 | M | 71 |
| 27 | M | 41 | > 5 cm | Adenocarcinoma | II | 27 | M | 70 |
| 28 | F | 45 | < 5 cm | Well | I | 28 | M | 42 |
| 29 | F | 59 | < 5 cm | Moderately | I | 29 | M | 41 |
| 30 | F | 55 | < 5 cm | Well | I | 30 | F | 36 |
| 31 | M | 47 | > 5 cm | Adenocarcinoma | II | 31 | M | 37 |
| 32 | M | 63 | < 5 cm | Well | I | 32 | M | 56 |
| 33 | M | 37 | < 5 cm | Well | II | 33 | M | 54 |
| 34 | F | 56 | > 5 cm | Moderately | III | 34 | F | 29 |
| 35 | F | 48 | > 5 cm | Poorly | IV | 35 | F | 36 |
| 36 | M | 46 | < 5 cm | Moderately | I | 36 | F | 33 |
| 37 | F | 48 | > 5 cm | Adenocarcinoma | II | 37 | M | 46 |
| 38 | M | 64 | > 5 cm | Poorly | III | 38 | F | 44 |
| 39 | F | 35 | < 5 cm | Moderately | I | 39 | M | 37 |
| 40 | M | 66 | < 5 cm | Well | II | 40 | F | 53 |
| 41 | F | 64 | < 5 cm | Well | I | 41 | M | 64 |
| 42 | M | 21 | < 5 cm | Moderately | I | 42 | M | 55 |
| 43 | F | 39 | < 5 cm | Poorly | II | 43 | F | 37 |
| 44 | F | 53 | < 5 cm | Moderately | I | 44 | M | 46 |
| 45 | M | 46 | > 5 cm | Poorly | IV | 45 | F | 47 |
| 46 | M | 55 | < 5 cm | Adenocarcinoma | III | 46 | M | 65 |
| 47 | F | 43 | < 5 cm | Poorly | II | 47 | F | 66 |
| 48 | F | 70 | < 5 cm | Adenocarcinoma | I | 48 | M | 38 |
| 49 | M | 68 | > 5 cm | Poorly | III | 49 | F | 67 |
| 50 | M | 46 | > 5 cm | Adenocarcinoma | II | 50 | F | 52 |
TNM: Tumor-node-metastasis.
p35 and EBI3 are highly expressed in colorectal cancer tissues
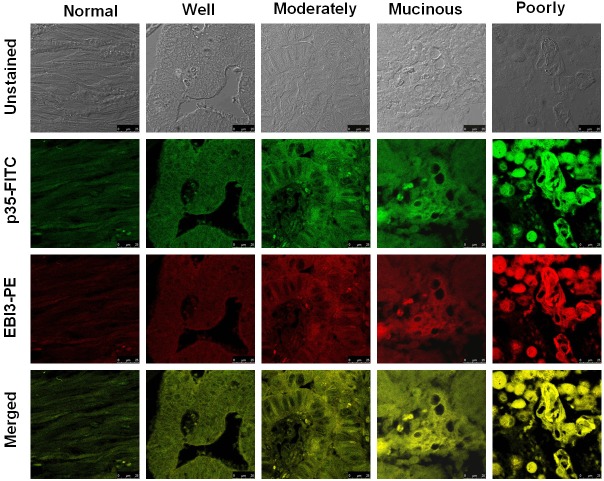
We first conducted immunostaining for detection of IL-35 expression in colorectal cancer sections using a FITC-conjugated anti-p35 antibody and a PE-conjugated anti-EBI3 antibody, respectively. It was found that both p35 and EBI3 were expressed in all CRC sections. Particularly, much higher levels of p35 and EBI3 were detected in poorly differentiated CRC samples as compared with that of well or moderately differentiated samples (Figure 1). Of note, p35 and EBI3 showed perfect co-localization in the tissue sections, demonstrating the expression of IL-35 in colorectal cancer cells.
Figure 1.
Detection of IL-35 expression in colorectal cancer tissues by immunostaining of EBI3 and p35. CRC sections were stained with FITC-conjugated anti-p35 antibody and PE-conjugated anti-EBI3 antibody, respectively, and the sections were then assessed under a fluorescent microscope by two pathologists. A perfect co-localization for the immunofluorescence of EBI3 and p35 was noted in all studied sections, indicating the expression of IL-35 in colorectal cancer cells. All images were taken under a confocal laser scanning microscope with 2000 x amplifications.
