Abstract
The association between incomplete revascularization (IR) and long-term mortality following stenting in the era of drug-eluting stents (DES) is not well understood. In this study, we test the hypothesis that IR is associated with a higher risk of long-term (5-year) mortality following stenting for multivessel coronary disease. Using data from New York State’s Percutaneous Coronary Intervention Reporting System, 21,767 patients with multivessel disease who underwent stenting between October 2003 and December 2005 were identified. Complete revascularization (CR) was achieved in 6,844 (31.4%) patients, and 14,923 (68.6%) patients were incompletely revascularized. The CR and IR patients were propensity-matched on a 1:1 ratio on the number of diseased vessels, the presence of total occlusion, type of stents, and the probability of achieving CR estimated using a logistic model with established risk factors as independent variables. Patients were followed for vital status until December 31, 2008 using the National Death Index. Differences in survival between the matched CR and IR patients were compared. Among the 6,511 pairs of propensity-matched patients, the 5-year survival rate for IR was lower compared to CR (79.3% vs. 81.4%, P=0.004), and the risk of death during follow-up was 16% higher for IR in comparison to CR (hazard ratio=1.16, 95% confidence interval: 1.06–1.27, P=0.001). In addition, subgroup analyses demonstrated that the association between IR and long-term mortality was not dependent on major patient risk factors. In conclusion, IR is associated with an increased risk of long-term mortality following stenting for multivessel disease in the DES era.
Keywords: coronary artery disease, long-term follow-up, mortality, stenting
Previous studies on the impact of incomplete revascularization (IR) on outcomes for percutaneous coronary intervention (PCI) with implantation of stents were primarily based on data from the era bare-metal stents (BMS). 1–7 More recent studies using data from the era of drug-eluting stents (DES) have in general found that IR was associated with a higher risk of major cardiac adverse events (MACE: death, repeat revascularization, or myocardial infarction) after PCI.8–17 However, findings regarding the impact of IR on mortality have been inconsistent across studies. Some studies have found that IR is not associated with an increased risk of mortality following PCI, 12–17 but other studies, including our previous study, reported that IR is associated with a higher risk of mortality.8–11 Moreover, the lengths of follow-up in the previous studies using the data from the DES era were usually no more than 3 years. Therefore there is a need for more studies that investigate the association between IR and mortality in longer follow-up periods. In this study, we examined the impact of IR on mortality for a longer follow-up period (up to 5 years) using data from a larger patient population. We hypothesized that incomplete revascularization was associated with a higher risk of long-term (5-year) mortality following stenting for multivessel disease in the DES era.
METHODS
The primary database used in this study is the New York State’s Percutaneous Coronary Intervention Reporting System (PCIRS). The PCIRS is by maintained by the New York State Department of Health, and contains detailed information on every PCI procedure performed in non-federal hospitals in the State since early 1990s. Data collected by the PCIRS relevant to this study include patient demographic variables, patients’ pre-procedural risk factors, detailed information about lesions in coronary arteries, including pre- and post-procedural stenosis for each lesion, the procedure performed and the intracoronary devices used for each lesion, major post-procedural complications, and the disposition at discharge. To ensure the completeness of the PCIRS database, data submitted by hospitals to the PCIRS are periodically matched to New York State’s hospital discharge database. To ensure the accuracy of the data reported by the hospitals, data validation is conducted regularly by the New York State Department of Health’s review agent by reviewing samples of medical records for cases reported to the PCIRS. The PCIRS was used to identify the study population and to track repeat PCI procedures after the index stenting procedures using patient identifiers including social security numbers, dates of birth, admission, procedure, and discharge.
Data from New York State’s Cardiac Surgery Reporting System (CSRS) were also used in the study. The CSRS registers all major cardiac surgical procedures performed in non-federal hospitals in the State. It employs similar data collection and auditing methods to that used in the PCIRS. In short, patient demographic variables, risk factors prior to surgery, procedural information, complications, and discharge status are reported to the CSRS by hospitals.
In addition, the National Death Index maintained by the National Center for Health Statistics was used in this study. The National Death Index collects all death certificate records in the United States, and it was used to ascertain the vital status of the study population in the follow-up period after hospital discharge.
The PCIRS and CSRS were used to identify the study population. The inclusion criteria were (1) patients had undergone stenting procedures with DES or BMS between Oct 1, 2003 and December 31, 2005 in New York, (2) had lesions with stenosis of at least 70% in at least two major epicardial arteries (left anterior descending artery and major diagonals, left circumflex artery and large marginal branches, and right coronary artery and right posterior descending artery), (3) had no lesions with stenosis ≥ 50% in the left main coronary artery, (4) had no history of PCI or CABG surgery before the index stenting procedures, (5) had no acute myocardial infarction within 24 hours prior to the index procedures, and (6) had not undergone CABG surgery in the index admission or within 30 days of discharge of the index procedure. A total of 21,767 patients who underwent stenting met the inclusion criteria and were included in this study.
