Abstract
Mesenchymal stem cells (MSCs) are capable of modulating the immune system and have been used to successfully treat a variety of inflammatory diseases in preclinical studies. Recent evidence has implicated paracrine signaling as the predominant mechanism of MSC therapeutic activity. We have shown in models of inflammatory organ failure that the factors secreted by MSCs are capable of enhancing survival, downregulating inflammation, and promoting endogenous repair programs that lead to the reversal of these diseases. As a marker of disease resolution, we have observed an increase in serum IL-10 when MSC-conditioned medium (MSC-CM) or lysate (MSC-Ly) is administered in vivo. Here we present an in vitro model of IL-10 release from blood cells that recapitulates this in vivo phenomenon. This assay provides a powerful tool in analyzing the potency of MSC-CM and MSC-Ly, as well as characterizing the interaction between MSC-CM and target cells in the blood.
Keywords: Mesenchymal stem cell, IL-10, Potency assay, Organ injury, Inflammation, Autoimmunity, Transplantation
1. Introduction
Bone marrow mesenchymal stem cells (MSCs) are resident nonhematopoietic progenitor cells that possess potent immunomodulatory abilities (1–3). Allogeneic MSC transplants have been used to successfully treat hematological (1, 4), cardiovascular (5, 6), as well as neurological (7, 8) and inherited diseases (9, 10) in preclinical studies, and allogeneic transplantation of MSCs has been given expanded access as a treatment for padiatric GvHD. Recent studies involving MSC transplantation have revealed that the therapeutic activity of these grafts is independent of differentiation, and paracrine interactions of MSCs with tissue and immune cells provide the majority of therapeutic benefit (11–15).
We have found administration of concentrated MSC-conditioned medium (MSC-CM) or MSC lysate (MSC-Ly) can reproduce the effects of an MSC graft in vivo and significantly increase serum IL-10 levels in two animal models (13, 14). IL-10 is a well-known antiinflammatory cytokine that can inhibit the secretion of proinflammatory cytokines and protect cells from apoptosis and necrosis in the context of acute inflammation (16–18). We have determined that MSC-CM and lysate also substantially enhance IL-10 secretion by peripheral blood mononuclear cells (PBMCs) in vitro and have developed an assay based on this discovery. This assay allows for rapid and reproducible assessment of the potency of MSCs and MSC molecular products in a manner relevant to animal and human testing of cell therapy.
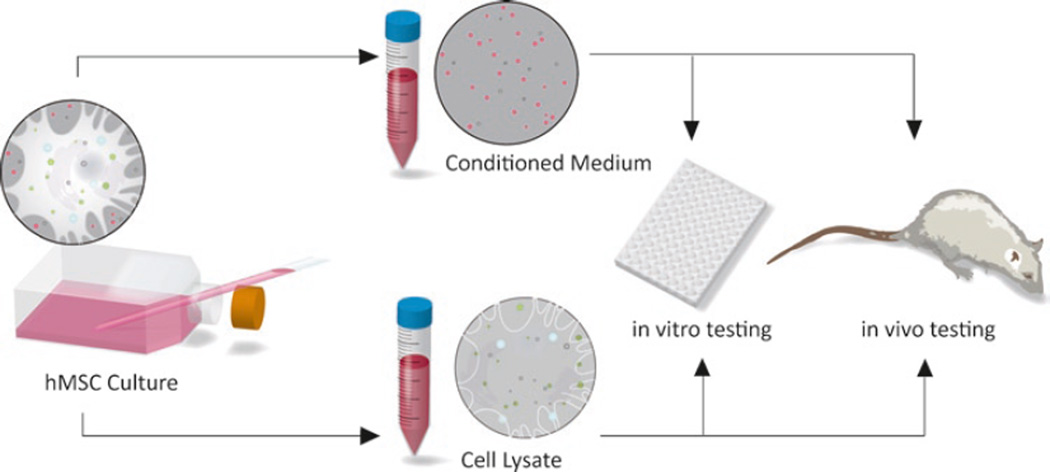
Conditioned medium and cell lysate are derived from human MSC cultures and can subsequently be used for both in vitro and in vivo experimentation (see Fig. 1). Below we provide methods for an ELISA-based IL-10 assay for in vitro testing, a powerful tool to aid in determining the efficacy and underlying paracrine mechanisms of MSC transplants and MSC-based therapies.
Fig. 1.
Schematic of in vitro and in vivo potency tests of MSC-derived materials.
2. Materials
2.1. Isolation of MSCs from Whole Bone Marrow and Maintenance of Culture
Whole bone marrow aspirate: can be obtained commercially (Lonza) or from a consented human donor.
