Abstract
The pathogenesis of amyotrophic lateral sclerosis (ALS) remains unclear. Accumulating evidence indicates that various miRNAs expressed in a spatially and temporally controlled manner in the nervous system have an important function in the development of neurodegenerative diseases. The present study aimed to determine the expression and cellular distribution of miRNA-9 in the spinal cord of G93A-SOD1 mutant mice at different time points (post-natal 95, 108 and 122 d). miRNA expression was evaluated by microarray analysis; differentially expressed miRNAs were validated by RT-qPCR. The cellular distribution of miRNA-9 was analyzed by in-situ hybridization. Microarray results indicated for the first time that various miRNAs were differentially expressed between the G93A-SOD1 mutant mice and the littermate control mice. miRNA-9 expression was upregulated at 95, 108, and 122 d as validated by microarray analysis, RT-qPCR, and ISH. ISH results also showed that the miRNA-9-positive cells mainly expressed in the cytoplasm were located in the dorsal horn and the ventral horn of the spinal cord. The majority of miRNA-9-positive cells were located in the ventral horn of the gray matter, the locus of neurodegeneration. These results indicated that the differential expression of miRNA-9 may have an important function in the pathogenesis of G93A-SOD1 transgenic mice.
Keywords: ALS, miRNA-9, differential expression
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder, which is characterized by the progressive and selective loss of motor neurons in the cerebral cortex, the brainstem, and the spinal cord [1,2]. However, effective treatments to reduce motor neuron degeneration are not yet available [3]. Sporadic (no family history) and genetic (inherited) forms of ALS have been reported. The majority of ALS cases are sporadic (SALS), and approximately 5 to 10% are familial (FALS). Mutations in the superoxide mutase 1 (SOD1) gene represent one of the most commonly identified causes of FALS, accounting for approximately 20% of the cases [4]. To date, the pathogenesis of ALS remains largely unknown.
microRNAs (miRNAs) are short (approximately 22 nucleotides in length), highly conserved, and single-stranded RNA molecules that regulate gene expression by either promoting the degradation or inhibiting the translation of target mRNAs [5]. miRNAs are involved in various physiological and pathological processes, such as tumorigenesis [1,6]. Studies [7-9] have demonstrated that miRNAs are associated with the development of the central nervous system and the pathogenesis of neurodegenerative diseases. However, few studies on miRNAs in ALS have been conducted. In the present study, the differential expression of miRNAs and the distribution of miRNA-9 in the spinal cord of G93A-SOD1 transgenic mice were investigated.
Materials and methods
Animals and tissues
Transgenic mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The mice express a low copy number of a mutant form of superoxide dismutase (SOD1) with glycine-93 replaced with alanine (G93A-SOD1), which can mimic the progression of human ALS symptoms. The mice in this study were housed under standard conditions: a constant temperature of 22 ± 1 °C; 40% relative humidity; 12 h / 12 h light/dark cycle; and free access to food and water. The mice were crossbred and PCR genotyping was performed using genomic DNA from the tails of newborn mice according to the genotyping protocol of Jackson Laboratory [10]. The Animal Ethics Committee of Weifang Medical University approved the experimental protocols. The mice were then divided in two groups: G93A-SOD1 transgenic and wild-type groups. The wild-type group was used as the control group. The mice from each group were sacrificed at early (95 d), middle (108 d), and late (122 d) stages. Spinal cord samples were collected immediately. Some of the samples were stored in liquid nitrogen for microarray analysis and RT-qPCR. The other samples were perfused intracardially with 4% paraformaldehyde in 0.1 M phosphate buffer and fixed in 4% paraformaldehyde for in-situ hybridization analysis.
Detection of miRNA expression
Exiqon miRCURYTM LNA array v.18.0 containing approximately 1200 capture probes and covering all of the mouse miRNAs was used to quantify genome-wide miRNA expression in the two groups. Each sample (1 μg) was 3-end-labeled with Hy3TM fluorescent labeling kit (Exiqon, Vedbaek) and hybridized on LNA arrays according to the manufacturer’s instructions. The slides were then scanned using Axon GenePix 4000B microarray scanner. The scanned images were imported in GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. The replicated miRNAs were averaged and the miRNAs with intensities ≥ 30 in all of the samples were chosen to calculate the normalization factor. The differentially expressed miRNAs were identified by fold change filtering. Hierarchical clustering was performed to identify the distinguishable miRNA expression profiling among the samples by using MEV software (v4.6, TIGR).
RNA isolation and cDNA synthesis
RT-qPCR was performed to validate the microarray results. Total RNA was isolated from the spinal cord derived from G93A-SOD1 and wild-type mice by using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The amount of RNA was quantified using an ND-1000 spectrophotometer (Nano-drop). RNA quality was verified by measuring the OD260/OD280 ratio. The total RNA (1 μg) was reverse transcribed to produce cDNA by using M-MLV transcriptase (Promega). The following primers were used in reverse transcription: (miRNA-9), 5’-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCATACA-3’; (U6), 5’-CGCTTCACGAATTTGCGTGTCAT-3’. The SuperScript III cDNA synthesis reaction was diluted at a ratio of 1:2 by using sterile water for RT-qPCR.
RT-qPCR
Each qPCR was performed for miRNA-9 and U6 in a final volume of 20 μl. Endogenous U6 expression was used as the control treatment. The reaction system comprised different components. For miRNA-9, the following substances were used: 10 μl of 2× SoFast TMEva Green Supermix mix (BIO-RAD, Singapore); 1 μl of miRNA-9 primer assay; 4 μl of diluted cDNA, and 4 μl of sterile water. For U6, the following materials were used: 10 μl of 2× SoFast TMEvaGreen Supermix mix (BIO-RAD); 0.5 μl of U6 primer assay; 4 μl of diluted cDNA; and 5 μl of sterile water. The following primers were used in qPCR: (miRNA-9) sense, 5’-GGGTCTTTGGTTATCTAGC-3’; antisense, 5’-TGCGTGTCGTGGAGTC-3’; (U6) sense, 5’-GCTTCGGCAGCACATATACTAAAAT-3’; and antisense, 5’-CGCTTCACGAATTTGCGTGTCAT-3’. Amplification was performed using the following program cycle: initial melting temperature, 95 °C for 5 min followed by 40 cycles of 95 °C for 5 s; 60 °C for 30 s; 72 °C for 30 s; and 80 °C for 10 s. Each cDNA sample was analyzed in triplicate. The difference in the relative expression of miRNA-9 between ALS and wild-type mice was calculated using the 2-ΔΔCt method.
