Abstract
The incretin - glucose-dependent insulinotropic polypeptide (GIP) - and the pro-inflammatory cytokine osteopontin are known to have important roles in the regulation of adipose tissue functions. In this work we show that GIP stimulates lipogenesis and osteopontin expression in primary adipocytes. The GIP-induced increase in osteopontin expression was inhibited by the NFAT (the transcription factor nuclear factor of activated T-cells) inhibitor A285222. Also, the NFAT kinase glycogen synthase kinase (GSK) 3 was upregulated by GIP. To test whether cAMP might be involved in GIP mediated effects on osteopontin a number of strategies were used. Thus, the β3-adrenergic receptor agonist CL316,243 stimulated osteopontin expression, an effects which was mimicked by OPC3911, a specific inhibitor of phosphodiesterase 3. Furthermore, treatment of phosphodiesterase 3B knock-out mice with CL316,243 resulted in a dramatic upregulation of osteopontin in adipose tissue which was not the case in wild-type mice. In summary, we delineate mechanisms by which GIP stimulate osteopontin in adipocytes. Given the established link between osteopontin and insulin resistance, our data suggest that GIP by stimulating osteopontin expression, also could promote insulin resistance in adipocytes.
Keywords: Osteopontin, GIP, adipocytes, NFAT, phosphodiesterase3B
Introduction
Obesity is a rapidly growing public health concern in the world. Along with cardiovascular disease, type 2 diabetes (T2D) is the most common co-morbidity of obesity. Over 80% of patients with T2D are overweight or obese. Adipocytes secrete numerous proteins, cytokines and chemokines collectively referred to as adipokines [1]. The adipokine secretion profile is altered in obesity, with increases, for example, in leptin and resistin and decreases in adiponectin [1]. Adipose tissues from obese subjects also contain significantly higher amounts of infiltrated macrophages and other immune cells [1–4]. The secretion of adipokines and pro-inflammatory cytokines from adipose tissue leads to a low grade systemic inflammation and contributes to insulin resistance in adipocytes, muscle and liver [1–4]. Osteopontin is a pro-inflammatory cytokine that has recently been shown to have a critical role in adipose tissue inflammation and insulin resistance. Osteopontin mRNA and protein expression is upregulated in adipose tissue from obese mice and humans [5–7]. Mice deficient in osteopontin have improved glucose tolerance as well as lower fasting plasma glucose, insulin and triglycerides after high fat diet regime compared to wild-type (wt) mice [8, 9] and neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance [10]. Also, the number of adipose tissue macrophages and the expression of pro-inflammatory cytokines were significantly reduced in osteopontin-deficient mice fed a high fat diet compared to wt mice [8, 9]. Although osteopontin appears to be an important mediator of obesity-induced insulin resistance, very little is known about molecular mechanisms involved in the regulation of osteopontin protein expression in adipocytes.
The incretin hormone glucose-dependent insulinotropic polypeptide (GIP) is produced by entero endocrine K-cells and is released into the circulation in response to nutrient stimulation [11, 12]. Impairment of GIP action is suspected to play a role in obesity and diabetes [11, 13]. The GIP receptor is a transmembrane heterotrimeric G-protein coupled receptor and is expressed by many cell types throughout the body including adipocytes [14] where it regulates several aspects of glucose and lipid metabolism. For example, GIP has been shown to increase delivery of triglycerides to the adipocyte by stimulating lipoprotein lipase, and to stimulate lipogenesis and glucose uptake in insulin dependent as well as independent manner. GIP has also been shown to stimulate lipolysis in some studies but has in others been shown to inhibit lipolysis ([11, 12] and references within, [15]).
In recent studies we have shown that GIP increases the expression of osteopontin in pancreatic β-cells and in adipocytes [16, Ahlqvist, unpublished]. In this work we investigate mechanisms whereby GIP induces osteopontin expression in adipocytes. We demonstrate roles for insulin signaling, phosphodiesterase 3B (PDE3B) and the transcription factor NFAT.
Materials and Methods
Animals
Male Sprague-Dawley rats (from B&K Universal Stockholm, Sweden) between 36 and 42 weeks of age were used for the isolation of primary adipocytes. C57BL/6 WT and PDE3B knock-out (KO) mice, originally generated as described in [17], were used for the real time RTPCR experiments on adipose tissue. All experiments have been approved by the Animal Ethics Committee, Lund, Sweden or by the NHLBI Animal Care and Use Committee, USA.
