Abstract
Vision is the most fundamental of our senses initiated when photons are absorbed by the rod and cone photoreceptor neurons of the retina. At the distal end of each photoreceptor resides a light-sensing organelle, called the outer segment, which is a modified primary cilium highly enriched with proteins involved in visual signal transduction. At the proximal end, each photoreceptor has a synaptic terminal, which connects this cell to the downstream neurons for further processing of the visual information. Understanding the mechanisms involved in creating and maintaining functional compartmentalization of photoreceptor cells remains among the most fascinating topics in ocular cell biology. This review will discuss how photoreceptor compartmentalization is supported by protein sorting, targeting and trafficking, with an emphasis on the best-studied cases of outer segment-resident proteins.
1. Introduction
Photoreceptors are elegant sensory neurons which generate electrical responses when stimulated by light. Photoreceptors have long served as an outstanding model for elucidating basic principles in sensory transduction and G protein signaling. One factor that contributed to the success of this field is the well-defined role of photoreceptors in vision, combined with the ability to assess signal inputs and outputs with high quantitative precision. Another factor is that all reactions responsible for capturing photons and producing electrical responses take place in the photoreceptor outer segment, a compartment exquisitely suited to perform these functions. Not surprisingly, the progress in understanding light signaling has been paralleled by studies of photoreceptor cell biology, in particular, outer segments. The significance of the latter has been further elevated in recent years by the extraordinary advances in ciliary biology and the realization that outer segments are not only a productive model for studying signal transduction, but also an excellent model for studying targeting, trafficking and the dynamic equilibria of ciliary proteins.
In this review, we will first discuss the structure-functional organization of photoreceptors and the process of outer segment morphogenesis, which will highlight the challenges and specifications of maintaining protein trafficking flow in these cells. We will then describe the mechanisms governing this flow, in regards to both membrane and soluble proteins, including signaling proteins which change their intracellular localization in response to light. When possible, we will emphasize parallels in mechanisms responsible for protein sorting into outer segments and other cilia. Another theme we will follow is how our current knowledge of photoreceptor cell biology arose from an interplay between the studies conducted in lower and higher vertebrates. Many of the most important findings in the field were obtained using amphibia and other cold-blooded species whose photoreceptors are relatively large, which makes studies of intracellular transport, membrane turnover and electrophysiological behavior more amenable. Over the past decade, technical advances in generating genetically manipulated frogs and fish have added a layer of utility for these model organisms. However, the development of transgenic and knockout mouse models provided additional advantages to study molecular and cellular mechanisms of photoreceptor function and has added the rod-dominant mouse model to the center stage of this field.
For further reading, the authors recommend several recent reviews covering the topic of photoreceptor cell biology (Deretic and Wang, 2012; Insinna and Besharse, 2008; Kennedy and Malicki, 2009; Sung and Chuang, 2010), as well as reviews on related topics in primary cilia (Garcia-Gonzalo and Reiter, 2012; Nachury et al., 2010; Pazour and Bloodgood, 2008).
2. Structure-functional organization of photoreceptors
Photoreceptors are the most abundant retinal neurons tightly aligned parallel to one another in the outer part of the retina, juxtapose to the retinal pigment epithelium (RPE), so that light must pass through the transparent inner retinal layers before reaching the light-sensitive photoreceptor outer segments. Though this configuration may seem counterproductive, it allows any light not captured by the photoreceptors to be absorbed by the RPE as opposed to the light being scattered or reflected back on to the photoreceptors. Curiously, invertebrate compound eyes in which photoreceptors are positioned the “right-way” round, still use melanosomes to absorb light that does not get absorbed by the visual pigment. These melanosomes are located both in the secondary pigment cells and the photoreceptors themselves immediately beneath the light-sensing rhabdoms (Summers et al., 1982). The two types of photoreceptor cells in the vertebrate retina are cones dedicated to high-resolution color vision and rods responsible for high-sensitivity photon detection under low light intensities. In the retinas of most vertebrate species, rods are the dominant photoreceptor type, though there are a few exceptions. Turtles, lizards, fish (i.e. zebrafish and goldfish) and the ground squirrel have cone-dominant retinas (Branchek and Bremiller, 1984; West and Dowling, 1975). In birds and mammals, rods and cones have a unique distribution across the retina with the central retina containing more cones then the periphery (Fei, 2003). An extreme case of this pattern is the fovea of birds, primates and humans, which contains only cones and is responsible for high acuity central vision (Curcio et al., 1987; Querubin et al., 2009; Yamada, 1969). In contrast, rods and cones in frogs are evenly distributed from the central to peripheral retina (Wilhelm and Gabriel, 1999). A defining feature of all photoreceptors is that they consist of four morphologically distinct compartments: the outer segment, the inner segment, the nuclear region and the synapse.
2.1. Outer segment is a ciliary organelle
The most distal photoreceptor compartment, adjacent to RPE, is the outer segment. The outer segment is the site where photons are absorbed and the visual signal is generated, a process known as phototransduction. In this review, we will discuss some of the phototransduction proteins, but recommend a number of comprehensive reviews (Arshavsky et al., 2002; Burns and Baylor, 2001; Fain et al., 2001)) and recent updates (Arshavsky and Burns, 2012; Kefalov, 2012; Luo et al., 2008; Palczewski, 2012) for in-depth discussions. The outer segment is a modified non-motile cilium, which is distinct from other cilia due to the presence of tightly-packed membrane disc stacks (Sjostrand, 1953), either separated from or being formed by a single plasma membrane. This organization allows the outer segment to achieve a very high density of the membranes containing visual pigments, rhodopsin or cone opsins, required for efficient light capture. As a result, the volumetric fraction of the outer segment containing cytoplasm is low compared to other parts of the photoreceptor cell.
In rods, each disc is a discrete self-contained flattened membrane vesicle with an intradiscal space of only ~6 nm (Gilliam et al., 2012; Nickell et al., 2007). Electron micrographs show that thin filamentous structures bridge adjacent discs and disc rims to the nearby plasma membrane (Corless and Schneider, 1987; Kajimura et al., 2000; Nickell et al., 2007; Roof and Heuser, 1982), presumably helping to maintain an orderly outer segment structure. Interestingly, both intradiscal thickness and disc spacing of ~32-35 nm (Gilliam et al., 2012; Nickell et al., 2007) are consistent across species, even though outer segment dimensions can be vastly different (see Table 1 in (Nickell et al., 2007) for a comprehensive comparison of EM data on disc measurements across species). For example, the volume of the rod outer segment in frogs is ~30-fold larger than in the mouse (Fig. 1). Differences in outer segment sizes are functionally important because the rate at which the photoresponse propagates is an inverse function of the outer segment volume (Arshavsky et al., 2002; Pugh and Lamb, 1993).
Fig. 1.
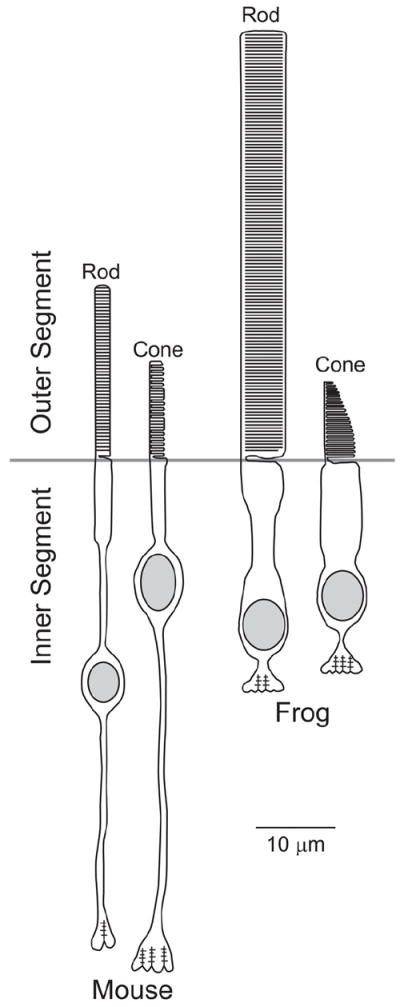
Schematic structures of mouse and frog photoreceptors drawn roughly to scale.
In rods, the outer segment can be divided into two separate membrane domains: the discs and the plasma membrane. Biochemical separation of these membranes into disc- and plasma membrane-enriched fractions have demonstrated differences in their protein composition (Molday and Molday, 1987) (Fig. 2). For example, the cGMP-gated channel and the Na/Ca/K exchanger, defining the electrical properties of these cells, are restricted to the plasma membrane (Bauer, 1988; Reid et al., 1990). In contrast the ABCA4 transporter, P(4)-ATPase Atp8a2, peripherin and rom-1 reside in discs where they perform various housekeeping functions (Coleman et al., 2009; Molday, 2004). Rhodopsin has been found in both the disc and the outer segment plasma membrane (Kamps et al., 1982; Molday and Molday, 1987). Proteins in the discs are either evenly distributed throughout the surface, called the lamellae, or reside in the highly curved rim. Proteins that are specifically localized to disc rims include peripherin, rom-1 and the ABCA4 transporter (Molday et al., 1987; Molday et al., 1999).
Fig. 2.
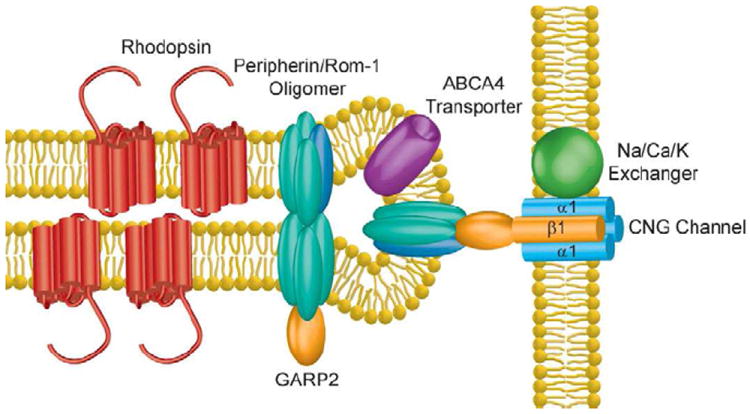
Membrane proteins residing in the rod disc rim and outer segment plasma membrane. Rhodopsin is primarily localized in the disc lamellae, though also present in the plasma membrane (not shown). The cGMP-gated (CNG) channel and the Na/Ca/K exchanger are localized to the plasma membrane. The GARP domain of the CNG channel β1-subunit associates with peripherin/rom-1 oligomeric complex located in the disc rim; this interaction is believed to tether the disc to the plasma membrane. Other peripherin/rom-1 oligomers are thought to interact with soluble GARP2, a splice variant of CNGβ1. The ABCA4 transporter is also localized within the disc rim.
The outer segment of cones differs from that of rods in structural organization and three-dimensional shape (Fig. 1). Cones owe their name to a conical shape of their outer segments. The narrowing of the cone outer segment is most prominent in lower vertebrates, e.g. in frogs it starts with a ~4.5 μm diameter base that rapidly tapers to a point over a length of 12 μm. The conical shape of the cone outer segment is less obvious in the mouse, where the outer segment base has a diameter of ~1.2 μm and only decreases to ~0.8 μm over the 14 μm length (Carter-Dawson and LaVail, 1979). Cone outer segments also consist of parallel disc-like membranes; however, unlike rod outer segment discs that are separated from the plasma membrane, the discs in cones are open, i.e. are contiguous with the plasma membrane. In cones of lower vertebrates, these open discs persist over the full outer segment length, while mammalian cones may contain discs separated from the plasma membrane like in rods (Anderson et al., 1978; Bunt, 1978; Carter-Dawson and LaVail, 1979; Cohen, 1970). For example, in mouse cones open discs are encountered in between stacks of 5-10 enclosed discs throughout the entire cone outer segment length (Anderson et al., 1978) (Fig. 3).
Fig. 3.
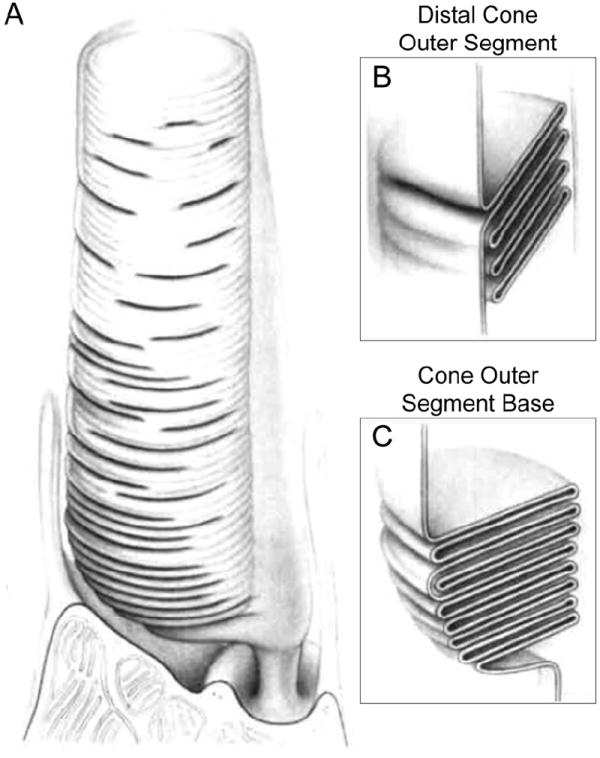
(A) Drawing of the entire outer segment and the distal inner segment portion of a mammalian cone. As described in the text, mammalian cone outer segments have only minor base-to-distal tapering. (B) A longitudinal section of the distal cone outer segment region, where one disc is continuous with the plasma membrane and several other discs are enclosed. (C) A longitudinal section of the cone outer segment base, where discs exist as contiguous plasma membrane evaginations. Image adapted from (Anderson et al., 1978).
The outer segment is connected to the inner segment through a single physical bridge called the connecting cilium. All outer segment-resident proteins are synthesized in the inner segment, so they must transverse through the connecting cilium to reach their final destination. Despite significant variation in photoreceptor sizes and shapes among rods and cones of different species, the diameter of the connecting cilium is remarkably consistent at ~0.3 μm (Besharse et al., 1985) and is the same as the diameter of the primary cilium (Garcia-Gonzalo and Reiter, 2012). Directly below the primary and connecting cilia resides the basal body, which acts as the organizing center where microtubules nucleate (Muresan et al., 1993; Troutt et al., 1990) (Fig. 4). The basal body itself contains nine triplet microtubules, of which two extend to form the axonemal structure, while the third terminates early and is used to anchor transition fibers that link the basal body to the plasma membrane (Fig. 4). The axonemal microtubules extend through the connecting cilium and at least one half the outer segment length (Kaplan et al., 1987; Knabe and Kuhn, 1997; Sale et al., 1988). Throughout the connecting cilium, microtubule doublets are cross-linked to the overlying plasma membrane by fibrous Y-link structures and rows of intramembrane particles (Besharse et al., 1985; Rohlich, 1975). At the distal axoneme the microtubules are reduced to singlets (Fig. 4) (Fisch and Dupuis-Williams, 2011; Insinna et al., 2008; Knabe and Kuhn, 1997; Roof et al., 1991; Steinberg and Wood, 1975). Unlike the rod axoneme, the cone axoneme, at least in frogs and fish, extends the full length of the outer segment and is discarded along with the cone outer segment tip during phagocytosis by the RPE (Bader et al., 2012; Eckmiller, 1996).
Fig. 4.
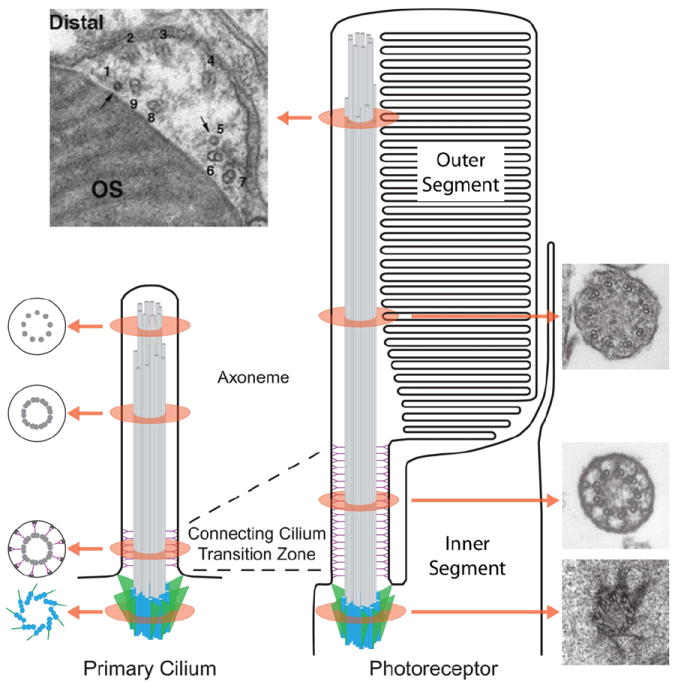
Schematic comparison of a primary cilium (left) and a photoreceptor outer segment (right). Drawings to the left of the primary cilium depict tangential sections through the subciliary compartments: basal body, transition zone, axoneme doublets, and axoneme singlets. Electron micrographs represent tangential sections through various ciliary components of the photoreceptor: the basal body of a mouse rod, reproduced from (Sedmak and Wolfrum, 2011); connecting cilium and axoneme doublets of rat rods, reproduced from (Besharse et al., 1985); and the axonemal transition from doublets to singlets of a zebrafish cone. The axonemal transition in the distal outer segment shows two singlets (1 and 5) and 7 doublets adjacent to the OS discs. Bar is 0.33 μm, reproduced from (Insinna et al., 2008).
2.2. Structure-functional organization of other photoreceptor compartments
The inner segment serves as the major housekeeping compartment of the photoreceptor cell. The inner segment stretches from the connecting cilium to the structure called the outer limiting membrane, which is a band of adherens junctions between photoreceptors and Muller glia cells located immediately above the photoreceptor nuclei. An interesting morphological feature of this compartment is that its distal edge protrudes thin microvilli, named calycal processes, which surround the proximal portion of the outer segment. The number of calycal processes varies among species. For example, a frog rod contains 16-20 individual calycal processes (Kinney and Fisher, 1978), a macaque rod contains 10 (Sahly et al., 2012), while a mouse rod has only one (Cohen, 1960). Though the exact function of calycal processes remains unknown, they contain actin filament bundles which may protect the outer segment against mechanical forces (Pagh-Roehl et al., 1992).
The inner segment contains two main sub-compartments, the ellipsoid and myoid. The ellipsoid is located directly below the connecting cilium and is packed with mitochondria that help satisfy the metabolic requirements of photoreceptors. Rods have thin elongated mitochondria while cones have short wider mitochondria, which allows an easy distinction between the respective inner segments in electron micrographs (Carter-Dawson and LaVail, 1979). The sequestration of mitochondria to the ellipsoid is a morphological adaptation proposed to bring them closer to the choroidal blood vessels for more efficient supply of oxygen required to produce ATP in direct proximity of the energy-demanding outer segments (Stone et al., 2008).
The ellipsoid region of the inner segment also conducts a high level of glucose metabolism (Linton et al., 2010; Okawa et al., 2008). Their plasma membrane, but not the plasma membrane of the outer segment, contains large amounts of the facilitative glucose transporter, Glut1, responsible for glucose delivery to the cell (Gospe et al., 2010). Once inside, glucose is phosphorylated by hexokinase which traps it inside the cell and initiates further steps of glycolytic degradation. Interestingly, unlike the majority of neurons that express the hexokinase I isoform, photoreceptors express hexokinase II (Reidel et al., 2011), the isoform with higher catalytic activity than hexokinase I particularly when associated with mitochondria (Wilson, 1995). Indeed, all hexokinase II in photoreceptors is found in the mitochondria-associated state (Reidel et al., 2011).
The myoid region, located below the ellipsoid, houses the biosynthetic membranes. The Golgi apparatus is located distal to the nucleus, while the endoplasmic reticulum is found both distal and proximal (Carter-Dawson and LaVail, 1979; Mercurio and Holtzman, 1982). In mice, clear separation between the ellipsoid and myoid is only distinguishable in cones. Mitochondria in mouse rods are so elongated that they form a less obvious ellipsoid region and can often be found in the myoid as well (Carter-Dawson and LaVail, 1979).
A dominant anatomical structure located in the photoreceptor inner segment is the ciliary rootlet, which is a large cytoskeleton-like arrangement extending from the basal body to the axon terminal (Cohen, 1960; Sjostrand, 1953; Spira and Milman, 1979). The mammalian ciliary rootlet is primarily composed of the protein rootletin, which forms parallel homodimers that organize into elongated polymers (Yang et al., 2002). In mouse photoreceptors, the vast majority of rootletin is confined to the inner segment (Reidel et al., 2011). In rootletin knockout mice, ciliated cells are devoid of rootlets, yet exhibit no obvious functional deficits (Yang et al., 2005).
