Abstract
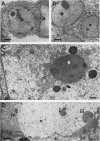
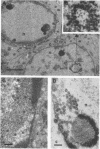
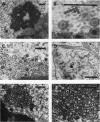
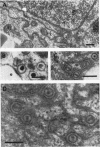
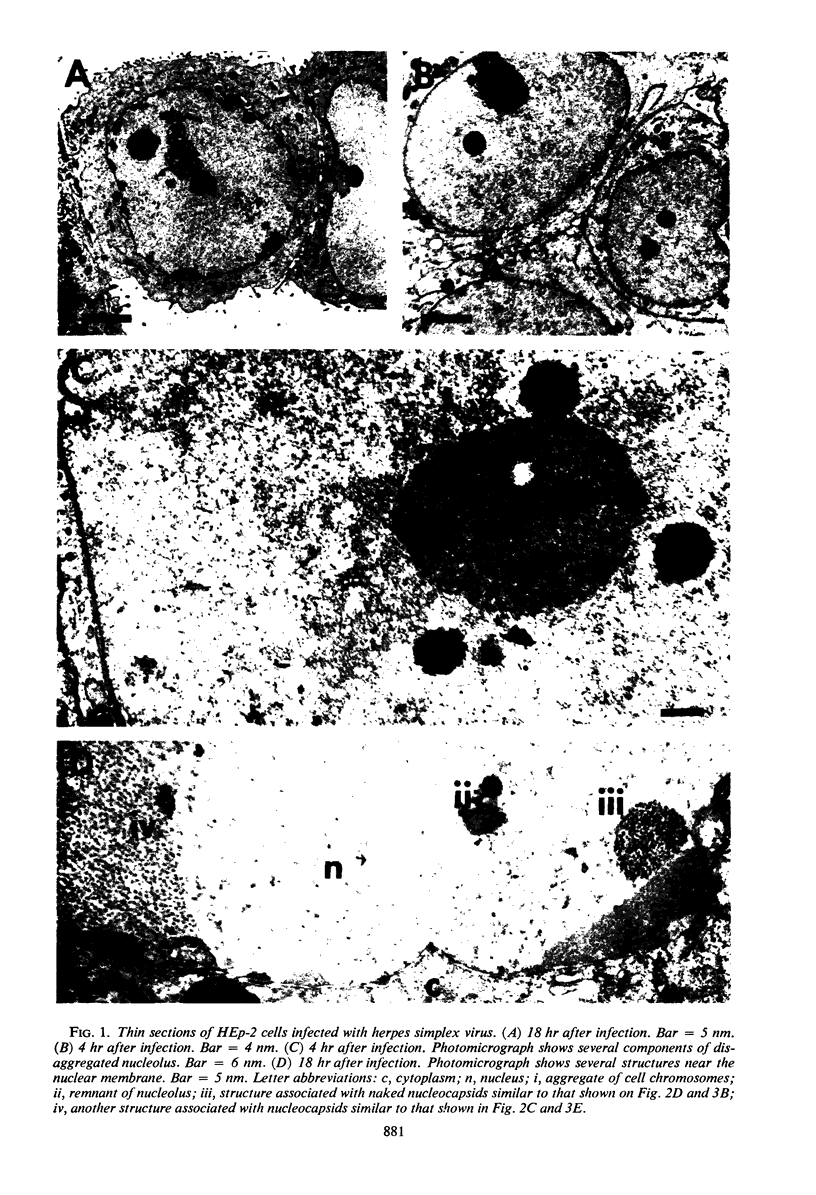
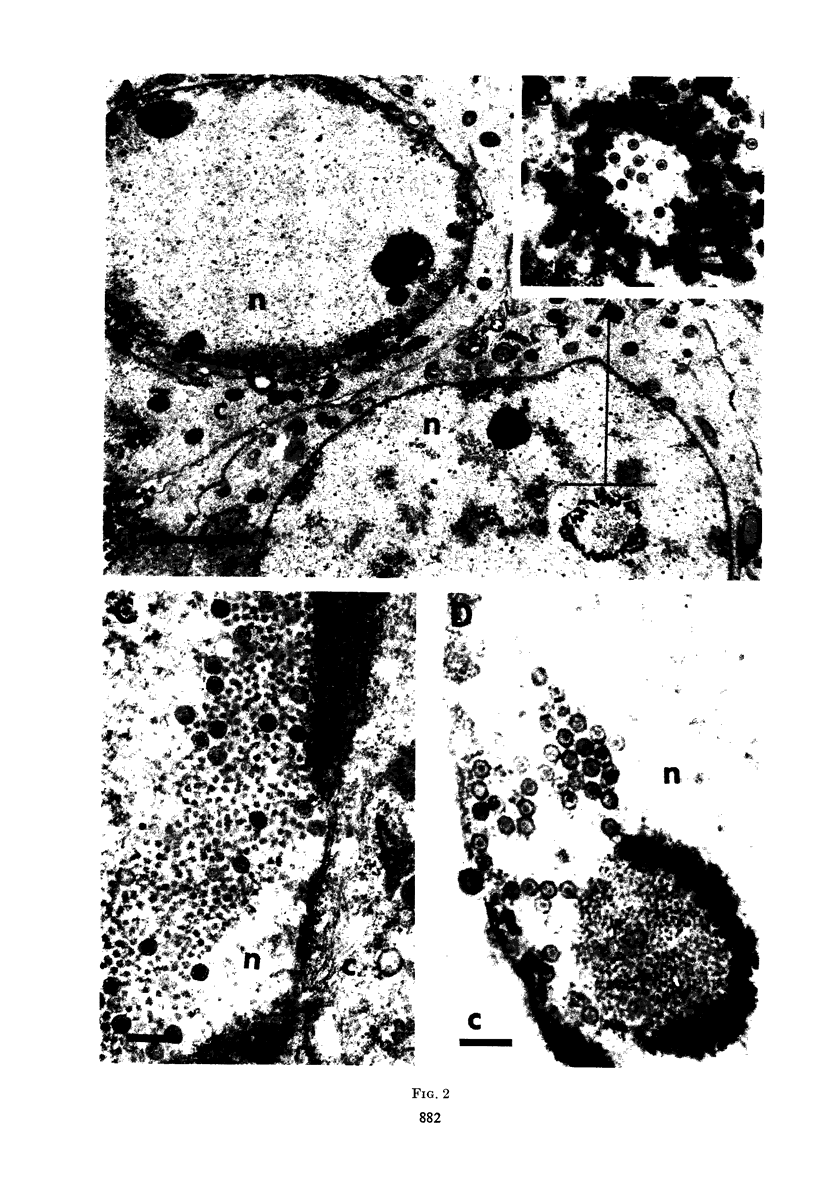
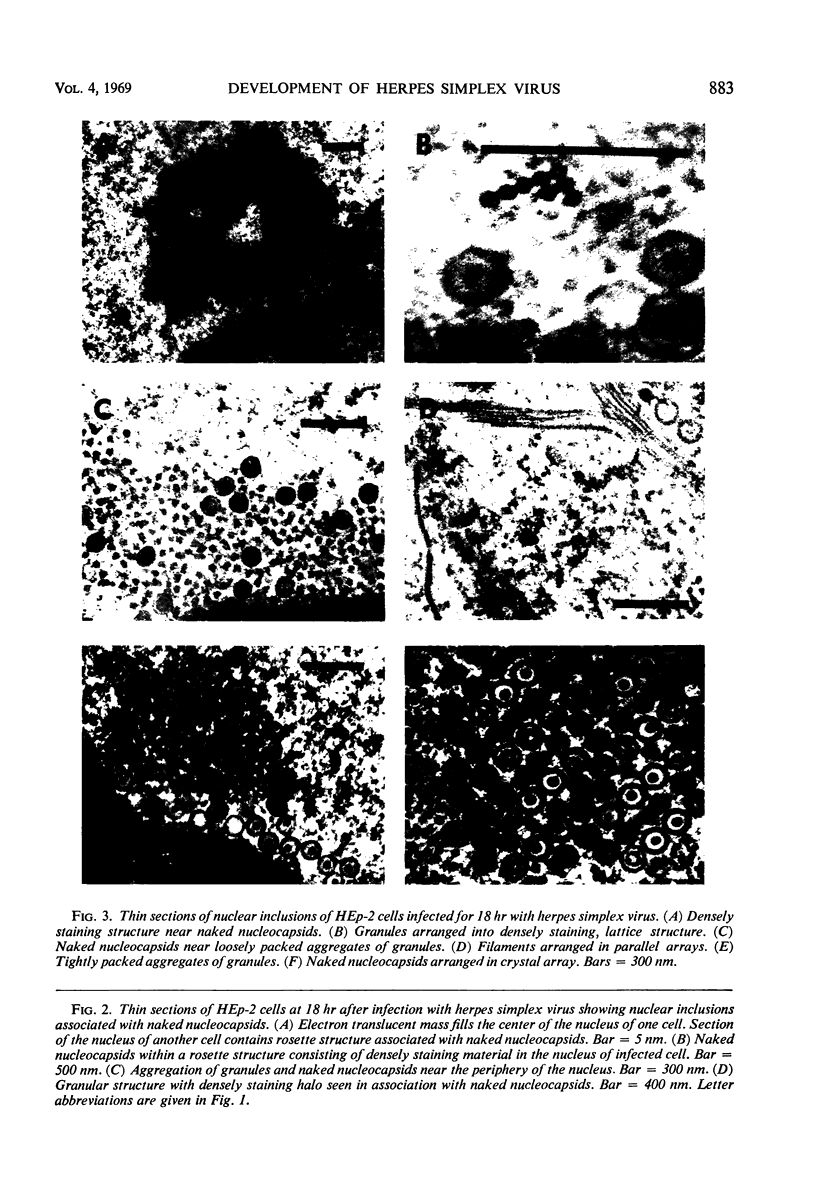
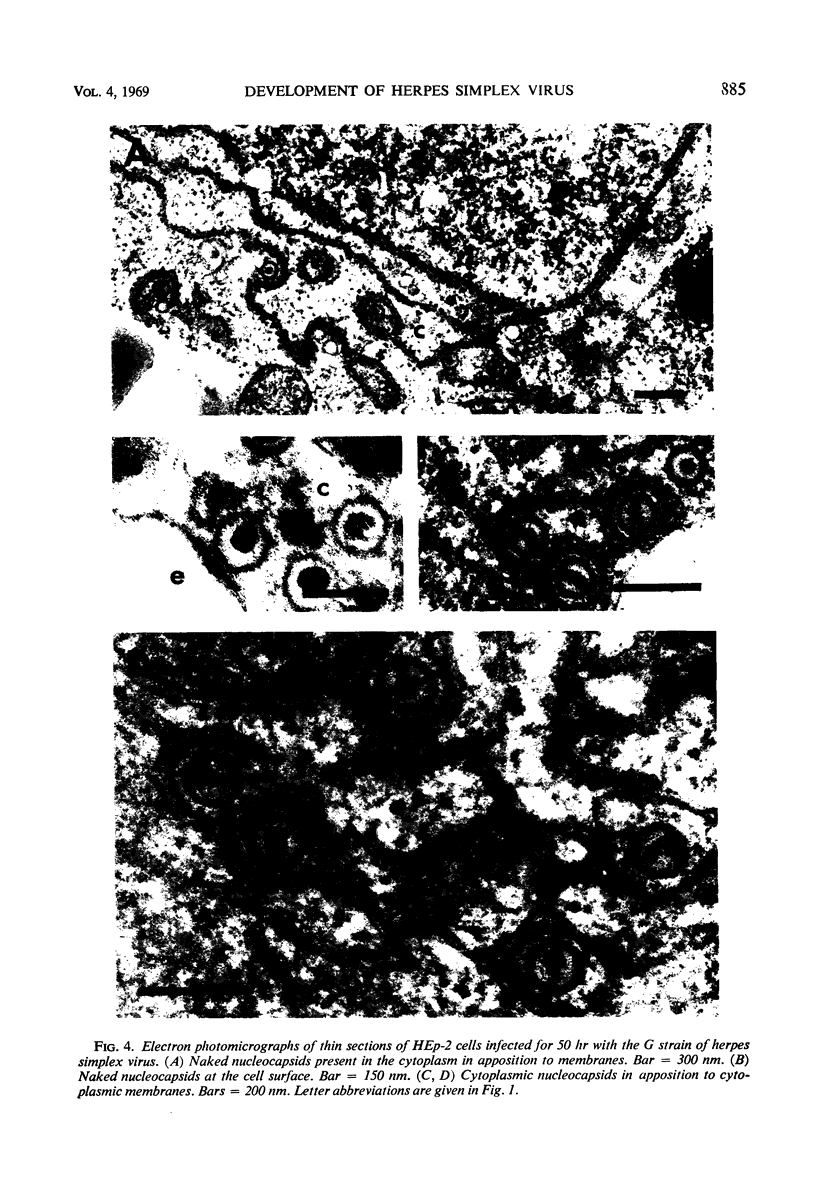
HEp-2 cells infected with two laboratory strains (mP and MP) and two freshly isolated strains (F and G) of herpes simplex virus were fixed at intervals between 4 and 50 hr postinfection and sectioned, and were then examined with the electron microscope. These studies revealed the following. (i) All four strains caused identical segregation of nucleoli and aggregation of host chromosomes at the nuclear membrane. (ii) The development of MP virus could not be differentiated from that of its parent mP strain. (iii) There were quantitative differences between laboratory (mP) and freshly isolated (F) type 1 strains. Thus, cells infected with F contained numerous nuclear crystals of nucleocapsids and relatively few cytoplasmic structures containing enveloped nucleocapsids. Conversely, cells infected with mP or with MP virus contained numerous cytoplasmic structures with enveloped nucleocapsids and relatively few nuclear crystals of nucleocapsids. (iv) There were qualitative differences between type 2 strain (G) isolated from genital