Chemicals and Reagents
Glucose-dependent insulinotropic polypeptide (SIGMA, St. Louis, MO) and Insulin (Novo Nordisk, Bagsvaerd, Denmark) were used for the treatment of adipocytes. The NFAT blocker A-285222 was kindly provided by Abbott Laboratories, Abbott Park, IL [18]. The anti-OPN rat monoclonal antibody MPIIIB101 was from the developmental studies hybridoma bank of the University of Iowa (Iowa City, IA). The anti-mouse osteopontin polyclonal antibody (O–17) was from IBL (Hamburg, Germany). The anti-phospho GSK3 Ser9/21 antibody was from Cell Signaling Technologies (Beverly, MA). The anti-GSK3 antibody was from Biosource (Amarillo, CA). Both GSK3 antibodies detect the alpha and beta isoforms of GSK3. The mouse monoclonal antibody to Beta-Actin (A5441) was purchased from SIGMA. Chemical reagents used in preparation of buffers were purchased from SIGMA and VWR International.
Isolation and treatment of primary adipocytes
Primary rat adipocytes were isolated from epididymal fat pads as previously described [19]. Adipocytes were diluted to a concentration of 12.5% (v/v) in serum free Dulbecco’s Modified Eagle Medium (SIGMA) containing 25 mM glucose, 100 U/mL penicillin, 100 µg/mL streptomycin and 0.5% bovine serum albumin (BSA). One milliliter of cell suspension was incubated with the compounds indicated in results, for 37°C, overnight with 5% CO2. Incubations were stopped by washing the adipocytes twice in BSA free Krebs-Ringer-Hepes (KRH) buffer containing 25 mM Hepes, 200 nM adenosine and 2 mM glucose. Adipocytes were lysed by vortexing in buffer containing 50 mM TES, 2 mM EGTA, 1 mM EDTA, 250 mM sucrose, 2% (v/v) Nonidet P-40, 40 mM phenylphosphate, 5 mM NaF, 1 mM dithioerythriol, 0.5 mM sodium orthovanadate, 10 µg/mL antipain, 10 µg/mL leupeptin, 1 µg/mL pepstatin A, pH 7.4. Lysates were centrifuged at 10000×g for 10 minutes at 4°C, and infranatants were transferred to new tubes. Protein concentrations were determined by the method of Bradford [20].
Differentiation and treatment of 3T3-L1 adipocytes
3T3-L1 fibroblasts (80,000–100,000 per 100 mm plate) were cultured in 10 ml of Dulbecco’s Modified Eagle Medium (DMEM) (SIGMA) containing 25 mM glucose, 100 U/mL penicillin, 100 µg/mL streptomycin and 10 % Fetal Calf Serum. Confluent cells were differentiated for 2 days in DMEM supplemented with 0.5 mM IBMX, 10 µg/ml insulin and 1 µM dexamethasone. Experiments were performed with 3T3-L1 adipocytes (80–95% differentiated) 12–14 days after the initiation of differentiation under humidified atmosphere (air/CO2—19:1). Differentiated adipocytes were incubated with GIP as indicated in results, for 37°C, overnight with 5% CO2. The cells were washed twice in Phosphate Buffered Saline (PBS), scraped and lysed by vortexing in buffer containing 50 mM TES, 2 mM EGTA, 1 mM EDTA, 250 mM sucrose, 2% (v/v) Nonidet P-40, 40 mM phenylphosphate, 5 mM NaF, 1 mM dithioerythriol, 0.5 mM sodium orthovanadate, 10 µg/mL antipain, 10 µg/mL leupeptin, 1 µg/mL pepstatin A, pH 7.4. Lysates were centrifuged at 10000×g for 10 minutes at 4°C, and infranatants were transferred to new tubes. Protein concentrations were determined by the method of Bradford [20].
Lipogenesis
Lipogenesis was measured as previously described [21]. One milliliter aliquots of 2% (v/v) adipocytes in KRH Buffer containing 0.55 mM glucose and 3.5% BSA, but without adenosine, were added to vials containing 0.4 µCi D-[6– 3H]-glucose (GE healthcare) and incubated for 30 minutes. The assay was stopped with a scintillation fluid containing 3 g/liter POPOP, 5 g/liter 2.5-diphenyl oxazole in toluol. The amount of 3H incorporated into the lipid based fraction was determined by scintillation counting.