In situ hybridization
5-DIG labeled miRCURY LNA™ detection probe (Product no. 88078-01, sequence: TCATACAGCTAGATAACCAAAGA, Exiqon) was used for ISH to detect the miRNA-9 expression in the spinal cord. The samples were perfused intracardially with 4% paraformaldehyde in 0.1 M phosphate buffer and fixed in 4% paraformaldehyde overnight at 4 °C. The samples were then dehydrated in 30% sucrose/PB O/N at 4 °C and cut into 10 μm sections from OCT embedded FrFr blocks and mounted on clean, non-contaminated SuperFrost/Plus slides. The samples were air dried for 2 h. The sections were then fixed in 4% paraformaldehyde/PBS (pH 7.2) at 4 °C for 10 min, digested with proteinase K (Roche Diagnostics) at 10 μg/mL at 37 °C for 10 min, acetylated for 10 min, and dehydrated in fresh gradient ethanol solutions. The hybridization mixture was heated (probe final concentration: 25 nM) at 65 °C for 5 min and chilled on ice. The sections were applied with 5 μl of hybridization mix, hybridized overnight 53 °C, and covered with Nescofilm in a humidified chamber with 50% formamide, 1× SSC. The cover slips were removed from 5× SSC at room temperature (RT). The sections were washed twice for 30 min at 53 °C in 50% formamide and 0.1% Tween-20, 1× SSC. The sections were washed thrice for 15 min in 0.2× SSC and PBS at RT. The sections were then blocked in blocking solution with 10% heat-inactivated goat serum and 0.1% Tween-20 for 1 h at RT and finally incubated for 1.5 h at RT in anti-dioxygenine antibody (anti-DIG-PA Fab fragments, Roche Diagnostics) diluted to 1:800 with the blocking solution. After the sample was washed twice for 20 min with 0.1% Tween-20 in PBS, phosphatase activity was determined using nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate p-toluidine as the stabilized solution (Eurobio) for 5 h. The sections were finally mounted with 80% glycerol.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). The homogeneity of variance was performed using SPSS 13.0 software. Independent sample t-tests were performed when normality and equality of variance were observed to compare the differences in miRNA-9 between ALS and wild-type mice. The significant level was defined as p < 0.05.
Results
Differential expression of miRNAs between the G93A-SOD1 transgenic mice and the wild-type mice
Among the 1227 detected miRNAs, 45, 67, and 205 miRNAs were upregulated by twofold and 78, 68, and 116 miRNAs were downregulated by twofold in the ALS transgenic mice than in the wild-type mice at 95, 108, and 122 d, respectively (Tables 1, 2,. 3, 4, 5 and 6).
Table 1.
miRNAs upregulated by more than twofold at 95 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-137-5p | 2.452403 | mmu-miR-532-5p | 2.289189 | mmu-miR-3970 | 2.793397 |
| mmu-miR-695 | 2.783668 | mmu-miR-1958 | 2.371464 | mmu-let-7a-5p | 3.236796 |
| mmu-miR-10a-5p | 2.111037 | mmu-miR-33-5p | 2.27027 | mmu-miR-21a-5p | 2.38365 |
| mmu-miR-330-5p | 2.085822 | mmu-miR-92a-2-5p | 2.601351 | mmu-miR-488-3p | 2.043919 |
| mmu-miR-872-5p | 2.008821 | mmu-miR-107-3p | 2.135071 | mmu-miR-181b-5p | 2.834276 |
| mmu-miR-1953 | 5.809685 | mmu-miR-1892 | 2.21982 | mmu-miR-145a-5p | 2.208494 |
| mmu-miR-30a-5p | 2.00104 | mmu-miR-9-5p | 2.18264 | mmu-miR-323-3p | 2.132593 |
| mmu-miR-541-3p | 2.531624 | mmu-miR-137-3p | 2.160253 | mmu-miR-338-3p | 3.961199 |
| mmu-miR-5115 | 2.591717 | mmu-miR-344b-3p | 2.579673 | mmu-miR-3068-3p | 2.391892 |
| mmu-miR-679-5p | 2.714137 | mmu-miR-219-5p | 5.587106 | mmu-miR-346-5p | 2.65124 |
| mmu-miR-136-5p | 2.135088 | mmu-miR-770-3p | 2.01685 | mmu-miR-214-3p | 3.488871 |
| mmu-miR-29b-3p | 3.202007 | mmu-miR-29c-3p | 3.104722 | mmu-miR-701-5p | 2.211149 |
| mmu-miR-5105 | 2.055702 | mmu-miR-29a-3p | 2.744773 | mmu-miR-28a-5p | 2.307286 |
| mmu-miR-128-3p | 2.706465 | mmu-let-7f-5p | 4.536757 | mmu-miR-758-5p | 3.254054 |
| mmu-miR-883b-5p | 2.334371 | mmu-miR-18a-3p | 2.041815 | mmu-miR-380-3p | 2.220855 |
Table 2.
miRNAs downregulated by more than twofold at 95 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-27b-3p | 0.476114 | mmu-miR-193a-3p | 0.279701 | mmu-miR-3069-3p | 0.384301 |
| mmu-miR-146b-5p | 0.428458 | mmu-miR-186-5p | 0.275878 | mmu-miR-433-3p | 0.196247 |
| mmu-miR-487b-3p | 0.478313 | mmu-miR-337-3p | 0.313496 | mmu-miR-1843a-5p | 0.415933 |
| mmu-miR-340-5p | 0.351139 | mmu-miR-25-5p | 0.325169 | mmu-miR-127-5p | 0.246626 |
| mmu-miR-34c-5p | 0.44376 | mmu-miR-543-3p | 0.176221 | mmu-miR-106b-5p | 0.336679 |
| mmu-miR-139-5p | 0.438943 | mmu-miR-411-3p | 0.208108 | mmu-miR-96-5p | 0.361927 |
| mmu-miR-382-5p | 0.279887 | mmu-miR-3096a-3p | 0.228304 | mmu-miR-7a-5p | 0.273277 |
| mmu-miR-1843a-3p | 0.412089 | mmu-miR-466a-3p | 0.49247 | mmu-miR-466a-3p | 0.404655 |
| mmu-miR-29b-1-5p | 0.419107 | mmu-miR-34b-5p | 0.300033 | mmu-miR-331-3p | 0.226319 |
| mmu-miR-182-5p | 0.138739 | mmu-miR-434-5p | 0.33917 | mmu-miR-190a-3p | 0.443964 |
| mmu-miR-210-3p | 0.232322 | mmu-miR-99b-5p | 0.175193 | mmu-miR-3096a-5p | 0.409574 |
| mmu-miR-132-5p | 0.371622 | mmu-miR-495-3p | 0.288044 | mmu-miR-30c-1-3p | 0.471495 |
| mmu-miR-3094-3p | 0.416216 | mmu-miR-379-5p | 0.226204 | mmu-miR-34a-5p | 0.416461 |
| mmu-miR-342-5p | 0.180964 | mmu-miR-153-3p | 0.256331 | mmu-miR-455-3p | 0.251744 |
| mmu-miR-154-3p | 0.452409 | mmu-miR-129-5p | 0.156382 | mmu-miR-873a-5p | 0.433559 |
| mmu-miR-190a-5p | 0.354376 | mmu-miR-344-3p | 0.346847 | mmu-miR-1839-3p | 0.283954 |
| mmu-miR-674-5p | 0.203119 | mmu-miR-3069-5p | 0.244422 | mmu-miR-3475 | 0.488254 |
| mmu-miR-212-3p | 0.406146 | mmu-miR-222-3p | 0.178797 | mmu-miR-339-5p | 0.257378 |
| mmu-miR-423-3p | 0.46661 | mmu-miR-3962 | 0.450364 | mmu-miR-125b-2-3p | 0.353998 |
| mmu-miR-338-5p | 0.342989 | mmu-miR-1843b-5p | 0.201995 | mmu-miR-10b-3p | 0.405634 |
| mmu-miR-212-5p | 0.383357 | mmu-miR-219-2-3p | 0.424644 | mmu-miR-185-5p | 0.223381 |
| mmu-miR-467b-5p | 0.360187 | mmu-miR-125a-3p | 0.431444 | mmu-miR-146a-5p | 0.28815 |
| mmu-miR-298-5p | 0.171883 | mmu-miR-708-3p | 0.256667 | mmu-miR-143-3p | 0.389059 |
| mmu-miR-376a-3p | 0.221935 | mmu-miR-320-3p | 0.33144 | mmu-miR-652-3p | 0.475185 |
| mmu-miR-342-3p | 0.362724 | mmu-miR-22-3p | 0.325647 | mmu-miR-328-3p | 0.41422 |
| mmu-miR-30d-5p | 0.419268 | mmu-miR-195a-5p | 0.288666 | mmu-miR-154-5p | 0.41497 |
Table 3.
