Abstract
Necrotizing enterocolitis (NEC) remains one of the most catastrophic comorbidities associated with prematurity. In spite of extensive research, the disease remains unsolved. The aims of this paper are to present the current state of the science on the pathogenesis of NEC, summarize the clinical presentation and severity staging of the disease, and highlight the nursing assessments required for early identification of NEC and ongoing care for infants diagnosed with this gastrointestinal disease. The distributions of systemic and intestinal clinical signs that are most sensitive to nursing assessment and associated with Bell Staging Criteria are presented. This descriptive data is representative of 117 cases of NEC diagnosed in low gestational age infants (<29 weeks gestation). The data highlights the clinical signs most commonly observed in infants with NEC, and thus, provides NICU nurses an evidence-based guide for assessment and care of infants with NEC.
Keywords: Necrotizing enterocolitis, prematurity, gastrointestinal disease, neonatal intensive care, nursing assessment
Necrotizing enterocolitis (NEC) is the most common life-threatening gastrointestinal emergency experienced by premature infants cared for in the Newborn Intensive Care Unit (NICU). It is a devastating gastrointestinal disease that is associated with severe sepsis, intestinal perforation, and significant morbidity and mortality1. The incidence of NEC is inversely correlated to gestational age and birth weight2-4. Low birth weight, premature infants are affected at a prevalence as high as 15% of all infants cared for in the NICU2-4. More than 11% of infants born at birth weights below 750 grams will develop NEC2-4. Though the majority of NEC cases are treated medically, an estimated 20 to 40% of infants will require urgent surgical intervention including exploratory laparotomy, bowel resection, and ostomy. The case fatality rate associated with surgical intervention is as high as 50%, and is highest among the smallest and most premature infants5-7. Infants who survive are prone to short bowel syndrome, parenteral nutrition-associated cholestasis, prolonged neonatal hospitalization, significantly impaired growth, and poor long-term neurodevelopment8-11. These infants require long term nursing care that is complicated and costly. The yearly additional hospital charges for NEC in the United States are estimated in excess of $6.5 million12. Epidemiologic risk factors and clinical predictors have been explored; however, only prematurity has been identified as a consistent risk factor associated with NEC2,13.
NEC is one of the major unsolved problems associated with premature birth. In order to establish the most pertinent nursing assessments required for early identification of NEC, this paper will (1) review the current state of the science on the pathogenesis of NEC, (2) summarize the clinical presentation and severity staging of the disease, and (3) discuss the nursing assessments required for early identification and care for infants diagnosed with this catastrophic gastrointestinal diagnosis.
State of the Science: Pathogenesis of Necrotizing Enterocolitis
NEC is characterized as an inflammatory disease of the newborn bowel. The pathogenesis of the disease, while not fully understood, is believed to be multifactorial in nature14-16. Factors related to intestinal ischemia and inflammation, enteral feeing, and aberrant bacterial colonization (i.e. infection) have been shown to play a role in the development of NEC in premature infants. To date, our knowledge of this disease has resulted from findings generated by investigators seeking to better understand the clinical epidemiology of the patient-specific risk factors associated with NEC, and the immune properties of the disease as understood in the context of the premature gastrointestinal (GI) system. Intestinal ischemia and inflammation, the role of enteral feedings, and aberrant bacterial colonization are three areas of scientific discovery that are likely to advance our knowledge of NEC in premature infants.
Intestinal Ischemia, Inflammation and the Premature GI System
Hypoxic-ischemic injury associated with inflammation of the newborn bowel has long been identified as an important component in the pathogenesis of NEC17,18. Based on histopathologic findings, it was previously believed that an ischemic event always preceded the development of NEC in the premature infant19. However, it has now been shown that ischemia is more likely to occur in association with an early and exaggerated inflammatory response of the GI villi that follows a neonatal intestinal insult1,20. Neonatal insults such as hypoxia and hypoperfusion, enteral feeding with infant formula, and eventually, aberrant colonization of the premature gut, result in injury to the epithelial cells and subsequent intestinal inflammation. Intestinal inflammation results in the release of inflammatory mediators, which often lead to an unfavorable response by the immature intestine, and in turn, determines the symptoms observed, the severity of the illness, and the clinical outcome for the patient1,21.
After years of study, prematurity remains the only consistently identified risk factor for NEC2. This is explained by the immaturity of the intestinal tract and associated immune response, attributes of the premature gut that make it prone to NEC1,22-24. Furthermore, the premature gut is developmentally immature in terms of motility, digestion and absorption, circulatory regulation, intestinal epithelial barrier function, and immune functions22. Premature infants present with inadequate intestinal motility, in large part, because intestinal motility develops during the third trimester. It is not until the 34th week of gestation that migrating motor complexes appear, which facilitate normal motility through the gut25. Normal motility is critical for digestion and absorption of nutrients, leading to ideal growth. In addition, motility is a critical aspect of preventing stasis and subsequent disease in the GI system. When motility is compromised, the intestinal epithelium is more likely to be exposed to potentially harmful substances, and thus potentially injured as in the pathogenesis of NEC.