SDS-PAGE and Western Blotting
Samples were mixed with Laemmli sample buffer, subjected to electrophoresis through 10% polyacrylamide gels, and subsequently transferred to Hybond C nitrocellulose membranes (GE healthcare, Amersham U.K.). Membranes were blocked with 10% milk in TBST (50 mM Tris pH 7.5, 150 mM NaCl and 0.1% (w/v) Tween-20) followed by incubation with primary antibodies overnight at 4°C. Membranes were washed and subsequently incubated for 1 hour at room temperature with a 1:10,000 dilution of HRP conjugated-antimouse IgG secondary antibody (GE healthcare) in 5% milk. After a second washing, membranes were incubated with ECL reagent (Pierce, Rockford, IL) for 5 minutes before image capture in a Fujifilm LAS-1000 CCD camera (GE healthcare). Western blots were quantified using Image Gauge software (Fujifilm).
Real time RT-PCR
Four-month old C57BL/6 WT and PDE3B knock-out (KO) mice [17] were subcutaneously injected with 1 mg/kg of CL316243 in PBS or with PBS alone. Epididymal fat pads were collected 24 h after injections. Total adipose tissue mRNA was isolated using mirVana miRNA Isolation Kit (Life Technologies, NY), according to the manufacturer’s protocol. Following primer sequences for SYBR Green real time RT-PCR were used: 18S, F: gatgtgaaggatgggaagtacag, R: cttcttggatacacccacagttc; osteopontin, F: cccggtgaaagtgactgatt, R: ttcttcagaggacacagcattc. Gene expression levels were quantified as a ratio of target transcripts to 18S mRNA.
Data Analysis
Data are presented as mean ± SEM for the indicated number of experiments. Statistical significance between the groups was determined by the student’s two tailed t-test, unless otherwise specified, using the Graphpad Prism 5 software package
Results
GIP induces lipogenesis in primary adipocytes
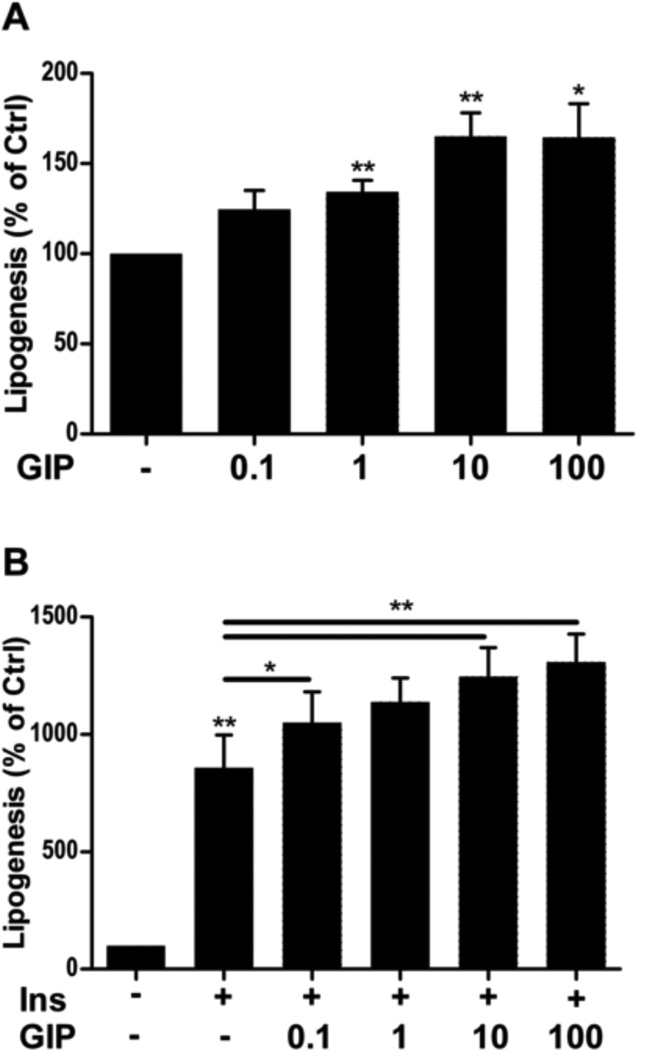
The effect of GIP on adipocyte lipogenesis was tested by stimulating cells for 10 minutes with or without various doses of GIP (0.1–100 nM) in the presence or absence of 1 nM insulin. GIP alone at doses 1–100 nM significantly induced lipogenesis, with 10–100 nM yielding approximately 50% increase in lipogenesis (Fig. 1A). GIP, in a dose dependent manner, potentiated the ability of 1 nM insulin to increase lipogenesis with a maximum effect seen at 10 nM, but clear effects already at 0.1 nM GIP (Fig. 1B).
Fig. 1.