The completeness of revascularization of stenting was determined by the degree of post-procedural stenosis in all lesions with pre-procedural stenosis ≥ 70% in major epicardial coronary vessels. CR was defined when the post-procedural stenosis in each of the lesions was reduced to < 50% in the index hospitalization or within 30 days in staged PCI procedures following discharge from the index hospitalization before the occurrence of a new MI. When CR was not achieved after the stenting procedure in the index admission or within 30 days of discharge, the revascularization was defined as incomplete revascularization (IR).18
The outcome variable is all-cause mortality after the index stenting procedure. Patients’ vital statuses were tracked by matching to the National Death Index on social security number, date of birth, and sex. The follow-up of vital status ended on December 31, 2008. For a patient who died before the end of 2008, the length of follow-up was calculated as the time interval from the date of the index procedure to the date of death. Otherwise, the follow-up ended on December 31, 2008 and the survival time was censored. The median length of follow-up was 3.9 years with an interquartile range of 3.4 – 4.6 years.
The framework of the analysis is propensity-matched survival analysis. First, the IR and CR patient groups were compared with respect to the distributions of baseline characteristics including demographics, pre-procedural risk factors, New York State PCI risk score for in-hospital mortality19 and type of stents (DES or BMS) using the Student’s t-test for continuous variables and Pearson’s chi-square test for categorical variables.
A logistic regression model was then fit to predict the probability of receiving CR using all available baseline risk factors.20 Using this propensity model, a propensity score (the log-odds of probability of receiving CR) was obtained for each patient. Next, we attempted to match each CR patient to an IR patient on the number of diseased vessels, the presence of total occlusion, type of stents (DES only, BMS only, or both), and the value of the propensity score. The matching caliper for the propensity score was set as the 0.6 times the standard deviation of its distribution.21 The standardized differences in means for continuous variables and in proportions for categorical variables between the matched IR and CR patients were calculated to examine how well the baseline risk factors in the 2 groups were balanced after matching.
The data from the propensity-matched patients were then used to compare the Kaplan-Meier survival curves for IR and CR by the log-rank test. In addition, the hazard ratio for death for IR in comparison to CR was obtained by fitting a Cox proportional hazards model. The analysis was repeated for each type of IR defined by the number of incompletely revascularized vessels, i.e., 1-vessel IR and at least 2-vessel IR.
Subgroup analysis was then conducted to test the significance of interactions between IR and pre-selected baseline risk factors such as age, ejection fraction, history of MI and congestive heart failure, diabetes, proximal left anterior descending artery disease, proximal vessel disease (stenosis ≥ 70% in proximal left anterior descending artery, right coronary, or left circumflex artery), the presence of a total occlusion, renal failure and New York State PCI risk score for in-hospital mortality (≥8 vs. <8). This analysis consists of fitting a Cox proportional hazards model for each of these risk factors. In each model, IR, the risk factor of interest, and their interaction term were included as independent variables, along with other significant predictors of survival (P<0.05) determined by a backward selection approach. The adjusted hazard ratio for death for IR vs. CR was then calculated for each level of the risk factor of interest, and the significance of the interaction term was examined at the α=0.05 level.
Since the majority of the patients only received DES, a sensitivity analysis was conducted by restricting the analysis to the DES patients to evaluate whether the impact of IR on long-term mortality in such patients is consistent with that observed in the entire study population. All statistical analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
In the study population of 21,767 patients, 18,374 (84.4%) received only DES, and 1,815 (8.3%) received only BMS. During the index hospitalization or within 30 days after discharge, a total of 6,844 (31.4%) patients were completely revascularized, and 14,923 (68.6%) patients were incompletely revascularized. There were variations in the prevalence of IR across providers. The interquartile ranges of prevalence of IR were 61.1% – 76.4% across hospitals and 60.7% – 81.1% across operators.
Table 1 shows that for IR was associated with older age, being non-Hispanic black or Hispanic, lower values of ejection fraction, 3-vessel disease, presence of total occlusion, history of a number of diseases such as MI, cerebrovascular disease, peripheral arterial disease, congestive heart failure, diabetes, renal failure, slightly higher New York State PCI risk score for in-hospital mortality and higher likelihood of implantation of BMS only.
Table 1.
Baseline patient characteristics of patients by completeness of revascularization.