It is thought that the immature intestinal epithelial cells that play a role in barrier and immune function within the gut mount an exaggerated inflammatory response to intestinal injury, which culminates in the diagnosis of NEC15. These characteristics of the preterm GI system, which make it vulnerable to intestinal ischemia, hyperosmolar injury, bacterial invasion, and subsequent inflammation, are likely to play a critical role in the development of NEC. These components of the pathophysiology of NEC lead to disruption of the intestinal mucosal integrity that is evident clinically as abdominal distension, acute feeding intolerance, cardiopulmonary compromise, bacteremia, and profound hemodynamic instability. Pneumatosis is the radiographic sign that is often present and, in the most severe cases, bowel perforation leads to pneumoperitoneum1. Caring for these infants is particularly challenging for nurses because it is difficult to differentiate NEC from other neonatal complications (i.e. sepsis, GI morbidities) as the early clinical signs are often vague or nonspecific. However, once diagnosed, the progression of disease is rapid with significant clinical consequences.
Enteral Feeding
The role that enteral feeding plays in the pathogenesis of NEC remains an important area of investigation. Studies have identified that the introduction of enteral feedings into the neonatal intestinal lumen causes a disruption of mucosal integrity, blood flow, and motility, playing a key role in the development of NEC26,27. Unabsorbed nutrients in the small and large intestine can lead to enteric bacterial proliferation. The enteric bacteria can produce intraluminal gas leading to distention, pneumatosis intestinalis, increased intraluminal pressure, and the resultant decreased blood flow28. Therefore, many preventative strategies for NEC have been focused on feeding strategies and understanding the characteristics of microorganisms present in the gastrointestinal tract. The time of initial enteral feeding, initial volume, rate of advancement, and type of feedings over the course of the neonatal period has been studied widely. However, much of the research in this area is conflicting, and no consensus has been reached regarding the optimal enteral feeding regimen and nutritional strategies that may prevent NEC27.
When to feed, what to feed, and how quickly to advance enteral feedings in the premature infant population are factors that vary widely from one institution to another29. Benefits have been shown following early enteral feeding, also known as gut-stim or trophic feeding, with breast milk30,31. In addition, enteral feeding with breast milk has been shown to be protective against NEC for both nutritional and immunologic reasons32. However, NEC has been found to occur in 90 to 95% of infants with a history of recent feeding volume advancement or reinitiation of enteral feedings33. A trial comparing rapid versus slow advancement of feeding was not completed due to an increase in the rate of NEC in the rapidly advancing group25. Conversely, other trials have found a protective effect against NEC in patients who had enteral feedings advanced more aggressively, therefore experiencing a shorter time to full feeding volumes and more rapid attainment of birth weight34-37. In short, best practices for enteral feeding are limited to the administration of breast milk early in the neonatal period. Beyond these factors, new knowledge and advances in neonatal care are needed to better establish stronger evidence based practice in enteral feeding.
Aberrant Bacterial Colonization
Abnormal bacterial colonization of the immature gut is a significant risk factor identified with NEC38. Prior to birth, the newborn intestine is essentially sterile. At birth, the newborn intestine is inoculated and under ideal circumstances, a normal intestinal immune defense system develops. The pathophysiology that underpins NEC leads to atypical immunity and inflammatory response24. Furthermore, this evidence has been corroborated by the fact that one of the consistently identified protective effects against NEC is feeding with breast milk. Breastfed infants have a lower incidence of NEC than formula-fed infants39,40. This finding has been explained by the notion that breastfeeding facilitates colonization of a balanced, non-pathogenic flora in the gut that helps prevent bacterial overgrowth, whereas formula feeding promotes abnormal, pathogenic bacterial growth. Aberrant colonization and bacterial overgrowth produces food-induced toxic by-products including unique microbial molecular patterns that are capable of altering the epithelial barrier and triggering an inflammatory cascade of the immature intestinal innate immune system. This specific inflammatory cascade has been hypothesized to be central in triggering the onset of pathogenesis of NEC24,32,38.
The significance of bacterial colonization and importance of balancing harmful and helpful bacteria in the premature gut is a topic of great interest to neonatal researchers and clinicians. This interest has underpinned studies exploring the effect of widespread antibiotics on the incidence of NEC41, the role of specific intestinal microbiota in the development of NEC42,43, and ultimately, the potential use of nutritional strategies such as pre- and probiotics for disease prevention44,45. Findings have shown that prolonged duration of initial empirical antibiotic treatment is significantly associated with increased rates of NEC in extremely low birth weight infants, presumably because these drugs disrupt the normal colonization of the neonatal intestinal microbiota41. Investigators have also shown that certain microbiota such as Lactobacillus and Bifidobacteria are protective against NEC, while other species such as Enterobacteriaceae, Clostridia, and Staphylococcus are commonly implicated in the pathogenesis of the disease46. That said, recent evidence generated on the most technologically advanced platform suggests that no one microorganism is predictive of the disease. Rather, a predominance of Proteobacteia is highly associated with NEC. According to this study, the limited diversity of total bacteria and abundance of pathogenic bacteria may contribute to the susceptibility of the premature infant gut to NEC42. Further study on the microbiological aspects of disease holds promise for new knowledge that may result in preventative strategies including the use of selected prebiotics and probiotics.
In sum, understanding the pathogenesis of NEC continues to evolve. New knowledge generated by multidisciplinary teams will contribute to our understanding of the immature gastrointestinal system, immune response, and nutritional aspects related to the pathogenesis of this disease.