lesions and type 1 strains. Thus, cells infected with the G strain contain numerous filaments in nuclei and unenveloped and partially enveloped nucleocapsids in the cytoplasm. Of particular interest is the finding that cytoplasmic membranes in apposition to nucleocapsids were thickened and bent as if they were enveloping the particle. The significance of the qualitative differences in the development of the four strains is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURELIAN L., ROIZMAN B. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. II. ALTERNATIVE SUPPRESSION OF SYNTHESIS OF INTERFERON AND VIRAL CONSTITUENTS. J Mol Biol. 1965 Mar;11:539–548. doi: 10.1016/s0022-2836(65)80009-2. [DOI] [PubMed] [Google Scholar]
- BARSKI G., ROBINEAUX R. Evolution of herpes simplex cellular lesions observed in vitro by phase contrast microcine-matography. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:632–636. doi: 10.3181/00379727-101-25042. [DOI] [PubMed] [Google Scholar]
- BEN-PORAT T., KAPLAN A. S. MECHANISM OF INHIBITION OF CELLULAR DNA SYNTHESIS BY PSEUDORABIES VIRUS. Virology. 1965 Jan;25:22–29. doi: 10.1016/0042-6822(65)90247-3. [DOI] [PubMed] [Google Scholar]
- Bedoya V., Rabson A. S., Grimley P. M. Growth in vitro of herpes simplex virus in human lymphoma cell lines. J Natl Cancer Inst. 1968 Sep;41(3):635–652. [PubMed] [Google Scholar]