| Variable | Incomplete Revascularization (n=14,923) |
Complete Revascularization (n=6,844) |
P Value |
|---|---|---|---|
| Age (Years) | <0.001 | ||
| <50 | 1,308(8.8%) | 707(10.3%) | |
| 50–59 | 3,235(21.7%) | 1,656(24.2%) | |
| 60–69 | 4,160(27.9%) | 1,951(28.5%) | |
| 70–79 | 4,042(27.1%) | 1,747(25.5%) | |
| ≥ 80 | 2,178(14.6%) | 783(11.4%) | |
| Sex | 0.57 | ||
| Female | 4,848(32.5%) | 2,250(32.9%) | |
| Male | 10,075(67.5%) | 4,594(67.1%) | |
| Race | <0.001 | ||
| Non-Hispanic white | 10,605(71.1%) | 5,373(78.5%) | |
| Non-Hispanic black | 1,577(10.6%) | 518(7.6%) | |
| Hispanic | 1,598(10.7%) | 534(7.8%) | |
| Other | 1,143(7.7%) | 419(6.1%) | |
| Body surface area (m2), mean (SD) | 2.01(0.27) | 2.02(0.26) | <0.001 |
| Body mass index group (kg/m2) | 0.09 | ||
| <18.5 | 146(1.0%) | 60(0.9%) | |
| 18.5–24.99 | 3,378(22.6%) | 1,467(21.4%) | |
| 25–29.99 | 5,888(39.5%) | 2,686(39.2%) | |
| ≥ 30 | 5,511(36.9%) | 2,631(38.4%) | |
| Ejection fraction | <0.001 | ||
| <20% | 884(5.9%) | 378(5.5%) | |
| 20–29% | 605(4.1%) | 142(2.1%) | |
| 30–39% | 1,120(7.5%) | 323(4.7%) | |
| ≥ 40% | 12,296(82.4%) | 5,994(87.6%) | |
| Missing | 18(0.1%) | 7(0.1%) | |
| Number of diseased vessels | <0.001 | ||
| 2 | 10,080(67.5%) | 6,115(89.3%) | |
| 3 | 4,843(32.5%) | 729(10.7%) | |
| Presence of total occlusion | 5,967(40.0%) | 684(10.0%) | <0.001 |
| Previous myocardial infarction | <0.001 | ||
| 1–7 days | 2,942(19.7%) | 1,309(19.1%) | |
| 8–20 days | 408(2.7%) | 116(1.7%) | |
| ≥ 21 days | 2,181(14.6%) | 529(7.7%) | |
| No myocardial infarction prior to procedures |
9,392(62.9%) | 4,890(71.4%) | |
| Cerebrovascular disease | 1,275(8.5%) | 489(7.1%) | <0.001 |
| Peripheral arterial disease | 1,273(8.5%) | 373(5.5%) | <0.001 |
| Hemodynamic state | 0.27 | ||
| Stable | 14,887(99.8%) | 6,832(99.8%) | |
| Unstable | 30(0.2%) | 12(0.2%) | |
| Shock | 6(0.0%) | 0(0.0%) | |
| Congestive heart failure | <0.001 | ||
| This admission | 1,266(8.5%) | 392(5.7%) | |
| Before this admission | 388(2.6%) | 121(1.8%) | |
| None | 13,269(88.9%) | 6,331(92.5%) | |
| Malignant ventricular arrhythmia | 77(0.5%) | 22(0.3%) | 0.05 |
| Chronic obstructive pulmonary disease | 981(6.6%) | 430(6.3%) | 0.42 |
| Diabetes requiring medication | 5,106(34.2%) | 1,947(28.4%) | <0.001 |
| Renal failure | <0.001 | ||
| Requiring dialysis | 352(2.4%) | 130(1.9%) | |
| Creatinine > 2.5 mg/dL (220 µmol/liter) | 226(1.5%) | 51(0.7%) | |
| No renal failure | 14,345(96.1%) | 6,663(97.4%) | |
| New York State PCI risk score for in- hospital mortality, mean (SD) |
3.9(3.1) | 3.3(2.8) | <0.001 |
| Type of stent | <0.001 | ||
| Bare-metal stents (BMS) only | 1,449(9.7%) | 366(5.3%) | |
| Drug-eluting stents (DES) only | 12,687(85.0%) | 5,687(83.1%) | |
| Both BMS and DES | 787(5.3%) | 791(11.6%) |
A total of 6,511 (95.1%) CR patients were propensity matched to IR patients using a 1:1 ratio. Table 2 shows that the baseline risk factors were well balanced between the matched IR and CR groups with standardized differences in the means and prevalences of such risk factors no more than 2.7% (all P values > 0.05).
Table 2.