Severity of Disease: Staging Criteria for NEC
Clinical presentation of NEC may vary among infants, which presents a challenge to clinicians aiming to diagnose the disease at the earliest and least severe stage of pathogenesis. The disease may present anywhere on the clinical spectrum, ranging from slow and insidious to rapid and progressive47,48. Systemic, intestinal, and radiological signs all play a role in diagnosis, but vary in observed presence and degree of involvement. Assigning disease severity based on staging criteria for NEC is important in the diagnosis and treatment of the disease. Bell Staging has traditionally been the standard in assigning severity of disease to NEC cases.
Bell Stages
Dr. Martin Bell proposed the original clinical criteria used to stage NEC cases in 1978. Three stages were outlined to enhance the recognition and diagnosis of NEC, and to provide the most effective treatment for each cohort of patients. The proposed staging criteria have been modified as our understanding of NEC has evolved to incorporate further specificity into each stage of disease (Table 1)26,47,49. Even with these subsequent modifications, it has recently been suggested that the Bell Staging criteria are outdated as a result of the increase in viability at lower gestational ages, the improvement in clinical management of medical NEC, and the occurrence of other acquired neonatal intestinal diseases that differ from premature infant NEC48. Bell Staging, however, continues to be used as the standard of practice to diagnose, stage, and treat NEC in the NICU.
Table 1.
Modified Bell Staging Criteria for NEC
| Stage | Classification | Systemic signs | Intestinal signs | Radiologic signs |
|---|---|---|---|---|
| IA | Suspected NEC | Temperature instability, apnea, bradycardia, lethargy |
Increased pregavage residuals, mild abdominal distention, emesis, guaiac-positive stool |
Normal or intestinal dilation, mild ileus |
| IB | Suspected NEC | Same as above | Bright red blood from rectum | Same as above |
| IIA | Proven NEC – mildly ill |
Same as above | Same as above, plus absent bowel sounds, with or without abdominal tenderness |
Intestinal dilation, ileus, pneumatosis intestinalis |
| IIB | Proven NEC – moderately ill |
Same as above, plus mild metabolic acidosis, mild thrombocytopenia |
Same as above, plus absent bowel sounds, definite abdominal tenderness, with or without abdominal cellulitis or right lower quadrant mass |
Same as IIA, plus portal venous gas, with or without ascites |
| IIIA | Advanced NEC – severely ill, bowel intact |
Same as IIB, plus hypotension, bradycardia, severe apnea, combined respiratory and metabolic acidosis, disseminated intravascular coagulation, and neutropenia |
Same as above, plus signs of generalized peritonitis, marked tenderness, and distention of abdomen |
Same as IIB, plus definite ascites |
| IIIB | Advanced NEC – severely ill, bowel perforated |
Same as IIIA | Same as IIIA | Same as IIB, plus pneumoperitoneum |
Adapted from Lee & Polin, 2003
Stage 1, or suspected NEC, includes patients who present with the mildest of symptoms. The diagnosis of NEC is often questionable, and should be suspected after other examinations rule out other gastrointestinal disorders. Systemic manifestations include temperature instability, lethargy, apnea, and bradycardia. The infant may feed poorly, have increasing pregavage residuals, vomit, present with a mildly distended abdomen, or pass stool with occult blood. Bowel loops may be distended on radiographic evaluation with mild ileus47.
Infants with a suspected NEC diagnosis who have disease that progresses to include the classic radiological sign of pneumatosis intestinalis are classified as Stage II, or proven, NEC cases (see Figure 1). This classification of patients present with signs more indicative of NEC than Stage I after other gastrointestinal disorders have been ruled out. Abdominal distention in these patients is marked (see Figure 2), and persistent occult or frank blood in the stool may be present. Radiological signs may include pneumatosis intestinalis , persistent or unchanging bowel loops, and the development of portal vein gas47.
Figure 1.
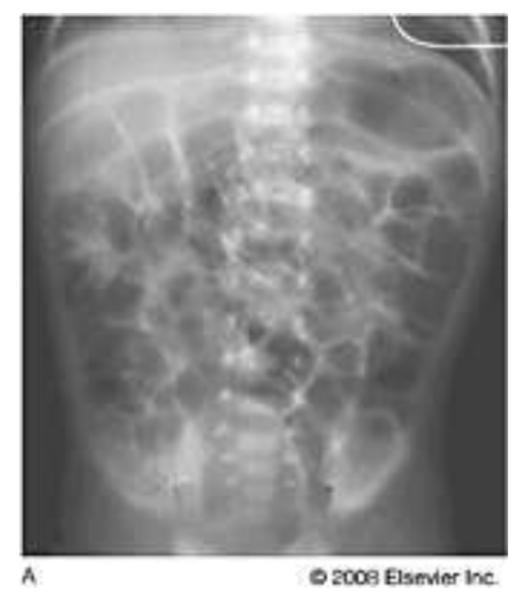
X-ray of neonate with pneumatosis intestinalis, observed in Bell Stage II Necrotizing Enterocolitis.
From Kim W-Y, Kim WS, Kim I-O, et al: Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol 2005;35:1056-1061.