- Couch E. F., Nahmias A. J. Filamentous structures of type 2 Herpesvirus hominis infection of the chorioallantoic membrane. J Virol. 1969 Feb;3(2):228–232. doi: 10.1128/jvi.3.2.228-232.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., James C. Biological and morphological aspects of the growth of equine abortion virus. J Bacteriol. 1966 Jul;92(1):250–257. doi: 10.1128/jb.92.1.250-257.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- EPSTEIN M. A. Observations on the mode of release of herpes virus from infected HeLa cells. J Cell Biol. 1962 Mar;12:589–597. doi: 10.1083/jcb.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Figueroa M. E., Rawls W. E. Biological markers for differentiation of herpes-virus strains of oral and genital origin. J Gen Virol. 1969 Mar;4(2):259–267. doi: 10.1099/0022-1317-4-2-259. [DOI] [PubMed] [Google Scholar]
- GRAY A., TOKUMARU T., SCOTT T. F. M. Different cytopathogenic effects observed in HeLa cells infected with herpes simplex virus. Arch Gesamte Virusforsch. 1958;8(1):59–76. doi: 10.1007/BF01242313. [DOI] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Zajac B. Early events in herpes simplex virus infection: a radioautographic study. J Virol. 1969 Jul;4(1):67–74. doi: 10.1128/jvi.4.1.67-74.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- LOVE R., RABSON A. S., WILDY P. CHANGES IN THE NUCLEOLUS OF NORMAL AND NEOPLASTIC CELLS INFECTED WITH RIBOVIRUSES AND DEOXYRIBOVIRUSES. Acta Unio Int Contra Cancrum. 