miRNAs upregulated by more than twofold at 108 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-34c-3p | 2.021835 | mmu-miR-26b-5p | 3.34617 | mmu-miR-376a-3p | 3.44564 |
| mmu-miR-3068-5p | 2.904925 | mmu-miR-125a-5p | 2.090313 | mmu-miR-342-3p | 11.08121 |
| mmu-miR-665-3p | 2.030384 | mmu-miR-101b-3p | 2.550437 | mmu-miR-219-5p | 5.073216 |
| mmu-miR-151-5p | 2.602618 | mmu-miR-369-3p | 2.406828 | mmu-let-7d-5p | 4.153295 |
| mmu-miR-465a-5p | 2.359133 | mmu-miR-128-3p | 9.513007 | mmu-miR-155-3p | 2.426607 |
| mmu-miR-136-3p | 2.516482 | mmu-miR-148b-3p | 4.393795 | mmu-miR-34b-5p | 4.448474 |
| mmu-miR-10a-5p | 2.711264 | mmu-miR-33-5p | 2.026692 | mmu-miR-434-5p | 2.278956 |
| mmu-miR-181a-5p | 4.833038 | mmu-miR-30c-5p | 2.643801 | mmu-miR-101a-3p | 2.115394 |
| mmu-miR-30a-5p | 4.727729 | mmu-miR-708-5p | 2.341402 | mmu-miR-140-5p | 6.475232 |
| mmu-miR-151-3p | 2.453423 | mmu-miR-1949 | 2.130279 | mmu-miR-377-3p | 3.186687 |
| mmu-let-7g-5p | 5.099158 | mmu-miR-210-3p | 2.678186 | mmu-miR-382-3p | 2.993366 |
| mmu-miR-744-5p | 3.089307 | mmu-miR-5099 | 3.283915 | mmu-miR-26a-5p | 3.481209 |
| mmu-miR-183-5p | 2.439446 | mmu-miR-98-5p | 2.275855 | mmu-miR-99a-5p | 2.218298 |
| mmu-miR-487b-3p | 2.050231 | mmu-miR-190a-5p | 2.904538 | mmu-miR-29c-3p | 3.181396 |
| mmu-miR-101a-3p | 4.40404 | mmu-miR-335-5p | 3.390922 | mmu-miR-320-3p | 2.008822 |
| mmu-miR-1224-5p | 2.698565 | mmu-miR-127-3p | 2.198304 | mmu-let-7i-5p | 2.472667 |
| mmu-miR-299a-3p | 2.703685 | mmu-miR-384-3p | 4.501741 | mmu-miR-126-3p | 7.362062 |
| mmu-miR-326-3p | 2.084344 | mmu-miR-181a-1-3p | 2.095356 | mmu-miR-195a-5p | 3.209104 |
| mmu-miR-5112 | 2.420771 | mmu-miR-192-5p | 2.014765 | mmu-miR-29a-3p | 3.794807 |
| mmu-miR-132-3p | 3.406183 | mmu-miR-9-5p | 3.190483 | mmu-miR-376b-3p | 3.904807 |
| mmu-miR-23a-3p | 4.193261 | mmu-miR-137-3p | 13.387 | mmu-miR-21a-5p | 2.047187 |
| mmu-miR-139-5p | 2.806742 | mmu-miR-344b-3p | 3.249742 | mmu-miR-344d-3p | 2.494471 |
| mmu-let-7d-3p | 3.009084 |
Table 4.
miRNAs downregulated by more than twofold at 108 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-711 | 0.420725 | mmu-miR-718 | 0.474571 | mmu-miR-3092-3p | 0.311307 |
| mmu-miR-149-5p | 0.492498 | mmu-miR-3102-3p.2-3p | 0.436533 | mmu-miR-3072-5p | 0.363899 |
| mmu-miR-543-5p | 0.390038 | mmu-miR-5103 | 0.387455 | mmu-miR-3473c | 0.486902 |
| mmu-miR-1247-5p | 0.450099 | mmu-miR-27a-5p | 0.483725 | mmu-miR-5119 | 0.346658 |
| mmu-miR-210-5p | 0.47395 | mmu-miR-204-5p | 0.448887 | mmu-miR-223-3p | 0.463509 |
| mmu-miR-5109 | 0.462526 | mmu-miR-292-5p | 0.498894 | mmu-miR-710 | 0.487984 |
| mmu-miR-302a-3p | 0.398372 | mmu-miR-1927 | 0.450318 | mmu-miR-883a-5p | 0.455241 |
| mmu-miR-3067-5p | 0.438496 | mmu-miR-320-5p | 0.458729 | mmu-miR-3067-3p | 0.272833 |
| mmu-miR-466c-5p | 0.499798 | mmu-miR-196a-2-3p | 0.495296 | mmu-miR-34a-5p | 0.345214 |
| mmu-miR-21a-3p | 0.478556 | mmu-miR-671-5p | 0.403675 | mmu-miR-3059-5p | 0.497316 |
| mmu-miR-804 | 0.461477 | mmu-miR-3085-3p | 0.480417 | mmu-miR-664-3p | 0.489577 |
| mmu-miR-296-5p | 0.475431 | mmu-miR-3110-5p | 0.413173 | mmu-miR-684 | 0.297214 |
| mmu-miR-20b-5p | 0.476646 | mmu-miR-874-5p | 0.48141 | mmu-miR-20a-5p | 0.414289 |
| mmu-miR-18b-3p | 0.390485 | mmu-miR-433-3p | 0.465635 | mmu-miR-674-3p | 0.392879 |
| mmu-miR-1306-5p | 0.447726 | mmu-miR-344i | 0.490352 | mmu-miR-10b-3p | 0.478392 |
| mmu-miR-5620-3p | 0.406891 | mmu-miR-764-5p | 0.260616 | mmu-miR-877-3p | 0.354168 |
| mmu-miR-5131 | 0.291022 | mmu-miR-127-5p | 0.359889 | mmu-miR-701-5p | 0.37725 |
| mmu-miR-351-5p | 0.480091 | mmu-miR-193b-3p | 0.440898 | mmu-miR-683 | 0.491099 |
| mmu-miR-3066-3p | 0.476217 | mmu-let-7f-5p | 0.456079 | mmu-miR-1907 | 0.44802 |
| mmu-miR-496a-5p | 0.351145 | mmu-miR-547-3p | 0.25347 | mmu-miR-1224-3p | 0.459508 |
| mmu-miR-1188-3p | 0.456196 | mmu-miR-3086-5p | 0.254644 | mmu-miR-3105-5p | 0.37575 |
| mmu-miR-324-3p | 0.440787 | mmu-miR-3092-5p | 0.355693 | mmu-miR-449c-3p | 0.431572 |
| mmu-miR-1193-3p | 0.396077 | mmu-miR-3544-3p | 0.496269 |
Table 5.