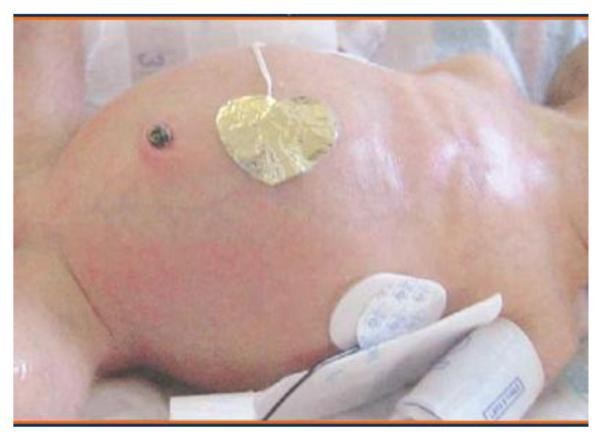
Figure 2.
Photograph of neonate with abdominal distention, commonly associated with necrotizing enterocolitis
Noerr, B. (2003). Beyond the basics: Advanced physiology and care concept: Cultivating clinical expertise. Adv Neonatal Care, 2003; 3,3 107-120
Advanced NEC cases are designated as Stage III, and includes those patients showing most or all symptoms present in Stages I and II. Stage III infants show a deterioration of vital signs, evidence of septic shock, or marked gastrointestinal bleeding. Bowel necrosis may occur by the time the diagnosis is made, at times requiring surgical intervention. In the advanced stage, pneumoperitoneum may be present on abdominal films in addition to the radiographic signs for Stages I and II. Cases of NEC in which few clinical signs are present before the patient develops pneumoperitoneum are rare; however, these patients are classified as Stage III47.
Modifications have been made to the Bell Staging criteria by researchers studying NEC aiming to more definitively define the differences in diagnosis stages. Walsh and Kliegman break each stage into two subcategories, and include signs that differentiate between milder and more severe courses of disease49. Newly included in the staging criteria are lab values indicative of acidosis, thrombocytopenia, neutropenia, and disseminated intravascular coagulation, in addition to intestinal signs of absent bowel sounds and abdominal tenderness. Ascites as demonstrated on abdominal ultrasound is also incorporated into the Bell Stage criteria modified by Walsh and Kliegman.
Alternate Classifications of Neonatal Intestinal Disease
Advances in perinatal treatment and premature infant viability have resulted in the proposal of further revisions to the Bell Stage classifications. Gordon and colleagues suggest redefining bedside and epidemiologic definitions that incorporate the trends in neonatology and pediatric surgery, which have emerged since Bell’s 1978 work48. This includes revising the classification system of intestinal disease to differentiate between infants born at birth weights below and above 1250 grams. Very low birth weight infants (<1250 g) suffering from intestinal disease can be broken into three groups: (1) feeding intolerance of prematurity; (2) medical acquired neonatal intestinal diseases (ANIDs): preterm NEC and viral enteritis of infancy (VEI); and (3) surgical ANIDs including spontaneous intestinal perforation (SIP), preterm NEC, and VEI 48. Four groups—ischemic NEC, VEI, cow’s milk protein allergy, and SIP—have been suggested for premature infants experiencing gastrointestinal pathology weighing more than 1250 grams48.
In reviewing the most recent research on neonatal intestinal disease, it is evident that NEC exists not as a single entity, but rather on a continuum that varies in presentation, complications, and management strategies. The Bell staging criteria are meant to be applied only to NEC cases. Diseases similar in presentation, such as SIP, should be excluded from Bell Staging in the diagnosis and management of these patients50. The new classification groups put forth by Gordon and colleagues have not been widely cited or incorporated into practice, to date, but provide an analysis of current neonatal intestinal disease diagnoses that warrant further discussion.
Long-term Outcomes Associated with NEC
Long-term outcomes of NEC are variable among infants based on severity of disease. Infants who survive NEC commonly experience long-term sequelae, which differ depending on clinical presentation and requirement of medical or surgical management1,14. Earlier detection and treatment may reduce the risk of morbidity and mortality.
GI Morbidities
Patients with NEC experience both short- and long-term GI morbidities. Due to the dysfunction or absence of bowel after surgical removal of necrotic intestine, infants face clinical problems associated with short bowel syndrome including malabsorption and failure to thrive. Approximately 25% of patients with NEC experience some degree of short bowel syndrome56. Factors that determine the severity of short bowel syndrome comprise the length of residual intestine after surgery, the anatomy of the remaining small bowel, the length of colon present, the age at resection, and the time allowed for adaptation56. Because patients with short bowel syndrome often require long-term parenteral nutrition, the incidence of catheter-related blood stream infections and complications associated with liver function are high57. Long-term parenteral nutrition, which is critical to the survival of surgically managed patients, can lead to hepatocellular damage, malabsorption of lipid-soluble vitamins, and mineral trace deficiencies. Secondary to NEC, patients may develop portal venous hypertension and hepatic failure58.
Up to 20% of NEC patients suffer from intestinal strictures caused by damaged intestinal mucosa, regardless of management strategy14,56. Management of strictures usually requires surgery. Strictures may have little impact on long-term GI morbidity, as they are often resected during ostomy reversal. However, this group of patients is already at high risk for short bowel syndrome, so careful attention must be paid to those who require further bowel resection56.