Surrounding the photoreceptor inner and outer segments is extracellular matrix, called the retinal interphotoreceptor matrix, which is composed of glycoproteins and proteoglycans (Adler and Klucznik, 1982; Adler and Severin, 1981; Beaty and Mello, 1987). The chondroitin sulfate-type proteoglycans that make up the interphotoreceptor matrix are primarily secreted by RPE cells (Tawara et al., 1989). The interphotoreceptor matrix can be subdivided into rod and cone-specific compartments, which are distinguished based on the presence of peanut agglutinin-binding glycoconjugates (PNA) in cones and wheat germ agglutinin-binding glycoconjugates (WGA) in rods and cones (Sameshima et al., 1987). The biochemically distinct cone component of the interphotoreceptor matrix, often referred to as the cone sheath, is primarily composed of chondroitin 6-sulfate proteoglycan. Cone sheath co-isolates with cone outer segments and is thought to contribute to retinal attachment to the RPE (Blanks et al., 1988; Hollyfield et al., 1989). Within the cone sheath, cone outer segments are encased by extensive RPE microvilli that travel as far as ~25 μm to reach cone outer segments located deeper in retina than rod outer segments (Steinberg and Wood, 1974).
The nuclear region is positioned between the inner segment and synaptic terminal. In mammalian retinas, photoreceptors form multiple rows of nuclei in the outer nuclear layer (9-11 nuclei per stack in the mouse). This feature reflects the outer segment diameter (~1.5 μm) being smaller than the nuclear diameter (~4 μm), so that the maximal density of outer segments is only achieved by stacking the nuclei on top of each other. Photoreceptors of lower vertebrates have more comparable diameters of outer segments and nuclei, so nuclear stacking is not nearly as prominent.
Cone and rod nuclei segregate to different sublaminae of the outer nuclear layer and can be separated by shape and histological staining. In the mouse retina, cone nuclei contain 1-3 clumps of irregularly shaped heterochromatin and are restricted to the outer third of the nuclear stack, most frequently right on the top. Rod nuclei have a single dense heterochromatin and represent the majority in each stack (Carter-Dawson and LaVail, 1979). Such a pattern, in which heterochromatin localizes in the nuclear center whereas euchromatin reside at the periphery, is characteristic of nocturnal mammalian species; the rods of diurnal mammals possess the nuclear architecture more common to other eukaryotic cells, with most heterochromatin positioned at the nuclear periphery and euchromatin situated in the center (Solovei et al., 2009).
The electrical responses generated by photoreceptors must be passed along to the next tier of neurons for integration and processing. This information transfer occurs in the outer plexiform layer where photoreceptors form synapses with bipolar and horizontal cells. Mammalian cone synapses, called pedicles, are large and flat. They lie side by side along the inner edge of the outer plexiform layer. Rod synapses, called spherules, are small and round. They are packed between and above cone pedicles.
Photoreceptor synapses are densely packed with synaptic vesicles, some of which are attached to a specialized structure called the synaptic ribbon (for a comprehensive review see (Mercer and Thoreson, 2011; Sterling and Matthews, 2005)). Synaptic ribbons are present in photoreceptors, retinal bipolar and inner hair cells – sensory neurons producing small graded voltage signals instead of action potentials. They are proposed to facilitate sustained vesicle release acting as conveyer belts; however, synaptic release may occur in their absence as well (e.g. (Dick et al., 2003; Zampighi et al., 2011)). In the frog retina, one cone terminal contains between 16 and 22 ribbons and one rod terminal – 13-15 (Gabriel and Wilhelm, 2001). In the mouse retina, rod synapses contain a single ribbon, while cone synapses from 20 to 42 (Haverkamp et al., 2000).
3. Outer segment morphogenesis and renewal
3.1. Major steps of outer segment formation
The major stages of outer segment morphogenesis are described in great detail (Greiner et al., 1983; Greiner et al., 1981; Knabe and Kuhn, 1997; Sedmak and Wolfrum, 2011; Whiteley and Young, 1985). This process is similar across all species and its initial stages closely parallel the morphogenesis of primary cilia in other cell types (Brechbuhl et al., 2008; Molla-Herman et al., 2010; Sorokin, 1962; Sorokin, 1968). As illustrated in Fig. 5, outer segment morphogenesis begins with the maturation of the basal body as it migrates towards the distal end of the inner segment. This basal body consists of the mother and daughter centrioles; however only the mother centriole is further modified to nucleate the microtubule doublets that form the axoneme (Marshall, 2008). In photoreceptors, the first step in cilium formation is the attachment of an intracellular ciliary vesicle to the distal end of the mother centriole. Sheet-like, distal appendages (transition fibers) projected from the mother centriole likely mediate this vesicle’s attachment. Axonemal extension occurs next and causes the ciliary vesicle to invaginate and form the ciliary sheath. At this stage, “periodic bead-like densities” which are characteristic of the photoreceptor connecting cilium, become visible (Besharse et al., 1985; Horst et al., 1987; Horst et al., 1990; Sedmak and Wolfrum, 2011), indicating that structural components of the mature connecting cilium begin to form. The basal body-ciliary vesicle structure then docks to the plasma membrane and the outer membrane of the sheath fuses with the plasma membrane. It is likely that upon this fusion, the ciliary sheath becomes the membrane region often referred to as the periciliary membrane.
Fig. 5.
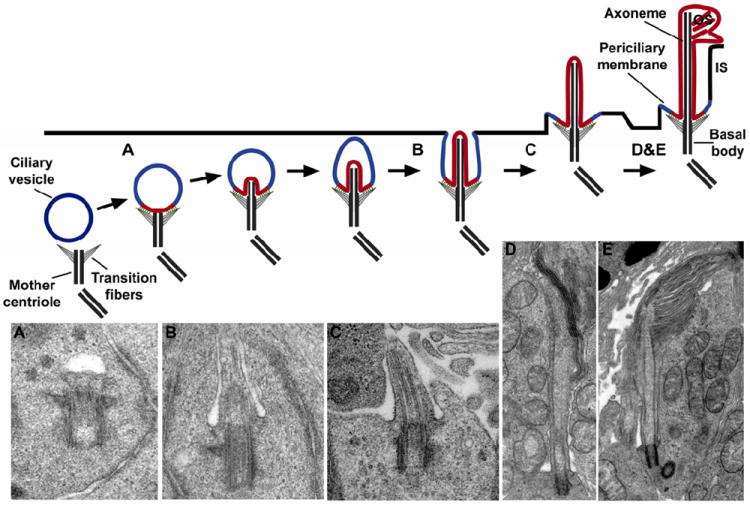
Schematic representation depicting the steps involved in outer segment formation. Outer segment morphogenesis begins when the mother centriole contacts a ciliary vesicle. Upon attachment, axonemal extension from the centriole causes the ciliary vesicle to invaginate and form the ciliary sheath. Fusion with the plasma membrane externalizes the developing outer segment and transforms the outer sheath into the periciliary membrane. Final stages of outer segment morphogenesis consist of disc formation and outer segment extension. (A-E) Electron micrographs of different stages of outer segment morphogenesis correlated with schematic diagram (Sedmak and Wolfrum, 2011). (A) The mother centriole attached to the ciliary vesicle in the cytoplasm of a differentiating photoreceptor. (B) The ciliary vesicle elongates to form the ciliary sheath. (C) The ciliary sheath fuses with the plasma membrane of the inner segment and the newly assembling outer segment emerges on the cell surface. (D-E) The axoneme extends into the outer segment and the first stacks of disc membranes appear.
The elaborate sequence of intracellular events that occurs prior to plasma membrane docking of the ciliary vesicle suggests that the outer segment’s identity as a ciliary organelle is defined prior to its emergence on the cell surface. The transition fibers that project from the basal body and mediate ciliary vesicle attachment may act to physically separate the membrane destined to enclose the outer segment from the plasma membrane enclosing the rest of the cell. This is likely to establish the membrane diffusion barrier and precluding lateral protein diffusion into the outer segment. If indeed the emergence of the outer segment coincides with the establishment of the membrane diffusion barrier, then the lipid and protein components of the outer segment plasma membrane and plasma membrane enclosing the rest of the cell never intermingle.
The subsequent stages of outer segment morphogenesis diverge considerably from that of primary cilia, due to the building of photoreceptor discs. It is presently unclear how disc formation is initiated in the developing photoreceptor and whether the underlying mechanism is the same as that for disc renewal in adult photoreceptors. Besides, it may differ across species. In mice and rats, the appearance of tubules and vesicles inside the prototypic outer segment precede the formation of organized and flattened discs (Besharse et al., 1985; Derobertis, 1956; Sedmak and Wolfrum, 2011). These vesicles and tubules are of varying size and orientation, with some arranged parallel with the axoneme. Conversely, neither vesicles nor tubules have been observed in cold-blooded vertebrate species, particularly in frogs (Kinney and Fisher, 1978; Nilsson, 1964; Stiemke et al., 1994). Outer segment morphogenesis then concludes with the lengthening of the axoneme and the stacking of tightly packed discs until the final length is reached, which is then maintained by the steady state equilibrium between basal disc formation and distal disc shedding.
3.2. Ongoing renewal of photoreceptor discs
Outer segments are constantly regenerated throughout the lifetime of a photoreceptor. It is believed that this phenomenon serves as a preventive mechanism as rapid turnover minimizes the accumulation of any damaged molecular components. A major source of damage is the constant photooxidative stress associated with light absorption in this compartment. Rod outer segment renewal occurs in an orderly fashion, which was first revealed by pulse/chase studies in rats, mice and frogs (Young, 1967). In these studies, newly synthesized radioactive protein molecules were first detected in the inner segment, the site of new protein synthesis, but soon after appeared as a discrete band in the basal portion of the outer segment. This band was displaced apically as new discs containing non-radioactive proteins were added, eventually causing the band to transverse the length of the outer segment. These studies demonstrated that the mouse rod outer segment is completely renewed once every 10-12 days, while the outer segment of frog rods is renewed at a slower rate of once every 6-7 weeks (Young, 1967). Similar studies showed that disc renewal in cones differs from that of rods (Anderson and Fisher, 1975; Young, 1971a; Young and Droz, 1968). No distinct radioactive band was observed, but rather the signal was evenly dispersed throughout the cone outer segment. Initially, this result was interpreted as evidence that cones do not renew their discs. However, studies in cone-dominant species, such as the ground squirrel, clearly demonstrated that cones undergo shedding as well (Anderson and Fisher, 1975; Long et al., 1986; Steinberg, 1974). In light of this evidence, the free dispersal of radioactive signal throughout cone outer segments indicates that the majority of protein molecules (primarily opsins) freely diffuse throughout the cone outer segment due to a contiguous disc structure, which allows the diffusion of proteins between lamellae.
In order to maintain the constant length of the photoreceptor outer segment, the generation of new discs is balanced by the phagocytosis of outer segment tips by RPE (Young and Bok, 1969). This active process requires the participation of both cell types. RPE possess microvilli that interdigitate between neighboring outer segment tips, so that one RPE cell contacts 30-50 rods and/or cones, depending on its location in the retina and the species examined (Snodderly et al., 2002; Young, 1971b). Although shedding without engulfment has never been observed (Williams and Fisher, 1987), photoreceptors likely designate membranes to be engulfed. For example, a recent study demonstrated that the outer leaflet of the plasma membrane at photoreceptor tips becomes enriched in phosphatidylserine immediately preceding disc shedding (Ruggiero et al., 2012). This phosphatidylserine exposure is reminiscent of the mechanism by which apoptotic cells are recognized by macrophages (Fadeel, 2004). Upon recognition of this and potentially additional signals, RPE cells internalize and digest outer segment tips (see (Finneman and Chang, 2008) for an in-depth discussion).
In many species, phagocytosis of rod outer segments occurs in a diurnal cycle with a burst happening directly after light onset (Basinger et al., 1976; Hollyfield et al., 1976; LaVail, 1976). This event is controlled by circadian rhythms in mammals (LaVail, 1980), by light exposure in Rana pipiens (Basinger et al., 1976), and by a combination of both in Xenopus laevis (Besharse et al., 1977). Cones shed discs in a diurnal manner as well, but the specific timing of this event shows considerable variation among species (Bobu et al., 2006; Fisher et al., 1983; Long et al., 1986; O’Day and Young, 1978; Young, 1977, 1978). Basal disc formation also appears to follow a diurnal pattern that compensates for membrane loss upon disc shedding. This was established by autoradiographic studies in frogs demonstrating an increase in rod disc formation upon light onset (Besharse et al., 1977).
3.3. Mechanism of disc formation
Two hypotheses are currently considered to explain how disc morphogenesis occurs in rod photoreceptors: an evagination model and a vesicular fusion model (Fig. 6 panels A and C, respectively). It should be noted that the bulk of evidence in support of each hypothesis has been obtained in different classes of vertebrate animals and, therefore, there may be species-dependent differences.
Fig. 6.
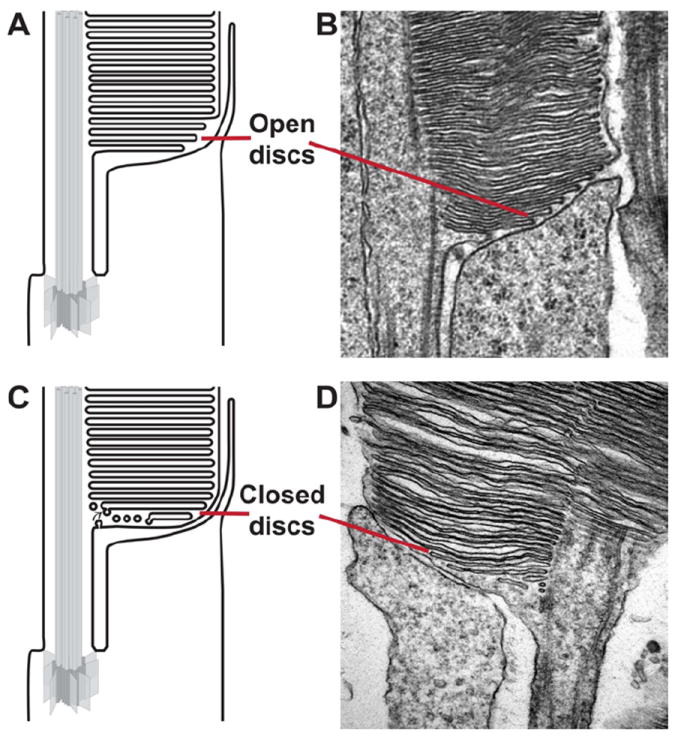
Schematic representations of the open disc, evagination model (A) and the closed disc, vesicle fusion models of rod disc formation (B). (C-D) Electron micrographs of two mouse rod are included to demonstrate the recent publication of images that support either model. Reprinted with permission from (Patil et al., 2012) and (Chuang et al., 2007).
The first model describing disc morphogenesis was the invagination model, postulating that both rod and cone discs are formed by invaginations of the plasma membrane at the base of the outer segment (Nilsson, 1964). It was subsequently replaced by the conceptually comparable evagination model, which states that discs are rather generated from outgrowths of the plasma membrane at the outer segment base (Steinberg et al., 1980). These evaginations are exposed to the extracellular space and appear on electron micrographs as open discs. An enclosed disc is formed when the lower membrane of an older evagination and the upper membrane of the adjacent younger evagination fuse at the rims. First evidence for this model came from electron micrographs of the basal outer segment region in rhesus monkeys and frogs showing the presence of open discs (Kinney and Fisher, 1978; Steinberg et al., 1980). Further support for this model came from studies in gecko and frog photoreceptors, using membrane-associating fluorescent dyes, such as Procion yellow and Lucifer yellow, which label most intensively the membranes of the newly formed discs (Laties et al., 1976; Matsumoto and Besharse, 1985). These lipophilic dyes accumulate in the membranes but do not cross them efficiently. Therefore, the pattern of their incorporation in rods indicates that nascent discs are open to the extracellular environment, but mature discs become enclosed and not accessible from the extracellular space. Consistent with disc formation occurring under light control, the prominence (Matsumoto and Besharse, 1985) and the width (Vaughan et al., 1989) of the fluorescent bands in frog rods increased upon light exposure. This correlation strongly suggests that the dye indeed incorporated into open discs. Unfortunately, the use of fluorescent dyes to examine whether nascent discs are open in mammals have failed, primarily because dyes produce intense staining of the interstitial space between their photoreceptors, making image analysis challenging (Laties et al., 1976).
The alternative model suggests that discs are formed by the fusion of vesicles and tubular cisternae at the outer segment base (Chuang et al., 2007; Miyaguchi and Hashimoto, 1992; Obata and Usukura, 1992). These vesicles may originate either from the plasma membrane of the outer segment or from transport vesicles moving through the connecting cilium (Chuang et al., 2007; Sung and Chuang, 2010). Nascent discs are then formed by the repeated fusion of vesicles at the outer segment base, followed by their flattening to form the mature disc shape. Morphological evidence for this hypothesis comes from electron micrographs of mouse rods that appear completely enclosed by the plasma membrane with vesicle-like structures observed at the base of the outer segment (Chuang et al., 2007) (Fig. 6B). EM cryofixation techniques have also yielded images of mouse outer segments with enclosed plasma membrane and vesicles of varying size at the outer segment base (Gilliam et al., 2012; Obata and Usukura, 1992). However, these images are in contradiction with those published in other studies of mouse rods, in which several apparently evaginating discs can be seen (Carter-Dawson and LaVail, 1979; Patil et al., 2012) (Fig. 6D). Supporters of each model suggest that this discrepancy in the EM appearance of the outer segment base in mouse rods may originate from technical artifacts of tissue fixation, which are described or discussed in multiple publications (Chuang et al., 2007; Kleinman and Ambati, 2008; Mustafi et al., 2009; Townes-Anderson, 1995; Yang et al., 2008).
Extensive morphological studies in the cones of ground squirrel, monkeys, and frogs indicated that cones form basal evaginating membranes to renew their outer segments. The prevailing theory for cone disc formation suggests that their open lamellar structure is achieved by incomplete rim formation of basal membrane evaginations (Arikawa et al., 1992; Farjo et al., 2006; Kinney and Fisher, 1978; Steinberg et al., 1980). As noted above, this results in either entirely open lamellar structure of amphibian cones, or a combination of enclosed discs and opened lamellae in mammalian cones. In addition to membrane evagination, subsequent invaginations of distal cone lamellae may play a role in generating the taper of cone outer segments (Eckmiller, 1990). It is also possible that outer segment tapering could be achieved by recycling membrane material from distal lamellae into newer, more basal lamellae (see (Corless, 2012) for a recent detailed update). The latter may play a significant role in reshaping the overall conical structure of the outer segment, following each shedding event (Bok, 1985).
3.4. Molecules implicated in disc formation
One of the major challenges in understanding the mechanism of outer segment morphogenesis is to identify critical molecules involved in this process. Clearly, both evagination and vesicular fusion models require participation of at least two types of proteins: those responsible for creating and maintaining highly curved disc edges and those responsible for membrane fusion.
Peripherin (also known as peripherin-2 or RDS) is perhaps the most frequently cited protein involved in disc formation (reviewed in (Conley and Naash, 2009; Goldberg, 2006)). Peripherin is a tetraspanin membrane protein that forms homo-tetramers and hetero-tetramers with its close homolog, rod outer segment protein 1 (Rom 1), as well as higher order oligomers consisting of multiple tetrameric structures (Goldberg and Molday, 1996; Loewen and Molday, 2000; Loewen et al., 2001). In rds mice, which have a spontaneous mutation severely truncating peripherin, rod outer segments fail to form and instead display rudimentary outer segment stumps completely lacking disc structures (Sanyal et al., 1980; Sanyal and Jansen, 1981; Travis et al., 1989). Immuno-EM studies showed that vesicles transporting rhodopsin to the outer segment accumulate around this stump, indicating that the failure to form discs results from the lack of peripherin rather than defects in the trafficking of rhodopsin (Usukura and Bok, 1987). rds heterozygotes (Sanyal et al., 1986) or transgenic mice expressing low levels of peripherin (Travis et al., 1992) form rod outer segments, however, they are filled with disorganized whorled membranes and eventually degenerate. Peripherin decorates disc rims and incisures throughout the rod outer segment, except for at the most basal outer segment region where it preferentially localizes to the axonemal side (Arikawa et al., 1992). In cone outer segments, peripherin localizes mostly to the axonemal side and is not found at the outer lamellar edge. The localization pattern and phenotypes associated with loss of peripherin suggest that it is required for forming and/or maintaining the hairpin like curvature of the disc rim. The intradiscal loop of peripherin contains an unpaired cysteine residue (C150) that is thought to form an intermolecular disulfide bridge connecting opposite faces of the disc membrane (Goldberg et al., 1998; Loewen and Molday, 2000). In vitro translation studies have demonstrated that expression of peripherin in the presence of microsomal vesicles results in these vesicles’ adopting a flattened morphology, which did not occur upon expression of the C150S peripherin mutant (Wrigley et al., 2000). It has also been suggested that peripherin may directly assist disc rim formation by fusing adjacent membranes together, since the C-terminus of peripherin contains a an amphipathic helix that functions as a fusogenic peptide in vitro (Boesze-Battaglia and Goldberg, 2002; Boesze-Battaglia et al., 1998; Edrington et al., 2007). However, it remains to be demonstrated whether peripherin mediates membrane fusion in vivo.