Baseline patient characteristics of propensity matched patients by completeness of revascularization.*
| Variable | Incomplete Revascularization (n=6,511) |
Complete Revascularization (n=6,511) |
|---|---|---|
| Age (Years) | ||
| <50 | 636(9.8%) | 674(10.4%) |
| 50–59 | 1,524(23.4%) | 1,591(24.4%) |
| 60–69 | 1,838(28.2%) | 1,850(28.4%) |
| 70–79 | 1,712(26.3%) | 1,652(25.4%) |
| ≥ 80 | 801(12.3%) | 744(11.4%) |
| Sex | ||
| Female | 2,191(33.7%) | 2,162(33.2%) |
| Male | 4,320(66.3%) | 4,349(66.8%) |
| Race | ||
| Non-Hispanic white | 5,030(77.3%) | 5,098(78.3%) |
| Non-Hispanic black | 508(7.8%) | 492(7.6%) |
| Hispanic | 546(8.4%) | 514(7.9%) |
| Other | 427(6.6%) | 407(6.3%) |
| Body surface area (m2), mean (SD) | 2.02(0.27) | 2.02(0.26) |
| Body mass index group (kg/m2) | ||
| <18.5 | 56(0.9%) | 60(0.9%) |
| 18.5–24.99 | 1,390(21.3%) | 1,393(21.4%) |
| 25–29.99 | 2,602(40.0%) | 2,560(39.3%) |
| ≥ 30 | 2,463(37.8%) | 2,498(38.4%) |
| Ejection fraction | ||
| <20% | 353(5.4%) | 349(5.4%) |
| 20–29% | 133(2.0%) | 130(2.0%) |
| 30–39% | 313(4.8%) | 308(4.7%) |
| ≥ 40% | 5,707(87.7%) | 5,717(87.8%) |
| Missing | 5(0.1%) | 7(0.1%) |
| Number of diseased vessels | ||
| 2 | 5,782(88.8%) | 5,782(88.8%) |
| 3 | 729(11.2%) | 729(11.2%) |
| Presence of total occlusion | 684(10.5%) | 684(10.5%) |
| Previous myocardial infarction | ||
| 1–7 days | 1,261(19.4%) | 1,247(19.2%) |
| 8–20 days | 122(1.9%) | 109(1.7%) |
| ≥ 21 days | 501(7.7%) | 506(7.8%) |
| No myocardial infarction prior to procedures |
4,627(71.1%) | 4,649(71.4%) |
| Cerebrovascular disease | 460(7.1%) | 461(7.1%) |
| Peripheral arterial disease | 371(5.7%) | 344(5.3%) |
| Hemodynamic state | ||
| Stable | 6,503(99.9%) | 6,501(99.8%) |
| Unstable or shock | 8(0.1%) | 10(0.2%) |
| Congestive heart failure | ||
| This admission | 382(5.9%) | 370(5.7%) |
| Before this admission | 123(1.9%) | 111(1.7%) |
| None | 6,006(92.2%) | 6,030(92.6%) |
| Malignant ventricular arrhythmia | 20(0.3%) | 19(0.3%) |
| Chronic obstructive pulmonary disease | 405(6.2%) | 409(6.3%) |
| Diabetes requiring medication | 1,922(29.5%) | 1,858(28.5%) |
| Renal failure | ||
| Requiring dialysis | 125(1.9%) | 119(1.8%) |
| Creatinine > 2.5 mg/dL (220 µmol/liter) | 51(0.8%) | 47(0.7%) |
| No renal failure | 6,335(97.3%) | 6,345(97.5%) |
| New York State PCI risk score for in- hospital mortality, mean (SD) |
3.4(2.7) | 3.3(2.8) |
| Type of stent | ||
| Bare-metal stents (BMS) only | 366(5.6%) | 366(5.6%) |
| Drug-eluting stents (DES) only | 5,687(87.3%) | 5,687(87.3%) |
| Both BMS and DES | 458(7.0%) | 458(7.0%) |
All p values > 0.10.
Table 3 shows that during the follow-up until December 31, 2008, 1,045 IR and 888 CR patients died among the 6,511 pairs. IR was associated 16% higher risk of death (hazard ratio = 1.16, 95% confidence interval (CI): 1.06 – 1.27, P=0.001). The 5-year Kaplan-Meier survival rate was 2.1% lower in the IR group (79.3% vs. 81.4%, P=0.004; Figure 1).
Table 3.
Hazard ratios for mortality for incomplete vs. complete revascularization.
| Patient Group | No. of Cases |
No. of Deaths |
Hazard Ratio (95% CI) |
P-Value |
|---|---|---|---|---|
| Incomplete revascularization | 6,511 | 1,045 | 1.16(1.06,1.27) | 0.001 |
| Complete revascularization | 6,511 | 888 | Reference | |
| Subgroups of incomplete revascularization |
||||
| 1 incompletely revascularized vessel | 5,413 | 846 | 1.13(1.02,1.25) | 0.01 |
| Matched completely revascularized Patients |
5,413 | 734 | Reference | |
| Multiple incompletely revascularized vessels |
1,098 | 199 | 1.27(1.03,1.57) | 0.03 |
| Matched completely revascularized patients |
1,098 | 154 | Reference |
CI=confidence interval.
Figure 1.