Neurodevelopmental Delays
Neurodevelopmental and growth delays in children with NEC have been documented9-11. A clear association now exists between NEC and neurodevelopmental impairment (NDI). The biological plausibility of cerebral white matter injury from proinflammatory cytokines illustrates how surgical patients are at increased risk for NDI, as they experience a surge of proinflammatory cytokines during surgery, and again potentially with recurrent sepsis and suboptimal nutrition9. Systematic reviews of studies comparing long-term outcomes of NEC patients found that the most common NDIs incurred include cerebral palsy, visual impairment, hearing impairment, cognitive impairment, and psychomotor impairment9,10. Patients with advanced stage disease requiring surgical intervention are at increased risk for the aforementioned NDIs 9-11. Of note, the age at follow-up to assess neurodevelopmental outcomes in NEC cases in the reported studies was approximately age 18 to 24 months. Data regarding outcomes following NEC several years after diagnosis and treatment is minimal. Research that more fully investigates long-term health outcomes following neonatal comorbidities, such as NEC, is necessary.
Caring for the Premature Infant at Risk of NEC: Critical Nursing Assessments
One of the greatest challenges in caring for premature infants at risk for NEC is its sudden and often unpredictable onset. Initial clinical signs of NEC can be vague and nonspecific and, therefore, are easily overlooked or misinterpreted by the neonatal intensive care team. Clinical predictors of NEC based on epidemiologic studies, while helpful in our knowledge of this disease, fall short of identifying a specific and highly predictive set of risk factors2-4. Furthermore, once clinical signs of NEC are present, the progression is rapid. At diagnosis, the disease has often progressed to an advanced stage. It is for these reasons that nurses caring for infants at risk of NEC be acutely aware of the critical nursing assessments relevant to the onset and progression of NEC.
In a retrospective analysis of 117 premature infants born prior to 29 weeks of gestation, diagnosed with NEC, and cared for in the NICU at a large academic medical center, Bell Staging criteria was utilized to assign disease severity. Table 2 shows the distribution of NEC cases stratified by NEC stages I through IIIB. Tables 3 and 4 present the distribution of systemic and intestinal clinical signs that were present in infants diagnosed with NEC, in total and stratified by specific NEC stage. The clinical signs that were measured correspond to the specific systemic and intestinal clinical signs that are relevant to the Bell Staging criteria. The data were collected retrospectively from the patient medical record. All variables were clearly defined for the data abstractors and measured within the 48 hour-window prior to the NEC diagnosis. The data serve to highlight the nurse-sensitive assessments that are most commonly present in the short window of time prior to NEC diagnosis at each stage of disease.
Table 2.
Distribution of NEC Cases by Bell Stage (n=117)
| Bell Stage | Distribution of Cases (n=117) |
|---|---|
| Stage I | 37 (31.5%) |
| Stage II | 28 (24%) |
| Stage IIIA | 15 (13%) |
| Stage IIIB | 37 (31.5%) |
Table 3.
Distribution of Systemic Signs by Bell Staging [% of cases]
|
Nurse-Sensitive Assessments (with definition) |
All NEC patients (n=117) |
Stage I (n=37) |
Stage II (n=28) |
Stage IIIA (n=15) |
Stage IIIB (n=37) |
|---|---|---|---|---|---|
|
Temperature Instability Temp ≤ 96.8°F (36°C) and/or ≥ 100.4°F (38°C) |
14.7% | 16.2% | 7.4% | 20% | 16.2% |
|
Bradycardia HR < 100 beats/minute |
54.3% | 64.8% | 66.7% | 73.3% | 27% |
|
Severe apnea, requiring increase in respiratory support Greater ventilator support to maintain oxygenation (i.e. increase in rate, transition from CPAP to IMV) |
49.6% | 32.4% | 46.4% | 73.3% | 59.5% |
|
Administration of respiratory medications initiated to support adequate oxygenation Administration of caffeine, aminophylline or theophylline |
63.2% | 78.4% | 78.6% | 60% | 37.8% |
|
Hypotension Decrease in mean BP of more than 4 mm Hg |
82.9% | 73% | 92.9% | 86.7% | 83.8% |
|
Severe hypotension, requiring vasopressor support Administration of dopamine, dobutamine, or epinephrine |
17.1% | 8.1% | 14.3% | 20% | 27% |
|
Respiratory acidosis pH < 7.25 and CO2 > 60 |
46.7% | 20.6% | 47.8% | 64.35 | 64.7% |
|
Metabolic acidosis TCO2 ≤ 18 |
11.9% | 9.7% | 13.6% | 28.6% | 5.9% |
|
Thrombocytopenia Platelets < 100,000 |
16.5% | 15.6% | 3.6% | 14.3% | 28.6% |
|
Neutropenia Neutrophils (polys) < 1,000 |
6.1% | 16.2% | 0 | 6.7% | 0 |
Table 4.