Phosphate-buffered saline (PBS) (see Note 1).
Ficoll-Paque density 1.077 g/mL (GE Healthcare).
Table-top centrifuge capable of 1,500 × g.
Hemocytometer and microscope, or other method capable of determining cell count.
Human MSC medium: α-minimum essential medium Eagle (Sigma Aldrich) supplemented with 15% (v/v) fetal bovine serum (HyClone), 2% (v/v) penicillin streptomycin solution (GIBCO), 1 µg/mL gentamicin (Sigma Aldrich), 1 ng/mL bFGF (R&D Systems). Store at 4°C.
37°C incubator.
2.2. Collection, Concentration, and Storage of MSC-CM
Conditioning medium: Dulbecco’s modified essential medium (Sigma Aldrich) supplemented with 0.5% (w/v) bovine serum albumin and 2% (v/v) penicillin streptomycin solution (GIBCO).
1× Trypsin prepared from 10 × 0.5% Trypsin-EDTA (GIBCO).
Hemocytometer.
For small volume of MSC-CM: 3 kDa centrifugal filters (Amicon Ultra Ultracel-3K, Cat No. UFC800396). Centrifuge capable of spinning at 4,000 × g.
For large volume of MSC-CM: Amicon pressure concentrator (no longer manufactured), pressurized nitrogen gas and 3 kDa regenerated cellulose ultrafiltration membranes (Millipore Ultracel).
+4°C freezer for storage.
2.3. Preparation of MSC Lysate
Sonicator.
Benchtop centrifuge capable of 1,500 × g.
+4°C freezer for storage.
2.4. Isolation of PBMCs from Whole Blood
Fresh whole blood from consenting donor.
PBS.
Ficoll-Paque density 1.077 g/mL (GE Healthcare).
C-10 medium: 500 mL 1× RPMI medium (GIBCO 1650), 50 mL inactivated fetal bovine serum, 6 mL MEM nonessential amino acids solution 10 mM 100× (GIBCO Cat No. 1140-050), 6 mL 100× sodium pyruvate (GIBCO 11360), 6 mL glutamate, 6 mL penicillin streptomycin solution (GIBCO 15140), 6 mL sodium bicarbonate, 3 mL betamercaptoethanol. Sterilize with corning bottle top filter or other means of sterilization. Store at 4°C.
Centrifuge capable of spinning at 1,500 × g.
96-Well plate.
37°C incubator.
2.5. Preparation of Samples and Stimulation with LPS
96-Well plate seeded with 50 µL per well at 2 × 106 PBMCs/mL.
MSC-CM and lysate, 50 µL per well.
LPS (Escherichia coli 0111:B4, Sigma-Aldrich L4391) at 30 µg/mL, 50 µL per well. Stock solution is diluted in C10 medium.
37°C incubator.
−80°C freezer.
2.6. ELISA and Analysis of Results
BD OptEIA™ human IL-10 ELISA Set and recommended buffers and solutions, or other human IL-10 ELISA Kit.
BD Falcon™ Microtest™ 96-well ELISA plate or other high-binding ELISA plate.
Plate reader (spectrophotometer) capable of reading at indicated wavelengths in ELISA Kit (450 nm with correction at 570 nm for BD OptEIA™ Human IL-10 ELISA set).
Microsoft Excel or other program capable of processing data from spectrophotometer.
3. Methods
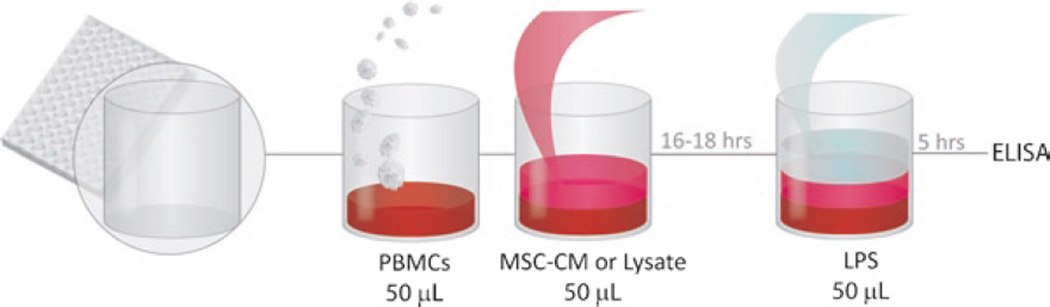
Overview: MSC-CM or lysate is prepared and added to PBMCs freshly isolated from whole blood and plated in a 96-well plate. As a mock cell control, we perform the assay with CM and/or lysates from fibroblasts. The plate is incubated overnight at 37°C for 16–18 h. The PBMCs are then stimulated with LPS for 5 h, at which time the plate is centrifuged and the supernatant stored for ELISA (see Fig. 2).