GIP induces lipogenesis on its own as well as potentiates insulin-induced lipogenesis in primary rat adipocytes. Primary rat adipocytes were stimulated with GIP at the indicated concentrations (nM) in the absence (A) or presence (B) of insulin (INS, 1 nM) for 30 minutes. Lipogenesis was determined as glucose incorporation into lipid relative to the untreated group. *p < 0.05, **p < 0.01. n = 5 per group. GIP: glucose-dependent insulinotropic polypeptide.
GIP upregulates osteopontin protein expression in primary adipocytes and 3T3-L1 adipocytes
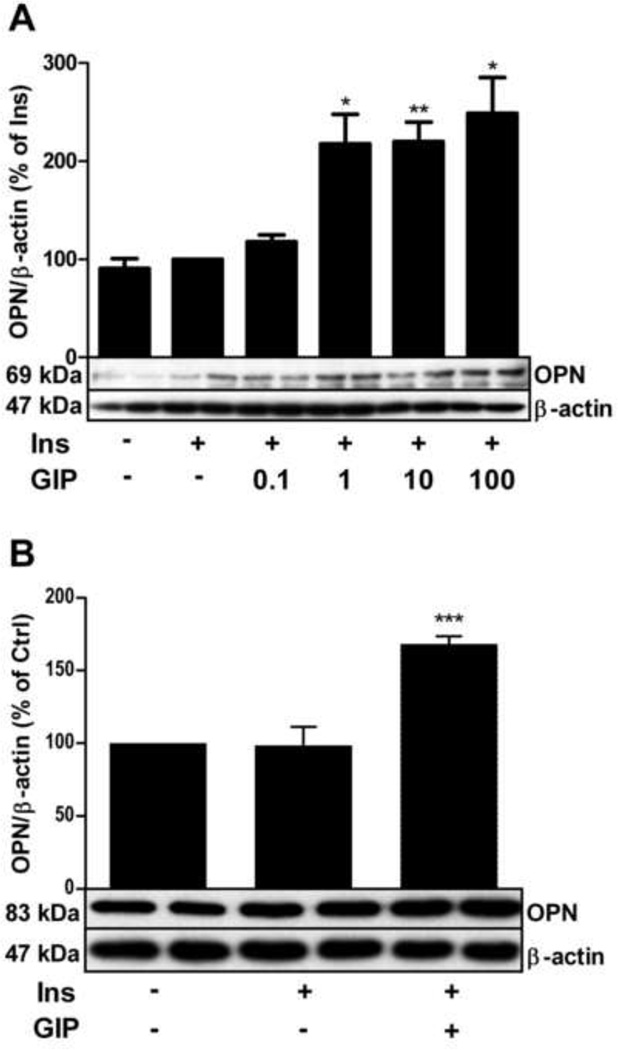
Osteopontin expression was significantly increased by incubation of rat adipocytes overnight with 1–100 nM GIP in the presence of 1 nM insulin (Fig. 2A, and Ahlqvist, unpublished). Incubation with insulin alone had no effect on osteopontin expression, but was required for the effect of GIP on osteopontin. The effect of GIP on osteopontin was also tested in another adipocyte model, mouse 3T3-L1 adipocytes. As shown in Fig. 2B, stimulation of mouse 3T3 L1 adipocytes with GIP also resulted in increased expression of osteopontin. Thus, the GIP response appears not to be model specific. In the following experiments we study mechanisms whereby GIP induces osteopontin expression using rat adipocytes as a model.
Fig. 2.
GIP upregulates osteopontin protein expression in primary adipocytes and 3T3-L1 adipocytes in the presence of insulin. 3T3 L1 adipocytes (A) and primary rat adipocytes (B) were stimulated with GIP at the indicated concentrations (nM) in A and 100 nM in B, in the presence or absence of insulin (INS, 1 nM) over night. Cells were lysed and osteopontin expression analyzed by western blotting. Representative blots are shown. *p < 0.05, **p < 0.01, ***p < 0.001 vs. non-treated controls. n=4 for both. GIP: glucose-dependent insulinotropic polypeptide, OPN: osteopontin
GIP-induced osteopontin expression is partially mediated by NFAT
The transcription factor NFAT has previously been shown to regulate osteopontin expression in the vasculature in response to high glucose and/or G protein-coupled receptor stimulation [22]. NFAT transcriptional activity is dependent on a balance between the phosphatase activity of calcineurin, which promotes translocation of NFAT to the nucleus and the activity of various kinases which phosphorylate NFAT leading to nuclear export instead [23, 24]. One important NFAT export kinase is glycogen synthase kinase (GSK) 3 [25, 26], a kinase which is negatively regulated upon phosphorylation by protein kinase B (PKB), a key target for insulin action [27].