miRNAs upregulated by more than twofold at 122 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-711 | 2.32896 | mmu-miR-678 | 3.872368 | mmu-miR-377-3p | 179.3738 |
| mmu-miR-31-5p | 2.533274 | mmu-miR-669g | 4.480121 | mmu-miR-382-3p | 2.885197 |
| mmu-miR-103-3p | 5.285574 | mmu-miR-670-5p | 4.910772 | mmu-miR-26a-5p | 3.440347 |
| mmu-miR-1198-5p | 2.528653 | mmu-miR-467d-3p | 5.19185 | mmu-miR-374c-3p | 2.945379 |
| mmu-miR-1961 | 30.98079 | mmu-miR-145a-3p | 2.526941 | mmu-miR-3081-5p | 3.50498 |
| mmu-miR-27b-3p | 18.45688 | mmu-miR-1949 | 3.034497 | mmu-miR-296-3p | 2.526625 |
| mmu-miR-3066-5p | 2.31918 | mmu-miR-2861 | 3.601861 | mmu-miR-467b-3p | 3.343922 |
| mmu-miR-96-3p | 6.419321 | mmu-miR-153-5p | 7.510829 | mmu-miR-3968 | 2.904469 |
| mmu-miR-210-5p | 7.216136 | mmu-miR-1251-3p | 7.230803 | mmu-miR-5616-3p | 5.676448 |
| mmu-miR-350-3p | 4.543493 | mmu-miR-3091-3p | 6.118372 | mmu-miR-125a-3p | 2.911635 |
| mmu-miR-138-1-3p | 4.547348 | mmu-miR-3086-3p | 3.337121 | mmu-miR-466a-5p | 2.196768 |
| mmu-miR-5109 | 2.051979 | mmu-miR-344e-3p | 2.026537 | mmu-let-7i-5p | 3.376316 |
| mmu-miR-10a-3p | 6.047581 | mmu-miR-709 | 3.271662 | mmu-miR-505-5p | 2.68932 |
| mmu-miR-665-3p | 2.318655 | mmu-miR-3096b-5p | 6.94822 | mmu-miR-465c-5p | 2.829785 |
| mmu-miR-465a-5p | 6.432397 | mmu-miR-5099 | 2.541044 | mmu-miR-206-3p | 3.862473 |
| mmu-miR-218-5p | 4.521491 | mmu-miR-467e-5p | 2.55806 | mmu-miR-99b-3p | 3.148833 |
| mmu-miR-695 | 3.717158 | mmu-miR-98-5p | 13.226 | mmu-miR-1899 | 2.613095 |
| mmu-miR-688 | 2.885817 | mmu-miR-221-3p | 2.531232 | mmu-let-7c-5p | 9.061984 |
| mmu-miR-291b-3p | 4.303074 | mmu-miR-212-3p | 2.484969 | mmu-let-7e-5p | 3.516478 |
| mmu-let-7b-3p | 6.085008 | mmu-miR-130b-3p | 6.847116 | mmu-miR-3095-3p | 5.609635 |
| mmu-miR-3082-5p | 2.352138 | mmu-miR-200c-5p | 5.341375 | mmu-miR-3064-5p | 45.63883 |
| mmu-miR-5117-3p | 2.985543 | mmu-miR-423-3p | 2.894901 | mmu-miR-216b-3p | 2.45094 |
| mmu-miR-5616-5p | 3.482754 | mmu-miR-466d-5p | 2.605069 | mmu-miR-29a-3p | 5.427003 |
| mmu-miR-293-3p | 8.437394 | mmu-miR-668-5p | 13.64906 | mmu-miR-5107-5p | 6.240363 |
| mmu-miR-466c-5p | 2.126331 | mmu-miR-466n-3p | 3.143261 | mmu-miR-686 | 3.791119 |
| mmu-miR-574-5p | 3.488874 | mmu-miR-467b-5p | 7.416311 | mmu-miR-693-5p | 2.720883 |
| mmu-miR-292-3p | 4.438217 | mmu-miR-5624-5p | 4.839843 | mmu-miR-375-3p | 2.614048 |
| mmu-miR-181a-5p | 13.3904 | mmu-miR-3963 | 2.914163 | mmu-miR-3544-3p | 15.35104 |
| mmu-miR-466e-5p | 5.671098 | mmu-miR-425-3p | 5.801479 | mmu-miR-30c-1-3p | 3.532289 |
| mmu-miR-130a-3p | 57.04854 | mmu-miR-540-5p | 4.883808 | mmu-miR-378b | 3.299737 |
| mmu-miR-804 | 14.94782 | mmu-miR-107-3p | 5.57924 | mmu-miR-3970 | 3.227847 |
| mmu-miR-1953 | 5.237508 | mmu-miR-122-3p | 2.114285 | mmu-miR-343 | 7.173264 |
| mmu-miR-30a-5p | 6.722681 | mmu-miR-1892 | 7.617978 | mmu-miR-712-5p | 23.03883 |
| mmu-miR-541-3p | 3.076973 | mmu-miR-1904 | 2.246417 | mmu-miR-877-5p | 5.172372 |
| mmu-miR-216a-3p | 4.261151 | mmu-miR-137-3p | 8.806756 | mmu-miR-376b-3p | 4.331379 |
| mmu-miR-344c-5p | 4.25928 | mmu-miR-214-5p | 3.644928 | mmu-miR-710 | 6.820602 |
| mmu-miR-532-3p | 2.731047 | mmu-miR-467c-3p | 2.159236 | mmu-let-7a-5p | 19.70974 |
| mmu-miR-5115 | 4.134992 | mmu-miR-3060-5p | 2.742718 | mmu-miR-21a-5p | 7.871505 |
| mmu-miR-5120 | 5.739049 | mmu-miR-1b-3p | 4.869725 | mmu-miR-301a-5p | 6.460626 |
| mmu-miR-679-5p | 2.444481 | mmu-miR-27a-5p | 37.60953 | mmu-miR-539-3p | 2.589723 |
| mmu-miR-27a-3p | 123.1872 | mmu-miR-410-3p | 17.4879 | mmu-miR-488-3p | 2.507628 |
| mmu-miR-136-5p | 12.56823 | mmu-miR-148b-5p | 4.865344 | mmu-miR-184-5p | 2.007527 |