Fig. 2.
Summary of in vitro inflammation assay for the testing of MSC-derived factors.
3.1. Isolation of MSCs from Whole Bone Marrow
Whole bone marrow centrifuged with Ficoll results in a pattern comparable to that of centrifuged whole blood; the corresponding “buffy coat” is enriched with MSCs. This layer is collected, counted, and plated on tissue culture polystyrene. The purity of MSCs isolated from whole bone marrow is relatively high due to their ability to differentially adhere to cell culture substrates compared to other hematopoietic marrow cells. Subsequent medium changes eliminate hematopoietic cells and other nonadherent cells. The identity of MSCs can be confirmed through phenotype and multipotency analysis using flow cytometry or differentiation media, respectively (see Note 3).
Wash bone marrow with equivalent volume of PBS, thus diluting the bone marrow 1:2. Prepare 5 mL Ficoll for each 10 mL of diluted bone marrow. Add diluted whole bone marrow slowly; avoid disturbance of the boundary between Ficoll and marrow (see Note 1).
Spin 30 min at 1,500 × g with no brake. Collect the resulting mononuclear cell layer and wash with 5 mL PBS.
Spin 10 min at 1,500 × g, high brake. Resuspend pellet in 1 mL hMSC medium. Determine cell count and seed at a density of 1–10 × 103 cells/cm2.
Let cells adhere and grow for 10 days. Perform first medium change at day 10; the following medium change is done at day 17. Subsequent medium changes should be performed at a frequency of every 3 or 4 days. Cells are typically used between passages 1–6.
3.2. Collection, Concentration, and Storage of MSC-CM
By convention, 1× MSC-CM is defined as conditioned medium from two million cells concentrated to 1 mL of volume. MSCs are incubated for 24 h in conditioning medium and concentrated to 1×. For small volumes, centrifugal filter tubes can be used. For larger volumes, the Amicon pressure concentrator and pressurized nitrogen gas offers a more efficient method.
-
1
Perform medium change on MSC cultures with two PBS washes. Add conditioning medium, incubate for 24 h (see Note 4).
-
2
Collect MSC-CM. Trypsinize cells; determine cell count and final volume of 1× MSC-CM.
For small volumes:
-
3
Add 4 mL PBS to centrifugal filter tube. Let centrifuge spin to 4,000 × g for a few minutes for the PBS to run through the filter to remove residual glycerin in the ultrafiltration membrane. Discard the PBS in both compartments of the centrifugal filters.
-
4
Add 4 mL MSC-CM under sterile conditions and spin at 4,000 × g for 5–15 min. Discard flow through and fill the centrifugal filter tube with new MSC-CM. Pipette the conditioned medium up and down to wash the filter and lessen congestion of the filter with proteins. Repeat process until the desired volume is reached.
For large volumes:
-
3
Sterilize the ultrafiltration membrane with 70% ethanol and let dry. Assemble pressure concentrator according to the manufacturer instructions. Place pressure concentrator onto stir plate and prepare a waste bottle.
-
4
Run 20 mL of PBS through the concentrator.
-
5
Discard waste and add MSC-CM. Monitor waste level in the bottle to determine the volume of MSC-CM.
-
6
Let run until desired final volume is reached. Disconnect and depressurize concentrator.
3.3. Preparation of MSC Lysate
While cell lysate can be obtained through lysate buffers and other chemical means, sonication, which causes physical disruption of the cell membrane, provides pure lysate without chemical contaminant and possible confounding factors.
Trypsize MSCs and pellet in tabletop centrifuge at 1,000 × g.
Discard supernatant and gently layer 1 mL PBS per 2 × 106 cells on top of cell pellet. To visualize lysis, do not resuspend cells into solution.
Sonicate cell pellet at 3 AU for 5 s with three pulses and collect in ice.
Centrifuge lysate at 2,000 × g for 2 min to precipitate membrane fragments. Retain the solution phase. The solution phase from this process is considered MSC-Ly.
3.4. Isolation of PBMCs from Whole Blood
Calculate the number of wells needed to conduct the assay to estimate the amount of blood needed, leaving extra wells for standards and controls. A total of 100,000 PBMCs will be required per well. Collect fresh whole blood from a consented donor. It is best to plate the PBMCs and add the MSC-CM or lysate on the day of the separation to ensure maximum viability (see Note 2). We have found 5 mL of blood to safely supply 10–15 million PBMCs, but this count varies from donor to donor.