1964;20:1384–1387. [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., JONES E. P., HOLDEN M., ROSE H. M. Intranuclear crystals of herpes simplex virus observed with the electron microscope. Virology. 1958 Jun;5(3):568–571. doi: 10.1016/0042-6822(58)90047-3. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Rabin E. R., Jenson A. B. Intracellular herpesvirus aggregate in the form of a pentagonal dipyramidal crystal-like structure. J Virol. 1968 Jan;2(1):78–80. doi: 10.1128/jvi.2.1.78-80.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Intranuclear formation of filaments in herpesvirus hominis infection of mice. Arch Gesamte Virusforsch. 1967;21(3):463–468. doi: 10.1007/BF01241746. [DOI] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER G. SEROLOGICAL COMPARISON OF THE HERPES VIRUSES. Br J Exp Pathol. 1964 Apr;45:135–141. [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. I. CHARACTERIZATION OF VIRAL PROGENY FROM CO-OPERATIVE INFECTION WITH MUTANTS DIFFERING IN CAPACITY TO MULTIPLY IN CANINE CELLS. J Mol Biol. 1965 Mar;11:528–538. doi: 10.1016/s0022-2836(65)80008-0. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr A physical difference between two strains of herpes simplex virus apparent on sedimentation in cesium chloride. Virology. 