| mmu-miR-183-5p | 2.440657 | mmu-miR-204-5p | 10.10235 | mmu-miR-697 | 3.568439 |
| mmu-miR-29b-3p | 26.68454 | mmu-miR-742-3p | 6.462112 | mmu-miR-181b-5p | 3.519909 |
| mmu-miR-2136 | 23.82247 | mmu-miR-219-5p | 58.88691 | mmu-miR-145a-5p | 3.35676 |
| mmu-miR-299a-3p | 8.800208 | mmu-miR-25-5p | 3.688824 | mmu-miR-362-3p | 2.638234 |
| mmu-miR-3474 | 2.005535 | mmu-miR-713 | 4.168932 | mmu-miR-20a-5p | 8.967211 |
| mmu-miR-133b-5p | 77.61893 | mmu-let-7d-5p | 6.013307 | mmu-miR-491-3p | 3.594207 |
| mmu-miR-714 | 9.375649 | mmu-miR-344-5p | 2.514492 | mmu-miR-880-5p | 10.76771 |
| mmu-miR-5105 | 2.236839 | mmu-miR-466a-5p | 12.01022 | mmu-miR-221-5p | 2.914677 |
| mmu-miR-5112 | 27.06149 | mmu-miR-346-3p | 6.306549 | mmu-miR-338-3p | 56.80957 |
| mmu-miR-204-3p | 2.780989 | mmu-miR-3095-5p | 3.169364 | mmu-miR-325-5p | 2.283128 |
| mmu-miR-1895 | 2.540623 | mmu-miR-5114 | 7.031087 | mmu-miR-691 | 3.144771 |
| mmu-miR-26b-5p | 2.114402 | mmu-miR-183-3p | 6.621648 | mmu-miR-196b-3p | 3.309794 |
| mmu-miR-3065-3p | 4.03445 | mmu-miR-669h-3p | 3.024023 | mmu-miR-3070b-3p | 2.775335 |
| mmu-miR-494-5p | 2.356306 | mmu-miR-331-5p | 4.581953 | mmu-miR-194-2-3p | 18.43107 |
| mmu-miR-106a-5p | 65.00243 | mmu-miR-677-5p | 3.246786 | mmu-miR-188-5p | 54.30583 |
| mmu-miR-128-3p | 12.73167 | mmu-miR-195a-3p | 2.184063 | mmu-miR-147-3p | 4.566976 |
| mmu-miR-761 | 9.989739 | mmu-miR-463-3p | 12.53996 | mmu-miR-5624-3p | 2.644001 |
| mmu-miR-883b-5p | 2.35211 | mmu-miR-1957a | 11.74711 | mmu-miR-346-5p | 4.163306 |
| mmu-miR-542-3p | 7.764003 | mmu-miR-1894-3p | 31.13534 | mmu-miR-30b-3p | 2.051475 |
| mmu-miR-1958 | 6.32935 | mmu-miR-1971 | 6.442928 | mmu-miR-701-5p | 5.823002 |
| mmu-miR-1893 | 3.991812 | mmu-miR-671-5p | 10.50069 | mmu-miR-28a-3p | 7.908172 |
| mmu-miR-466b-5p | 3.301444 | mmu-miR-499-3p | 172.2427 | mmu-miR-1907 | 5.036028 |
| mmu-miR-7a-2-3p | 2.046015 | mmu-miR-107-5p | 5.46512 | mmu-miR-3105-5p | 2.774986 |
| mmu-miR-466m-5p | 23.50902 | mmu-miR-665-5p | 3.522869 | mmu-miR-489-5p | 6.494757 |
| mmu-miR-5625-3p | 3.090129 | mmu-miR-503-5p | 3.001966 | mmu-miR-5621-5p | 8.468883 |
| mmu-miR-29b-1-5p | 3.860122 | mmu-miR-140-5p | 3.422258 | ||
| mmu-miR-743b-5p | 10.07274 | mmu-miR-142-3p | 222.1103 |
Table 6.
miRNAs downregulated by more than twofold at 122 d
| Name | Fold change | Name | Fold change | Name | Fold change |
|---|---|---|---|---|---|
| mmu-miR-495-5p | 0.339321 | mmu-miR-190a-5p | 0.193533 | mmu-miR-874-5p | 0.398086 |
| mmu-miR-3068-5p | 0.247305 | mmu-miR-10b-5p | 0.266441 | mmu-miR-3069-3p | 0.361669 |
| mmu-miR-290-3p | 0.252711 | mmu-miR-338-5p | 0.138908 | mmu-miR-652-5p | 0.42407 |
| mmu-miR-1843b-3p | 0.37422 | mmu-miR-135a-5p | 0.24666 | mmu-miR-433-3p | 0.186811 |
| mmu-miR-151-5p | 0.453855 | mmu-miR-712-3p | 0.286408 | mmu-miR-1843a-5p | 0.378559 |
| mmu-miR-291a-5p | 0.38995 | mmu-miR-127-3p | 0.419585 | mmu-miR-193b-3p | 0.432846 |
| mmu-miR-29a-5p | 0.444011 | mmu-miR-324-3p | 0.320562 | mmu-miR-7a-5p | 0.473021 |
| mmu-miR-3084-3p | 0.106545 | mmu-miR-138-5p | 0.283583 | mmu-miR-331-3p | 0.346048 |
| mmu-miR-28a-5p | 0.311724 | mmu-miR-144-3p | 0.125605 | mmu-miR-375-5p | 0.340044 |
| mmu-miR-361-5p | 0.467213 | mmu-miR-718 | 0.454415 | mmu-miR-3072-5p | 0.14226 |
| mmu-miR-196a-1-3p | 0.368253 | mmu-let-7i-3p | 0.377898 | mmu-miR-3473c | 0.350358 |
| mmu-miR-296-5p | 0.23509 | mmu-miR-3102-3p.2-3p | 0.246599 | mmu-miR-451a | 0.348975 |
| mmu-let-7g-5p | 0.347148 | mmu-miR-3098-3p | 0.350802 | mmu-miR-483-3p | 0.396762 |
| mmu-miR-744-5p | 0.164894 | mmu-miR-370-3p | 0.316776 | mmu-miR-34a-5p | 0.157207 |
| mmu-miR-146b-5p | 0.321076 | mmu-miR-5103 | 0.253431 | mmu-miR-3059-5p | 0.293724 |