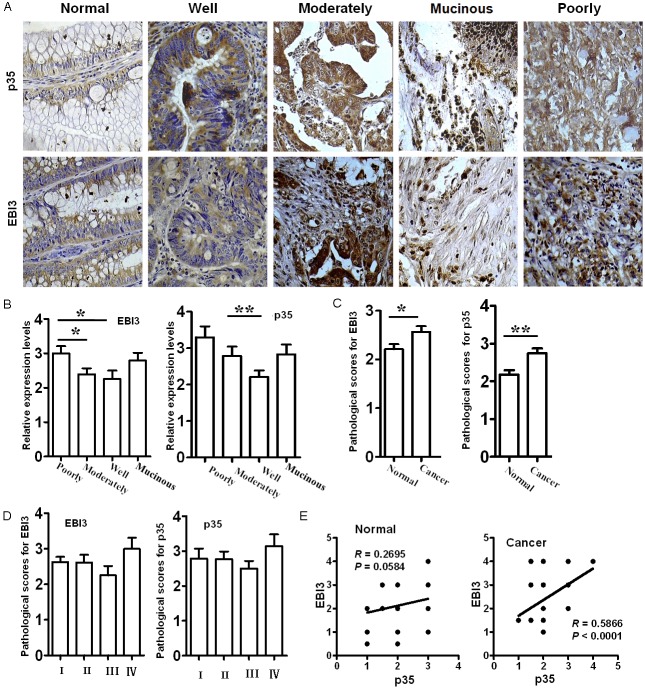
For quantitative analysis of IL-35 expression levels in different types of colorectal cancer tissues, we did immunohistochemical analysis of p35 and EBI3 as described. In line with the above results, cancer sections from all patients displayed positive reactivity for p35 and EBI3 as shown in Figure 2A. It was interestingly noted that the expression levels for p35 and EBI3 in tumors were correlated to the extent of cell differentiation, in which the highest expression was detected in poorly differentiated tumor samples, while well differentiated tumor samples showed the lowest expression, and the expression levels for moderately differentiated tumor samples were in the between of these two types of tumors (Figure 2B). However, no correlation was detected in terms of clinical stage, age and gender, and tumor size with p35 and EBI3 expressions (data not shown). Of note, high levels of p35 and EBI3 expressions were also detected in patients with mucinous adenocarcinoma (Figure 2B). The sections were next scored for immunoreactive area as detailed earlier based on the intensity of staining. In consistent with the expression data, the patients showed significantly higher scores than that of controls for both p35 and EBI3 (Figure 2C). Next, we examined p35 and EBI3 expression in different stages of tumor samples, and much higher levels of p35 and EBI3 expressions were noted in stage IV tumor samples (Figure 2D). Furthermore, p35 was noted to be positively correlated to EBI3 in colorectal cancer tissues, but not in normal tissues after Pearson correlation analysis (Figure 2E).
Figure 2.
Quantitative analysis of EBI3 and p35 expression in colorectal cancer tissues by immunohistochemistry. Immunohistochemical analysis was employed for quantitative analysis of p35 and EBI3 expression in CRC tissues. A. Representative images for the immunohistochemical staining of EBI3 and p35 in different types of CRC tissues. All images were taken under a light microscope with 400 x amplifications. B. Bar graphic figures showing the relative expression levels of EBI3 and p35 assessed in all sections. C. Pathological scores assessed based on the intensity of immunoreactive area of all sections analyzed. D. Bar graphic figures showing the differences of EBI3 and p35 expression in different stages of CRC sections. E. Results for correlation analysis of EBI3 and p35 in normal and CRC sections. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
IL-35 levels are correlated to colorectal cancer progression and prognosis
The above expression data prompted us to examine IL-35 levels in cancer tissues. For this purpose, we conducted ELISA analysis of IL-35 using CRC lysates. In line with the expression data, IL-35 levels were almost 1-fold higher in colorectal cancer tissues than that of control tissues (Figure 3A), and a similar correlation for IL-35 levels and the extent of differentiation of cancer cells (Figure 3B), as well as the clinical stages of tumors (Figure 3C) was noted. Particularly, higher levels of IL-35 were highly correlated to patients with colorectal cancer (Figure 3D). Next, we analyzed the differences of serum IL-35 levels between cancer patients and normal controls. Although serum IL-35 was likely less significantly as compared with that in cancer tissues, a similar trend was also noted (Figure 3E-G), and serum IL-35 was positively correlated to that in cancer tissues (Figure 3H). More importantly, a significant reduction for serum IL-35 was noted in all patients after tumor resection (Figure 3I). All together, those data suggest that IL-35 could be a valuable biomarker for assessing colorectal cancer progression and prognosis.