Prominin 1 is a pentaspan transmembrane protein that appears to play a critical role in disc morphogenesis as well. Outer segments of prominin 1 knockout mice display elongated and misoriented discs (Yang et al., 2008; Zacchigna et al., 2009). In differentiated cells, prominin 1 is found in microvilli and primary cilia tips where it is implicated in stabilization of curved membrane protrusions (Huttner and Zimmerberg, 2001; Iglic et al., 2006). In mice, prominin 1 is located at the outer segment base, whereas in frogs it is located at the rod outer segment base and along the open cone outer segment rims (Han et al., 2012; Maw et al., 2000; Zacchigna et al., 2009). This propensity to localize at the open disc membranes, which contrasts peripherin’s localization at the edges of closed discs, was proposed to serve as indirect evidence for open discs to exist in rods, thereby supporting the evagination hypothesis of disc morphogenesis (Han et al., 2012; Yang et al., 2008). Prominin 1 was reported to interact with protocadherin 21 (Yang et al., 2008) and protocadherin 21 knockout yields a phenotype closely resembling the prominin 1 knockout (Rattner et al., 2001). Interestingly, protocadherin 21 undergoes a proteolytic cleavage at the outer segment base, an event that has been suggested to render disc formation irreversible (Rattner et al., 2004). However, the specific roles of prominin 1 and protocadherin 21 in disc morphogenesis remain to be elucidated.
In support of the vesicular fusion model, Sung and colleagues (Chuang et al., 2007) have presented intriguing data on the Smad Anchor for Receptor Activation (SARA) protein. SARA is a phosphoinositide-binding protein first characterized for its critical role in regulating fusion processes in early endosomes (Hu et al., 2002). In mouse photoreceptors, SARA is concentrated near the axonemal space at the base of the outer segment. It was proposed that rhodopsin-containing vesicles recruit SARA using rhodopsin’s C-terminus, which then mediates an interaction between these vesicles and nascent discs containing PI3P. Reduction in SARA expression resulted in accumulation of vesicles at the base of the outer segment and mislocalization of rhodopsin throughout the entire rod cell. Typically, vesicular fusion is mediated by SNARE proteins and requires the pairing of a v-SNARE protein on the vesicle membrane with a t-SNARE protein on the target membrane. In mouse photoreceptors, the t-SNARE protein, syntaxin 3, was shown to interact and co-localize with SARA at the outer segment base, suggesting that SNARE-mediated fusion may drive disc formation under the control of SARA (Chuang et al., 2007). The nature of the cognate v-SNARE in this model remains to be established, although VAMP 2, distributed throughout the entire mammalian photoreceptor (Kwok et al., 2008), was suggested to fulfill this role (Chuang et al., 2007). It is fair to note that the presence of SNARE proteins at the outer segment base is also generally consistent with the evagination hypothesis, which requires membrane fusion as well.
Interestingly, the expression of syntaxin 3 in frog rods is limited to the plasma membrane surrounding all non-outer segment parts of the cell and not inside the outer segment base (Baker et al., 2008; Mazelova et al., 2009b). This difference may be viewed as indirect evidence for mouse and frog rods utilizing alternative disc formation mechanisms.
We should also mention that rhodopsin comprising ~50% of the outer segment membrane mass plays an important, although indirect role in disc morphogenesis. Rhodopsin knockout mice fail to form proper outer segments and instead produce thin elongated structures with a small content of unorganized membranes (Humphries et al., 1997; Lem et al., 1999). This phenotype may be explained by a simple fact that outer segments lacking rhodopsin are devoid of building material required for their structure. Consistently, mice expressing one copy of the rhodopsin gene have reduced rod outer segment diameters (Liang et al., 2004; Makino et al., 2012), while mice overexpressing rhodopsin in rods have enlarged discs (Wen et al., 2009). It is also plausible that the absence of rhodopsin may abolish the major trafficking pathway utilized to carry outer segment-specific lipids and proteins necessary for supporting outer segment morphogenesis.
4. Protein sorting and trafficking
Photoreceptors are among the most interesting differentiated cells to study intracellular protein sorting and trafficking both because they are highly polarized neurons and because the rate at which they perform constant renewal of their outer segment membranes is enormous. Furthermore, being a ciliary structure, the outer segment with its distinct set of transmembrane and lipidated proteins serves as a productive model for studying protein targeting to cilia. Conversely, mechanisms discovered in primary and other sensory cilia can apply to the photoreceptor outer segment, thereby stimulating studies in both fields.
4.1. The protein composition of plasma membrane is different between outer segments and the rest of the cell
One defining feature of the photoreceptor plasma membrane, shared with other ciliated cells, is that it is electrically contiguous yet separated into two domains with distinct protein compositions: one enclosing the outer segment and the connecting cilium and another enclosing the rest of the cell (Baker et al., 2008; Spencer et al., 1988; Steinberg et al., 1980; Wolfrum and Schmitt, 2000). This separation is believed to be established by a membrane diffusional barrier that prevents free exchange of integral membrane proteins. The subdivision is functionally analogous to that between the axonal initial segment and the rest of the axon in mature neurons, or to the division between apical and basolateral membrane compartments of polarized epithelial cells (Caudron and Barral, 2009). One idea is that the membrane barrier is formed by a fibrous cytoskeletal network that lines the membrane so closely that it impedes diffusion of transmembrane proteins. Another idea is that it is formed by anchoring transmembrane proteins to the cytoskeletal matrix, thereby putting a physical barrier for protein diffusion both inside and outside the membrane. In-depth discussion of this topic can be found in recent reviews (Breslow and Nachury, 2011; Garcia-Gonzalo and Reiter, 2012; Nachury et al., 2010).
The initial hypothesis on localization of the membrane diffusional barrier in photoreceptors came from freeze-fracture EM studies of the connecting cilium, which revealed a highly organized array of particles within its membrane (Rohlich, 1975). Transverse sections through this area showed that these particles are connected by fibrous Y-links from the ciliary plasma membrane to the axoneme (Fig. 4). Similar particles are found in the membrane of the transition zone, an area extending ~100 nm from the base of many (but not all) cilia (Gilula and Satir, 1972; Weiss et al., 1977). It was suggested that these particles may restrict diffusion of membrane proteins (Spencer et al., 1988), however, the ~25 nm spacing between individual particles is not dense enough to restrict the diffusion of proteins typically not exceeding ~5 nm in diameter. Despite this argument, these particles may simply be the only visible feature of a more complex network, including membrane and/or cytoskeletal elements, which prohibit membrane protein diffusion.
An alternative hypothesis is that the membrane diffusion barrier is located outside the connecting cilium. Studies performed in primary cilium indicate that the barrier extends from the cilium base into the surrounding plasma membrane. The most convincing evidence in this respect was obtained using a lipid-anchored fluorescent protein, glycosylphosphatidylinositol (FP-GPI) (Vieira et al., 2006). In polarized epithelial cells, FP-GPI is localized to the apical plasma membrane, but does not diffuse into the cilium. Importantly, the area devoid of FP-GPI forms a ring of 0.5-0.7 μm in diameter around the cilium base, clearly extending past the transition zone. Whether the extent of the membrane barrier may include the apical membrane of the photoreceptor inner segment has yet to be defined.
Despite recent progress in identifying putative molecular components of the diffusional barrier in the primary cilium, the molecular composition of this barrier in photoreceptors remains a subject of ongoing investigation. One protein candidate discussed in this context is Cep290/NPHP6, a 290 kDa cilia-centrosomal protein that forms large coiled-coiled structures up to ~380 nm in length (Fraser et al., 1973). Cep290/NPHP6 is a component of the microtubule-membrane linkage within the transition zone of all cilia analyzed so far, including photoreceptors where it is found at the base of the connecting cilium (Betleja and Cole, 2010; Rachel et al., 2012; Williams et al., 2011). Recent studies of the Chlamydomonas flagella showed that Cep290/NPHP6 prevents the entry of plasma membrane proteins into the ciliary membrane (Craige et al., 2010). Knockdown of Cep290/NPHP6 results in loss of the transition zone Y-shaped connectors as well as decreased levels of the ciliary cation channel, polycystin-2. Mutations of Cep290/NPHP6 are associated with a syndromic ciliopathy called Joubert syndrome (Sayer et al., 2006; Valente et al., 2006), and are the most common cause of the childhood recessive blindness known as Leber’s congenital amaurosis (Cremers et al., 2002).
Another strong candidate revealed in the studies of primary cilium is septin 2, which was recently shown to localize to the primary cilium base (Hu et al., 2010). The deletion of septin 2 resulted in decreased retention of ciliary-resident membrane proteins, directly implicating this protein in creating and maintaining the diffusional barrier. However, septin 2 has yet to be studied in photoreceptors.
In addition to the concept that a meshwork of specific proteins is responsible for impeding lateral membrane diffusion, the membrane barrier may have a unique lipid composition resembling that of lipid rafts. For example, the analysis of the barrier region surrounding the primary cilium with Laurdan, a fluorescent probe assessing the fluidity of the membrane environment, revealed that the lipid bilayer in this region is more stiff than the surrounding apical membrane and the membrane enclosing the cilium (Vieira et al., 2006). A similar idea that the high cholesterol content of the inner segment plasma membrane region surrounding the connecting cilium prevents protein diffusion into and out of the outer segment was expressed in early studies of photoreceptor lipids (e.g. (Andrews and Cohen, 1983)). However, the exact interplay between proteins and lipids in forming the diffusional barrier in all ciliary organelles remains to be fully understood.
4.2. Rhodopsin transport
Rhodopsin constitutes the majority of the outer-segment resident proteins. To calculate the number of rhodopsin molecules transported through the connecting cilium of a given rod every second, we need to consider the total number of rhodopsin molecules in the outer segment and the rate of outer segment renewal. A mouse rod outer segment is estimated to contain between 5·107 and 7·107 rhodopsins (Lyubarsky et al., 2004; Nickell et al., 2007) and is completely renewed within 10 days (Young, 1967). Therefore, ~80 rhodopsin molecules have to be synthesized and delivered each second (Williams, 2002)(the actual number varies diurnally, as discussed in Section 3.2). Larger outer segments of amphibian rods contain up to 3·109 rhodopsin molecules (Pugh and Lamb, 2000) and renew within 6-8 weeks (Young, 1967); therefore, they need to replenish ~700 rhodopsins per second. This continuous demand for rhodopsin renewal emphasizes the cell’s requirement for a highly efficient mechanism of its outer segment delivery, which also provides an excellent model to study ciliary receptor transport.
Like all membrane proteins, rhodopsin follows an intracellular path from the site of its synthesis in the ER to the Golgi complex and trans-Golgi network where it is sorted into vesicles destined for the outer segment. Rhodopsin contains an intracellular targeting signal located at its C-terminus, which includes the last four amino acids comprising the VXPX motif (Deretic et al., 1998; Li et al., 1996; Sung et al., 1994; Tam et al., 2000). Deletion or mutations of these amino acids result in mislocalization of rhodopsin from the outer segment. Furthermore, these mutations or deletions in humans lead to the most severe forms of retinal degeneration (Berson et al., 2002). Rhodopsin also contains a predicted FR targeting signal within its intracellular H-8 α-helix. FR signal (a Phe-Arg amino acid doublet) was originally characterized for the ciliary receptor, smoothened (Corbit et al., 2005), and was shown to play a critical role in rhodopsin targeting to the primary cilium of cultured cells (Wang et al., 2012), but it remains to be tested whether mutations of this signal lead to rhodopsin mistargeting in photoreceptors.
Rhodopsin sorting in the trans-Golgi network and the subsequent transport of rhodopsin-containing vesicles to the base of the connecting cilium involves an elegant interplay among three small GTPases; Arf4, Rab11 and Rab8. Each of these GTPases is believed to have a distinct function by either sorting rhodopsin into transport vesicles, targeting the vesicles towards the basal body, or delivering vesicles to the connecting cilium base.
A breakthrough in mechanistic understanding of rhodopsin sorting into transport vesicles was made by Deretic and colleagues when they showed that Arf4 interacts with rhodopsin’s VXPX targeting motif (Deretic et al., 2005). Disrupting the Arf4-rhodopsin interaction by antibodies against either Arf4 or rhodopsin’s C-terminus prevented trans-Golgi vesicle budding in an ex vivo assay, thereby demonstrating that Arf4 binding is required to initiate this process. Though members of the Arf GTPase family have been long-known to regulate vesicle transport of lipids and proteins from their site of synthesis to their site of action (Donaldson and Jackson, 2011), these studies were the first to identify a small GTPase involved in sorting cilia-resident cargo. Since these studies, Arf4 has been implicated in sorting other ciliary membrane receptors, including polycystin-1 and polycystin-2, which also contain a VXPX targeting motif (Geng et al., 2006; Ward et al., 2011). This suggests that the Arf4-dependent transport mechanisms may be conserved in many ciliated cells.
The most recent update on specific stages in rhodopsin transport vesicle formation and trafficking can be found in (Wang et al., 2012); see Fig. 7 reproduced from this paper for illustration. Upon binding to rhodopsin Arf4 recruits ASAP1, a multi-functional protein that assists several subsequent steps of rhodopsin trafficking. ASAP1 contains a number of functional domains: BAR, pleckstrin homology, Arf-GAP, proline-rich and SH3 (Nie et al., 2006; Randazzo and Hirsch, 2004). The BAR domain mediates membrane curvature (Jian et al., 2009; Nie et al., 2006) suggesting that ASAP1 functions as a membrane-deforming coat protein used to create rhodopsin transport vesicles. ASAP1 also contains the Arf GTPase activating domain involved in Arf4 release from the budding rhodopsin transport vesicle (Mazelova et al., 2009a; Wang et al., 2012). A recent study demonstrated that ASAP1 also binds to the FR targeting sequence of rhodopsin (Wang et al., 2012). Mutating the predicted FR targeting sequence in rhodopsin prevented ASAP1 binding and redirected rhodopsin targeting in cultured cells from the cilium to the surrounding membrane. Following binding to rhodopsin, ASAP1 recruits two additional proteins, Rab11 and FIP3 (Inoue et al., 2008; Mazelova et al., 2009a).
Fig. 7.
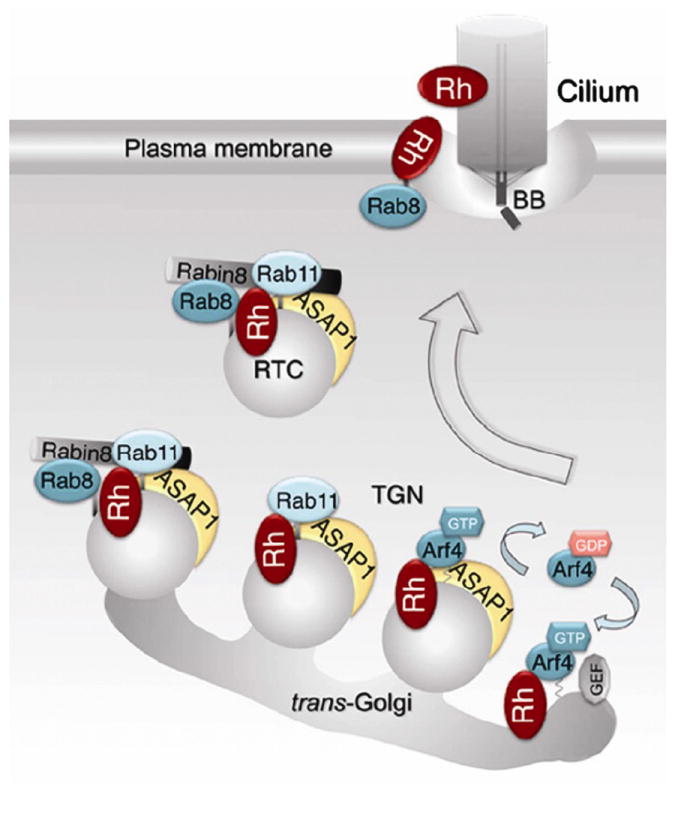
Molecular interactions taking place during rhodopsin transport to the outer segment. The figure is reproduced with permission from (Wang et al., 2012). At the trans-Golgi, GTP-bound Arf4 interacts with rhodopsin and they recruit ASAP1 into the ternary complex. ASAP1 likely initiates membrane deformation through its BAR domain while mediating GTP-hydrolysis of Arf4, which then dissociates from the trans-Golgi. ASAP1 then selectively binds Rab11, which also associates with rhodopsin. ASAP1 and Rab11 recruit Rabin8 and Rab8. On rhodopsin transport carriers (RTCs), ASAP1 serves as a scaffold for the Rab11/Rabin8/Rab8 complex, which controls the activation of Rab8. Activated Rab8 regulates RTCs fusion and the delivery of rhodopsin across the membrane diffusion barrier surrounding the connecting cilium.
The newly formed rhodopsin transport vesicles are now endowed with ASAP1, Rab11 and FIP3 and ready to be delivered to the connecting cilium base. These vesicles, presumably targeted by Rab11, are carried along the microtubules linked to the basal body. This process is facilitated by the dynein motor, which is shown to interact with rhodopsin C-terminus (Tai et al., 1999) and/or FIP3 (Horgan et al., 2010a, b). At the basal body, the microtubules change polarity, so the dynein motor cannot assist in the vesicles’ final transition to the cilium base. Therefore, it is possible that the final stage of the vesicles’ transport is assisted by a kinesin motor.
Along the vesicle’s route to the basal body, ASAP1 recruits the GDP-bound form of Rab8 (Deretic and Wang, 2012), which is activated by its guanine nucleotide exchange factor (GEF) Rabin8 near the basal body (Hattula et al., 2002; Nachury et al., 2007). Once activated, Rab8 plays a critical role in vesicle docking and fusion at the base of the photoreceptor connecting cilium (Deretic et al., 1995; Moritz et al., 2001a) and primary cilium (Bryant et al., 2010; Knodler et al., 2010; Nachury et al., 2007; Westlake et al., 2011). A study in primary cilium showed that Rab11 plays an active role in Rab8 activation by interacting with Rabin8 and stimulating its GEF activity toward Rab8 (Knodler et al., 2010).
Interestingly, photoreceptors (and other ciliated cells) contain an additional protein reported to possess GEF activity toward Rab8 – the retinitis pigmentosa GTPase regulator, RPGR. RPGR is present in the connecting cilium of photoreceptors and the transition zone of other cilia (Hong et al., 2003; Patil et al., 2012). Initially, RPGR was thought to be a GEF for Ran GTPase (Renault et al., 2001). However, a study by Khanna and colleagues (Murga-Zamalloa et al., 2010) demonstrated that RPGR interacts with Rab8a and facilitated the GDP/GTP exchange on Rab8a at a rate only slightly below that of Rabin8. The authors further demonstrated that some of the RPGR mutations found in human patients with X-linked retinitis pigmentosa (Breuer et al., 2002; Shu et al., 2007) result in an up to 50% reduction in its GEF activity. The presence of two Rab8 guanine exchange factors suggests that an additional level of Rab8 control may be needed to maintain the high level of vesicular trafficking and membrane expansion unique to the outer segment.
The fusion site of rhodopsin transport vesicles was most thoroughly investigated in frogs. Pulse-chase and immunohistochemistry studies located these vesicles either close to or fusing within the base of the connecting cilium (Besharse and Pfenninger, 1980; Defoe and Besharse, 1985; Papermaster et al., 1985). This membrane region, termed the periciliary ridge complex, consists of nine symmetrically arrayed ridges and grooves that extend laterally ~0.4-1 μm along the plasma membrane with the connecting cilium at the center (Papermaster, 2002; Papermaster et al., 1985; Peters et al., 1983). Although not shown in direct experiments, it is generally assumed that the periciliary ridge resides on the ciliary side of the diffusion barrier. While the periciliary ridge complex has only been imaged in frog photoreceptors it has been discussed that a similar structure exists in all cilia but is less anatomically distinct (Nachury et al., 2010). The absence of a profound periciliary ridge complex in mammalian rods could reflect a significantly lower volume of the vesicular trafficking flow into their outer segments. An alternative hypothesis is that vesicles are transported directly from the inner segment through the axonemal shaft to the base of the outer segment for fusion (Chuang et al., 2007; Sung and Chuang, 2010). However, evidence of vesicles present in the connecting cilium remains controversial and has not been documented for other types of cilia. A recent study, employing an EM method called cryo-electron tomography, identified that the axonemal shaft of the mouse connecting cilium does not contain vesicles but rather contains low-contrast particles bounded by microtubules, which are likely to impede any vesicular transport (Gilliam et al., 2012). On the other hand, the authors observed small vesicles in the space between the axoneme and the plasma membrane at the proximal portion of the connecting cilium, which were interpreted as vesicles just about to fuse with the plasma membrane. Interestingly, the number of these vesicles was significantly increased in the proximal connecting cilium of the mice lacking BBS4, a component of the BBSome protein complex involved in cilia morphogenesis and maintenance (Gilliam et al., 2012).
4.3. Photoreceptor distribution of membrane proteins lacking targeting motifs
One interesting consequence of photoreceptors maintaining a large flux of post-Golgi transport vesicles delivered to the outer segment is that membrane-associated proteins lacking specific targeting information tend to accumulate in this compartment. This pattern, particularly striking for frog rods containing very large outer segments, was first noted by Papermaster and colleagues (Moritz et al., 2001b; Tam et al., 2000). They demonstrated that deletion of the VXPX targeting motif from a rhodopsin C-terminal construct transgenically expressed in Xenopus rods resulted in only a partial mislocalization of this construct from the outer segment.