| mmu-miR-325-3p | 0.361389 | mmu-miR-298-5p | 0.330357 | mmu-miR-3971 | 0.388255 |
| mmu-miR-487b-3p | 0.318543 | mmu-miR-376a-3p | 0.104594 | mmu-miR-873a-5p | 0.355127 |
| mmu-miR-101a-3p | 0.189311 | mmu-miR-30d-5p | 0.322125 | mmu-miR-1943-3p | 0.368966 |
| mmu-miR-1224-5p | 0.360472 | mmu-miR-193a-3p | 0.297904 | mmu-miR-3475 | 0.238778 |
| mmu-miR-431-5p | 0.474967 | mmu-miR-186-5p | 0.460699 | mmu-miR-3104-3p | 0.280163 |
| mmu-miR-615-5p | 0.35682 | mmu-miR-543-3p | 0.282108 | mmu-miR-92a-3p | 0.493385 |
| mmu-miR-3105-3p | 0.422037 | mmu-miR-411-3p | 0.250887 | mmu-miR-467a-5p | 0.136188 |
| mmu-miR-326-3p | 0.394951 | mmu-miR-155-3p | 0.396201 | mmu-miR-674-3p | 0.487958 |
| mmu-miR-23a-3p | 0.461305 | mmu-miR-5626-5p | 0.33411 | mmu-miR-125b-2-3p | 0.254925 |
| mmu-miR-340-5p | 0.339629 | mmu-miR-379-5p | 0.288851 | mmu-miR-10b-3p | 0.211123 |
| mmu-miR-34c-5p | 0.074025 | mmu-miR-153-3p | 0.37579 | mmu-miR-185-5p | 0.357345 |
| mmu-miR-139-5p | 0.353424 | mmu-miR-129-5p | 0.469387 | mmu-miR-143-3p | 0.162113 |
| mmu-let-7d-3p | 0.362006 | mmu-miR-3069-5p | 0.430008 | mmu-miR-652-3p | 0.256424 |
| mmu-miR-382-5p | 0.19934 | mmu-miR-1843b-5p | 0.44958 | mmu-miR-328-3p | 0.347203 |
| mmu-miR-491-5p | 0.368848 | mmu-miR-219-2-3p | 0.243031 | mmu-miR-541-5p | 0.280503 |
| mmu-miR-1843a-3p | 0.130198 | mmu-miR-3072-3p | 0.372183 | mmu-miR-379-3p | 0.373429 |
| mmu-miR-101b-3p | 0.163835 | mmu-miR-383-5p | 0.393393 | mmu-miR-9-3p | 0.214284 |
| mmu-miR-369-3p | 0.49817 | mmu-miR-3085-3p | 0.241654 | mmu-miR-324-5p | 0.189889 |
| mmu-miR-341-3p | 0.444242 | mmu-miR-5621-3p | 0.2397 | mmu-miR-741-3p | 0.329126 |
| mmu-miR-148b-3p | 0.483882 | mmu-miR-708-3p | 0.195404 | mmu-miR-154-5p | 0.372162 |
| mmu-miR-182-5p | 0.289559 | mmu-miR-320-3p | 0.415748 | mmu-miR-551b-3p | 0.322774 |
| mmu-miR-3107-5p | 0.367641 | mmu-miR-22-3p | 0.384327 | mmu-miR-329-5p | 0.452029 |
| mmu-miR-210-3p | 0.305306 | mmu-miR-195a-5p | 0.262231 | mmu-miR-361-3p | 0.345876 |
| mmu-miR-132-5p | 0.366162 | mmu-miR-3110-5p | 0.267735 |
Among the differentially expressed miRNAs, miRNA-9 is highly expressed in the nervous system. miRNA-9 is involved in proliferation and differentiation of neural stem cells (NSCs). In the G93A-SOD1 transgenic mice, expression changes in NSCs were detected. Therefore, miRNA-9 was chosen as the investigation target. Microarray results showed that miRNA-9 was upregulated at the three time points. Heat map and hierarchical clustering analysis showed a clear distinction between the transgenic group and the control group (Figure 1).
Figure 1.
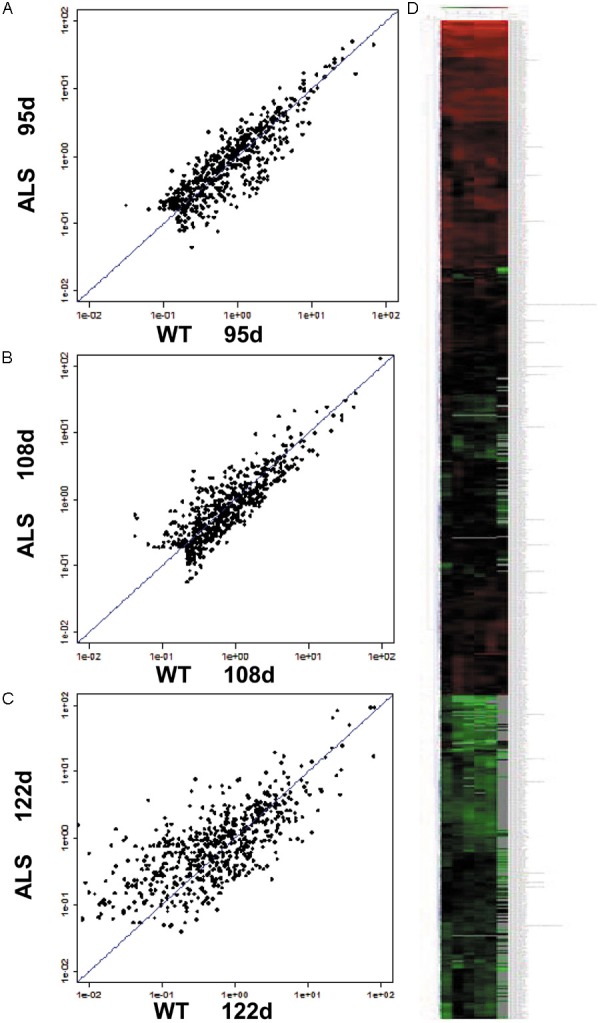
Differential expression of miRNAs in the spinal cord of ALS and wild-type mice. A-C: Scatter plots of expressed miRNAs at 95, 108, and 122 d, respectively. D: Heat map and hierarchical clustering. The heat map diagram shows the result of the two-way hierarchical clustering of miRNAs. Each row represents an miRNA and each column represents a sample. The miRNA clustering tree is shown on the left and the sample clustering tree is shown on top. The color scale shown at the top illustrates the relative expression level of an miRNA in a specific slide: red represents a relatively high expression level; green represents relatively low expression levels.
miRNA target prediction
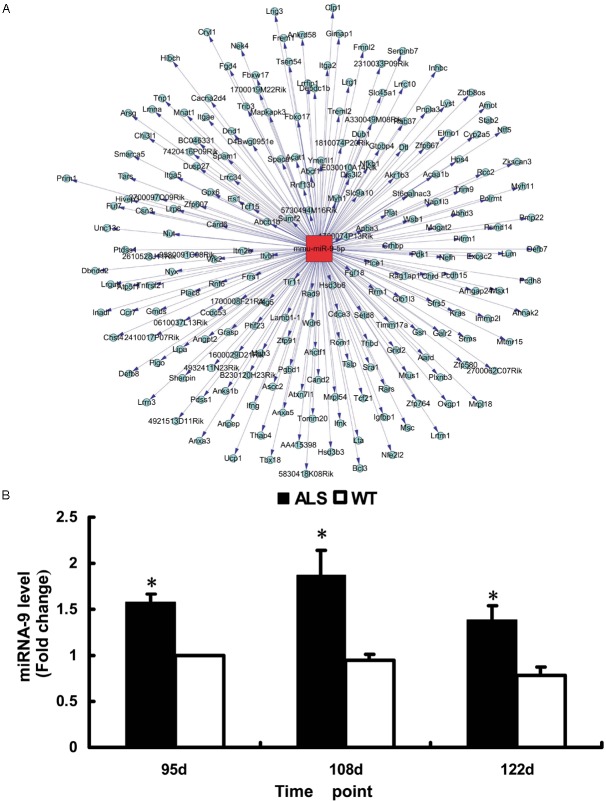
To investigate the possible mechanisms of miRNA-9 pathogenesis in ALS, we used miRanda, TargetScan, and Microcosm to search for the putative protein-coding gene targets of miRNA-9 (Figure 2). miRNA-9 was predicted to contain target sites in the TLX and Hes1. miRNA-9 could also regulate the proliferation and differentiation of NSCs by binding to the complementary sequences of the 3’ UTR.