Figure 3.
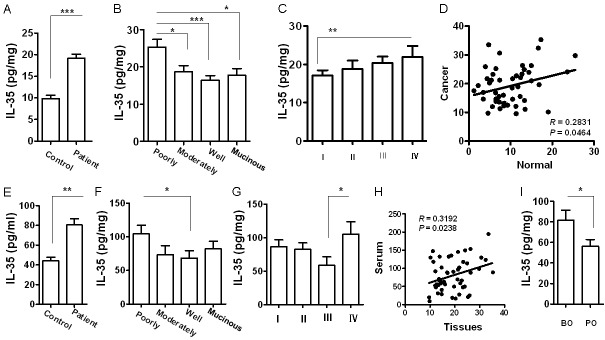
ELISA analysis of IL-35 levels in colorectal cancer lysates and serum samples. A. A bar graphic figure showing the difference of IL-35 levels between control and CRC lysates. B. IL-35 levels in different types of CRC lysates. C. IL-35 levels in different stages of CRC samples. D. Results for correlation analysis of IL-35 levels between CRC tissues and normal tissues. E. Serum IL-35 levels in controls and CRC patients. F. Serum IL-35 levels in patients with different types of colorectal cancer. G. Comparison of serum IL-35 levels between patients with different stages of colorectal cancer. H. Results for correlation analysis of IL-35 levels between serum samples and CRC lysates. I. Comparison of serum IL-35 levels in patients before and after surgical resection of tumors. BO, before operation; PO, post operation. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Serum IL-35 is associated with higher peripheral Tregs in colorectal cancer patients
Given that increased number for Treg cells is a characteristic feature for the extent of colorectal cancer malignancy and progression, we therefore next, examined the correlation between IL-35 levels and Treg numbers in the peripheral blood for dissecting the mechanisms underlying IL-35 promotion of CRC progression. To this end, we first gated CD4+CD25+ cells and then analyzed Foxp3 expression in the gated cells as shown in Figure 4A. As expected, CRC patients showed almost 1-fold higher Foxp3+ Treg cells than that of control subjects (Figure 4B). Particularly, significantly higher proportion of Foxp3+ Treg cells was noted in patients with poorly differentiated tumors as compared with that of patients with moderately or well differentiated tumors (Figure 4C), and patients in stage I displayed the lowest number for Treg cells, while highest number of Treg cells was detected in patients in stage IV (Figure 4D). Next, we conducted correlation analysis between serum IL-35 levels and peripheral Treg numbers. Remarkably, serum IL-35 levels were positively correlated to higher number of peripheral Treg cells in colorectal cancer patients (Figure 4E). Together, our data suggest that IL-35 could be a potent factor for induction of peripheral Treg cells in colorectal cancer patients, and through which it promotes CRC progression associated with poor prognosis.
Figure 4.
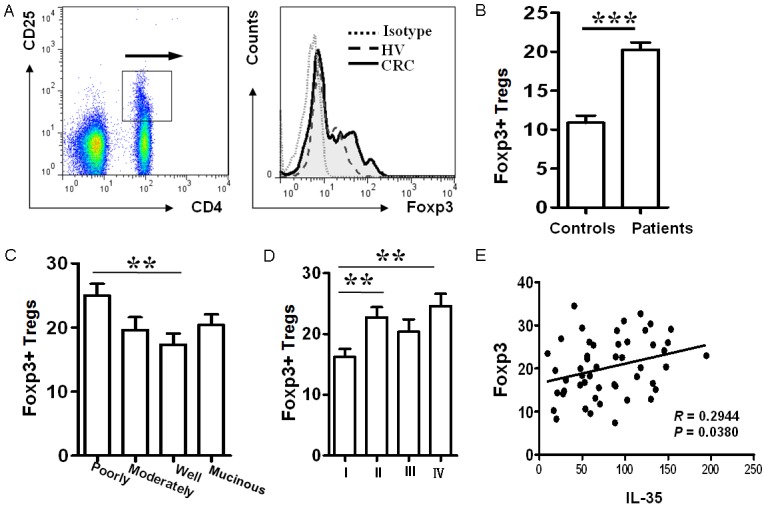
Serum IL-35 levels associate with the number of peripheral Treg cells in colorectal cancer patients. A. A paradigm for flow cytometry analysis of the peripheral Foxp3+ Treg cells. B. Comparison of peripheral Foxp3+ Treg cells between normal controls and CRC patients. A total of 50 patients and 50 matched controls were analyzed. C. The distribution of peripheral Treg cells in patients with different types of colorectal cancer. D. Comparison for the number of peripheral Treg cells between patients with different stages of colorectal cancer. E. Serum IL-35 levels are highly correlated to the number of peripheral Treg cells in CRC patients. **, p < 0.01; ***, p < 0.001.