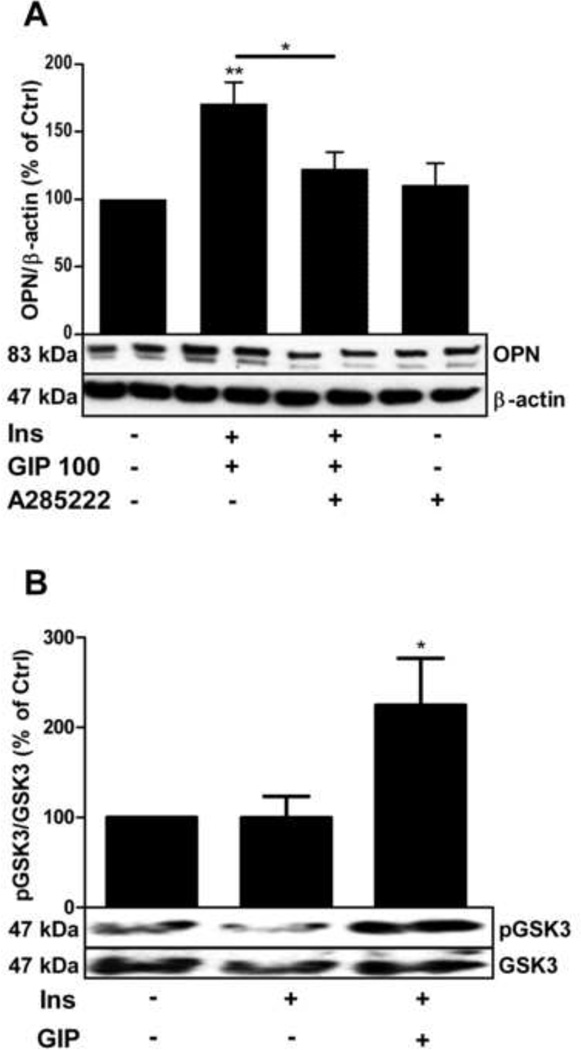
The role of NFAT in GIP-induced osteopontin expression was studied using the NFAT inhibitor A285222, a compound that effectively blocks NFAT nuclear accumulation and transcriptional activity in several cell types (i.e. immune, vascular, pancreatic acinar cells) [18, 22, 28]. As shown in Fig. 3A, A285222 (1 µM) inhibited GIP-induced osteopontin expression, suggesting that NFAT is activated upon GIP stimulation in adipocytes and that it plays a role in the regulation of GIP-induced osteopontin expression in these cells. Incubation with A-285222 in the absence of GIP and insulin had no effect on basal osteopontin expression. Interestingly, under the same stimulatory conditions shown to increase osteopontin expression (100 nM GIP in the presence of insulin), an increase in GSK3 phosphorylation was observed (Fig. 3B). The effect of GIP on GSK3 phosphorylation, which is associated with reduced kinase activity and hence would result in decreased NFAT nuclear export, may represent one mechanism by which GIP stimulation engages NFAT-signaling in adipocytes.
Fig. 3.
GIP-induced osteopontin expression is partially mediated by NFAT and involves inhibition of the NFAT export kinase GSK3. lA. Primary rat adipocytes were stimulated overnight by GIP (100 nM) in the presence of insulin (INS, 1 nM) with or without a 30 minute pre-treatment with the NFAT inhibitor A-285222 (1 µM) over night. Cells were lysed and osteopontin expression analyzed by western blotting. Representative blots are shown, n=7. B. Adipocytes were stimulated by GIP (100 nM) in presence or absence of insulin (INS, 1 nM) over night, cells were lysed and GSK3 phosphorylation was analyzed by western blotting. Representative blots are shown. .*p < 0.05, **p < 0.01 n=9. GIP: glucose-dependent insulinotropic polypeptide, GSK3: glycogen synthase kinase 3, NFAT: the transcription factor nuclear factor of activated T-cells, OPN: osteopontin
The synergism between GIP and insulin signaling on lipogenesis and the fact that insulin was required for the GIP-induction of osteopontin suggest cross-talk between these two signaling pathways in adipocytes. It is also possible that GIP may mediate some of its effects on osteopontin by increasing intracellular cAMP. In adipocytes, β-adrenergic receptor agonists increase cAMP and have been shown to increase phosphorylation of PKB as well as to potentiate the ability of insulin to induce phosphorylation of PKB [29]. Thus, we looked at the ability of cAMP, a major second messenger engaged by GIP stimulation [15], to induce osteopontin expression.