1961 Sep;15:75–79. doi: 10.1016/0042-6822(61)90079-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr Demonstration of a surface difference between virions of two strains of herpes simplex virus. Virology. 1963 Feb;19:198–204. doi: 10.1016/0042-6822(63)90009-6. [DOI] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Siegert R., Falke D. Elektronenmikroskopische Untersuchungen über die Entwicklung des Herpesvirus hominis in Kulturzellen. Arch Gesamte Virusforsch. 1966;19(2):230–249. [PubMed] [Google Scholar]
- Siminoff P., Menefee M. G. Normal and 5-bromodeoxyuridine-inhibited development of herpes simplex virus. An electron microscope study. Exp Cell Res. 1966 Nov-Dec;44(2):241–255. doi: 10.1016/0014-4827(66)90429-0. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Roizman B. Herpes simplex virus products in productive and abortive infection. 3. Differentiation of infectious virus derived from nucleus and cytoplasm with respect to stability and size. J Virol. 1968 Oct;2(10):979–985. doi: 10.1128/jvi.2.10.979-985.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D. H., WILDY P., RUSSELL W. C. QUANTITATIVE ELECTRON MICROSCOPY STUDIES ON THE GROWTH OF HERPES VIRUS USING THE TECHNIQUES OF NEGATIVE STAINING AND ULTRAMICROTOMY. Virology. 1964 Dec;24:523–538. doi: 10.1016/0042-6822(64)90204-1. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]