Figure 2.
A: Network of miRNA-9 regulatory genes. B: Expression of miRNA-9 in the spinal cord of ALS and wild-type mice. Results of RT-qPCR indicate that the expression of miRNA-9 was upregulated (n = 3). *p < 0.05 vs. wild-type littermates.
Differential expression and distribution of miRNA-9 in the spinal cord of the ALS transgenic mice
RT-qPCR was performed to validate the microarray results. The results indicated that miRNA-9 levels in the transgenic mice were significantly higher than those in the control subjects at different time points (p < 0.05; Figure 2). The results of RT-qPCR were similar to the microarray results.
In-situ hybridization was performed to investigate the distribution of miRNA-9 in the spinal cord. The results indicated that miRNA-9 was localized in the cytoplasm of the positive cells. The miRNA-9-positive cells were mainly detected in the gray matter of the ventral horn and the dorsal horn of the spinal cord in ALS and wild-type mice at different time points. The majority of the miRNA-9-positive cells were located in the ventral horn of the gray matter, the locus of neurodegeneration. The number of miRNA-9-positive cells was significantly increased at post-natal 95, 108, and 122 d in the ALS mice compared with the wild-type mice (p < 0.05; Figure 3).
Figure 3.
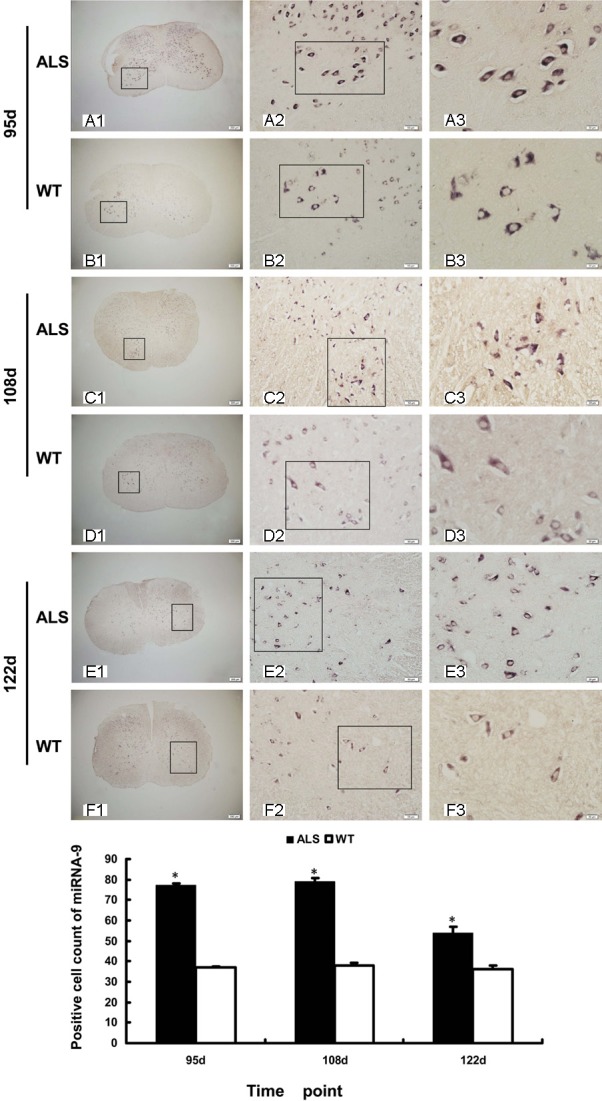
Expression and cellular distribution of miRNA-9 in the spinal cord of ALS and wild-type mice. Results of ISH show that the miRNA-9-positive cells were mainly detected in the gray matter of the ventral horn and the dorsal horn of the spinal cord. The majority of the miRNA-9-positive cells were located in the ventral horn of the gray matter. A1-A3: Represent the distribution of miRNA-9-positive cells in the ALS mice at 95 d. B1-B3: Represent the distribution of miRNA-9-positive cells in the WT mice at 95 d. C1-C3: Represent the distribution of miRNA-9-positive cells in the ALS mice at 108 d. D1-D3: Represent the distribution of miRNA-9-positive cells in the WT mice at 108 d. E1-E3: Represent the distribution of miRNA-9-positive cells in the ALS mice at 122 d. F1-F3: Represent the distribution of miRNA-9-positive cells in the WT mice at 122 d. Scale bar = 200, 50, 20 μm. The total number of miRNA-9-positive cells was counted (n = 3). *p < 0.05 vs. wild-type littermates.
In addition to injury on the motor neurons in the spinal cord, injury on the motor neurons in the brain can occur in ALS, in which muscle atrophy and paralysis possibly occur. Therefore, the expression of miRNA-9 was also detected in the brain and the gastrocnemius. miRNA-9-positive cells were also detected in the cerebral cortex and the hippocampi but were not found in the gastrocnemius (data not shown).
Discussion
miRNAs have an important function in many cellular processes. miRNAs can interact with specific sequences of target mRNA (mainly in the 3’ UTR), recruiting the RNA-induced silencing complex (RISC). On the basis of complete or incomplete miRNA-mRNA complementation, miRNAs can lead to degradation or translational suppression of target mRNA [11]. Therefore, miRNAs are a part of a novel regulatory pathway [12].
Early studies on the development of the nervous system evaluated the consequences of the decrease in the Dicer gene (which results in the absence of mature miRNAs) during neurogenesis. In zebrafish, the complete absence of Dicer leads to critical defects in the general morphology of the central nervous system and the peripheral nervous system; neuronal differentiation is also impaired [13]. miRNAs also have an important function in the pathogenesis of neurodegenerative diseases such as Alzheimer’s [14], Parkinson’s [15], and Huntington’s diseases [16]. In ALS, a few studies on miRNAs have been reported. miRNA-206, a skeletal muscle-specific miRNA, delays the progression of ALS and promotes the regeneration of the neuromuscular synapses in mice [17]. In the present study, microarray results showed for the first time that numerous miRNAs were differentially expressed in the spinal cord at different time points in the G93A-SOD1 transgenic mice. The function of these differentially expressed miRNAs should be investigated in future studies.
miRNA-9 is highly expressed in the nervous system and conserved among species, exhibiting 100% similarity between Drosophila melanogaster and vertebrates [18]. Studies with different model systems have revealed that miRNA-9 regulates neurogenesis by acting on neural or non-neural cell lineages. For example, miRNA-9 suppresses the expression of TLX, an essential regulator of NSC self-renewal, and maintains the adult NSCs in an undifferentiated and self-renewable state, thereby regulating the proliferation and distribution of NSCs [19]. Increased miRNA-9 expression reduces the proliferation of the mouse NSCs and accelerates neural differentiation. Antisense knockdown of miRNA-9 leads to the proliferation of NSCs [20]. In addition, the overexpression of miRNA-9 decreases the levels of Hes1, which regulates proliferation and differentiation characteristics of NSCs. The overexpression of miRNA-9 also promotes cell cycle exit and neuronal differentiation. By contrast, the knockdown of miRNA-9 inhibits neuronal differentiation [21]. miRNA-9 can promote the neural differentiation of MSCs in the bone marrow by targeting Zfp521Z [22]. The loss of miRNA-9 suppresses proliferation but promotes the migration of hNPCs cultured in vitro [23]. These findings have indicated that miRNA-9 may regulate neural differentiation.