Osteopontin is upregulated in adipocytes treated with the β3-adrenergic receptor agonist CL316243 (CL), or with the PDE3 inhibitor OPC 3911 and in adipose tissue from PDE3B KO mice
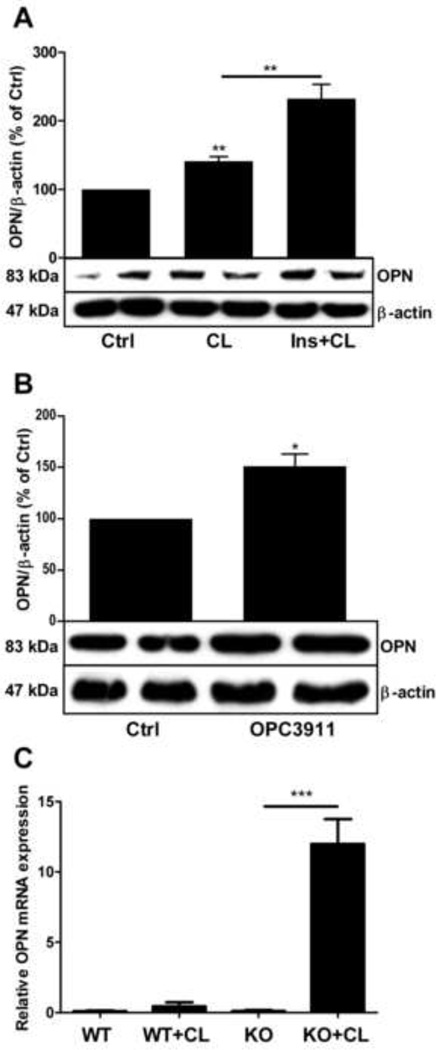
As shown in Fig. 4A, incubation of adipocytes with CL alone induced an upregulation of osteopontin expression and this effect was potentiated by insulin. Furthermore, selective inhibition of PDE3, a major cAMP hydrolyzing enzyme in adipocytes, also led to upregulation of osteopontin expression (Fig. 4B). A connection between PDE3B and the regulation of osteopontin is further supported by findings in PDE3B KO mice. Injection of CL into PDE3B KO mice resulted in an extensive upregulation of osteopontin mRNA in adipose tissue whereas no such effect of CL was obtained in control mice (Fig. 4C).
Fig. 4.
Osteopontin is upregulated in adipocytes treated with CL316243 (CL), a β3-adrenergic receptor agonist, by OPC 3911, a PDE3 inhibitor, and in adipose tissue from PDE3B KO mice treated with CL in vivo. A. Primary rat adipocytes were stimulated with the β3-adrenergic agonist CL316,243 (10 nM) in the presence or absence of insulin (INS, 1 nM), n=4. B. Adipocytes were incubated with or without the PDE3 inhibitor OPC 3911 (10 µM). Cells were lysed and OPN expression analyzed by western blotting. Representative blots are shown, n=4. C. Four-month old C57BL6 PDE3B knock-out mice were subcutaneously injected with 1 mg/kg of CL316243 in PBS or with PBS alone. Epididymal fat pads were collected 24 h after injections. Total mRNA was prepared and gene expression was measured using real time RT-PCR as described in methods. Gene expression levels were quantified as a ratio of target transcripts to 18S mRNA. Data are expressed as means ± SEM (n=7–8). *p < 0.05, **p < 0.01, ***p < 0.001. OPN: osteopontin, PDE3B: phosphodiesterase 3B
Thus, it appears as if GIP utilizes insulin as well as cAMP signaling components to regulate osteopontin protein expression.
Discussion
In this work we show that mechanisms whereby GIP induces upregulation of osteopontin expression in adipocytes involve crosstalk with insulin signaling networks, the transcriptional factor NFAT and the cAMP/PDE3B system. Our results provide a better understanding of the molecular mechanism that control the expression of osteopontin, which is emerging as a key regulator of adipocytes function [8–10, 30].