Wash blood with equivalent volume of PBS, thus diluting the blood 1:2. Prepare 5 mL Ficoll for each 10 mL of diluted blood. Gently layer diluted blood on the Ficoll column (see Note 1).
Spin 30 min at 1,500 × g with no brake. Collect buffy coat and wash with 5 mL PBS.
Spin 10 min at 1,500 × g. Resuspend pellet in 1 mL C-10 medium and determine cell count.
Dilute or concentrate to two million cells/mL. Seed in 96-well plate at 50 µL per well (100,000 cells per well). Incubate at 37°C for storage if MSC-CM or lysate cannot be added immediately.
3.5. Preparation of Samples and Stimulation with LPS
Add 50 µL of MSC-CM or lysate to each well in the blood plate and incubate at 37°C for 16–18 h.
Prepare LPS solution. Vortex well before dilution. Prepare 6 mL per plate of LPS diluted in C-10. Add 50 µL to each well, thus yielding a final concentration of 10 µg/mL in the wells, and incubate at 37°C or 5 h.
Spin plates at 1,500 × g for 10 min to settle PBMCs to the bottom of the wells. Collect supernatant in a new 96-well plate and store at −80°C (see Note 5).
3.6. ELISA and Analysis of Results
Perform ELISA for human IL-10 according to the manufacturer instructions. Use spectrophotometer to read plates at wavelengths indicated by ELISA Kit and import raw data into program of choice.
Perform linear regression to generate standard curve and equation.
Convert raw absorbance data into concentration values using linear regression equation and generate bar graph. High IL-10 secretion by PBMCs indicates high potency of treatment.
3.7. Conclusion
MSCs are promising candidates for cell-based immunomodulatory therapy. They can be easily isolated from bone marrow aspirates, expanded 50 population doublings in 10 weeks with minimal loss in potency, and to date have not been found to cause adverse immune responses in allogenic transplantation recipients (19). Despite controversial theories regarding the primary therapeutic mechanism of action, the uses of MSC treatments have become diverse (9). Currently ongoing clinical trials exist for the use of MSC transplants in steroid refractory graft vs. host disease (20), periodontitis (21), and severe chronic myocardial ischemia (9, 22) among others. In our laboratory, we have demonstrated the effective use of MSC-CM and MSC-Ly in treating multiple organ dysfunction syndrome. To harness both the secreted and intracellular metabolism of MSCs, we have also created an MSC extracorporeal device for the treatment of organ failure (13, 14).
By quantifying the potency of MSC-CM or MSC-Ly, this method enables optimization of dosage, growth, and storage conditions, as well as treatment procedures for clinical use of MSCs and MSC-based products. In future development of these products, the antiinflammatory activity that these cells possess can be measured reliably and reproducibly with this assay, providing for better consistency and more rigorous release criteria. The assay also provides a valuable tool in elucidating the mechanism underlying MSC immunomodulation. The speed at which MSC transplantation conveys therapeutic activity (~hours) is considerably faster than that of other cell transplants for regeneration purposes (~days to weeks), an inconsistency that can only be explained if other hypotheses for the mechanism of action other than engraftment and differentiation are considered. The time scale of these results could plausibly be explained by the occurrence of MSC lysis and the release of what would be paracrine factors during transplantation. This potency assay can account for and facilitate decomposing the “lysate effect.”
Table 1.
Immunophenotype of human MSCs (19)
| Positive | Negative | Inducible |
|---|---|---|
| CD13, CD29, CD44, CD49a, b, c, e, f, CD51, CD54, CD58, CD71, CD73, CD90, CD102, CD105, CD106, CDw119, CD120a, CD120b, CD123, CD124, CD126, CD127, CD140a, CD166, P75, TGF-bIR, TGF-bIIR, HLA-A, B, C, SSEA-3, SSEA-4, D7, PD-L1 | CD3, CD4, CD6, CD9, CD10, CD11a, CD14, CD15, CD18, CD21, CD25, CD31, CD34, CD36, CD38, CD45, CD49d, CD50, CD62E, L, S, CD80, CD86, CD95, CD117, CD133, SSEA-1, ABO | HLA-DR |
CD cluster of differentiation; TGF transforming growth factor; HLA human leukocyte antigen; SSEA stage-specific embryonic antigen; ABO blood group antigens
Table 2.