Distribution of Intestinal Signs by Bell Staging [% of cases]
|
Nurse-Sensitive Assessments (with definition) |
All NEC patients (n=117) |
Stage I (n=37) |
Stage II (n=28) |
Stage IIIA (n=15) |
Stage IIIB (n=37) |
|---|---|---|---|---|---|
|
Increased pregavage residuals Increase in prefeeding gastric residuals from baseline |
72.4% | 78.4% | 81.5% | 93.3% | 51.4% |
|
Abdominal distention Increase in abdominal girth measured as part of the abdominal exam |
82.1% | 81.1% | 78.6% | 100% | 78.4% |
|
Emesis Nurse assessment and documentation of emesis |
38.5% | 32.4% | 42.9% | 46.7% | 37.8% |
|
Guaiac-positive stool Presence of fecal occult blood, as measured via Guaiac test |
34.5% | 29.7% | 63% | 26.7% | 21.6% |
|
Frank blood from rectum Nurse assessment and documentation of frank blood from rectum |
14.7% | 10.8% | 40.7% | 0 | 5.4% |
|
Absent bowel sounds Nurse assessment and documentation of absent bowel sounds |
23.9% | 13.5% | 21.4% | 40% | 29.7% |
|
Abdominal tenderness Nurse assessment and documentation of abdominal tenderness |
10.3% | 0 | 3.6% | 33.3% | 16.2% |
|
Right Lower Quadrant (RLQ) mass Nurse assessment and documentation of abdominal mass, specifically in the right lower quadrant |
0.9% | 2.7% | 0 | 0 | 0 |
Assessments and Treatment
Early identification of developing NEC in preterm infants is essential to reduce the devastating effects of the disease. Nurses are in a unique position to assess early signs and symptoms of NEC, when they are present. Nurses are often the first to respond to acute changes in clinical status and physical assessments, initiating the series of events to diagnose and treat NEC. Immediate medical management and intervention of bowel rest, gastric decompression, and introduction of antibiotic treatment is crucial to decreasing morbidity and mortality in these infants1,14. An important component of the medical management of NEC is close observation and serial abdominal exams. Surgical intervention may be prevented through careful assessments, early intervention, and medical management. The role of nurses in performing critical assessments and their awareness of early identifiers contributes greatly to early recognition and management of long-term medical and surgical sequelae of NEC.
Systemic Signs
Clinical signs and symptoms of NEC at disease onset are relatively nonspecific and often common to other neonatal disease processes. These may include temperature instability, apnea, bradycardia, episodes of oxygen desaturation, and signs of lethargy or irritability14. Laboratory tests may point toward nonspecific indicators of an inflammatory process. Commonly, an absolute neutropenia or a leukocytosis with a bandemia is present. Evolving thrombocytopenia and metabolic acidosis are often markers of disease progression1. Additional clinical signs include glucose instability, hyponatremia and coagulation abnormalities. Finally, research has shown that cytokines such as platelet activating factor, tumor necrosis factor, and selected pro- and anti-inflammatory mediators have been associated with NEC21,51-54. Advanced stages of this disease process include a combination of hypotension, bradycardia, severe apnea, combined respiratory and metabolic acidosis, coagulation abnormalities, and neutropenia14.
Table 3 describes the distribution of systemic symptoms over a cohort of NEC patients (n=117), ranging from Bell Stage I to Stage IIIB. Findings most significant across all stages of NEC were hypotension (82.9%), administration of respiratory medications associated with increased respiratory support needs (63.2%), and bradycardia (54.3%). Hypotension, defined as a decrease in mean BP of more than 4 mm/Hg from baseline, remained one of the leading symptoms present prior to NEC diagnosis, in all stages of disease. This descriptive data emphasizes the importance of nurse assessment pertaining to subtle changes in systemic signs, such as cardiovascular and respiratory clinical status, as indicative of a gastrointestinal disease onset, as in the case of NEC.
Intestinal Signs
Intestinal signs indicative of NEC, whether early or late in disease progression, include increasing pregavage residuals, bilious aspirates, abdominal distention, emesis, guaiac-positive stool, absent bowel sounds, abdominal tenderness, and a right lower quadrant mass26. The manifestations of NEC occur over a spectrum, and can include some, all, or none of the mentioned intestinal signs. Table 4 describes the distribution of intestinal symptoms over a cohort of NEC patients, ranging from Bell Stage I to Stage IIIB. Findings most significant across all stages of NEC were evidence of increased pregavage residuals (72.4%) and abdominal distention (82.1%). In infants who developed Stage IIIA NEC, 100% presented with abdominal distention and 93.3% were noted to have an increase in pregavage residuals prior to disease onset. Interestingly, prior to Stage IIIB NEC, 78.4% of infants presented with abdominal distention and 51.4% had evidence of increased pregavage residuals.
Discussion
This descriptive data may highlight differences in the pathogenesis that underpins medical versus surgical treatment needs associated with NEC in low gestational age neonates. The data further emphasize the importance of nursing assessments such as routinely measuring the abdominal girth of an infant, and assessing for pregavage residuals prior to gavage feeding. These nursing assessments, which are part of a through abdominal exam, are not to be overlooked, as changes from baseline are highly likely to be associated with patients who develop advanced stages of NEC. In addition, the inclusion of assessment of emesis, abdominal masses or tenderness, auscultation of bowel sounds in all four quadrants of the abdomen, and testing stool for occult blood are important. These components of a through abdominal assessment, as well as precise cardiovascular and respiratory nursing assessments, as summarized in Table 5, are critical to identifying clinical signs associated with NEC.
Table 5.
Priority Nursing Assessments for Prevention of NEC
| Physiologic System | |||
|---|---|---|---|
| Generalized | Cardiovascular and Respiratory | Gastrointestinal | |
|
Nursing
assessments and clinical signs associated with NEC |
Temperature
Behavior
|
Vital signs
|
Feeding intolerance
Abdominal exam
Stool frequency and characteristics
|
Conclusion
Despite advances in neonatal intensive care and significant gains in premature infant survival, NEC remains one of the most significant complications of premature birth. Its pathogenesis and, more important, its prevention, remain unsolved. Infants who develop NEC are at increased risk of death, infection, and long-term health consequences that result in aberrant growth and neurodevelopment. The onset of the disease is often insidious, yet progression is rapid. Findings from the NICU nurse’s physical assessments are often the first clues to diagnosis. NICU nurses must be highly knowledgeable about NEC and the risk factors associated with this neonatal disease. Furthermore, NICU nurses must hone their assessment skills such that they are able to identify the earliest signs of NEC and strategically intervene early in the course of this devastating gastrointestinal disease.