A detailed study in our laboratory (Baker et al., 2008), also performed with transgenic Xenopus, examined this phenomenon further by comparing the targeting of two structurally related transmembrane proteins found in photoreceptors, R9AP and syntaxin-3. R9AP is predominantly localized to the outer segment, with a minor fraction present in the plasma membrane of the inner segment mostly in the synaptic region (Baker et al., 2008; Hu and Wensel, 2002; Martemyanov et al., 2003). In contrast, syntaxin-3 in frog rods is localized to all parts of the plasma membrane except that enclosing the outer segment (Baker et al., 2008; Mazelova et al., 2009b). We were unable to identify an outer segment targeting motif within R9AP, but instead found that its normal localization pattern is essentially indistinguishable from that of randomly-chosen untargeted membrane reporter constructs. In contrast, syntaxin-3 was shown to contain critical targeting information encoded within its SNARE-homology domain. Removal of this domain, or its replacement with the corresponding sequence from R9AP, resulted in an intracellular distribution pattern resembling that of R9AP or untargeted reporter constructs.
We also demonstrated that untargeted membrane protein constructs found predominantly in rod and cone outer segments, are not found in the cilia of other cell types. This suggests that the outer segment biased delivery of untargeted proteins is not determined simply by the ciliary nature of this organelle. Rather, the outer segment bias reflects the fact that this compartment receives a lion’s share of all transport vesicles produced in photoreceptor cells. The fact that outer segments serve as a “default” trafficking destination in Xenopus photoreceptors has a critical consequence. Each membrane protein or protein complex destined for other parts of the cell must encode a distinct targeting signal in order to avoid the vast default trafficking flow to the outer segment. This adds a whole new dimension to understanding protein targeting and trafficking in photoreceptors because the vast majority of membrane proteins in these cells reside outside the outer segment.
Recently, our laboratory tested whether the predominant outer segment delivery of untargeted proteins is unique to frogs or can apply to all species, particularly those who have small outer segments and may require fewer transport vesicles to sustain outer segment integrity. For these studies, we analyzed the intracellular localization of the well-characterized C-terminal targeting motif of rhodopsin and its untargeted mutant expressed in frog and mouse rods. These constructs contained rhodopsin’s palmitoylated C-terminal, either with or without the VXPX targeting motif, fused to GFP and a single-pass transmembrane domain. Both constructs were expressed transgenically in frog rods or by in vivo electroporation in mouse rods (Fig. 8). As expected, the targeted construct was localized exclusively to outer segments in both species (panels A and D). However, the localization patterns of the construct lacking the targeting motif were different. While frog rods displayed the classical default pattern with the majority of GFP fluorescence in the outer segment and small spillage outside (Fig. 8B), the untargeted construct was distributed throughout the entire mouse rod cell (Fig. 8E).
Fig. 8.
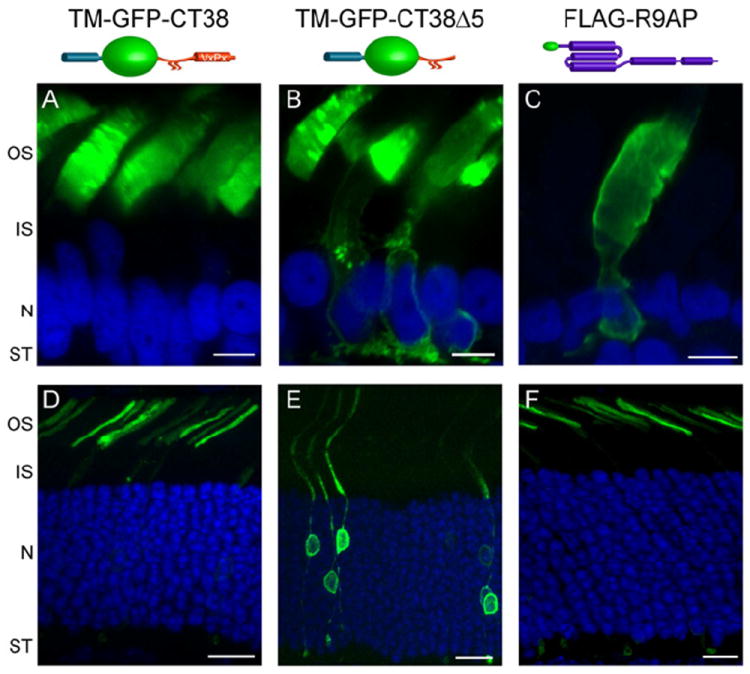
Comparison of R9AP expression with non-targeted and targeted constructs in frog and mouse rods. Immunofluorescent expression of an outer segment targeted construct (a single pass transmembrane domain from the activin receptor fused to a GFP and rhodopsin’s C-terminal 38 amino acids) is exclusive localized to the outer segment in both frog (A) and mouse (D) rods. In contrast, the outer segment un-targeted construct (the same activin-GFP-rhodopsin backbone, but lacking C-terminal VXPX targeting sequence) is localized throughout the frog (B) and mouse (E) rod plasma membrane. As described in the text, the distribution of untargeted construct prefers frog outer segments compared to mouse outer segments. Scale bar is 5 μm (A-C) and 10 μm (D-F).
The most parsimonious explanation for this difference is that the majority of post-Golgi transport vesicles in mouse rods are not intended for the outer segment, perhaps reflecting the drastic difference in outer segment volume and trafficking requirements between these species. Rod outer segments in mice have ~40-fold smaller volume than in frogs (25×1.5 μm vs. 60×6 μm) and sustain ~10-fold lower rhodopsin renewal rate (see Section 4.2). Therefore, default delivery of outer segment-specific proteins is not a viable option for mammalian photoreceptors. One immediate implication of this finding is that it calls for re-evaluating the outer segment targeting of R9AP, whose distribution in frog rods is very similar to that of untargeted proteins (Fig. 8C), whereas in mouse rods it is strikingly different (Fig. 8F). One possibility is that R9AP targeting is species-specific; another is that we previously overlooked specific R9AP targeting in frog rods due to the striking similarity between targeted and default distribution of R9AP in this case. Experiments exploring these possibilities are being actively pursued in our laboratory.
4.4. Outer segment transport of other resident membrane proteins
In contrast to rhodopsin, surprisingly little is known about outer segment targeting of other transmembrane proteins residing specifically in this compartment. It is not even clear whether delivery of other proteins is using components of the rhodopsin trafficking pathway, or several unique pathways coexist. Apart from the opsins, the only photoreceptor-specific protein containing the VXPX targeting motif is retinol dehydrogenase (prRDH or RDH8). This motif has been shown to be required for the outer segment delivery of this enzyme (Luo et al., 2004).
Peripherin is the only outer segment protein that was clearly demonstrated to contain a targeting signal other than VXPX (Tam et al., 2004). Transgenic expression of a reporter construct fused to multiple fragments of peripherin’s C-terminus revealed a 20 amino acid sequence with outer segment targeting capability. Interestingly, this targeting sequence overlapped with an amphipathic helix demonstrated to function as a fusogenic peptide in vitro and proposed to participate in disc rim formation (Boesze-Battaglia and Goldberg, 2002; Boesze-Battaglia et al., 1998; Edrington et al., 2007). A recent study from our laboratory has further refined the original 20 amino acid peripherin targeting sequence down to 10 residues not overlapping with the fusogenic sequence. Furthermore, a single valine (position 332 in most vertebrate peripherin sequences) was shown to be critical for rod outer segment targeting of peripherin in both frogs and mice (Salinas et al., 2013).
It is hard to overlook that both peripherin and rhodopsin contain a valine residue critical for their targeting. The difference is that rhodopsin targeting also relies on a second indispensable residue, a proline within the VXPX sequence. The significance of both proteins containing a critical valine is currently unclear and awaits further studies of accessory proteins sorting peripherin into post-Golgi transport vesicles headed to the outer segment. One of the first studies to examine whether peripherin and rhodopsin are delivered in the same vesicles was performed in degenerating photoreceptors of detached cat retinas (Fariss et al., 1997). The authors showed that peripherin accumulates in intracellular vesicles while rhodopsin accumulates in the plasma membrane. Although results obtained with degenerating photoreceptors are not always easy to interpret, this finding may be viewed as evidence that under normal conditions peripherin and rhodopsin utilize separate transport pathways. Other experiments showed that peripherin was reliably localized into the rudimentary outer segment stumps of the rhodopsin knockout mouse (Lee et al., 2006), thus establishing that peripherin can be delivered independently of rhodopsin. However, this important observation is insufficient to distinguish whether peripherin is travelling in the same vesicles as rhodopsin under normal conditions. Ongoing studies in our laboratory are aimed at identifying proteins that recognize peripherin’s targeting sequence and ensure its delivery to the outer segment.
The difficulty in identifying outer segment targeting signals was highlighted by a recent attempt to find such a signal in guanylate cyclase (Karan et al., 2011). The authors concluded that guanylate cyclase targeting could be mediated by either multiple signals present in its C-terminus, or by co-transport with another protein. An example of how multi-protein complex formation may dictate outer segment targeting was obtained in our recent study (Gospe et al., 2011) of the RGS9-Gβ5 GTPase activating complex for transducin, which is tethered on the surface of disc membranes by the aforementioned R9AP protein. We have found that RGS9-Gβ5 contains an endogenous targeting signal completely excluding it from the outer segment, yet RGS9-Gβ5 association with R9AP overrides this signal and assures the complex delivery to the outer segment.
Another approach to identifying outer segment targeting signals is to look for parallel patterns with protein targeting to other cilia types (primary, sensory and motile). This is how the ASAP1-interacting FR signal was first noted in rhodopsin (Corbit et al., 2005). Overall, this approach was more successful when applied in the reciprocal direction: the VXPX targeting sequence was found in a few ciliary proteins, such as polycystin-1, polycystin-2 and olfactory cyclic nucleotide gated channel, CNGβ1b, and shown to be critical for their ciliary targeting (Geng et al., 2006; Jenkins et al., 2006; Ward et al., 2011). Another targeting sequence that is both necessary and sufficient for ciliary localization was identified within the 18 C-terminal residues of fibrocystin (Follit et al., 2010), a large single-pass transmembrane protein localized to cilia and centrosomes (Ward et al., 2003). However, none of the outer segment-specific proteins contain identifiable homology to this fibrocystin sequence.
Several ciliary GPCR receptors (e.g. somatostatin receptor 3 and serotonin receptor 6) have a targeting signal encoded in their intracellular i3 loop, with the consensus sequence AX[S/A]XQ (Berbari et al., 2008). This sequence is sufficient to redirect non-ciliary receptors, such as somatostatin receptor 5 and serotonin receptor 7, to the primary cilium. In contrast, mutating the i3 loop did not prevent localization of ciliary receptors to the primary cilia, suggesting that additional targeting information is encoded elsewhere (Berbari et al., 2008). Although the i3 loop consensus sequence is present in rhodopsin and all three cone opsins, whether it plays a role in outer segment targeting has yet to be validated.
4.5. IFT transport
Axonemal precursors are concentrated near the basal body and must be delivered to the distal end of the cilium for assembly. This task is performed by the intraflagellar transport machinery (IFT), the mechanism responsible for building and maintaining all ciliary organelles. IFT was discovered by Rosenbaum and colleagues using video-enhanced microscopy of Chlamydomonas flagella, which revealed large particles moving rapidly up and down the flagella length (Kozminski et al., 1993). IFT particles are large protein complexes carried by kinesin-2 and cytoplasmic dynein-1b/-2 motors (Cole et al., 1998; Kozminski et al., 1995; Pazour et al., 1999; Piperno and Mead, 1997; Porter et al., 1999). The IFT particle is composed of at least 20 proteins, named according to their masses, arranged in two sub-complexes functioning as adaptors between motors and ciliary cargo (reviewed in (Taschner et al., 2012)). The core of the IFT-A particle consists of six proteins and functions in the retrograde aspect of IFT. The IFT-B particle contains 14 proteins and is required for the anterograde aspect of IFT. Both particles tend to be concentrated near the basal body (Baker et al., 2003; Pazour et al., 2002). The cycle of intraflagellar transport can be divided into four phases: 1) cargo assembly onto IFT particles near the basal body; 2) anterograde transport to the tip of the cilia mediated by kinesin; 3) cargo exchange and IFT-associated motor switching in the ciliary tip; 4) retrograde transport back to the basal body mediated by dynein.
The first indication that IFT takes place in photoreceptors was obtained upon the conditional deletion of Kif3A, a subunit of the heterotrimeric kinesin-II. This knockout resulted in opsin mislocalization, disorganization of outer segments and, eventually, cell death (Marszalek et al., 2000). Similar results have been obtained by disrupting kinesin-II function in mouse photoreceptors using different Cre drivers, although the timing and expression level of the Cre recombinase affected the severity of the phenotype (Avasthi et al., 2009; Jimeno et al., 2006). Mutations in Kif3A or Kif3B that prevent proper outer segment formation are also seen in zebrafish (Insinna et al., 2009; Zhao and Malicki, 2011). A second motor engaged in anterograde IFT is another kinesin, Kif17 (Evans et al., 2006; Snow et al., 2004). In zebrafish photoreceptors, Kif17 co-localizes with IFT proteins along the axoneme of the connecting cilium and its knockdown prevents outer segment development (Insinna et al., 2008). Subunits of both kinesin-II and Kif17 co-immunoprecipitate with IFT proteins in retinal extracts, further supporting their role in outer segment morphogenesis (Baker et al., 2003; Insinna et al., 2008). In sensory cilia, Kif17 is the predominant kinesin motor identified in the distal portion of the cilium where the axoneme converts from doublets to singlets (Fig. 4) (Evans et al., 2006; Insinna et al., 2008; Snow et al., 2004). Based on this pattern, it has been hypothesized that kinesin-II carries IFT particles along the microtubule doublets, whereas Kif17 takes over as they turn into singlets, although the exact interplay between these motors requires further elucidation (Malicki and Besharse, 2012).
In mammals, the canonical IFT retrograde motor, cytoplasmic dynein-2, was demonstrated to be expressed in tissues containing ciliated cells (Mikami et al., 2002). The same study localized cytoplasmic dynein-2 to the connecting cilium of photoreceptors. Knockdown of the cytoplasmic dynein-2 complex in zebrafish demonstrated that it is essential for outer segment maintenance (Krock et al., 2009). The other major form of dynein is cytoplasmic dynein-1 which may be involved in IFT as well. In zebrafish, the dynein-1 heavy chain was localized to the outer segment axoneme and its mutation led to multiple photoreceptor defects, including abnormal outer segments (Insinna et al., 2010). A third dynein heavy chain, dynein-3, was recently reported in the sensory cilia of C. elegans, but like dynein-1, its function and interactions with the IFT complex remains to be investigated (Hao et al., 2011a).
A critical role for the IFT particle in maintaining the outer segment was revealed in a mouse model of polycystic kidney disease, Tg737orpk, which contains a mutation in the IFT-B protein, IFT88. Along with drastically shortened renal cilia (Pazour et al., 2000), these mice also displayed abnormal development of photoreceptor outer segments leading to opsin mislocalization and cell death (Pazour et al., 2002). The ability of a single mutation in IFT88 to cause both renal and retinal diseases led to the current awareness that disruptions in IFT and associated proteins cause syndromic ciliopathies (reviewed in (Davis and Katsanis, 2012; Waters and Beales, 2011)). Although rare, these mutations can be devastating. For example, a mutation in the IFT-A particle protein, IFT144 (also known as WDR19) is associated with retinal, renal, skeletal and other abnormalities known as Jeune and Sensenbrenner syndromes (Bredrup et al., 2011). A series of subsequent studies used the power of zebrafish genetics to demonstrate that mutations in multiple IFT components can all prevent proper outer segment formation (Bahadori et al., 2003; Davis et al., 2011; Hudak et al., 2010; Krock and Perkins, 2008; Omori et al., 2008; Sukumaran and Perkins, 2009; Tsujikawa and Malicki, 2004).
Although the IFT core machinery is highly conserved across cell types and organisms, its cargo varies depending on the specialized function of each cilium. One set of cargo universal to all cilia are the IFT components themselves. After all, while the IFT complex is moving to the tip of the cilium utilizing the IFT-B particle and driven by kinesin, the dynein motor and the IFT-A particle serve as cargo. The opposite occurs during retrograde transport. Given that outer segments are constantly renewed, the retrograde aspects of IFT in photoreceptors may be less critical than in other cilia, except for IFT particle/kinesin recovery. What may be relevant to photoreceptor renewal is the delivery of another type of IFT cargo – the structural components of the axoneme (Hao et al., 2011b; Marshall and Rosenbaum, 2001; Qin et al., 2004). While outer segment membranes are added at the base, microtubules can be elongated only at the tips. Therefore, species whose axoneme extends all the way to the outer segment tip may require reliable tubulin delivery to the distal outer segment, following each phagocytic event. The only axonemal component identified in photoreceptors so far is tubulin, which is a presumed IFT cargo in these cells. The same argument applies to the initial outer segment/axoneme elongation during photoreceptor morphogenesis.
The most often-cited IFT cargo in photoreceptors is rhodopsin. The first line of supporting evidence came from mislocalization of rhodopsin in mouse models with disrupted IFT subunits or motors (Marszalek et al., 2000; Pazour et al., 2002). However, rhodopsin mislocalization in these mice occurs when outer segment defects become apparent, so the causality between the two phenomena is ambiguous. Recently, Pazour and colleagues designed an inducible knockout mouse disrupting another IFT protein, IFT20, which interacts with rhodopsin directly (Keady et al., 2011). The loss of IFT20 caused rhodopsin to accumulate in the Golgi within 48 hours of induction, suggesting that IFT20 may play a direct role in outer segment trafficking of rhodopsin. However, IFT20 is the only IFT protein located at the Golgi complex and at the base of the cilium, but not within the cilium (Sedmak and Wolfrum, 2010). This localization pattern suggests that IFT20 may function in both trans-Golgi trafficking and vesicular docking events for rhodopsin transport vesicles, but not in carrying IFT cargo toward the tip of the outer segment axoneme (Follit et al., 2006; Keady et al., 2011).
Recent live-cell imaging showed that rhodopsin transport into primary cilium of cultured cells is dependent on Kif3a and occurs on the same time scale as IFT88 transport into the same cilium (Trivedi et al., 2012). Additionally, live FRAP experiments in mouse rods expressing a GFP-tagged rhodopsin demonstrated that recovery of the GFP-rhodopsin signal after photobleaching of the connecting cilium is strongly dependent on Kif3a.
Another outer segment resident protein proposed to be carried by IFT is guanylate cyclase 1. Initial evidence came from its mislocalization to the inner segment in mice deficient for IFT88 (Insinna and Besharse, 2008). Follow up studies showed that IFT88 co-immunoprecipiates with guanylate cyclase from bovine retinal extracts and that this interaction was formed or stabilized by the chaperone proteins, MRJ and HSC70 (Bhowmick et al., 2009). The same may apply to other proteins transported in the same vesicle as guanylate cyclase.
These results provide a strong support for the role of IFT in rhodopsin and cyclase trafficking. However, there is a major difference from the traditionally portrayed IFT in which the cargo is carried along the entire length of the axoneme and delivered at the tip of the cilium. In contrast, both of these proteins are incorporated into nascent photoreceptor discs. Therefore, IFT is only needed to facilitate their transport from the basal body to the outer segment base. This may further suggest that the anterograde movement of IFT components beyond the outer segment base is required for allowing the IFT particle to reach the axoneme tip, where the kinesin-to-dynein motor switch takes place to return the IFT particle and kinesin back to the basal body.
4.6. Outer segment delivery of lipidated proteins
Many signaling proteins located in photoreceptor outer segments are tightly associated with disc membranes via lipid modifications (reviewed in (Burns and Arshavsky, 2005; Wensel, 2008)). This mode of attachment allows for rapid lateral diffusion of proteins along the membrane surface, which assures frequent and efficient encounters of interacting proteins leading to a rapid onset of light responses.
The following outer segment-specific proteins contain lipid modifications. The rod-specific cGMP phosphodiesterase (PDE) is farnesylated and geranylgeranylated on its α- and β-subunits, respectively (Anant et al., 1992), whereas the cone-specific PDE is likely geranylgeranylated on both of its α-subunits (Li et al., 1990). Transducin α-subunit (Gαt) is acylated by a heterogeneous group of fatty acids in rods (Kokame et al., 1992; Lobanova et al., 2007; Neubert et al., 1992) and homogeneously myristoylated in cones (Rosenzweig et al., 2007). Heterogeneous protein acylation in rods is also documented for recoverin (Dizhoor et al., 1992) and is likely to apply to guanylate cyclase activating proteins, GCAPs (Peshenko et al., 2012). This rod-specific pattern of protein acylation is likely to reflect a general property of N-myrisoyl transferase in these cells, which acylates protein substrates with both C14 and C12 fatty acyl groups. The γ-subunits of transducin in rods and cones (Gγ1 and Gγ8, respectively) are farnesylated (Chen et al., 2003; Fukada et al., 1990). Also farnesylated is the rod-specific isoform of opsin kinase, GRK1 (Anant and Fung, 1992; Inglese et al., 1992). In contrast, the cone-specific isoform of opsin kinase, GRK7, is geranylgeranylated (Hisatomi et al., 1998). Finally, the retinol dehydrogenase RDH8 is presumably palmitoylated (Luo et al., 2004).