In ALS, the proliferation and differentiation of NSCs and neural progenitor cells (NPCs) have been debated. Previous studies demonstrated that an increase in the proliferation of NPCs is observed in the ependymal zone surrounding the central canal (EZ) in the spinal cord; increased de novo neurogenesis from NPCs is also observed during ALS-like disease onset and progression [24]. In another study, NSCs are intravenously engrafted in the central nervous system of ALS-affected animals and their wild-type counterparts, resulting in a higher cell grafting efficiency in the ALS animals than in the wild-type animals; the two main fates observed in these animals are neuronal and astrocytic [25]. In SOD1G93AG1H mice, the number of nestin-positive NPCs is greatly increased, the majority of nestin-positive NPCs co-expresses the astrocyte marker GFAP, and a small number of GFAP co-expresses the neuronal marker NeuN [26]. In wobbler mouse, the percentage of neurons obtained from in vitro differentiation of NPCs is significantly higher than that from the healthy mice [27].
Our results indicated that miRNA-9 levels were upregulated at 95, 108, and 122 d in the G93A-SOD1 transgenic mice compared with the control mice. The miRNA-9-positive cells were detected in the ventral horn and the dorsal horn of the spinal cord. The majority of miRNA-9-positive cells were located in the ventral horn of the spinal cord, the locus of neurodegeneration. However, the function of miRNA-9 as a regulator in the differentiation of NSCs and NPCs in ALS remains unclear. The increase in expression and the distribution characteristics of miRNA-9 may be involved in the differentiation of the neurons derived from NSCs and NPCs in ALS.
ALS is the third most common neurodegenerative disease occurring in adulthood (after Alzheimer’s and Parkinson’s diseases). To date, no effective therapies are available for patients with ALS. The transplantation of the NSCs derived from the central nervous system is a promising therapeutic strategy to treat ALS [28]. The directional differentiation of the NSCs into neurons should be determined and could promote effective treatment. Therefore, the regulatory mechanisms of miRNAs in the proliferation and differentiation of the NSCs should be elucidated to provide a new treatment method.
Acknowledgements
The microarray experiments were performed by KangChen Bio-tech, Shanghai, China. This study was supported by the National Natural Science Foundation of China (Grant No. 81271413), the Shandong Province Science and Technology Development Program of China (Grant No. 2012GSF11827), the Shandong Province Natural Science Foundation of China (Grant No. ZR2012HQ021), the Muscular Dystrophy Association (Grant Nos. 157511 and 254530), the ALS Therapy Alliance (Grant No. 2013D001622), the Shandong Province Taishan Scholar Project, and the Shandong Province Education Department of China (Grant Nos. J12LK51 and J11LF16).
Disclosure of conflict of interest
None.
References
- 1.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 2.Wei J, Wang F, Kong LY, Xu S, Doucette T, Ferguson SD, Yang Y, McEnery K, Jethwa K, Gjyshi O, Qiao W, Levine NB, Lang FF, Rao G, Fuller GN, Calin GA, Heimberger AB. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013;73:3913–26. doi: 10.1158/0008-5472.CAN-12-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi L, Gan L, Luo C, Lien L, Liu R. Temporal Response of Neural Progenitor Cells to Disease Onset and Progression in Amyotrophic Lateral Sclerosis-Like Transgenic Mice. Stem Cells Dev. 2007;16:579–88. doi: 10.1089/scd.2006.0120. [DOI] [PubMed] [Google Scholar]
- 4.Kulshreshtha D, Vijayalakshmi K, Alladi PA, Sathyaprabha TN, Nalini A, Raju TR. Vascular Endothelial Growth Factor Attenuates Neurodegenerative Changes in the NSC-34 Motor Neuron Cell Line Induced by Cerebrospinal Fluid of Sporadic Amyotrophic Lateral Sclerosis Patients. Neurodegener Dis. 2011;8:322–30. doi: 10.1159/000323718. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008;9:831–42. doi: 10.1038/nrg2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–21. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HK, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, Vanderburg C, Krichevsky AM. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum Mol Genet. 2013;22:3077–92. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- 9.Sibley CR, Seow Y, Curtis H, Weinberg MS, Wood MJ. Silencing of Parkinson‘s disease-associated genes with artificial mirtron mimics of miR-1224. Nucleic Acids Res. 2012;40:9863–9875. doi: 10.1093/nar/gks712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H, Wang X. Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;420:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–76. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–77. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 13.Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 14.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer‘s disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82:283–9. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson R, Zuccato C, Belyaev ND, Guest DJ, Cattaneo E, Buckley NJ. A microRNA-based gene dysregulation pathway in Huntington‘s disease. Neurobiol Dis. 2008;29:438–45. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–9. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan SL, Ohtsuka T, Gonzalez A, Kageyama R. MicroRNA9 regulates neural stem cell differentiation by controlling Hes1 expression dynamics in the developing brain. Genes Cells. 2012;17:952–61. doi: 10.1111/gtc.12009. [DOI] [PubMed] [Google Scholar]
- 22.Han R, Kan Q, Sun Y, Wang S, Zhang G, Peng T, Jia Y. MiR-9 promotes the neural differentiation of mouse bone marrow mesenchymal stem cells via targeting zinc finger protein 521. Neurosci Lett. 2012;515:147–52. doi: 10.1016/j.neulet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6:323–335. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24:34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitrecic D, Nicaise C, Gajovic S, Pochet R. Distribution, differentiation, and survival of intravenously administered neural stem cells in a rat model of amyotrophic lateral sclerosis. Cell Transplant. 2010;19:537–48. doi: 10.3727/096368910X498269. [DOI] [PubMed] [Google Scholar]
- 26.Juan L, Dawei Z, Julie AD. Increased number and differentiation of neural precursor cells in the brainstem of superoxide dismutase 1(G93A) (G1H) transgenic mouse model of amyotrophic lateral sclerosis. Neurol Res. 2007;29:204–9. doi: 10.1179/174313206X152519. [DOI] [PubMed] [Google Scholar]
- 27.DiFebo F, Curti D, Botti F, Biella G, Bigini P, Mennini T, Toselli M. Neural precursors (NPCs) from adult L967Q mice display early commitment to “in vitro” neuronal differentiation and hyperexcitability. Exp Neurol. 2012;236:307–18. doi: 10.1016/j.expneurol.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Pandya RS, Mao LL, Zhou EW, Bowser R, Zhu Z, Zhu Y, Wang X. Neuroprotection for amyotrophic lateral sclerosis: role of stem cells, growth factors, and gene therapy. Cent Nerv Syst Agents Med Chem. 2012;12:15–27. doi: 10.2174/187152412800229152. [DOI] [PMC free article] [PubMed] [Google Scholar]