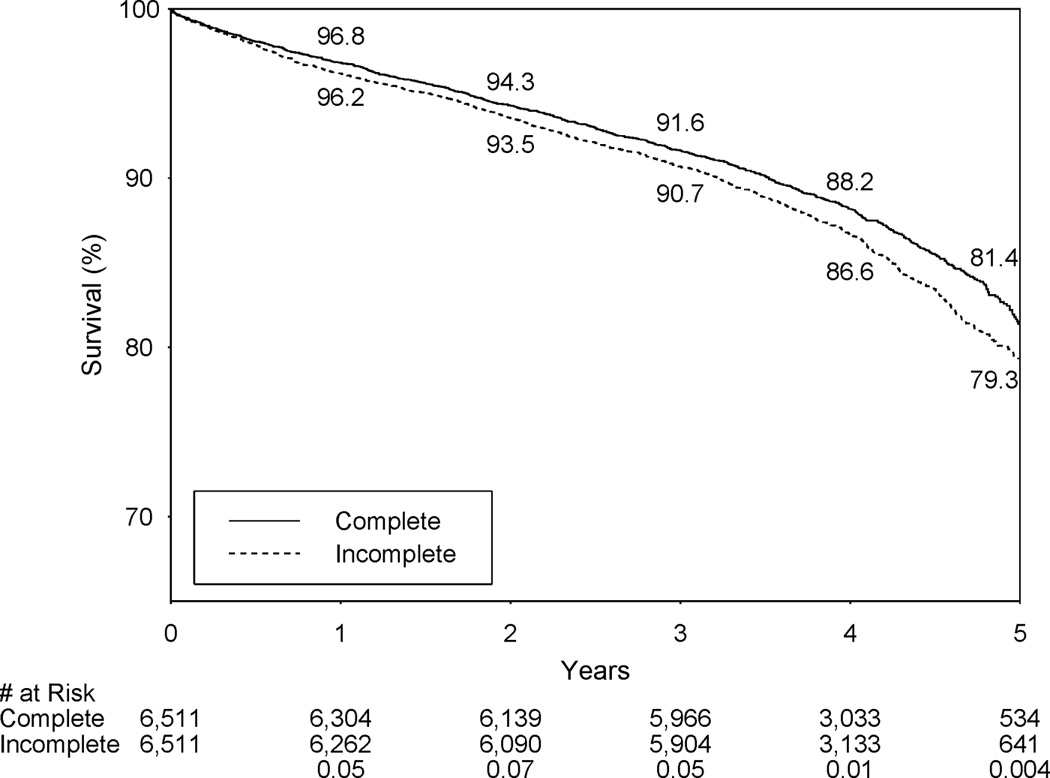
Kaplan-Meier survival curves for incomplete vs. complete revascularization in propensity-matched patients.
Table 3 shows that each type of IR was associated with a significantly higher risk of death than CR, and the respective hazard ratios for the death for 1-vessel and multiple-vessel IR were 1.13 (P=0.01) and 1.27 (P=0.03). The respective 5-year survival rates for 1-vessel IR and matched CR patients were 79.8% and 81.4% (P=0.03), and the respective 5-year survival rates for multiple-vessel IR and matched CR patients were 77.0% and 81.0% (P=0.04).
Table 4 shows that among the 20 subgroups examined, IR was associated with significantly higher risk of death than CR in 13 subgroups (P<0.05). In addition, none of the interactions between IR and the selected risk factors was statistically significant (all P values ≥ 0.09). Thus there was no evidence that the association between IR and long-term mortality was dependent on these risk factors.
Table 4.
Hazard ratios for mortality for incomplete vs. complete revascularization in selected subgroups of patients.*
| No. of Deaths/Cases |
5-Year KM Survival (%) |
Adjusted† Hazard Ratio for Death |
||||||
|---|---|---|---|---|---|---|---|---|
| Patient Group | CR | IR | CR | IR | P- Value | Hazard Ratio (95% CI) |
P-Value | P-Value for Interaction |
| Age (Years) | 0.85 | |||||||
| < 80 | 654/5767 | 769/5710 | 85.0 | 82.7 | 0.01 | 1.16(1.04,1.28) | 0.007 | |
| ≥ 80 | 234/744 | 276/801 | 56.5 | 56.4 | 0.13 | 1.18(0.99,1.41) | 0.07 | |
| Ejection fraction | 0.16 | |||||||
| < 40% | 175/787 | 229/799 | 71.8 | 64.0 | 0.01 | 1.31(1.08,1.60) | 0.007 | |
| ≥ 40% | 711/5717 | 815/5707 | 82.8 | 81.5 | 0.03 | 1.12(1.01,1.24) | 0.03 | |
| Prior myocardial infarction |
0.34 | |||||||
| Yes | 332/1862 | 384/1884 | 76.6 | 74.6 | 0.03 | 1.22(1.05,1.42) | 0.008 | |
| No | 556/4649 | 661/4627 | 83.4 | 81.3 | 0.01 | 1.12(1.00,1.25) | 0.06 | |
| Congestive heart failure |
0.42 | |||||||
| Yes | 195/481 | 218/505 | 51.3 | 50.5 | 0.51 | 1.07(0.88,1.30) | 0.49 | |
| No | 693/6030 | 827/6006 | 84.0 | 82.0 | 0.002 | 1.17(1.06,1.30) | 0.002 | |
| Diabetes mellitus | 0.52 | |||||||
| Yes | 324/1858 | 397/1922 | 76.9 | 72.6 | 0.04 | 1.20(1.03,1.39) | 0.02 | |
| No | 564/4653 | 648/4589 | 83.1 | 82.1 | 0.02 | 1.13(1.01,1.26) | 0.04 | |
| Proximal left anterior descending artery stenosis ≥ 70% |
0.47 | |||||||
| Yes | 273/1973 | 263/1594 | 81.6 | 78.0 | 0.14 | 1.09(0.92,1.30) | 0.30 | |
| No | 615/4538 | 782/4917 | 81.3 | 79.8 | 0.005 | 1.18(1.06,1.31) | 0.003 | |
| Proximal coronary artery stenosis ≥ 70% |
0.10 | |||||||
| Yes | 591/4037 | 610/3575 | 80.5 | 78.7 | 0.07 | 1.09(0.97,1.22) | 0.13 | |
| No | 297/2474 | 435/2936 | 82.7 | 80.2 | 0.002 | 1.28(1.10,1.48) | 0.001 | |
| Total coronary occlusion |
0.82 | |||||||
| Yes | 81/684 | 109/684 | 84.4 | 82.1 | 0.07 | 1.19(0.89,1.59) | 0.24 | |
| No | 807/5827 | 936/5827 | 81.0 | 78.9 | 0.006 | 1.15(1.04,1.26) | 0.004 | |
| Renal failure | ||||||||
| Yes | 86/166 | 103/176 | 37.4 | 37.4 | 0.50 | 1.39(1.04,1.86) | 0.03 | 0.21 |
| No | 802/6345 | 942/6335 | 82.6 | 80.7 | 0.003 | 1.14(1.04,1.25) | 0.007 | |
| New York State PCI risk score for in- hospital mortality |
0.09 | |||||||
| ≥ 8 | 230/479 | 233/493 | 44.5 | 45.2 | 0.76 | 1.01(0.84,1.22) | 0.92 | |
| < 8 | 658/6032 | 812/6018 | 84.5 | 82.4 | <0.001 | 1.21(1.09,1.35) | <0.001 | |
IR=incomplete revascularization; CR=complete revascularization; CI=confidence interval.