While lipid modifications allow retention of signaling proteins on the disc membrane, they do not provide a means for specific protein targeting to the outer segment. Two types of mechanisms responsible for this targeting are discussed in the current literature. First, it is possible that proteins undergoing lipid modifications become attached to the ER/Golgi where they are sorted into transport vesicles destined for outer segment delivery (Section 4.2). Since only one lipidated protein, photoreceptor retinol dehydrogenase, contains the VXPX targeting motif (Luo et al., 2004), the majority of these proteins would need to be somehow packaged into the transport vesicles containing outer segment-targeted transmembrane proteins. For example, a double knockout of both guanylate cyclase isoforms, GC1 and GC2, resulted in reduced protein levels and impaired outer segment delivery for multiple lipidated proteins: GCAP1, transducin, PDE and rhodopsin kinase in cones; GCAP1, GCAP2 and PDE in rods (Baehr et al., 2007; Karan et al., 2008a; Karan et al., 2008b). The authors hypothesized that these lipidated proteins are normally delivered to the outer segment via association with guanylate cyclase-bearing transport vesicles.
Another mechanism for the outer segment delivery of lipidated proteins involves lipid-binding proteins acting as “trafficking chaperones”. These chaperones bind lipidated protein cargo in the inner segment and enable their diffusion through the photoreceptor cytoplasm by hiding the lipid moiety within an intramolecular cavity. The cargo proteins are eventually unloaded under a tight control by small GTPases of the Arf-like (Arl) family. Protein release is thought to take place either directly at the base of connecting cilium (e.g. (Wright et al., 2011)), or at the transport vesicles heading to this destination (e.g. (Karan et al., 2010; Zhang et al., 2012)).
Currently, two trafficking chaperones, PrBP/δ and UNC119, have been identified in photoreceptors. PrBP/δ, also known as the δ-subunit of PDE (Gillespie et al., 1989), is a prenyl-binding protein initially shown to solubilize membrane-bound rod PDE (Florio et al., 1996) and subsequently demonstrated to assist PDE delivery to rod outer segments (Norton et al., 2005; Zhang et al., 2007). PrBP/δ knockout resulted in reduced outer segment transport of PDE, along with another farnesylated protein, rhodopsin kinase (Zhang et al., 2007). Biochemical studies showed that PrBP/δ specifically binds farnesylated rather than geranylgeranylated proteins (Zhang et al., 2004). However, the outer segment delivery of transducin βγ-subunit complex (Gβ1γ1), farnesylated on Gγ1, was unaffected by the PrBP/δ knockout, indicating that additional mechanisms are involved. In fact, Gβ1γ1 has its own trafficking chaperone, phosducin, a protein concealing the farnesyl group into an intramolecular cleft, thereby increasing Gβ1γ1 solubility (Gaudet et al., 1996; Yoshida et al., 1994). Phosducin knockout resulted in incomplete delivery of Gβ1γ1 to rod outer segments (Sokolov et al., 2004) (see further discussion of the role of phosducin in transducin trafficking in Section 6.3). PrBP/δ also interacts with several ubiquitously expressed Ras, Rab and Rho GTPases (Hanzal-Bayer et al., 2002; Marzesco et al., 1998; Nancy et al., 2002), suggesting that PrBP/δ is a multifunctional protein facilitating membrane extraction, cytosolic sequestration and intracellular transport of many prenylated proteins.
The other trafficking chaperone identified in photoreceptors is UNC119 (Higashide et al., 1996), which binds to acylated proteins and assists their delivery to outer segments, sensory cilia and primary cilia (Wright et al., 2011; Zhang et al., 2011). The major binding partner of UNC119 in photoreceptors is Gαt (Gopalakrishna et al., 2011; Zhang et al., 2011). UNC119 knockout resulted in a partial Gαt mislocalization from outer segments of dark-adapted rods, emphasizing its role in Gαt outer segment delivery (Zhang et al., 2011). In addition, Gαt knockout mice have reduced levels of UNC119 protein even though UNC119 transcript remains unchanged, suggesting that UNC119 is protected by it interactions with Gαt (Sinha et al., 2013). We will further discuss the function of UNC119 in Section 6.3 in the context of light-driven transducin translocation in rods.
Both PrBP/δ and UNC119 contain an immunoglobulin-like fold that creates a hydrophobic pocket to accommodate the lipid moiety of their associated cargo (Hanzal-Bayer et al., 2002; Ismail et al., 2011; Wright et al., 2011; Zhang et al., 2011). While the mechanistic details of lipidated cargo association with PrBP/δ and UNC119 are still missing, the mechanism of cargo release is now well-understood. Cargo release takes place upon PrBP/δ and UNC119 interaction with the GTP-bound forms of small GTPases Arl2 or Arl3 (Hanzal-Bayer et al., 2002; Linari et al., 1999; Veltel et al., 2008b; Wright et al., 2011). Both Arl3 and Arl2 can bind and release cargo from PrBP/δ (Ismail et al., 2011); however, only Arl3 binding can release cargo from UNC119 (Ismail et al., 2012). Arl3 knockout resulted in a typical ciliopathy, including photoreceptor degeneration (Schrick et al., 2006). Despite the close homology between PrBP/δ and UNC119, these proteins utilize opposite strategies for cargo release. Arl2 or Arl3 binding to PrBP/δ allosterically displaces its farnyslated cargo by reducing the size of the hydrophobic pocket, essentially squeezing the lipid moiety from PrBP/δ (Ismail et al., 2011). In contrast, Arl3 binding to UNC119 expands the hydrophobic pocket letting go of its myristoylated cargo (Ismail et al., 2012).
The idea that PrBP/δ and UNC119 release their cargo at the base of connecting cilium implies that the guanyl exchange factor (GEF) for Arl3/Arl2 has to reside either right there or along the path of transport vesicles ultimately fusing at that location. However, the molecular identity of this GEF in photoreceptors or elsewhere remains unknown. In contrast, the GTPase activating protein, at least for Arl3, was identified as RP2 (Veltel et al., 2008a), a lipidated protein frequently mutated in X-linked retinitis pigmentosa patients (Avidor-Reiss et al., 2004; Schwahn et al., 1998). RP2 is enriched at the inner/outer segment junction, although is also present at multiple additional cellular locations (Evans et al., 2010; Holopainen et al., 2010).
In some cases, appreciable fractions of acylated phototransduction proteins are not entirely confined to the outer segment. For example, rod outer segments contain only a quarter of total cellular GCAP2 (Dizhoor et al., 1995; Otto-Bruc et al., 1997; Strissel et al., 2005), although the functional significance of this pattern is unknown. An even more intriguing example relates to localization of the Ca2+-binding protein, recoverin, which regulates the activity of rhodopsin kinase in the outer segment (Chen et al., 1995; Chen et al., 2012; Kawamura, 1993; Klenchin et al., 1995; Makino et al., 2004). Another function of recoverin is to modulate photoreceptor synaptic output via a yet-to-be-identified mechanism (Sampath et al., 2005). In dark-adapted rods, recoverin is present in all subcellular compartments, whereas sustained illumination causes its redistribution from outer segments to synaptic terminals with little change elsewhere (Strissel et al., 2005). The functional consequences of recoverin absence from light-adapted outer segments have not yet been addressed experimentally. However, it is logical to assume that it may increase the amount of rhodopsin kinase available to phosphorylate rhodopsin, thereby contributing to photoreceptor light adaptation. The role of recoverin translocation to the synapse is even less clear.
This light-dependent redistribution of recoverin is likely to be driven by the “myristoyl switch” mechanism. The Ca2+-bound form of recoverin has high membrane affinity, while the Ca2+-free recoverin is soluble due to an insertion of its lipid moiety into an intramolecular cleft (Zozulya and Stryer, 1992). Therefore, when Ca2+ in the outer segment decreases upon illumination, recoverin is expected to dissociate from the disc membranes, diffuse throughout the rod cytoplasm and eventually re-associate with membranes in other cellular compartments maintaining higher Ca2+ concentrations.
4.7. Protein targeting to other photoreceptor compartments
Protein transport to photoreceptor compartments other than the outer segment is poorly understood. However, some insights have been gained by studies of proteins residing in the plasma membrane surrounding the cell body and synaptic proteins, particularly in regards to the identity of targeting signals encoded within their sequences.
Transgenic Xenopus studies performed in our laboratory revealed that two plasma membrane proteins, syntaxin 3 and the facilitative glucose transporter Glut1, contain targeting information used to actively exclude these proteins from the rod outer segment (Baker et al., 2008; Gospe et al., 2010). This information is encoded within the SNARE homology domain of syntaxin 3 and the cytosolic C-terminal tail of Glut1. Transgenic expression of either protein lacking the respective targeting sequence resulted in their mislocalization to the outer segment, matching the default distribution pattern for untargeted proteins. These two targeting regions have no evident structural similarities suggesting that multiple mechanisms may participate in protein targeting to the non-outer segment part of the plasma membrane. What is similar between these two targeting regions is that they participate in protein-protein interactions. This suggests that they may either directly bind to components of vesicular trafficking machinery (such as Rab GTPases or coat proteins) or interact with these components indirectly via additional binding partners encoding the actual targeting signal. This dilemma illustrates our limited knowledge of the exact mechanisms that dictate inner segment plasma membrane targeting.
The knowledge of protein targeting and trafficking to photoreceptor synaptic terminals is also very limited. As described in Section 2.2, photoreceptors contain ribbon synapses, which are large electron dense plates that function to organize synaptic vesicles for precise and sustained release. The ribbon is made of RIBEYE polymers (Magupalli et al., 2008; Schmitz et al., 2000) and many Cytoskeletal matrix assembled at the Active Zone (CAZ) proteins. These proteins organize into two distinct compartments: the ribbon including RIBEYE, CtBP1, Kif3A, Piccolo and RIM1 proteins and the active zone including RIM2, Munc 13-1, CAST, and the L-type voltage gated Ca2+ channels (tom Dieck and Brandstatter, 2006). Bassoon, a multi-domain scaffolding protein, localizes to the border between the two compartments (Brandstatter et al., 1999; tom Dieck et al., 2005) and is believed to play a critical role in tethering the ribbon to the active zone (Dick et al., 2003).
The ribbon is a dynamic structure that assembles and disassembles in response to changing illumination (Regus-Leidig et al., 2009; Spiwoks-Becker et al., 2004). Ribbons are constructed by assembling precursor spheres containing protein complexes of RIBEYE, Bassoon and Piccolo (Regus-Leidig et al., 2009). These spheres are non-membranous ~130 nm electron dense particles surrounded by vesicles and believed to serve as the transport units for ribbon proteins. This transport mechanism is distinct from the mechanisms described for conventional synapses where proteins including Piccolo, Bassoon, syntaxin 1, SNAP-25 and N-cadherin are transported in ~80 nm dense core vesicles (Shapira et al., 2003; Zhai et al., 2001). The assembly site of the ribbon precursor spheres and molecular motors responsible for their transport remain unknown. The active zone components are likely to be transported separately from the ribbons, however, the underlying mechanism remains unknown as well.
Interestingly, the kinesin-2 subunit, KIF3A, essential for IFT is also a prominent component of the ribbon (Muresan et al., 1999). Expression of a dominant negative KIF3B construct in zebrafish cones resulted in their failure to form ribbons (Insinna et al., 2009). However, a conditional KIF3A photoreceptor knockout in mice disrupted IFT (causing shortening of outer segments and mislocalization of opsin) without affecting the localization of synaptic vesicle- or ribbon-specific proteins (Avasthi et al., 2009; Marszalek et al., 2000). Clearly, understanding whether KIF3A plays any transport role in the ribbon synapse bears further investigation.
Another striking feature of the photoreceptor synapse is the massive accumulation of pre-synaptic vesicles. Studies performed at conventional synapses in the central nervous system demonstrated that synaptic vesicle components organize into heterogeneous pools of transport vesicles in the cell soma before being carried down the axon by molecular motors. Once in the synapse, these precursor vesicles must be sorted, likely in an endosomal compartment, to create mature synaptic vesicles (reviewed in (Bonanomi et al., 2006; Santos et al., 2009)). The motors required for the transport of synaptic vesicle precursors are principally kinesin-3, with kinesin-1 and myosin-V also playing a role (Hirokawa et al., 2010). Both of the kinesin-3 subunits, KIF1A and KIF1Bβ, use a protein adaptor, DENN/MADD, to interact with the synaptic vesicle marker, Rab3A (Niwa et al., 2008). In photoreceptors, Rab3A activity is also necessary for the delivery of vesicles to the ribbon synapse for release (Tian et al., 2012), though its exact involvement in vesicle transport from the Golgi to the synapse is not known.
5. Outer segment accessibility for soluble molecules
The large size of photoreceptor outer segments makes these cells a particularly instructive model to investigate the diffusion of soluble proteins between the ciliary lumen and rest of the cellular cytoplasm. Studies using photobleaching of transgenically expressed GFP in mouse rods, or photoactivation of a GFP analog in frog rods, established that soluble proteins can rapidly equilibrate between the cytoplasm portions of the outer and inner segments through the connecting cilium. GFP equilibrated between the rod inner and outer segments with a half-time of ~30 sec in mice (Nair et al., 2005a) and ~7 min in frogs (Calvert et al., 2010). Both values are reasonably close to the theoretical predictions accounting for the overall anatomy of each cell and diffusional hindrance in the respective subcellular compartments ((Calvert et al., 2010); see also (Calvert et al., 2006) for a simplified version of this analysis).
These observations are consistent with all cytoskeletal elements inside and around the connecting cilium being at least 25 nm apart (e.g. the space between microtubule doublets, or Y-links; (Rohlich, 1975)), which is not dense enough to form a barrier for diffusion of soluble proteins. Furthermore, these experiments indicate that the base of the outer segment does not contain a barrier for soluble protein diffusion that would not be observed in electron micrographs, as suggested in early studies (e.g. (Wolfrum et al., 2002)).
A remarkable phenomenon related to the ability of soluble proteins to populate the outer segment cytoplasmic space was revealed in the recent study by Calvert and colleagues (Najafi et al., 2012). They expressed monomers, dimers and trimers of GFP in Xenopus rods and found that the relative outer segment abundance of these proteins was inversely proportional to their size. For example, the outer segment fraction of the GFP trimer was ~3-times smaller than the corresponding fraction of the GFP monomer. They next used GFP photoactivation to show that, despite this difference in the outer segment fractions of GFP isoforms, the rates at which they diffuse into the outer segment are statistically indistinguishable. Therefore, the difference in the outer segment abundance for these molecules is not explained by a size-dependent diffusion barrier at the outer segment base. Instead, it is explained by the phenomenon of steric volume exclusion, which postulates that the aqueous (i.e. cytoplasmic) volume available to a given molecule depends on the size and shape of this molecule and on the shape of the aqueous space.
Steric volume exclusion, well-known in physical chemistry (Giddings et al., 1968), has been previously overlooked in studies of outer segments and other cilia. This phenomenon is particularly prominent in outer segments because the cytoplasmic thickness between discs is comparable to the size of average proteins. We will discuss in Section 6.2 how steric volume exclusion can lead to significant protein disequilibrium between the inner and outer segments, using arrestin as an example. It is important to stress that steric volume exclusion is predicted to affect the intracellular distribution of soluble proteins not just in frogs, but in all species, including mammals. This is because accessibility of soluble proteins to the outer segment is determined primarily by the fine structure of thin intradiscal spaces, which are remarkably similar among all species, and not so much by the outer segment volume or diameter.
6. Light-dependent translocation of photoreceptor signaling proteins
An interesting phenomenon related to trafficking of soluble and lipidated proteins in photoreceptors is the massive light-induced translocation of several phototransduction proteins between the outer segment and the rest of the cell. In Section 4.6 we discussed light-induced redistribution of recoverin, however, most attention in the field has been devoted to light-induced redistribution of transducin and arrestin (Fig. 9). Given the significant number of detailed reviews on this topic (Arshavsky and Burns, 2012; Artemyev, 2008; Burns and Arshavsky, 2005; Calvert et al., 2006; Gurevich et al., 2011; Slepak and Hurley, 2008), we will briefly overview the functional aspects of this phenomenon and concentrate on the underlying trafficking mechanisms.
Fig. 9.
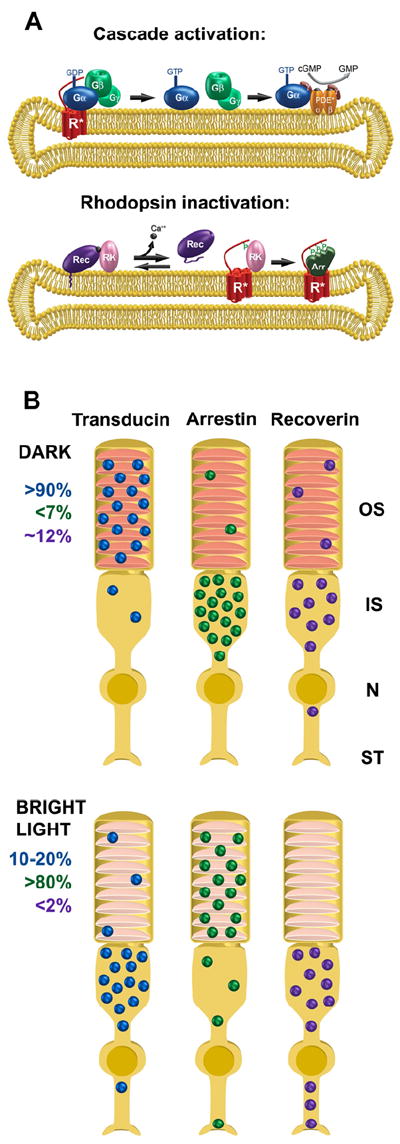
(A) The role of translocating proteins in visual signal transduction. The visual signal is initiated by the photoexcitation of rhodopsin (R*) which activates the heterotrimeric G protein transducin by catalyzing the GDP/GTP exchange on the α-subunit (Gα) followed by Gα dissociation from the βγ-subunit (Gβ and Gγ). Gα bound to GTP stimulates cGMP phosphodiesterase (PDE) leading to decline of cGMP and the onset of the photoresponse. These reactions are illustrated on the upper membrane disc. The reactions responsible for inactivation of R* are illustrated on the lower disc. They include R* phosphorylation by rhodopsin kinase (RK) followed by arrestin (Arr) binding. The RK activity is regulated by the Ca2+-binding protein recoverin (Rec), which binds to and inhibits RK at high Ca2+. The drawings are modified with permission from (Calvert et al., 2006). (B) Schematic illustration of transducin, arrestin and recoverin distribution in dark- and light-adapted rods. The numbers on the left, color-coded to the corresponding translocating proteins, represent the percentage of the proteins found in the outer segments. The subcellular rod compartments are abbreviated on the right: OS – outer segment; IS – inner segment; N – nucleus; ST – synaptic terminal.
6.1. Functional role of light-driven protein translocation
Light-driven protein translocation is viewed as an adaptive mechanism enabling photoreceptors to optimize their performance at different levels of ambient illumination. Let us first consider the case of transducin, which is confined to the outer segments of dark-adapted rods and translocates to other cellular compartments in bright light. Transducin is a G protein mediating visual signaling between photoexcited rhodopsin (R*) and the effector enzyme in the phototransduction cascade, PDE (Fig. 9A). During the course of a typical photoresponse, a single molecule of R* activates multiple transducin molecules, which amplifies the signal in this cascade. Because transducin activation rate is proportional to its outer segment concentration ((Heck and Hofmann, 2001; Leskov et al., 2000); reviewed in (Arshavsky et al., 2002)), the high outer segment content of transducin in the dark enables rods to produce highly amplified photoresponses, including those evoked by single photons.
Continuous bright light causes the majority of transducin in rods (but not cones) to translocate from the outer segment (Fig. 9B). This reduces transducin activation rate, as experimentally validated by demonstrating that the extent of transducin translocation correlates with a reduction in signal amplification in the phototransduction cascade (Sokolov et al., 2002). This mechanism may enable rods to produce useful light responses under background illumination saturating photoresponses of completely dark-adapted rods.
Another major translocating protein is arrestin, a protein contributing to the deactivation of the photoresponse by binding to phosphorylated R*, thereby terminating transducin activation (Fig. 9A). Light exposure triggers arrestin translocation in both rods and cones in the direction opposite to transducin (Fig. 9B). Dark-adapted outer segments contain only a small fraction of total cellular arrestin, whereas light-adapted outer segments accumulate nearly the entire cellular arrestin pool (Broekhuyse et al., 1985). Arrestin translocation may contribute to photoreceptor light-adaptation as well. The increase in the outer segment arrestin concentration may expedite photoresponse recovery by speeding the deactivation of R*. This increase may be also critical for replenishing the free outer segment arrestin depleted by binding to R*. While both hypothesis are plausible, neither has been tested in direct experiments.