Discussion
IL-35, a member of the IL-12 family of cytokines, is well documented as an anti-inflammatory cytokine implicated in the regulation of autoimmune diseases and allograft rejection. For example, previous studies revealed that IL-35 produced by Treg cells is potent to suppress the functionality of Th1, Th17 and Th2 cells [13]. More recently, IL-35 has been further recognized to play a pivotal role in the pathogenesis of tumor development, progression and prognosis. In general, IL-35 expression is considered with immunosuppression along with tumor progression and poor prognosis. However, contradictory results have also been reported; for example, it has been noted that ectopic IL-35 expression in human cancer cells suppresses cell growth by inducing cell cycle arrest at the G1 phase along with enhanced apoptosis [19]. Therefore, the exact impact of IL-35 on tumorigenesis, particularly on colorectal cancer (CRC) progression and prognosis, is yet to be fully addressed.
In the present report, we conducted expression analysis of IL-35 in 50 CRC patients and 50 age-matched healthy controls. We demonstrated experimental evidence indicating that IL-35 is highly expressed in vast majority of colorectal cancer cells. We next analyzed IL-35 expression levels by immunohistochemical analysis and ELISA analysis of cancer tissue samples. Our data have consistently revealed that the expression levels for IL-35 are associated with the extent of CRC malignancy and clinical stage, in which much higher levels of IL-35 were detected in poorly differentiated CRC tissues, while well differentiated CRC tissues manifested the lowest IL-35 expression as compared with that of poorly or moderately differentiated CRC tissues. Correlation analysis suggested that IL-35 is positively correlated to CRC malignancy and progression. To further confirm the results in CRC tissues, we analyzed serum IL-35 levels. Similar as the data obtained from cancer tissues, CRC patients manifested 1-fold higher serum IL-35 than that of control subjects, and higher levels of serum IL-35 were associated with the extent of CRC differentiation and clinical stage. A noteworthy finding is that patients underwent a significant reduction for serum IL-35 after surgical resection of tumor tissues. All together, our data support that IL-35 could be a valuable biomarker essential for assessing CRC progression and prognosis after surgical resection and chemotherapy in clinical settings.
To address the mechanisms underlying IL-35 promotion of CRC progression associated with poor prognosis, we performed correlation analysis between serum IL-35 levels and peripheral Treg production. Remarkably, serum IL-35 levels were highly correlated to the number of peripheral Treg cells in CRC patients. Of interestingly note, IL-35 has been found to mediate potent induction of Treg cells [13]. Given the role of Treg cells played in tumorigenesis and cancer progression, we thus assume that IL-35 enhances CRC progression at least partly by inducing Treg production.
As aforementioned, other than Treg cells, colorectal cancer cells also express high levels of IL-35. IL-35 derived from CRC cells may recruit Treg cells or other immune cells with suppressive function into the cancerous milieu, and through which it promotes CRC progression. In line with this assumption, tumor-derived IL-35 has been found to increase CD11b+Gr1+ myeloid cell accumulation in the tumor microenvironment and, thereby, promotes tumor angiogenesis [24]. Also, Olson and colleagues characterized a population of CD8+CTLA-4+ IL-35-secreting tumor Ag-specific Tregs in prostate cancer patients, which prevents Ag-specific effector responses by an IL-35-dependent mechanism [31].
In consistent with published data, we noted that IL-35 levels in the tumor tissues and peripheral blood are negatively correlated to colorectal cancer cell differentiation. Unex-pectedly, IL-35 levels have also been found to be positively correlated to the clinical stage of CRC patients. Given the role of IL-35 played in protection of tumor cells against immunity, this observation in fact further supports our original hypothesis. Indeed, as the disease evolves, higher levels of IL-35 in the tumor microenvironment are likely to be a prerequisite to ensure CRC progression and metastasis.