In addition to incomplete revascularization, patient group and interaction between incomplete revascularization and patient group, models adjusted for age, body mass index, ejection fraction group, previous myocardial infarction, cerebrovascular disease, peripheral arterial disease, congestive heart failure, chronic obstructive pulmonary disease, diabetes and renal failure.
In the sensitivity analysis that was restricted to the 5,687 (87.3%) pairs of IR and CR patients who received only DES, the 5-year survival rate for IR was 78.7% in comparison to 80.9% for CR (P<0.001), and IR was related to significantly higher risk of death than CR (hazard ratio=1.20, 95% CI: 1.09–1.33, P<0.001). In addition, each type of IR was associated with significantly higher risk of death in DES patients. The respective hazard ratios for death were 1.16 (95% CI: 1.04–1.29, P=0.008) for 1-vessel and 1.43 (95% CI: 1.12–1.81, P=0.004) for multiple-vessel IR patients, in comparison to the matched CR patients.
DISCUSSION
In this study, we found that IR was associated with a 16% (hazard ratio=1.16, 95% CI: 1.06–1.27, P=0.001) higher risk of death during a 5-year follow-up after stenting in patients with multivessel disease in the DES era and the difference in mortality increased over time. We also found that the association between IR and mortality was not dependent on patient risk factors.
The increased risk of death in IR patients observed in this study is consistent with our previous studies that examined the impact of IR on 18-month mortality after stenting with DES (hazard ratio=1.23, 95% CI: 1.04–1.45, P=0.01)9 and 7-year mortality after stenting with BMS hazard ratio=1.12, 95% CI: 1.01–1.26, P=0.04).18 The 95% of CI of the hazard ratio in this study has substantial overlaps with the 95% CIs of the hazard ratios in the 2 previous studies. A strong impact of IR on mortality was found in a single-center study by Tamburino et al that included 508 patients.11 During the follow-up period that averaged 27 months, the risk of cardiac death in the IR patients was 2.70 times the risk in the CR patients (adjusted hazard ratio=2.70, 95% CI: 1.09–6.67, P=0.03).11 In another single-center study of 679 patients by Lehmann et al10, the risk of death for IR was 2 times the risk for CR in a 2.5-year follow-up (adjusted hazard ratio=1.96, 95% CI: 1.08–3.57, P=0.03).10 A common characteristic of these 2 studies is that these are single-center studies with small sample sizes, and the estimated impact of IR on mortality is imprecise as indicated by the wide 95% CIs for hazard ratios.10, 11 Therefore, caution needs to be taken when interpreting the results of these 2 studies. In a study with larger sample size, Genereux et al examined the impact of residual Synergy Between PCI With Taxus and Cardiac Surgery (SYNTAX) score on 1-year mortality following PCI in 2,686 patients enrolled in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial.8 The residual SYNTAX score was used to quantify the extent and complexity of any residual stenosis after PCI. It was found that a one-unit increase of the residual SYNTAX score was associated with a 5% increase in all-cause mortality (hazard ratio=1.05, 95% CI: 1.02–1.09, P=0.006).8
On the other hand, some studies found that IR was associated with higher risk of composite outcome (MACE) but did not find significant differences in mortality following PCI between IR and CR patients in the DES era.12–15 However, except for the study by Kim et al12, all 5 other studies found a trend indicating that IR was associated with higher risk of long-term mortality following PCI. 13–17 The adjusted hazard ratios for death for IR in comparison to CR in these 5 studies ranged from 1.15 (95% CI: 0.64–2.08, P=0.64) in a single-center study by Song et al of 873 patients followed for 35 month15 to 1.51 (95% CI 0.78–2.94, P=0.08) in another single-center study by Chung et al of 845 patients followed for 4 years.16
To better evaluate the results of our study in relation to the other studies, a few strengths and limitations are worth discussing. A major strength of this study is that it is a large population-based study utilizing data from real-world practice. Including all eligible patients in all hospitals in New York reduces the impact of providers’ practice patterns on the results and makes the results more generalizable to other settings. Second, the validity of this study is ensured by using high-quality data of the PCIRS and the CSRS, in which the completeness and accuracy of data are achieved by rigorous data auditing. Last, the chance of loss to follow-up for vital status is minimized in this study through matching to the National Death Index that includes all records of death certificates in the United States.