Acknowledgments
Work conducted at Brigham and Women’s Hospital, Boston, MA The authors acknowledge support from the American Nurses Foundation Research Grant (2008-112) and Specialized Center of Research Study of Bronchopulmonary Dysplasia (HL 72931)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henry MCW, Moss RL. Necrotizing enterocolitis. Ann Rev Med. 2009;60:111–24. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 2.Moss RL, Kalish LA, Duggan C, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: a multi-institutional study. J Perinatol. 2008;28(10):665–674. doi: 10.1038/jp.2008.119. [DOI] [PubMed] [Google Scholar]
- 3.Llanos AR, Moss ME, Pinzon MC, et al. Epidemiology of neonatal necrotizing enterocolitis: a population based study. Paediatr Perin Epidemiol. 2002;16:342–349. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 4.Chandler JC, Hebra A. Necrotizing enterocolitis in infants with very low birth weight. Semin Pediatr Surg. 2000;9:63–72. doi: 10.1016/s1055-8586(00)70018-7. [DOI] [PubMed] [Google Scholar]
- 5.Petty JK, Ziegler MM. Operative strategies for necrotizing enterocolitis: the prevention and treatment of short-bowel syndrome. Semin Pediatr Surg. 2005;14:191–198. doi: 10.1053/j.sempedsurg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Tam PKH. Necrotizing enterocolitis-surgical management. Semin Neonatol. 1997;2:297–305. [Google Scholar]
- 7.Henry MCW, Moss RL. Surgical therapy for necrotizing enterocolitis: bringing evidence to the bedside. Semin Pediatr Surg. 2005;14:181–190. doi: 10.1053/j.sempedsurg.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Soraisham AS, Amin HJ, Al-Hindi MY, Singhal N, Sauve RS. Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight less than or equal to 1250 g? J Paediatr Child Health. 2006;42:499–504. doi: 10.1111/j.1440-1754.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 9.Schulske SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161(6):583–590. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 10.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F193–F198. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 12.Bisquera JA, Cooper TR, Berseth CR. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birthweight infants. Pediatrics. 2002;109(3):423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 13.Gregory KE. Clinical predictors of necrotizing enterocolitis in premature infants. Nurs Res. 2008;57:260–270. doi: 10.1097/01.NNR.0000313488.72035.a9. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention, and management. Drugs 2008. 2008;68(9):1227–1238. doi: 10.2165/00003495-200868090-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32(2):70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Lin PW, Stoll BJ. Necrotizing enterocolitis. Lancet. 2006;368:1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 17.Crissinger KD, Berney DL, Valesquez OR, Gonsalez E. An animal model of necrotizing enterocolitis induced by infant formula and ischemia in developing piglets. Gastroenterology. 1994;106:1215–1222. doi: 10.1016/0016-5085(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 18.Crissinger KD. Regulation of hemodynamics and oxygenation in developing intestine: insight into the pathogenesis of necrotizing enterocolitis. Acta Pædiatrica. 2008;83(s396):8–10. doi: 10.1111/j.1651-2227.1994.tb13233.x. [DOI] [PubMed] [Google Scholar]
- 19.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 20.Nowicki PT. Ischemia and necrotizing enterocolitis: where, when, and how. Semin Pediatr Surg. 2005;14:152–158. doi: 10.1053/j.sempedsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Crissinger KD. Understanding necrotizing enterocolitis – promising directions. Pathophys. 1999;5:247–256. [Google Scholar]
- 23.Neu JN, Chen M, Beierle E. Intestinal innate immunity: how does it related to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:137–144. doi: 10.1053/j.sempedsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotizing enterocolitis. Semin Fetal Neonatal Med. 2006;11:369–377. doi: 10.1016/j.siny.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111:529–534. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–459. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietz J, Achanti B, Lilien L, Stepka EC, Mehta SK. Prevention of necrotizing enterocolitis in preterm infants: a 20-year experience. Pediatrics. 2007;119:e164–e170. doi: 10.1542/peds.2006-0521. [DOI] [PubMed] [Google Scholar]
- 28.Kleigman RM. The relationship of neonatal feeding practices and the pathogenesis and prevention of necrotizing enterocolitis. Pediatrics. 2003;111:671–672. doi: 10.1542/peds.111.3.671. [DOI] [PubMed] [Google Scholar]
- 29.Hans DM, Pylipow M, Long JD, Thureen PJ, Georgieff MK. Nutritional practices in the Neonatal Intensive Care Unit: analysis of a 2006 neonatal nutrition survey. Pediatrics. 2009;123:51–57. doi: 10.1542/peds.2007-3644. [DOI] [PubMed] [Google Scholar]
- 30.Berseth CL, Nordyke C. Enteral nutrients promote postnatal maturation of intestinal motor activity in preterm infants. Am J Physiol Gastrointest Liver Physiol. 1993;264:G1046–G1051. doi: 10.1152/ajpgi.1993.264.6.G1046. [DOI] [PubMed] [Google Scholar]