Another, emerging theme in the field is that light-induced protein translocation may play a broader adaptive/neuroprotective role than merely regulating the sensitivity of phototransduction. For example, it has been proposed that transducin translocation in daytime allows rods to save energy when they contribute little to vision (Burns and Arshavsky, 2005; Chertov et al., 2011; Fain, 2006). Protein translocation may also be neuroprotective by reducing the overall activity of the phototransduction cascade, since excessive light signaling is viewed as a pro-apoptotic factor in photoreceptors, particularly in rodent models (Fain, 2006; Organisciak and Vaughan, 2010).
An additional, potentially neuroprotective mechanism is the light-driven translocation of Grb14, a relatively low-abundant signaling protein thought to prevent dephosphorylation of the insulin receptor by counteracting the activity of protein tyrosine phosphatase-1B (Bereziat et al., 2002). In dark-adapted rods, Grb14 is confined to the cell somas and synaptic terminals, while illumination causes its partial redistribution to outer segments (Rajala et al., 2009). The authors suggested that this process facilitates the activity of the insulin receptor in the outer segment, which in turn protects photoreceptor cells against stress-induced apoptosis ((Rajala and Anderson, 2010); see also (Punzo et al., 2009; Punzo et al., 2012)).
6.2. The mechanism of light-dependent arrestin translocation
While potential involvement of molecular motors in facilitating light-dependent translocation of transducin and arrestin was discussed in several earlier studies (e.g. (Peterson et al., 2005; Reidel et al., 2006)), the prevailing current opinion in the field is that both proteins move by diffusion (Arshavsky and Burns, 2012; Artemyev, 2008; Calvert et al., 2006; Gurevich et al., 2011; Kerov and Artemyev, 2011; Nair et al., 2005a; Rosenzweig et al., 2007; Slepak and Hurley, 2008). Direct experiments (Calvert et al., 2010; Nair et al., 2005a) and theoretical calculations (Calvert et al., 2010; Calvert et al., 2006) demonstrated that it takes no more than a few minutes for an average-sized soluble protein to equilibrate between the cytoplasmic spaces of outer segments and the rest of the cell by diffusion (see also Section 5). This is sufficiently rapid to explain the experimentally observed kinetics of arrestin and transducin translocation, which also occurs on the timescale of several minutes (Elias et al., 2004; Sokolov et al., 2002; Strissel et al., 2006). However, diffusion alone cannot explain the directionality of protein translocation because it results in equilibration of soluble proteins throughout the entire photoreceptor cytoplasm. Therefore, any protein translocation mechanism requires combining diffusion with a means of light-dependent retention of arrestin and transducin in individual subcellular compartments.
Understanding the mechanism of arrestin translocation requires first to consider a conundrum related to its distribution in dark adapted photoreceptors. Despite arrestin being a soluble protein predicted to be evenly distributed throughout the entire photoreceptor cytoplasm, dark-adapted rod outer segments contain a disproportionally smaller amount of arrestin than the amount of cytoplasm. This arrestin disequilibrium was first noted in early immunostaining studies (e.g. (Broekhuyse et al., 1985)) and subsequently quantified using several alternative techniques. Pugh and colleagues used transgenic Xenopus to demonstrate that rod outer segments contained ~3-fold larger cellular fraction of a cytoplasmic marker, soluble GFP, than the fraction of GFP-tagged arrestin (Peet et al., 2004). A study from our laboratory used semi-quantitative Western blotting to measure arrestin amounts in thin serial tangential sections of the photoreceptor layer from a mouse retina (Strissel et al., 2006). A conservative estimate from these measurements indicated that dark-adapted rod outer segments contain <7% of the total cellular arrestin. This amount is smaller than the predicted outer segment fraction of the photoreceptor cytoplasmic volume. An alternative approach to quantify arrestin in isolated osmotically-intact rod outer segments yielded a somewhat higher, but still low value of ~14% (Song et al., 2011). In our opinion, the latter number may be somewhat overestimated since mouse outer segments obtained by the technique used in that study tend to retain attached inner segment fragments (Gilliam et al., 2012).
One plausible explanation why outer segments contain less than predicted arrestin in the dark is that the majority of arrestin is associated with cellular structures located in the photoreceptor soma, for example with the tubulin cytoskeleton binding arrestin with low affinity (Hanson et al., 2007a; Nair et al., 2005a). Indeed, tubulin is strikingly more abundant in inner than outer segments (for quantitative analysis, see Figure 4 in (Song et al., 2007)). Other potential binding partners for arrestin are: enolase 1 (Smith et al., 2011), NSF (Huang et al., 2010)), or LGN (Kerov et al., 2005b; Nair et al., 2005b). Yet, none of these proteins is likely to be expressed in amounts sufficient to bind appreciable fractions of arrestin, which is among the most abundant proteins in rods, expressed at ~80% level of rhodopsin (Song et al., 2011; Strissel et al., 2006).
An alternative explanation for the predominantly inner segment localization of arrestin in dark-adapted rods is provided by the principle of steric volume exclusion described in Section 5. Calvert and colleagues (Najafi et al., 2012) compared the actual size and shape of an arrestin-GFP molecule with those of GFP monomer, dimer and trimer and concluded that when each molecule is completely equilibrated throughout the rod cytoplasm, outer segments contain 3-fold more GFP monomer than either arrestin-GFP or GFP trimer. Provided that arrestin GFP and GFP trimer have comparable molecular sizes, the principle of steric volume exclusion is sufficient to explain the arrestin disequilibrium described in (Peet et al., 2004) without involving any contribution from low-affinity arrestin binding sites in the inner segments.
Although the actual arrestin molecule is smaller than arrestin-GFP, arrestin forms dimers and tetramers at physiologically high concentrations (Hanson et al., 2007b; Imamoto et al., 2003; Schubert et al., 1999), so that the majority of arrestin in intact photoreceptors is predicted to exist as oligomers (Kim et al., 2011). Molecular details of arrestin oligomerization and its putative physiological role are described in a comprehensive review by Gurevich and colleagues (Gurevich et al., 2011). Steric volume exclusion predicts that outer segments should contain ~1.5-fold less arrestin dimers than monomers and ~3-fold less arrestin tetramers than monomers (Najafi et al., 2012). Therefore, a combination of steric volume exclusion with arrestin oligomerization may be sufficient to explain why photoreceptor outer segments contain a very small fraction of arrestin in the dark. Yet, the available experimental evidence is incomplete to reject a hypothesis that includes both mechanisms: steric volume exclusion and arrestin binding to inner segment structure elements.
We can move forward to the description of arrestin translocation mechanism. The most straightforward hypothesis is that arrestin translocation is driven entirely by its binding to large quantities of phosphorylated R* produced in light-exposed outer segments (Mangini et al., 1994). In this case, arrestin distribution between the outer segment and the rest of the cell would be determined by three factors: the relative cytoplasmic volumes of these compartments (taking steric volume exclusion into account), the amount of phosphorylated R* in the outer segment generated by light, and the rate of arrestin diffusion between these compartments. A modification of this hypothesis, incorporating the role of inner segment binding sites, was described by Slepak and colleagues as the ‘competitive double-sink’ model (Nair et al., 2005a). The low affinity of these sites makes their interaction with arrestin highly dynamic, so arrestin can dissociate from these sites and diffuse around the cell, ultimately being trapped by phosphorylated R* in the outer segments upon light exposure. According to this hypothesis, arrestin re-translocation from outer segments in the dark is also based on the diffusion-driven equilibrium: the thermal decay of R* causes arrestin release into photoreceptor cytoplasm, followed by re-association of a large fraction of arrestin with the low-affinity binding sites in inner segments.
Regardless of whether the arrestin disequilibrium in the dark is explained by steric volume exclusion or inner segment binding sites, the mechanism for arrestin translocation driven by its binding to R* postulates that the relationship between translocating arrestin and phosphorylated R* produced at any given light intensity is both linear and stoichiometric. However, experimental validation of this assumption by our laboratory yielded a surprising result (Strissel et al., 2006). Quantitative analysis of the amounts of R* produced and arrestin translocated to outer segments in rods exposed to light of variable intensity and duration yielded a highly non-linear relationship. About one third of total arrestin translocated into outer segments as light intensity reached a critical threshold producing just over 1,000 R*/rod/sec (Fig. 10). Under these conditions, the molar ratio between translocated arrestin and R* was ~30:1, a much higher value than could be achieved by a formation of their stoichiometric complex. Additional arrestin translocation was achieved at light intensities above this threshold, which was linear and stoichiometric in regards to R* (Fig. 10).
Fig.10.
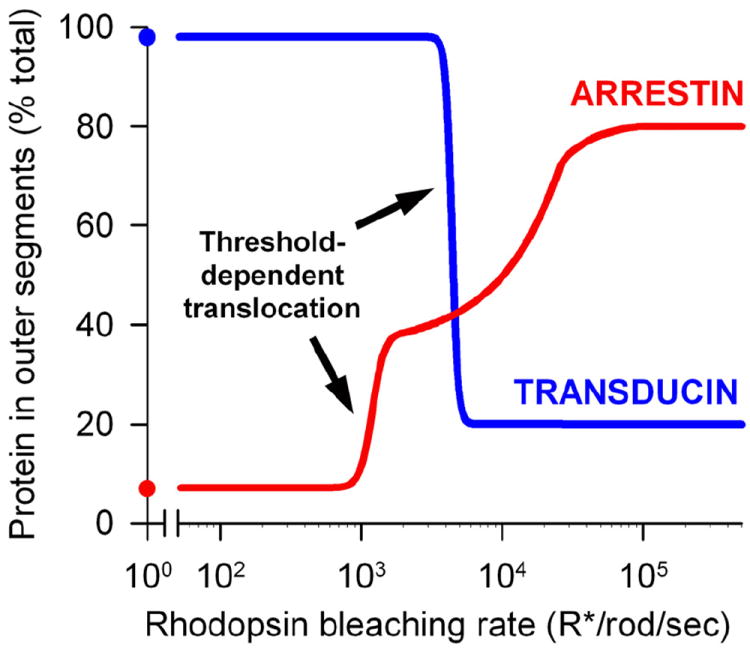
Light-dependency of transducin and arrestin translocation in mouse rods. The rod outer segment contents of arrestin and transducin in living anesthetized mice kept in the dark or subjected to 30-40 minutes of continuous illumination of indicated light intensity. Note that the value for the threshold light intensity triggering transducin translocation is updated from ~10,000 R*/rod/sec estimated in an earlier report (Sokolov et al., 2002) to ~4,000 R*/rod/sec directly measured in the subsequent studies (Lobanova et al., 2007; Lobanova et al., 2010). The data for arrestin are taken from (Strissel et al., 2006).
Further experiments indicated that arrestin translocation threshold is not determined by the absolute amount of R* produced, but rather by the activation level of the phototransduction cascade (Strissel et al., 2006). The threshold was reduced to light intensity producing under 400 R*/rod/sec in rods of R9AP knockout mice, whose photoresponses last longer than normally. In contrast, the threshold was completely eliminated in rods of transducin α-subunit knockout mice unable to produce photoresponses at all. Importantly, the additional, threshold-independent arrestin translocation in bright light was preserved in both mutant mice, consistent with R* phosphorylation and arrestin binding being unaffected by either knockout.
These results led us to propose an alternative ‘threshold, light-dependent release’ model of arrestin translocation (Strissel et al., 2006). This model suggests that the threshold-dependent phase of arrestin translocation is triggered by an intracellular signal downstream from phototransduction causing arrestin release from the inner segment binding sites, followed by its equilibration between the inner and outer segments by diffusion. The additional, threshold-independent translocation in this model is explained by the traditional R*-trapping mechanism. Another explanation for the threshold, incorporating the concept of steric volume exclusion developed after that study was published, is that a signal downstream from phototransduction causes arrestin oligomers’ disassembly leading to the outer segment population with smaller arrestin monomers.
In agreement with either version of this model, arrestin translocation in bright light was shown to be preserved in mice in which the ability of R* to bind arrestin was severely impaired – rhodopsin kinase knockout mice or mice expressing rhodopsin without phosphorylation sites (Mendez et al., 2003; Zhang et al., 2003). Notably, the amount of translocating arrestin in these animals appeared smaller than in wild-type mice, consistent with the threshold-dependent translocation component being preserved, while the R* trapping-dependent component being abolished.
Despite the lack of alternative experimental measurements of the stoichiometry between R* production and arrestin translocation, the methodology used in the (Strissel et al., 2006) study was questioned by supporters of the ‘competitive double-sink’ model (e.g. (Gurevich et al., 2011)). They pointed out that Western blot quantification used in that study may be compromised by limited signal linearity and protein detection thresholds. Generally speaking, these are credible concerns. However, they seem to overlook one critical experiment reported in (Strissel et al., 2006). As described above, the light intensity threshold for arrestin translocation in R9AP knockout mice was shifted to ~3-times lower light intensity than in wild type mice. Because producing less R* cannot increase the Western blot signal originating from stoichiometrically bound arrestin, this observation argues that arrestin translocation threshold is not explained by artifacts of the Western blot analysis. Little is known about the molecular mechanism defining this threshold. It has recently been hypothesized that phosphoinositide signaling downstream from rhodopsin and that both PLC and PKC are involved (Orisme et al., 2010), however details of these mechanisms remain to be elucidated.
From the functional perspective, the advantage of the threshold-dependent arrestin translocation mechanism is that it causes a preemptive increase of free arrestin concentration in outer segments. Such an increase is expected to be adaptive because outer segments acquire additional arrestin ready to quench large amounts of R* produced in bright light. In contrast, arrestin translocation driven by the depletion of its free outer segment pool by binding to R* represents a reactive mechanism predicting outer segments to have the highest amount of free arrestin in the dark. This amount could be rapidly depleted at the onset of bright illumination and minutes would be required for arrestin diffusion to replenish the exhausted free outer segment arrestin pool. The adaptive role of such a reactive mechanism is questionable since R* can be thermally inactivated at this timescale without binding arrestin.
6.3. The mechanism of light-dependent transducin translocation
The mechanism of transducin translocation is now understood in significant detail (Arshavsky and Burns, 2012; Artemyev, 2008; Calvert et al., 2006; Slepak and Hurley, 2008). It is ultimately based on the difference in membrane affinities of transducin’s αβγ heterotrimer and its individual subunits, Gαt and Gβ1γ1. As described in Section 4.6, each Gαt and Gβ1γ subunit contains a single lipid modification, a farnesyl residue on Gγ1 and an acyl residue on Gαt. The combined action of these lipid modifications enables tight association of transducin heterotrimer with the membranes of photoreceptor discs in the dark. However, transducin activation by R* causes a separation of the GTP-bound Gαt from Gβ1γ1 (Fig. 11A). Because each separated subunit contains only a single lipid moiety, their membrane affinities are much lower than the affinity of the heterotrimer. This allows Gαt and Gβ1γ1 to dissociate from the membrane and to distribute throughout the photoreceptor cytosol by diffusion.
Fig. 11.
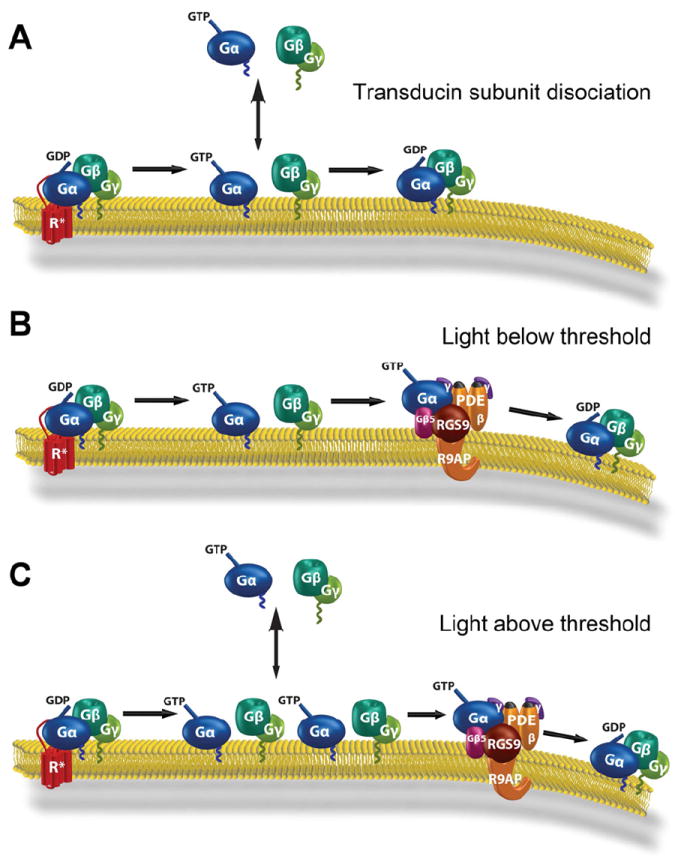
The mechanism of transducin translocation. (A) Transducin dissociation from membranes and translocation require the activation and separation of its functional subunits. (B) In light intensity below transducin translocation threshold produces less activated transducin than PDE. (C) In light intensity above transducin translocation threshold there is more activated transducin than PDE. This excess activated transducin, neither retained on the membrane by PDE nor rapidly deactivated by RGS9, dissociates from the membrane to the cytosol and ultimately diffuse out of the rod outer segment. Reproduced with permission from (Arshavsky and Burns, 2012).
On the surface, this model predicts a linear relationship between the amount of translocated transducin and R* produced. Yet, the actual light-dependency of transducin translocation is characterized by a distinct threshold reached at the light intensity generating ~4,000 R*/sec in mouse rods (Lobanova et al., 2007) (Fig. 10). The lack of translocation at light intensities below this threshold can be explained by a rapid association of activated Gαt with its membrane-bound effector, PDE. As long as the amount of activated Gαt does not exceed the amount of PDE on the same disc surface, no membrane dissociation of Gαt would occur. Additionally, PDE serves as a co-factor for the RGS9·Gβ5·R9AP GTPase activating complex (Skiba et al., 2000), which inactivates Gαt within a fraction of a second. This is followed by transducin subunit re-association, leaving little time for Gβ1γ1 to dissociate as well (Fig. 11B). However, Gαt activation in excess of PDE at light intensities producing over 4,000 R*/rod/sec (a condition possible since rods contain less PDE than transducin) generates a pool of transducin molecules that are free to dissociate from the disc membranes because they are neither retained by PDE nor rapidly deactivated by RGS9 (Fig. 11C).
Unfortunately, this hypothesis cannot be validated by knocking out PDE because photoreceptors lacking PDE undergo severe retinal degeneration (Bowes et al., 1990; Tsang et al., 1996). Therefore, major predictions of this hypothesis were supported by demonstrating that the threshold for transducin translocation is dependent on the lifetime of activated transducin and/or on its affinity for PDE. A transgenic replacement of the PDE γ-subunit with its W70A mutant, binding Gαt with low affinity and not facilitating Gαt deactivation by RGS9 (Slepak et al., 1995; Tsang et al., 1998), shifted the translocation threshold to dimmer light (Lobanova et al., 2007). A similar shift was observed in rods lacking RGS9 (Kerov et al., 2005a; Lobanova et al., 2007). In both mice, the lifetime of activated Gαt was longer than normally, which explains why the steady-state concentration of activated transducin saturating PDE was achieved at a dimmer light. Conversely, RGS9 overexpression in rods, shortening the lifetime of activated Gαt, shifted the threshold to a higher light intensity (Lobanova et al., 2007), just as the hypothesis predicts.
Direct measurements indicate that the rates at which Gαt and Gβ1γ1 translocate from rod outer segments (Sokolov et al., 2002) are comparable with the diffusion rate of soluble GFP (Calvert et al., 2010; Nair et al., 2005a), each occurring on the timescale of several minutes. This observation does not appear intuitive because lipidated Gαt and Gβ1γ1 have to bypass hundreds of tightly packed disc membranes as they diffuse from the outer segment. What prevents them from membrane re-association while in route? The answer involves several molecular adaptations affecting each transducin subunit.
An interesting property of Gαt is that its lipidated N-terminus is surrounded by several negatively charged amino acid residues (Kosloff et al., 2008). Such an arrangement imposes an electrostatic repulsion between Gαt and the membrane counteracting the attraction provided by the acyl group, which ultimately reduces the membrane affinity of Gαt. This electrostatic repulsion could accelerate the detachment of Gαt from the membrane and minimize its subsequent interactions with discs upon diffusion. Once Gαt dissociates from the membrane, its solubility is thought to be further increased by an insertion of the acylated N-terminus into an intramolecular cavity (Preininger et al., 2012).
Another molecular adaptation facilitating the light-dependent translocation of Gαt is the nature of its acyl group. Unlike the majority of N-terminally acylated proteins, Gαt is modified predominantly by the C12 or C14:2 lipids (Section 4.6), which have lower membrane affinities than the myristoyl group, C14 (Peitzsch and McLaughlin, 1993). Experiments in rats demonstrated that the extent of Gαt translocation is inversely proportional to the hydrophobicity of its acyl modification (Lobanova et al., 2007). While most of myristoylated Gαt remained in rod outer segments, the Gαt species modified with C12 and C14:2 moieties translocated with high efficiency. In contrast, the Gαt mutant containing an additional N-terminal lipid modification barely translocated at all (Kerov and Artemyev, 2011).