In summary, we demonstrated evidence that IL-35 is highly expressed in colorectal cancer cells. High levels of IL-35 in CRC tissues and peripheral blood are correlated to the extent of CRC malignancy and clinical stage. Particularly, CRC patients undergo a significant reduction of serum IL-35 after surgical resection of tumors. Mechanistic study revealed that serum IL-35 is correlated to the production of peripheral Treg cells, while IL-35 derived from colorectal cancer cells may recruit Treg cells and other immunosuppressive cells into the tumor microenvironment, and through which, IL-35 promotes CRC progression associated with poor prognosis. All together, our data support that IL-35 could be a valuable biomarker for assessing CRC progression and prognosis in clinical settings.
Acknowledgements
We are grateful to the nurses, doctors and staffs at the Department of Surgery of Affiliated Hospital of GDMC in Zhanjiang and the Department of Surgery of Second Clinical Medical School of GDMC for collection of study subjects and clinical data. This work was supported by the National Natural Science Foundation of China (30971779, 81273237), the Key Project of Science and Technology Innovation of Education Department of Guangdong Province (2012KJCX0059), the Science and Technology Project of Dongguan (2012105102016, 20131051010006) and the Science and Technology Innovation Fund of Guangdong Medical College (STIF201110).
Disclosure of conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 2.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 5.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol. 2011;187:462–471. doi: 10.4049/jimmunol.1100259. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead GS, Wilson RH, Nakano K, Burch LH, Nakano H, Cook DN. IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. J Allergy Clin Immunol. 2012;129:207–215. e201–205. doi: 10.1016/j.jaci.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141:1875–1886. doi: 10.1053/j.gastro.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 11.Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devergne O, Coulomb-L’Hermine A, Capel F, Moussa M, Capron F. Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedobitek G, Pazolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198:310–316. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- 18.Poleganov MA, Bachmann M, Pfeilschifter J, Muhl H. Genome-wide analysis displays marked induction of EBI3/IL-27B in IL-18-activated AML-derived KG1 cells: critical role of two kappaB binding sites in the human EBI3 promotor. Mol Immunol. 2008;45:2869–2880. doi: 10.1016/j.molimm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun. 2013;430:364–369. doi: 10.1016/j.bbrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 21.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 22.Nishino R, Takano A, Oshita H, Ishikawa N, Akiyama H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y. Identification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer. Clin Cancer Res. 2011;17:6272–6286. doi: 10.1158/1078-0432.CCR-11-0060. [DOI] [PubMed] [Google Scholar]
- 23.Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Bregeaud L, Perennec M, Brousse N, Kastelein R, Devergne O. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217–1228. doi: 10.1016/S0002-9440(10)62340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol. 2013;190:2415–2423. doi: 10.4049/jimmunol.1202535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Lv JW, Yang P, Yu Q, Pang J, Wang Z, Guo H, Liu S, Hu J, Li J, Leng J, Huang Y, Ye Z, Wang CY. Loss of dicer exacerbates cyclophosphamide-induced bladder overactivity by enhancing purinergic signaling. Am J Pathol. 2012;181:937–946. doi: 10.1016/j.ajpath.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, Zhang Y, Xu J, Zhang S, Yu Q, Pang J, Rao X, Kuczma M, Marrero MB, Fulton D, Kraj P, Su Y, Wang CY. SUMO1 regulates endothelial function by modulating the overall signals in favor of angiogenesis and homeostatic responses. Am J Transl Res. 2013;5:427–440. [PMC free article] [PubMed] [Google Scholar]
- 27.Rao X, Zhong J, Zhang S, Zhang Y, Yu Q, Yang P, Wang MH, Fulton DJ, Shi H, Dong Z, Wang D, Wang CY. Loss of methyl-CpG-binding domain protein 2 enhances endothelial angiogenesis and protects mice against hind-limb ischemic injury. Circulation. 2011;123:2964–2974. doi: 10.1161/CIRCULATIONAHA.110.966408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski CW, Oti FE, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Muller MW, Giese NA, Friess H, Kleeff J. Cannabinoids in pancreatic cancer: correlation with survival and pain. Int J Cancer. 2008;122:742–750. doi: 10.1002/ijc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong J, Yang P, Muta K, Dong R, Marrero M, Gong F, Wang CY. Loss of Jak2 selectively suppresses DC-mediated innate immune response and protects mice from lethal dose of LPS-induced septic shock. PLoS One. 2010;5:e9593. doi: 10.1371/journal.pone.0009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen XQ, Liu XF, Liu WH, Guo W, Yu Q, Wang CY. Comparative analysis of dendritic cell numbers and subsets between smoking and control subjects in the peripheral blood. Int J Clin Exp Pathol. 2013;6:290–296. [PMC free article] [PubMed] [Google Scholar]
- 31.Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012;189:5590–5601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]