The demonstration that GIP as an effective stimulus for osteopontin expression in adipocytes is in agreement with findings in pancreatic islets, β-cells [16]. It is also in line with recent data in adipose tissue showing that a common variant of the GIPR (rs10423928) was associated with reduced GIP receptor function and lower osteopontin mRNA levels, along with better insulin sensitivity (Ahlqvist, unpublished). Conversely, a number of proinflammatory genes were upregulated in 3T3-L1 adipocytes overexpressing the GIP receptor [31]. The finding that an NFAT inhibitor prevented GIP-induced upregulation of osteopontin is in agreement with studies demonstrating a role for NFAT as a regulator of osteopontin expression in other systems. For example in arterial smooth muscle NFAT was shown to bind to the promoter region of osteopontin and drive its expression in response to diabetes-induced hyperglycemia in vivo, as well as in isolated cells in vitro [22]. Furthermore, NFAT was recently shown to regulate a number of genes in adipocytes of importance for the regulation of lipolysis [32]. NFAT has been shown to be regulated by GSK3-dependent phosphorylation in adipocytes as inhibition of GSK3 resulted in increased NFAT transcriptional activity [23]. In this work we show that GSK3 was phosphorylated and thereby inhibited by GIP in adipocytes. Thus, GIP potentially, via cAMP-dependent mechanisms, recruits insulin signaling components such as PKB, an upstream negative regulator of GSK3 activity. This per se, could result in decreased NFAT re-phosphorylation and hence nuclear export, increased net nuclear accumulation of NFAT and thereby increased osteopontin transcription. Indeed, activation of the GIP receptor, which is a member of the B-family of G protein-coupled receptors, results in the stimulation of PKB [11].
The finding that a β3-adrenergic receptor agonist mimicked the action of GIP on osteopontin expression is in agreement with GIP as a stimulator of cAMP production and PKA activation. CL induced an upregulation of osteopontin expression in the absence as well as presence of insulin whereas the GIP effect required the presence of insulin. Mechanisms explaining this difference have not been established but most likely depend upon additional signaling pathways recruited by CL. For example, we have previously shown that CL alone is able to phosphorylate and activate PKB [29], a key mediator of insulin action. Thus it is possible that CL to a larger extent than GIP is able to cross-talk with insulin signaling components and that this crosstalk is essential for mediating the effects on NFAT phosphorylation and osteopontin expression.
In the context of cAMP signaling mechanisms we show that PDE3B seems to have a key role in the regulation of osteopontin expression, not only in isolated adipocytes but also in adipose tissue in vivo. Thus, injection of CL into PDE3B KO mice induced a dramatic increase in osteopontin mRNA expression in adipose tissue as was not the case in wild-type mice. These results are in agreement with the finding that a selective inhibitor of PDE3 increased osteopontin expression in isolated adipocytes although this did not require stimulation of cAMP production as was the case in the in vivo situation. These data indicate an important role for a cAMP pool controlled by PDE3B in the regulation of osteopontin expression.
In summary, in this work we demonstrate a connection between GIP and osteopontin in adipocytes and identify several mechanisms involved in this context. The results should be considered in light of the fact that GIP as well as osteopontin are important targets in the pathophysiology of obesity and diabetes.
Highlights.
GIP stimulates lipogenesis and osteopontin expression in primary adipocytes
GIP-induced osteopontin expression is NFAT-dependent
Osteopontin expression is PDE3-dependent
Osteopontin expression is increased in PDE3B KO mice
Acknowledgement
We thank Ann Kristin Holmén Påhlbrink and Eva Ohlson for excellent technical assistance. This work was supported by the Swedish Research Council (Project grants 2010-3362 to ED, 6589 and 2009-1039 to LG and 2011-3900 to MFG), by an Advanced Research Grant from the European Research Council to LG (GA269045), by a grant from Knut & Alice Wallenberg foundation and by LUDC (Lund University Diabetes Centre). Grants were also obtained from the following foundations: Swedish Diabetes Association; Novo Nordisk Foundations, Denmark; The Swedish Society of Medicine and Albert Påhlsson. EG and VM were supported by the NHLBI Intramural Research Program.