There are several limitations of our study. Other recent studies have shown that fractional flow reserve is better than anatomy-based evaluation to achieve optimal prognoses following PCI. 22–24 Also, data were not available to assess residual ischemia using scintigraphy or residual Syntax score. Second, we only had access to all-cause mortality, and the results may have been different if cardiac mortality had been used as an outcome. Third, in our observational study, achieving CR in stenting was not a random event. Therefore, treatment selection bias is a concern and is confirmed by the uneven distributions of some patient risk factors between IR and CR patients. To minimize the impact of selection bias, we propensity-matched IR and CR patients and were able to achieve comparable distributions of risk factors between IR and CR patients. However, we could not evaluate if the propensity-matched patients have comparable distributions of other variables such as lesion length and vessel size that were not available in our data. Therefore, there could be some residual bias even after we conducted the propensity matching. Also, data from the first 3 years following the first DES approval in the United States were used to achieve long-term follow-up in this study. However, a newer generation of DES has been approved since then, and caution needs to be exercised when generalizing the results to current practice.
Acknowledgments
We thank the New York State Cardiac Advisory Committee for their encouragement and support of this study; and Kimberly Cozzens, Cynthia Johnson, and the cardiac catheterization laboratories of the participating hospitals for their tireless efforts to ensure the timeliness, completeness, and accuracy of the registry data.
Funding sources: This work was supported by the National Institutes of Health (RC1HL099122).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
Disclaimer: The views expressed are those of the authors and do not necessarily reflect those of the New York State Department of Health. An abstract based on part of this study was presented at the American College of Cardiology – 2012 Scientific Sessions, Chicago, IL: March 24–27, 2012.
References
- 1.Mariani G, De Servi S, Dellavalle A, Repetto S, Chierchia S, D'Urbano M, Repetto A, Klersy C, Grp RS. Complete or incomplete percutaneous coronary revascularization in patients with unstable angina in stent era: Are early and one-year results different? Catheter Cardiovasc Interv. 2001;54:448–453. doi: 10.1002/ccd.1309. [DOI] [PubMed] [Google Scholar]
- 2.van den Brand MJ, Rensing BJ, Morel MA, Foley DP, de Valk V, Breeman A, Suryapranata H, Haalebos MM, Wijns W, Wellens F, Balcon R, Magee P, Ribeiro E, Buffolo E, Unger F, Serruys PW. The effect of completeness of revascularization on event-free survival at one year in the arts trial. J Am Coll Cardiol. 2002;39:559–564. doi: 10.1016/s0735-1097(01)01785-5. [DOI] [PubMed] [Google Scholar]
- 3.Ijsselmuiden AJJ, Ezechiels JP, Westendorp ICD, Tijssen JGP, Kiemeneij F, Slagboom T, van der Wieken R, Tangelder GJ, Serruys PW, Laarman GJ. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: A randomized comparison. Am Heart J. 2004;148:467–474. doi: 10.1016/j.ahj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Nikolsky E, Gruberg L, Patil CV, Roguin A, Kapeliovich M, Petcherski S, Boulos M, Grenadier E, Amikam S, Linn S, Markiewicz W, Beyar R. Percutaneous coronary interventions in diabetic patients: Is complete revascularization important? J Invasive Cardiol. 2004;16:102–106. [PubMed] [Google Scholar]
- 5.McLellan CS, Ghali WA, Labinaz M, Davis RB, Galbraith PD, Southern DA, Shrive FM, Knudtson ML, Investigators A. Association between completeness of percutaneous coronary revascularization and postprocedure outcomes. Am Heart J. 2005;150:800–806. doi: 10.1016/j.ahj.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, Racz M, Holmes DR, King SB, Walford G, Ambrose JA, Sharma S, Katz S, Clark LT, Jones RH. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation. 2006;113:2406–2412. doi: 10.1161/CIRCULATIONAHA.106.612267. [DOI] [PubMed] [Google Scholar]