Unlike Gαt, electrostatic properties of Gβ1γ1 enhance its membrane binding, which is expected to impede both Gβ1γ1 membrane release and its subsequent diffusion. This electrostatic attraction is somewhat compensated by Gγ1 modification with a farnesyl isoprenoid residue (Section 4.6), which is shorter and less lipophilic than the more prominent geranylgeranyl group. The significance of Gγ1 farnesylation for Gβ1γ1 translocation was revealed by demonstrating that a point mutation causing Gγ1 to become geranylgeranylated instead leads to a major slowdown in Gβ1γ1 translocation (Kassai et al., 2005). Another mechanism enhancing Gβ1γ1 solubility is the binding to phosducin, a major photoreceptor-specific protein interacting with multiple types of G protein βγ-subunits (Beetz and Hein, 2011). Gβ1γ1 binding to phosducin results in the insertion of the farnesyl moiety into a cleft formed between these two molecules (Loew et al., 1998), which makes the entire complex highly soluble (Yoshida et al., 1994). Experimental validation of phosducin’s role in transducin translocation was obtained by demonstrating that transducin translocation is suppressed in rods of phosducin knockout mice (Sokolov et al., 2004). Taken together, these multiple molecular adaptations counteract the unfavorable electrostatic properties of Gβ1γ1. However, the rate of Gβ1γ1 translocation in saturating light is still ~2.5-times slower than the translocation of Gαt (Sokolov et al., 2002).
The mechanism underlying transducin return to rod outer segments in the dark remains less understood, though recent studies argue that it is also achieved through diffusion assisted by protein interactions promoting the solubility of transducin subunits. While not directly demonstrated, it is commonly assumed that transducin subunits relocated to the inner segment reform highly insoluble heterotrimers. This further assumes that transducin return to the outer segment relies on specific subunit separation mechanisms.
A protein recently shown to assist the return of Gαt to the outer segment is UNC119 described in Section 4.6 (Gopalakrishna et al., 2011; Zhang et al., 2011). Experiments with UNC119 knockout mice revealed that, in addition to a portion of Gαt being constitutively mislocalized from outer segments, Gαt re-translocation to outer segments in the dark is slower than in wild type controls (Zhang et al., 2011). While it is clearly established that binding to UNC119 both keeps Gαt in the soluble form (Section 4.6) and prevents it from binding to Gβ1γ1, the mechanism of the Gαt association with UNC119 in the inner segment remains a subject of active investigation. One study (Zhang et al., 2011) proposed that this interaction results from transducin’s spontaneous activation by GTP, leading to subunit separation and Gαt trapping by UNC119. The authors showed that UNC119 elutes Gαt from membranes only in the presence of GTP and pointed out that the rate of spontaneous, R*-independent transducin activation (Ramdas et al., 1991) is close to the rate of transducin return (Sokolov et al., 2002). The latter argument, however, may be reconsidered in light of a recent report (Belcastro et al., 2012) that under conditions of lower overall rhodopsin bleaching than employed in (Sokolov et al., 2002), transducin re-translocation is ~10-fold faster (½ time of ~25 min) than initially measured. Another study argued that UNC119 can sequester Gαt from the membrane-bound heterotrimer (Gopalakrishna et al., 2011), suggesting that the complex between Gαt and UNC119 can be formed without a delay imposed by the spontaneous transducin activation rate. Another interesting question regarding the role of UNC119 in Gαt re-translocation is that experiments described in Section 4.6 argue that UNC119 discharges its acylated cargo proteins at the outer segment base, whereas re-translocating Gαt populates the entire outer segment length. This potential discrepancy awaits a complete mechanistic explanation. Finally, we should mention that transducin re-translocation is suppressed but not completely abolished in UNC119 knockout mice. Therefore, additional proteins, for example UNC119B (Zhang et al., 2011), are likely to be involved as well.
The return of Gβ1γ1 also appears to be assisted by other proteins, particularly phosducin. Based on the fact that phosphorylation of phosducin reduces its affinity for Gβ1γ1, Willardson and colleagues (Lee et al., 2004) proposed that phosducin phosphorylation in the outer segment enables it to release bound Gβ1γ1. Consistently, Sokolov and colleagues demonstrated that the rate of transducin return in rods is significantly delayed by mutating major phosphorylated sites on phosducin (Belcastro et al., 2012). However, specific mechanisms governing the cycle of phosducin phosphorylation and dephosphorylation in specific subcellular compartments remain far from understood. Another protein that may be potentially involved in Gβ1γ1 re-translocation is PrBP/δ, also described in Section 4.6 (Norton et al., 2005; Zhang et al., 2007), although no direct evidence in support of this have been reported so far.
6.4. Transducin translocation requires photoresponse saturation: why transducin does not translocate in cones
An important functional consequence of the threshold-dependent transducin translocation mechanism is that it is triggered upon complete light-saturation of the phototransduction cascade. Indeed, transducin activation in excess of PDE cannot cause any additional reduction in cGMP concentration, even if photoreceptors would contain some non-hydrolyzed cGMP under these conditions. This likely explains why the threshold for transducin translocation in rods corresponds to the intensity of background illumination completely saturating their photoresponses (Nakatani et al., 1991).
Cones are different from rods in that they are not saturated by bright light, which implies that transducin in cones is never activated in excess of PDE, at least not longer than momentarily. Consistent with this logic, no transducin translocation in cones of wild type mice was observed in all (Coleman and Semple-Rowland, 2005; Elias et al., 2004; Kennedy et al., 2004; Lobanova et al., 2010; Rosenzweig et al., 2007), but one (Chen et al., 2007) studies. On the other hand, transgenic manipulations slowing phototransduction deactivation in cones caused both saturation of their light responses and robust transducin translocation from cone outer segments (Lobanova et al., 2010). The single most important property of cones preventing their saturation by bright light is a very high spontaneous inactivation rate of their visual pigments. A more in-depth discussion of this and other biochemical mechanisms that enable cones to avoid response saturation under broad illumination conditions see a recent review (Arshavsky and Burns, 2012).
Overall, the presence or absence of transducin translocation appears to be an evolutionary adaptation specific for each photoreceptor type. Rods contribute little if anything to daylight vision. Therefore, transducin translocation facilitates their transition into a deeply light-adapted, energy-saving and perhaps neuroprotective state during most of the day. In contrast, cones adapt to ambient light of essentially any intensity on the timescale of just a few seconds, which means that biochemical conditions enabling transducin translocation are rarely, if ever achieved. Thus, the absence of transducin translocation in cones is essentially a “byproduct” of their superior ability to produce responses to light under all natural conditions.
7. Concluding remarks
In this review, we have summarized many aspects of protein transport mechanisms employed by the photoreceptor cell, highlighting the best-studied examples of membrane, soluble and lipidated protein transport. Clearly, the advances in elucidating rhodopsin trafficking mechanisms have provided the groundwork for how membrane proteins are transported to the outer segment. However, understanding how other outer segment-resident proteins are delivered to this compartment is just beginning. Even less is known about targeting and transport of proteins destined to reside in other photoreceptor compartments. Given that defects in protein targeting and trafficking lead to multiple forms of visual impairment, identifying and understanding all pathways involved in photoreceptor protein delivery is among the most exciting directions in ocular cell biology.
Acknowledgments
We thank Joseph C. Besharse, Uwe Wolfrum, Ching-Hwa Sung and Paulo A. Ferreira for providing high-resolution images of illustrations used in the figures. We also would like to thank Peter D. Calvert for helpful discussions. The experimental work by the authors is supported by grants from the NIH: EY022508 (JNP); EY022862 (RYS); EY020542 (SAB); EY010336 (VYA); EY012859 (VYA); EY05722 (VYA); and an unrestricted grant from Research to Prevent Blindness to Duke University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler AJ, Klucznik KM. Proteins and glycoproteins of the bovine interphotoreceptor matrix: composition and fractionation. Exp Eye Res. 1982;34:423–434. doi: 10.1016/0014-4835(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Adler AJ, Severin KM. Proteins of the bovine interphotoreceptor matrix: tissues of origin. Exp Eye Res. 1981;32:755–769. doi: 10.1016/0014-4835(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Anant JS, Fung BK. In vivo farnesylation of rat rhodopsin kinase. Biochem Biophys Res Commun. 1992;183:468–473. doi: 10.1016/0006-291x(92)90505-f. [DOI] [PubMed] [Google Scholar]
- Anant JS, Ong OC, Xie HY, Clarke S, O’Brien PJ, Fung BK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. [PubMed] [Google Scholar]
- Anderson DH, Fisher SK. Disc shedding in rodlike and conelike photoreceptors of tree squirrels. Science. 1975;187:953–955. doi: 10.1126/science.1145180. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. [PubMed] [Google Scholar]
- Andrews LD, Cohen AI. Freeze-fracture studies of photoreceptor membranes: new observations bearing upon the distribution of cholesterol. J Cell Biol. 1983;97:749–755. doi: 10.1083/jcb.97.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol. 1992;116:659–667. doi: 10.1083/jcb.116.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Burns ME. Photoreceptor signaling: Supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- Artemyev NO. Light-dependent compartmentalization of transducin in rod photoreceptors. Mol Neurobiol. 2008;37:44–51. doi: 10.1007/s12035-008-8015-2. [DOI] [PubMed] [Google Scholar]
- Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Marc RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–14298. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Bader JR, Kusik BW, Besharse JC. Analysis of KIF17 distal tip trafficking in zebrafish cone photoreceptors. Vision Res. 2012;75:37–43. doi: 10.1016/j.visres.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadori R, Huber M, Rinner O, Seeliger MW, Geiger-Rudolph S, Geisler R, Neuhauss SC. Retinal function and morphology in two zebrafish models of oculo-renal syndromes. Eur J Neurosci. 2003;18:1377–1386. doi: 10.1046/j.1460-9568.2003.02863.x. [DOI] [PubMed] [Google Scholar]
- Baker SA, Freeman K, Luby-Phelps K, Pazour GJ, Besharse JC. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J Biol Chem. 2003;278:34211–34218. doi: 10.1074/jbc.M300156200. [DOI] [PubMed] [Google Scholar]
- Baker SA, Haeri M, Yoo P, Gospe SM, 3rd, Skiba NP, Knox BE, Arshavsky VY. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–498. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194:1074–1076. doi: 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Evidence for two functionally different membrane fractions in bovine retinal rod outer segments. J Physiol. 1988;401:309–327. doi: 10.1113/jphysiol.1988.sp017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty NB, Mello RJ. Extracellular mammalian polysaccharides: glycosaminoglycans and proteoglycans. J Chromatogr. 1987;418:187–222. doi: 10.1016/0378-4347(87)80009-9. [DOI] [PubMed] [Google Scholar]
- Beetz N, Hein L. The physiological roles of phosducin: from retinal function to stress-dependent hypertension. Cellular and molecular life sciences : CMLS. 2011;68:599–612. doi: 10.1007/s00018-010-0550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcastro M, Song H, Sinha S, Song C, Mathers PH, Sokolov M. Phosphorylation of phosducin accelerates rod recovery from transducin translocation. Invest Ophthalmol Vis Sci. 2012;53:3084–3091. doi: 10.1167/iovs.11-8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereziat V, Kasus-Jacobi A, Perdereau D, Cariou B, Girard J, Burnol AF. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J Biol Chem. 2002;277:4845–4852. doi: 10.1074/jbc.M106574200. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002;43:3027–3036. [PubMed] [Google Scholar]
- Besharse JC, Forestner DM, Defoe DM. Membrane assembly in retinal photoreceptors. III. Distinct membrane domains of the connecting cilium of developing rods. J Neurosci. 1985;5:1035–1048. doi: 10.1523/JNEUROSCI.05-04-01035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977;75:507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Pfenninger KH. Membrane assembly in retinal photoreceptors I. Freeze-fracture analysis of cytoplasmic vesicles in relationship to disc assembly. J Cell Biol. 1980;87:451–463. doi: 10.1083/jcb.87.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betleja E, Cole DG. Ciliary trafficking: CEP290 guards a gated community. Curr Biol. 2010;20:R928–931. doi: 10.1016/j.cub.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Bhowmick R, Li M, Sun J, Baker SA, Insinna C, Besharse JC. Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic. 2009;10:648–663. doi: 10.1111/j.1600-0854.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks JC, Hageman GS, Johnson LV, Spee C. Ultrastructural visualization of primate cone photoreceptor matrix sheaths. J Comp Neurol. 1988;270:288–300. doi: 10.1002/cne.902700209. [DOI] [PubMed] [Google Scholar]
- Bobu C, Craft CM, Masson-Pevet M, Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci. 2006;47:3109–3118. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Goldberg AF. Photoreceptor renewal: a role for peripherin/rds. Int Rev Cytol. 2002;217:183–225. doi: 10.1016/s0074-7696(02)17015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Lamba OP, Napoli AA, Jr, Sinha S, Guo Y. Fusion between retinal rod outer segment membranes and model membranes: a role for photoreceptor peripherin/rds. Biochemistry. 1998;37:9477–9487. doi: 10.1021/bi980173p. [DOI] [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture Invest Ophthalmol Vis Sci. 1985;26:1659–1694. [PubMed] [Google Scholar]
- Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Brandstatter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, Nitschke P, Gilissen C, Haugen OH, Sanders JS, Stolte-Dijkstra I, Mans DA, Steenbergen EJ, Hamel BC, Matignon M, Pfundt R, Jeanpierre C, Boman H, Rodahl E, Veltman JA, Knappskog PM, Knoers NV, Roepman R, Arts HH. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet. 2011;89:634–643. doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Nachury MV. Primary cilia: how to keep the riff-raff in the plasma membrane. Curr Biol. 2011;21:R434–436. doi: 10.1016/j.cub.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Breuer DK, Yashar BM, Filippova E, Hiriyanna S, Lyons RH, Mears AJ, Asaye B, Acar C, Vervoort R, Wright AF, Musarella MA, Wheeler P, MacDonald I, Iannaccone A, Birch D, Hoffman DR, Fishman GA, Heckenlively JR, Jacobson SG, Sieving PA, Swaroop A. A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am J Hum Genet. 2002;70:1545–1554. doi: 10.1086/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse RM, Tolhuizen EF, Janssen AP, Winkens HJ. Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res. 1985;4:613–618. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt AH. Fine structure and radioautography of rabbit photoreceptor cells. Invest Ophthalmol Vis Sci. 1978;17:90–104. [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- Calvert PD, Schiesser WE, Pugh EN., Jr Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol. 2010;135:173–196. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chen CK, Inglese J, Lefkowitz RJ, Hurley JB. Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Chen CK, Woodruff ML, Chen FS, Chen Y, Cilluffo MC, Tranchina D, Fain GL. Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J Neurosci. 2012;32:15998–16006. doi: 10.1523/JNEUROSCI.1639-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ng PS, Faull KF, Lee RH. Cone photoreceptor betagamma-transducin: posttranslational modification and interaction with phosducin. Invest Ophthalmol Vis Sci. 2003;44:4622–4629. doi: 10.1167/iovs.03-0420. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu M, Sezate SA, McGinnis JF. Light threshold-controlled cone alpha-transducin translocation. Invest Ophthalmol Vis Sci. 2007;48:3350–3355. doi: 10.1167/iovs.07-0126. [DOI] [PubMed] [Google Scholar]
- Chertov AO, Holzhausen L, Kuok IT, Couron D, Parker E, Linton JD, Sadilek M, Sweet IR, Hurley JB. Roles of glucose in photoreceptor survival. J Biol Chem. 2011;286:34700–34711. doi: 10.1074/jbc.M111.279752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Zhao Y, Sung CH. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI. The ultrastructure of the rods of the mouse retina. Am J Anat. 1960;107:23–48. doi: 10.1002/aja.1001070103. [DOI] [PubMed] [Google Scholar]
- Cohen AI. Further studies on the question of the patency of saccules in outer segments of vertebrate photoreceptors. Vision Res. 1970;10:445–453. doi: 10.1016/0042-6989(70)90001-5. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JA, Kwok MC, Molday RS. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem. 2009;284:32670–32679. doi: 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Semple-Rowland SL. GC1 deletion prevents light-dependent arrestin translocation in mouse cone photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46:12–16. doi: 10.1167/iovs.04-0691. [DOI] [PubMed] [Google Scholar]
- Conley SM, Naash MI. Focus on molecules: RDS. Exp Eye Res. 2009;89:278–279. doi: 10.1016/j.exer.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corless JM. Cone outer segments: a biophysical model of membrane dynamics, shape retention, and lamella formation. Biophys J. 2012;102:2697–2705. doi: 10.1016/j.bpj.2012.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless JM, Schneider TG. Patterns of interdisk connections within the lamellar domains of retinal rod outer segment disks: observations relevant to the axial propagation of incisures. Exp Eye Res. 1987;45:883–905. doi: 10.1016/s0014-4835(87)80104-5. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FP, van den Hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Jr, Packer O, Hendrickson AE, Kalina RE. Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science. 1987;236:579–582. doi: 10.1126/science.3576186. [DOI] [PubMed] [Google Scholar]
- Davis EE, Katsanis N. The ciliopathies: a transitional model into systems biology of human genetic disease. Curr Opin Genet Dev. 2012;22:290–303. doi: 10.1016/j.gde.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, Muzny DM, Young AC, Wheeler DA, Cruz P, Morgan M, Lewis LR, Cherukuri P, Maskeri B, Hansen NF, Mullikin JC, Blakesley RW, Bouffard GG, Gyapay G, Rieger S, Tonshoff B, Kern I, Soliman NA, Neuhaus TJ, Swoboda KJ, Kayserili H, Gallagher TE, Lewis RA, Bergmann C, Otto EA, Saunier S, Scambler PJ, Beales PL, Gleeson JG, Maher ER, Attie-Bitach T, Dollfus H, Johnson CA, Green ED, Gibbs RA, Hildebrandt F, Pierce EA, Katsanis N. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet. 2011;43:189–196. doi: 10.1038/ng.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defoe DM, Besharse JC. Membrane assembly in retinal photoreceptors. II. Immunocytochemical analysis of freeze-fractured rod photoreceptor membranes using anti-opsin antibodies. J Neurosci. 1985;5:1023–1034. doi: 10.1523/JNEUROSCI.05-04-01023.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. doi: 10.1242/jcs.108.1.215. [DOI] [PubMed] [Google Scholar]
- Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci U S A. 1998;95:10620–10625. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Wang J. Molecular assemblies that control rhodopsin transport to the cilia. Vision Res. 2012 doi: 10.1016/j.visres.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci U S A. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derobertis E. Electron Microscope Observations on the Submicroscopic Organization of the Retinal Rods. J Biophys Biochem Cy. 1956;2:319–330. doi: 10.1083/jcb.2.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ericsson LH, Johnson RS, Kumar S, Olshevskaya E, Zozulya S, Neubert TA, Stryer L, Hurley JB, Walsh KA. The NH2 terminus of retinal recoverin is acylated by a small family of fatty acids. J Biol Chem. 1992;267:16033–16036. [PubMed] [Google Scholar]
- Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Cloning, sequencing, and expression of a 24-kDa Ca2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmiller MS. Distal invaginations and the renewal of cone outer segments in anuran and monkey retinas. Cell Tissue Res. 1990;260:19–28. doi: 10.1007/BF00297486. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Renewal of the ciliary axoneme in cone outer segments of the retina of Xenopus laevis. Cell Tissue Res. 1996;285:165–169. doi: 10.1007/s004410050632. [DOI] [PubMed] [Google Scholar]
- Edrington TCt, Lapointe R, Yeagle PL, Gretzula CL, Boesze-Battaglia K. Peripherin-2: an intracellular analogy to viral fusion proteins. Biochemistry. 2007;46:3605–3613. doi: 10.1021/bi061820c. [DOI] [PubMed] [Google Scholar]
- Elias RV, Sezate SS, Cao W, McGinnis JF. Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis. 2004;10:672–681. [PubMed] [Google Scholar]
- Evans JE, Snow JJ, Gunnarson AL, Ou G, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J Cell Biol. 2006;172:663–669. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet. 2010;19:1358–1367. doi: 10.1093/hmg/ddq012. [DOI] [PubMed] [Google Scholar]
- Fadeel B. Plasma membrane alterations during apoptosis: role in corpse clearance. Antioxidants & redox signaling. 2004;6:269–275. doi: 10.1089/152308604322899332. [DOI] [PubMed] [Google Scholar]
- Fain GL. Why photoreceptors die (and why they don’t) Bioessays. 2006;28:344–354. doi: 10.1002/bies.20382. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs JS, Nagel BA, Quiambao AB, Nash ZA, Fliesler SJ, Naash MI. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol. 2006;173:59–68. doi: 10.1083/jcb.200509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y. Development of the cone photoreceptor mosaic in the mouse retina revealed by fluorescent cones in transgenic mice. Mol Vis. 2003;9:31–42. [PubMed] [Google Scholar]