Abbreviations
- CL
CL316,243
- GIP
glucose-dependent insulinotropic polypeptide
- GSK
glycogen synthase kinase
- NFAT
nuclear factor of activated T-cells
- PDE
phosphodiesterase
- PKA
protein kinase A
- PKB
protein kinase B
- T2D
type 2 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rasouli N, Kern PAJ. Adipocytokines and the metabolic complications of obesity. Clin Endocrinol Metab. 2008 Nov;93(11 Suppl 1):S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance and dyslipidemia. Curr Diab Rep. 2009;9:26–32. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 Dec 14;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 5.Kiefer FW, Zeyda M, Todoric J, et al. Endocrinology. 2008 Mar;149(3):1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Ambrosi J, Catalán V, Ramírez B, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin Endocrinol Metab. 2007 Sep;92(9):3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 7.Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin Invest. 2007 Oct;117(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiefer FW, Neschen S, Pfau B, et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011 Aug;54(8):2132–2142. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman J, Miles PD, Ofrecio JM, et al. Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mce. PLoS One. 2010 Nov 12;5(11):e13959. doi: 10.1371/journal.pone.0013959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiefer FX, Zeyda M, Gollinger K, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010 Apr;59(4):935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP) Vitam Horm. 2009;80:409–471. doi: 10.1016/S0083-6729(08)00615-8. [DOI] [PubMed] [Google Scholar]
- 12.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci. 2000;66(2):91–103. doi: 10.1016/s0024-3205(99)00314-8. [DOI] [PubMed] [Google Scholar]
- 13.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002 Jul;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 14.Yip RG, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology. 1998 Sep;139(9):4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 15.Mohammad S, Ramos LS, Buck J, et al. Gastric inhibitory peptide controls adipose insulin sensitivity via activation of cAMP-response element-binding protein and p110β isoform of phosphatidylinositol 3-kinase. J. Biol Chem. 2011 Dec 16;286(50):43062–43070. doi: 10.1074/jbc.M111.289009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyssenko V, Eliasson L, Kotova O, et al. Pleiotropic effects of GIP on islet function involve osteopontin. Diabetes. 2011 Sep;60(9):2424–2433. doi: 10.2337/db10-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YH, Park S, Hockman S, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin Invest. 2006 Dec;116(12):3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trevillyan JM, Chiou XG, Chen YW, et al. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. Biol Chem. 2001 Dec 21;276(51):48118–48126. doi: 10.1074/jbc.M107919200. [DOI] [PubMed] [Google Scholar]
- 19.Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes, I. Cell preparation, manipulation, and predictability in behavior. J. Biol Chem. 1985;260(28):15122–15129. [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Moody AJ, Stan MA, Stan M, Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974;6(1):12–16. doi: 10.1055/s-0028-1093895. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Ohman J, et al. Nuclear factor of activated T cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Biol. 2010 Feb;30(2):218–224. doi: 10.1161/ATVBAHA.109.199299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002 Apr;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson LM, Nilsson-Ohman J, Zetterqvist AV, Gomez MF. Nuclear factor of activated T-cells transcription factors in the vasculature: the good guys or the bad guys? Curr Opin Lipidol. 2008 Oct;19(5):483–490. doi: 10.1097/MOL.0b013e32830dd545. [DOI] [PubMed] [Google Scholar]
- 25.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear Export of NF-ATc Enhanced by Glycogen Synthase Kinase-3. Science. 1997 Mar 28;275:1930–1933. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson J, Nilsson LM, Chen YW, et al. High glucose activates nuclear factor of activated T cells in native vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2006 Apr;26(4):794–800. doi: 10.1161/01.ATV.0000209513.00765.13. [DOI] [PubMed] [Google Scholar]
- 27.Cross DA, Watt PW, Shaw M, et al. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997 Apr 7;406(1–2):211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 28.Awla D, Zetterqvist AV, Abdulla A, et al. NFATc3 regulates trypsinogen activation, neutrophil recruitment and tissue damage in experimental acute pancreatitis. Gastroenterology. doi: 10.1053/j.gastro.2012.07.098. in press. [DOI] [PubMed] [Google Scholar]
- 29.Zmuda-Trzebiatowska E, Manganiello V, Degerman E. Novel mechanisms of the regulation of protein kinase B in adipocytes; implications for protein kinase A, Epac, phosphodiesterases 3 and 4. Cell Signal. 2007 Jan;19(1):81–86. doi: 10.1016/j.cellsig.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Zeyda M, Gollinger K, Todoric J, Kiefer FW, Keck M, Aszmann O, Prager G, Zlabinger GJ, Petzelbauer P, Stulnig TM. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011 Jun;152(6):2219–2227. doi: 10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 31.Nie Y, Ma RC, Chan JC, Xu H, Xu G. Glucose-dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose-dependent insulinotropic peptide receptor-overexpressing adipocytes. FASEB J. 2012 Jun;26(6):2383–2393. doi: 10.1096/fj.11-196782. [DOI] [PubMed] [Google Scholar]
- 32.Holowachuk EW. Nuclear factor of activated T cell (NFAT) transcription proteins regulate genes involved in adipocyte metabolism and lipolysis. Biochem Biophys Res Commun. 2007 Sep 21;361(2):427–432. doi: 10.1016/j.bbrc.2007.07.026. [DOI] [PubMed] [Google Scholar]