- 7.Kalarus Z, Lenarczyk R, Kowalczyk J, Kowalski O, Gasior M, Was T, Zebik T, Krupa H, Chodor P, Polonski L, Zembala M. Importance of complete revascularization in patients with acute myocardial infarction treated with percutaneous coronary intervention. Am Heart J. 2007;153:304–312. doi: 10.1016/j.ahj.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu K, Parise H, Mehran R, Serruys PW, Stone GW. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: The residual syntax (synergy between pci with taxus and cardiac surgery) score. J Am Coll Cardiol. 2012;59:2165–2174. doi: 10.1016/j.jacc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannan EL, Wu C, Walford G, Holmes DR, Jones RH, Sharma S, King SB. Incomplete revascularization in the era of drug-eluting stents: Impact on adverse outcomes. JACC Cardiovasc Interv. 2009;2:17–25. doi: 10.1016/j.jcin.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann R, Fichtlscherer S, Schachinger V, Held L, Hobler C, Baier G, Zeiher AM, Spyridopoulos I. Complete revascularization in patients undergoing multivessel pci is an independent predictor of improved long-term survival. J Interv. Cardiol. 2010;23:256–263. doi: 10.1111/j.1540-8183.2010.00556.x. [DOI] [PubMed] [Google Scholar]
- 11.Tamburino C, Angiolillo DJ, Capranzano P, Dimopoulos K, La Manna A, Barbagallo R, Tagliareni F, Mangiafico S, Guzman LA, Galassi AR, Bass TA. Complete versus incomplete revascularization in patients with multivessel disease undergoing percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv. 2008;72:448–456. doi: 10.1002/ccd.21666. [DOI] [PubMed] [Google Scholar]
- 12.Kim YH, Park DW, Lee JY, Kim WJ, Yun SC, Ahn JM, Song HG, Oh JH, Park JS, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ. Impact of angiographic complete revascularization after drug-eluting stent implantation or coronary artery bypass graft surgery for multivessel coronary artery disease. Circulation. 2011;123:2373–2381. doi: 10.1161/CIRCULATIONAHA.110.005041. [DOI] [PubMed] [Google Scholar]
- 13.Rosner GF, Kirtane AJ, Genereux P, Lansky AJ, Cristea E, Gersh BJ, Weisz G, Parise H, Fahy M, Mehran R, Stone GW. Impact of the presence and extent of incomplete angiographic revascularization after percutaneous coronary intervention in acute coronary syndromes: The acute catheterization and urgent intervention triage strategy (acuity) trial. Circulation. 2012;125:2613–2620. doi: 10.1161/CIRCULATIONAHA.111.069237. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz L, Bertolet M, Feit F, Fuentes F, Sako EY, Toosi MS, Davidson CJ, Ikeno F, King SB., 3rd Impact of completeness of revascularization on long-term cardiovascular outcomes in patients with type 2 diabetes mellitus: Results from the bypass angioplasty revascularization investigation 2 diabetes (bari 2d) Circ Cardiovasc Interv. 2012;5:166–173. doi: 10.1161/CIRCINTERVENTIONS.111.963512. [DOI] [PubMed] [Google Scholar]
- 15.Song YB, Lee SY, Hahn JY, Choi SH, Choi JH, Lee SH, Hong KP, Park JE, Gwon HC. Complete versus incomplete revascularization for treatment of multivessel coronary artery disease in the drug-eluting stent era. Heart Vessels. 2012;27:433–442. doi: 10.1007/s00380-011-0173-x. [DOI] [PubMed] [Google Scholar]
- 16.Chung JW, Park KH, Lee MH, Park KW, Park JS, Kang HJ, Koo BK, Kwon YW, Kim HS. Benefit of complete revascularization in patients with multivessel coronary disease in the drug-eluting stent era. Circ J. 2012;76:1624–1630. doi: 10.1253/circj.cj-11-1285. [DOI] [PubMed] [Google Scholar]
- 17.Head SJ, Mack MJ, Holmes DR, Jr, Mohr FW, Morice MC, Serruys PW, Kappetein AP. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: A subgroup analysis of 3-year syntax data. Eur J Cardiothorac Surg. 2012;41:535–541. doi: 10.1093/ejcts/ezr105. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Dyer AM, King SB, 3rd., Walford G, Holmes DR, Jr, Stamato NJ, Venditti FJ, Sharma SK, Fergus I, Jacobs AK, Hannan EL. Impact of incomplete revascularization on long-term mortality after coronary stenting. Circ Cardiovasc Interv. 2011;4:413–421. doi: 10.1161/CIRCINTERVENTIONS.111.963058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Hannan EL, Walford G, Ambrose JA, Holmes DR, Jr, King SB, 3rd, Clark LT, Katz S, Sharma S, Jones RH. A risk score to predict in-hospital mortality for percutaneous coronary interventions. J Am Coll Cardiol. 2006;47:654–660. doi: 10.1016/j.jacc.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Gu XS, Rosenbaum PR. Comparison of multivariate matching methods: Structures, distances, and algorithms. J Comp Graph Stat. 1993;2:405–420. [Google Scholar]
- 22.De Bruyne B. Multivessel disease: From reasonably incomplete to functionally complete revascularization. Circulation. 2012;125:2557–2559. doi: 10.1161/CIRCULATIONAHA.112.106872. [DOI] [PubMed] [Google Scholar]
- 23.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 24.Gössl M, Faxon DP, Bell MR, Holmes DR, Gersh BJ. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ Cardiovasc Interv. 2012;5:597–604. doi: 10.1161/CIRCINTERVENTIONS.111.965509. [DOI] [PubMed] [Google Scholar]