- 31.Kamitsuka MD, Horton MK, Williams MA. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250-1500 grams and less than 35 weeks of gestation. Pediatrics. 2000;105:379–384. doi: 10.1542/peds.105.2.379. [DOI] [PubMed] [Google Scholar]
- 32.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. J FASEB. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 33.Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205–18. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Book LS, Herbst JJ, Jung AL. Comparison of fast and slow feeding rate schedules to the development of necrotizing enterocolitis. J Pediatr. 1976;89(3):463–466. doi: 10.1016/s0022-3476(76)80552-5. [DOI] [PubMed] [Google Scholar]
- 35.Rayyis SF, Ambalavaanan N, Wright L, Carlo WA. Randomized trial of slow versus fast feed advancements on the incidence of necrotizing enterocolitis in very low birth weight infants. J Pediatr. 1999;134(3):293–297. doi: 10.1016/s0022-3476(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 36.Salhotra A, Ramji S. Slow versus fast enteral feed advancements in very low birth weight infants. A randomized controlled trial. Indian Pediatr. 2004;41:435–441. [PubMed] [Google Scholar]
- 37.Caple J, Armentrout D, Huseby V, Halbardier B, Garcia J, Sparks JW, et al. Randomized controlled trial of slow versus rapid feeding advancement in preterm infants. Pediatrics. 2004;114:1597–1600. doi: 10.1542/peds.2004-1232. [DOI] [PubMed] [Google Scholar]
- 38.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42:S46–52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 39.Schanler RJ. The use of human milk for premature infants. Pediatr Clin North Am. 2001;48:207–219. doi: 10.1016/s0031-3955(05)70295-9. [DOI] [PubMed] [Google Scholar]
- 40.Lucas A, Cole T. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 41.Cotton CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkstorm MV, Hall L, Soderlund S, Hakansson EG, Hakansson S, Domellof M. Intestinal flora in very low-birth weight infants. Acta Paediatr. 2009;98(11):1762–7. doi: 10.1111/j.1651-2227.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 44.Lin HC, Su BH, Chen AC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 45.Anderson T, Lord A, Shotkoski N, O’Keefe C. The use of probiotics for the prevention of necrotizing enterocolitis in the premature infant. ICAN: Inf Child Adol Nut. 2009;10:246–252. [Google Scholar]
- 46.Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Ped Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 47.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon PV, Swanson JR, Attridge JT, Clark R. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell’s criteria? J Perinatol. 2007;27:661–671. doi: 10.1038/sj.jp.7211782. [DOI] [PubMed] [Google Scholar]
- 49.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17(4):213–288. doi: 10.1016/0045-9380(87)90031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell NJ. Letters to the editor: emerging trends in neonatal intestinal disease. J Perinatol. 2008;28:383. doi: 10.1038/jp.2008.13. [DOI] [PubMed] [Google Scholar]
- 51.Edelson MB, Bagwell CE, Rozycki HJ. Circulation pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103(4):766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 52.Rabinowitz SS, Dzakpasu P, Piecuch S, Leblanc P, Valencia G, Kornecki E. Platelet-activating factor in patients at risk for necrotizing enterocolitis. J Pediatrics. 2001;138(1):81–86. doi: 10.1067/mpd.2001.110132. [DOI] [PubMed] [Google Scholar]
- 53.Hsueh W, Caplan MS, Sun X, Tan X, MacKendrick W, Gonzalez-Crussi R. Platelet-activating factor, tumor necrosis factor, hypoxia and necrotizing enterocolitis. Acta Paed Supp. 1994;396:11–17. doi: 10.1111/j.1651-2227.1994.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 54.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma cytokine levels in necrotizing enterocolitis. Acta Paed Supp. 1994;396:18–20. doi: 10.1111/j.1651-2227.1994.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 55.Ren Y, Lin CL, Li Z, Chen XY, Huang X, Lui V, et al. Up-regulation of macrophage migration inhibitory factor in infants with acute neonatal necrotizing enterocolitis. Histopathology. 2005;46(6):659–667. doi: 10.1111/j.1365-2559.2005.02159.x. [DOI] [PubMed] [Google Scholar]
- 56.Henry MCW, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2008;17(2):98–109. doi: 10.1053/j.sempedsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Goulet O, Sauvat F. Short bowel syndrome and intestinal transplantation in children. Curr Opin Clin Nutr Metab Care. 2006;9(3):304–314. doi: 10.1097/01.mco.0000222116.68912.fc. [DOI] [PubMed] [Google Scholar]
- 58.Bradshaw WT. Necrotizing enterocolitis: etiology, presentation, management, and outcomes. J Perinat Neonatal Nurs. 2009;23:87–94. doi: 10.1097/JPN.0b013e318196fefb. [DOI] [PubMed] [Google Scholar]
- 59.Kim W-Y, Kim WS, Kim I-O, et al. Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol. 2005;35:1056–1061. doi: 10.1007/s00247-005-1533-4. [DOI] [PubMed] [Google Scholar]
- 60.Noerr B. Beyond the basics: Advanced physiology and care concept: Cultivating clinical expertise. Adv Neonatal Care. 2003;3(3):107–120. [Google Scholar]