Differentiation media for MSCs (23)
| Differentiation medium |
Composition |
|---|---|
| Osteogenic | IMDM with 0.1 µM dexamethasone (Sigma-Aldrich, St Louis, MO), 0.2 mM ascorbic acid (AsA; Sigma-Aldrich), 10 mM-glycerol phosphate (Sigma-Aldrich) |
| Chondrogenic | High-glucose DMEM (Bio-fluid, Rockville, MD) with 0.1 M dexamethasone, 50 g/mL AsA, 100 g/mL sodium pyruvate(Sigma-Aldrich), 40 g/mL proline (Sigma-Aldrich), 10 ng/mL TGF-1, and 50 mg/mL ITS premix (Becton Dickinson; 6.25 g/mL insulin, 6.25 g/mL transferrin, 6.25 ng/mL selenius acid, 1.25 mg/mL bovine serum albumin (BSA), and 5.35 mg/mL linoleic acid) |
| Adipogenic | IMDM with 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), 1 M hydrocortisone (Sigma-Aldrich), 0.1 mM indomethacin(Sigma-Aldrich), and 10% rabbit serum (Sigma-Aldrich) |
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (R01 DK43371), MIT Class of 1972 Fund, and the Shriners Hospitals for Children.
Footnotes
It is recommended that the bone marrow or blood be diluted 1:1 with PBS, as the larger volume provides a greater margin of error during the collection of the buffy coat.
We found that blood stored for prolonged periods before use tends to be activated during storage and can thereby confound the results of the assay. Freshly obtained PBMCs are preferred.
Phenotype and multipotency analysis.
While the identities of relevant cell surface markers of human MSCs remain controversial, Table 1 offers a certain immunophenotype of MSCs. It is possible to label for certain cell surface markers and conduct flow cytometry for isolation.
MSCs have been shown to be capable of differentiating into bone, cartilage, adipose cells, and myoblasts in vivo (9). Lee et al. developed differentiation media in which, under the right conditions, MSCs can be observed to differentiate into these tissues in vitro (23). Table 2 includes specialized media used to encourage differentiation of MSCs. MSC differentiation kits are also commercially available from vendors.
It is also possible to perform multipotency tests in vivo through subcutaneous transplantation of MSCs. While it is beyond the scope of this paper to discuss in vivo assays, the reader may find it worthwhile to refer to the work of Bianco et al. concerning the formation of ectopic bone marrow using subcutaneous transplants of MSCs (24).
This volume is typically 15 mL for Corning T-175 Flasks. For other containers, a similar volume per cell ratio should be achieved, although small variations are negligible as the final definition of 1× and 10× medium is based on cell count and not initial volume.
Despite centrifugation, the supernatant may still be contaminated with PBMCs during the collection process. Freezing before the ELISA is recommended to lyse all remaining cells.
References
- 1.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363.9419:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;10.4:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30.1:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 4.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81.10:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 5.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107.18:9040–9042. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 6.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 7.Jin HK, Carter JE, Hungtley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. Journal of Clinical Investigation. 2002;109.9:1183–1191. doi: 10.1172/JCI14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Experimental Neurology. 2002;174.1:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 9.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: An update on clinical trials with mesenchymal stem cells. Journal of Cellular Physiology. 2007;211.1:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nature Medicine. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 11.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. The FASEB Journal. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 12.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. PNAS. 2006;104.5:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnarid T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation Research. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 14.Parekkadan B, VanPoll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2.9:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47.5:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 16.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey É. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine. 2008;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. Journal of Experimental Medicine. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, Mcleroy P, Nibhanupudy B, Lim S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney International. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 19.Parekkadan B. Massachusetts Institute of Technology, Doctoral Thesis. 2008. Cellular and molecular immunotherapeutics derived from the bone marrow stroma. [Google Scholar]
- 20.Lazarus H, Koc O, Devine S, Curtin P, Maziarz R, Holland H, Shpall E, McCarthy P, Atkinson K, Cooper B. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biology of Blood and Marrow Transplantation. 2005;11.5:389–398. doi: 10.1016/j.bbmt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Baba S. Clinical trials of regeneration for periodontal tissue. Home – ClinicalTrials.gov. [18 Aug 2009];2005 Web. < http://clinicaltrials.gov/ct2/show/NCT00221130>.
- 22.Kastrup J. Stem cell therapy for vasculogenesis in patients with severe myocardial ischemia. Home – ClinicalTrials.gov. [18 Aug 2009];2005 Web. < http://clinicaltrials.gov/ct2/show/NCT00260338>.
- 23.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 24.Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gs a-mutated skeletal progenitor cells. The Journal of Clinical Investigation. 1998;101.8:1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]