- Finneman SC, Chang Y. Photoreceptor—RPE Interactions. Physiology and Molecular Mechanisms. In: Tombran-Tink J, Barnstable CJ, editors. Visual Transduction and Non-Visual Light Perception. Humana Press; 2008. pp. 67–86. [Google Scholar]
- Fisch C, Dupuis-Williams P. Ultrastructure of cilia and flagella - back to the future! Biol Cell. 2011;103:249–270. doi: 10.1042/BC20100139. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Pfeffer BA, Anderson DH. Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci. 1983;24:844–856. [PubMed] [Google Scholar]
- Florio SK, Prusti RK, Beavo JA. Solubilization of membrane-bound rod phosphodiesterase by the rod phosphodiesterase recombinant delta subunit. J Biol Chem. 1996;271:24036–24047. doi: 10.1074/jbc.271.39.24036. [DOI] [PubMed] [Google Scholar]
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser RD, Gillespie JM, Macrae TP. Tyrosine-rich proteins in keratins. Comp Biochem Physiol B. 1973;44:943–947. doi: 10.1016/0305-0491(73)90244-7. [DOI] [PubMed] [Google Scholar]
- Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990;346:658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Wilhelm M. Structure and function of photoreceptor and second-order cell mosaics in the retina of Xenopus. Int Rev Cytol. 2001;210:77–120. doi: 10.1016/s0074-7696(01)10004-5. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- Giddings JC, Kucera E, Russell CP, Myers MN. Statistical Theory for Equilibrium Distribution of Rigid Molecules in Inert Porous Networks. Exclusion Chromatography. J Phys Chem. 1968;72:4397. [Google Scholar]
- Gillespie PG, Prusti RK, Apel ED, Beavo JA. A soluble form of bovine rod photoreceptor phosphodiesterase has a novel 15-kDa subunit. J Biol Chem. 1989;264:12187–12193. [PubMed] [Google Scholar]
- Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell. 2012;151:1029–1041. doi: 10.1016/j.cell.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF. Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol. 2006;253:131–175. doi: 10.1016/S0074-7696(06)53004-9. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Loewen CJ, Molday RS. Cysteine residues of photoreceptor peripherin/rds: role in subunit assembly and autosomal dominant retinitis pigmentosa. Biochemistry. 1998;37:680–685. doi: 10.1021/bi972036i. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry. 1996;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna KN, Doddapuneni K, Boyd KK, Masuho I, Martemyanov KA, Artemyev NO. Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J Biol Chem. 2011;286:28954–28962. doi: 10.1074/jbc.M111.268821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe SM, 3rd, Baker SA, Arshavsky VY. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci. 2010;123:3639–3644. doi: 10.1242/jcs.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe SM, 3rd, Baker SA, Kessler C, Brucato MF, Winter JR, Burns ME, Arshavsky VY. Membrane attachment is key to protecting transducin GTPase-activating complex from intracellular proteolysis in photoreceptors. J Neurosci. 2011;31:14660–14668. doi: 10.1523/JNEUROSCI.3516-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner JV, Bodley HD, Weidman TA, Peace DG. Pericentriolar processes of photoreceptor cell basal bodies in the mammalian retina. Z Mikrosk Anat Forsch. 1983;97:309–318. [PubMed] [Google Scholar]
- Greiner JV, Weidman TA, Bodley HD, Greiner CA. Ciliogenesis in photoreceptor cells of the retina. Exp Eye Res. 1981;33:433–446. doi: 10.1016/s0014-4835(81)80094-2. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA, Gurevich EV. The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res. 2011;30:405–430. doi: 10.1016/j.preteyeres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Anderson DW, Papermaster DS. Prominin-1 localizes to the open rims of outer segment lamellae in Xenopus laevis rod and cone photoreceptors. Invest Ophthalmol Vis Sci. 2012;53:361–373. doi: 10.1167/iovs.11-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol. 2007a;368:375–387. doi: 10.1016/j.jmb.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Van Eps N, Francis DJ, Altenbach C, Vishnivetskiy SA, Arshavsky VY, Klug CS, Hubbell WL, Gurevich VV. Structure and function of the visual arrestin oligomer. EMBO J. 2007b;26:1726–1736. doi: 10.1038/sj.emboj.7601614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzal-Bayer M, Renault L, Roversi P, Wittinghofer A, Hillig RC. The complex of Arl2-GTP and PDE delta: from structure to function. EMBO J. 2002;21:2095–2106. doi: 10.1093/emboj/21.9.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Efimenko E, Swoboda P, Scholey JM. The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes? PLoS ONE. 2011a;6:e20995. doi: 10.1371/journal.pone.0020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011b;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Heck M, Hofmann KP. Maximal rate and nucleotide dependence of rhodopsin-catalyzed transducin activation: initial rate analysis based on a double displacement mechanism. J Biol Chem. 2001;276:10000–10009. doi: 10.1074/jbc.M009475200. [DOI] [PubMed] [Google Scholar]
- Higashide T, Murakami A, McLaren MJ, Inana G. Cloning of the cDNA for a novel photoreceptor protein. J Biol Chem. 1996;271:1797–1804. doi: 10.1074/jbc.271.3.1797. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hisatomi O, Matsuda S, Satoh T, Kotaka S, Imanishi Y, Tokunaga F. A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett. 1998;424:159–164. doi: 10.1016/s0014-5793(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Besharse JC, Rayborn ME. The effect of light on the quantity of phagosomes in the pigment epithelium. Exp Eye Res. 1976;23:623–635. doi: 10.1016/0014-4835(76)90221-9. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Varner HH, Rayborn ME, Osterfeld AM. Retinal attachment to the pigment epithelium. Linkage through an extracellular sheath surrounding cone photoreceptors. Retina. 1989;9:59–68. [PubMed] [Google Scholar]
- Holopainen JM, Cheng CL, Molday LL, Johal G, Coleman J, Dyka F, Hii T, Ahn J, Molday RS. Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptor cells. Biochemistry. 2010;49:7439–7447. doi: 10.1021/bi1005249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DH, Pawlyk B, Sokolov M, Strissel KJ, Yang J, Tulloch B, Wright AF, Arshavsky VY, Li T. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–2421. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 binds dynein light intermediate chain 2 and its overexpression fragments the Golgi complex. Biochem Biophys Res Commun. 2010a;394:387–392. doi: 10.1016/j.bbrc.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010b;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- Horst CJ, Forestner DM, Besharse JC. Cytoskeletal-membrane interactions: a stable interaction between cell surface glycoconjugates and doublet microtubules of the photoreceptor connecting cilium. J Cell Biol. 1987;105:2973–2987. doi: 10.1083/jcb.105.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motil Cytoskeleton. 1990;17:329–344. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci U S A. 2002;99:9755–9760. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chuang JZ, Xu K, McGraw TG, Sung CH. SARA, a FYVE domain protein, affects Rab5-mediated endocytosis. J Cell Sci. 2002;115:4755–4763. doi: 10.1242/jcs.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SP, Brown BM, Craft CM. Visual Arrestin 1 acts as a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J Neurosci. 2010;30:9381–9391. doi: 10.1523/JNEUROSCI.1207-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak LM, Lunt S, Chang CH, Winkler E, Flammer H, Lindsey M, Perkins BD. The intraflagellar transport protein ift80 is essential for photoreceptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Invest Ophthalmol Vis Sci. 2010;51:3792–3799. doi: 10.1167/iovs.09-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MM, Rancourt D, Farrar GJ, Kenna P, Hazel M, Bush RA, Sieving PA, Sheils DM, McNally N, Creighton P, Erven A, Boros A, Gulya K, Capecchi MR, Humphries P. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr Opin Cell Biol. 2001;13:478–484. doi: 10.1016/s0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Iglic A, Hagerstrand H, Veranic P, Plemenitas A, Kralj-Iglic V. Curvature-induced accumulation of anisotropic membrane components and raft formation in cylindrical membrane protrusions. J Theor Biol. 2006;240:368–373. doi: 10.1016/j.jtbi.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Imamoto Y, Tamura C, Kamikubo H, Kataoka M. Concentration-dependent tetramerization of bovine visual arrestin. Biophys J. 2003;85:1186–1195. doi: 10.1016/S0006-3495(03)74554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J, Glickman JF, Lorenz W, Caron MG, Lefkowitz RJ. Isoprenylation of a protein kinase. Requirement of farnesylation/alpha-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J Biol Chem. 1992;267:1422–1425. [PubMed] [Google Scholar]
- Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Baye LM, Amsterdam A, Besharse JC, Link BA. Analysis of a zebrafish dync1h1 mutant reveals multiple functions for cytoplasmic dynein 1 during retinal photoreceptor development. Neural Dev. 2010;5:12. doi: 10.1186/1749-8104-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC. Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn. 2009;238:2211–2222. doi: 10.1002/dvdy.21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SA, Chen YX, Miertzschke M, Vetter IR, Koerner C, Wittinghofer A. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J. 2012;31:4085–4094. doi: 10.1038/emboj.2012.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SA, Chen YX, Rusinova A, Chandra A, Bierbaum M, Gremer L, Triola G, Waldmann H, Bastiaens PI, Wittinghofer A. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat Chem Biol. 2011;7:942–949. doi: 10.1038/nchembio.686. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem. 2009;284:1652–1663. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno D, Feiner L, Lillo C, Teofilo K, Goldstein LS, Pierce EA, Williams DS. Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci. 2006;47:5039–5046. doi: 10.1167/iovs.06-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura N, Harada Y, Usukura J. High-resolution freeze-etching replica images of the disk and the plasma membrane surfaces in purified bovine rod outer segments. J Electron Microsc (Tokyo) 2000;49:691–697. doi: 10.1093/oxfordjournals.jmicro.a023860. [DOI] [PubMed] [Google Scholar]
- Kamps KM, De Grip WJ, Daemen FJ. Use of a density modification technique for isolation of the plasma membrane of rod outer segments. Biochim Biophys Acta. 1982;687:296–302. doi: 10.1016/0005-2736(82)90558-2. [DOI] [PubMed] [Google Scholar]
- Kaplan MW, Iwata RT, Sears RC. Lengths of immunolabeled ciliary microtubules in frog photoreceptor outer segments. Exp Eye Res. 1987;44:623–632. doi: 10.1016/s0014-4835(87)80134-3. [DOI] [PubMed] [Google Scholar]
- Karan S, Frederick JM, Baehr W. Involvement of guanylate cyclases in transport of photoreceptor peripheral membrane proteins. Adv Exp Med Biol. 2008a;613:351–359. doi: 10.1007/978-0-387-74904-4_41. [DOI] [PubMed] [Google Scholar]
- Karan S, Frederick JM, Baehr W. Novel functions of photoreceptor guanylate cyclases revealed by targeted deletion. Mol Cell Biochem. 2010;334:141–155. doi: 10.1007/s11010-009-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S, Tam BM, Moritz OL, Baehr W. Targeting of mouse guanylate cyclase 1 (Gucy2e) to Xenopus laevis rod outer segments. Vision Res. 2011;51:2304–2311. doi: 10.1016/j.visres.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008b;48:442–452. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai H, Aiba A, Nakao K, Nakamura K, Katsuki M, Xiong WH, Yau KW, Imai H, Shichida Y, Satomi Y, Takao T, Okano T, Fukada Y. Farnesylation of retinal transducin underlies its translocation during light adaptation. Neuron. 2005;47:529–539. doi: 10.1016/j.neuron.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22:921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ. Rod and cone visual pigments and phototransduction through pharmacological, genetic, and physiological approaches. J Biol Chem. 2012;287:1635–1641. doi: 10.1074/jbc.R111.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–2138. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41:915–928. doi: 10.1016/s0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- Kerov V, Artemyev NO. Diffusion and light-dependent compartmentalization of transducin. Mol Cell Neurosci. 2011;46:340–346. doi: 10.1016/j.mcn.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerov V, Chen D, Moussaif M, Chen YJ, Chen CK, Artemyev NO. Transducin activation state controls its light-dependent translocation in rod photoreceptors. J Biol Chem. 2005a;280:41069–41076. doi: 10.1074/jbc.M508849200. [DOI] [PubMed] [Google Scholar]
- Kerov VS, Natochin M, Artemyev NO. Interaction of transducin-alpha with LGN, a G-protein modulator expressed in photoreceptor cells. Mol Cell Neurosci. 2005b;28:485–495. doi: 10.1016/j.mcn.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kim M, Hanson SM, Vishnivetskiy SA, Song X, Cleghorn WM, Hubbell WL, Gurevich VV. Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry. 2011;50:2235–2242. doi: 10.1021/bi1018607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney MS, Fisher SK. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Vol. 201. Royal Society; 1978. The photoreceptors and pigment epithelium of the larval Xenopus retina: morphogenesis and outer segment renewal; pp. 149–167. [DOI] [PubMed] [Google Scholar]
- Kleinman ME, Ambati J. Fifty years later: the disk goes to the prom. J Clin Invest. 2008;118:2681–2684. doi: 10.1172/JCI36515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin VA, Calvert PD, Bownds MD. Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J Biol Chem. 1995;270:16147–16152. doi: 10.1074/jbc.270.27.16147. [DOI] [PubMed] [Google Scholar]
- Knabe W, Kuhn HJ. Ciliogenesis in photoreceptor cells of the tree shrew retina. Anat Embryol (Berl) 1997;196:123–131. doi: 10.1007/s004290050085. [DOI] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N terminus of photoreceptor G-protein alpha-subunit. Nature. 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Alexov E, Arshavsky VY, Honig B. Electrostatic and lipid anchor contributions to the interaction of transducin with membranes: Mechanistic implications for activation and translocation. J Biol Chem. 2008;283:31197–31207. doi: 10.1074/jbc.M803799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock BL, Mills-Henry I, Perkins BD. Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest Ophthalmol Vis Sci. 2009;50:5463–5471. doi: 10.1167/iovs.09-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok MC, Holopainen JM, Molday LL, Foster LJ, Molday RS. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion. Mol Cell Proteomics. 2008;7:1053–1066. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties AM, Bok D, Liebman P. Procion yellow: a marker dye for outer segment disc patency and for rod renewal. Exp Eye Res. 1976;23:139–148. doi: 10.1016/0014-4835(76)90197-4. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- Lee BY, Thulin CD, Willardson BM. Site-specific phosphorylation of phosducin in intact retina. Dynamics of phosphorylation and effects on G protein beta gamma dimer binding. J Biol Chem. 2004;279:54008–54017. doi: 10.1074/jbc.M405669200. [DOI] [PubMed] [Google Scholar]
- Lee ES, Burnside B, Flannery JG. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskov IB, Klenchin VA, Handy JW, Whitlock GG, Govardovskii VI, Bownds MD, Lamb TD, Pugh EN, Jr, Arshavsky VY. The gain of rod phototransduction: reconciliation of biochemical and electrophysiological measurements. Neuron. 2000;27:525–537. doi: 10.1016/s0896-6273(00)00063-5. [DOI] [PubMed] [Google Scholar]
- Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci U S A. 1996;93:14176–14181. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TS, Volpp K, Applebury ML. Bovine cone photoreceptor cGMP phosphodiesterase structure deduced from a cDNA clone. Proc Natl Acad Sci U S A. 1990;87:293–297. doi: 10.1073/pnas.87.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Fotiadis D, Maeda T, Maeda A, Modzelewska A, Filipek S, Saperstein DA, Engel A, Palczewski K. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J Biol Chem. 2004;279:48189–48196. doi: 10.1074/jbc.M408362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Hanzal-Bayer M, Becker J. The delta subunit of rod specific cyclic GMP phosphodiesterase, PDE delta, interacts with the Arf-like protein Arl3 in a GTP specific manner. FEBS Lett. 1999;458:55–59. doi: 10.1016/s0014-5793(99)01117-5. [DOI] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, Song H, Miyagishima KJ, Stearns GW, Lindsay K, Wei J, Chertov AO, Peters TA, Caffe R, Pluk H, Seeliger MW, Tanimoto N, Fong K, Bolton L, Kuok DL, Sweet IR, Bartoletti TM, Radu RA, Travis GH, Zagotta WN, Townes-Anderson E, Parker E, Van der Zee CE, Sampath AP, Sokolov M, Thoreson WB, Hurley JB. Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci U S A. 2010;107:8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Finkelstein S, Song H, Tsang SH, Chen C-K, Sokolov M, Skiba NP, Arshavsky VY. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J Neurosci. 2007;27:1151–1160. doi: 10.1523/JNEUROSCI.5010-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanova ES, Herrmann R, Finkelstein S, Reidel B, Skiba NP, Deng WT, Jo R, Weiss ER, Hauswirth WW, Arshavsky VY. Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light. J Neurosci. 2010;30:6815–6824. doi: 10.1523/JNEUROSCI.0613-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew A, Ho YK, Blundell T, Bax B. Phosducin induces a structural change in transducin beta gamma. Structure. 1998;6:1007–1019. doi: 10.1016/s0969-2126(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem. 2000;275:5370–5378. doi: 10.1074/jbc.275.8.5370. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Moritz OL, Molday RS. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J Biol Chem. 2001;276:22388–22396. doi: 10.1074/jbc.M011710200. [DOI] [PubMed] [Google Scholar]
- Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp Eye Res. 1986;43:193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- Luo DG, Xue T, Yau KW. How vision begins: an odyssey. Proc Natl Acad Sci U S A. 2008;105:9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Marsh-Armstrong N, Rattner A, Nathans J. An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase. J Neurosci. 2004;24:2623–2632. doi: 10.1523/JNEUROSCI.5302-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Magupalli VG, Schwarz K, Alpadi K, Natarajan S, Seigel GM, Schmitz F. Multiple RIBEYE-RIBEYE interactions create a dynamic scaffold for the formation of synaptic ribbons. J Neurosci. 2008;28:7954–7967. doi: 10.1523/JNEUROSCI.1964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA. Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol. 2004;123:729–741. doi: 10.1085/jgp.200308994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Wen XH, Michaud NA, Covington HI, DiBenedetto E, Hamm HE, Lem J, Caruso G. Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS ONE. 2012;7:e37832. doi: 10.1371/journal.pone.0037832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J, Besharse JC. Kinesin-2 family motors in the unusual photoreceptor cilium. Vision Res. 2012 doi: 10.1016/j.visres.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangini NJ, Garner GL, Okajima TI, Donoso LA, Pepperberg DR. Effect of hydroxylamine on the subcellular distribution of arrestin (S-antigen) in rod photoreceptors. Vis Neurosci. 1994;11:561–568. doi: 10.1017/s0952523800002467. [DOI] [PubMed] [Google Scholar]
- Marshall WF. Basal bodies platforms for building cilia. Curr Top Dev Biol. 2008;85:1–22. doi: 10.1016/S0070-2153(08)00801-6. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Martemyanov KA, Lishko PV, Calero N, Keresztes G, Sokolov M, Strissel KJ, Leskov IB, Hopp JA, Kolesnikov AV, Chen CK, Lem J, Heller S, Burns ME, Arshavsky VY. The DEP domain determines subcellular targeting of the GTPase activating protein RGS9 in vivo. J Neurosci. 2003;23:10175–10181. doi: 10.1523/JNEUROSCI.23-32-10175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzesco AM, Galli T, Louvard D, Zahraoui A. The rod cGMP phosphodiesterase delta subunit dissociates the small GTPase Rab13 from membranes. J Biol Chem. 1998;273:22340–22345. doi: 10.1074/jbc.273.35.22340. [DOI] [PubMed] [Google Scholar]
- Matsumoto B, Besharse JC. Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Invest Ophthalmol Vis Sci. 1985;26:628–635. [PubMed] [Google Scholar]
- Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, Kumaramanickavel G, John S, Nancarrow D, Roper K, Weigmann A, Huttner WB, Denton MJ. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9:27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009a;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009b;122:2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Thoreson WB. The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci. 2011;28:453–471. doi: 10.1017/S0952523811000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM, Holtzman E. Smooth endoplasmic reticulum and other agranular reticulum in frog retinal photoreceptors. J Neurocytol. 1982;11:263–293. doi: 10.1007/BF01258247. [DOI] [PubMed] [Google Scholar]
- Mikami A, Tynan SH, Hama T, Luby-Phelps K, Saito T, Crandall JE, Besharse JC, Vallee RB. Molecular structure of cytoplasmic dynein 2 and its distribution in neuronal and ciliated cells. J Cell Sci. 2002;115:4801–4808. doi: 10.1242/jcs.00168. [DOI] [PubMed] [Google Scholar]
- Miyaguchi K, Hashimoto PH. Evidence for the transport of opsin in the connecting cilium and basal rod outer segment in rat retina: rapid-freeze, deep-etch and horseradish peroxidase labelling studies. J Neurocytol. 1992;21:449–457. doi: 10.1007/BF01191508. [DOI] [PubMed] [Google Scholar]
- Molday RS. Molecular organization of rod outer segments. In: Williams DS, editor. Photoreceptor cell biology and inherited retinal degenerations. World Scientific Publishing; 2004. pp. 259–300. [Google Scholar]
- Molday RS, Hicks D, Molday L. Peripherin. A rim-specific membrane protein of rod outer segment discs. Invest Ophthalmol Vis Sci. 1987;28:50–61. [PubMed] [Google Scholar]
- Molday RS, Molday LL. Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol. 1987;105:2589–2601. doi: 10.1083/jcb.105.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, Warren R, Loewen C, Molday L. Cyclic GMP-gated channel and peripherin/rds-rom-1 complex of rod cells. Novartis Found Symp. 1999;224:249–261. doi: 10.1002/9780470515693.ch14. discussion 261-244. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, Saunier S, Spassky N, Bastin P, Benmerah A. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant Rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001a;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Papermaster DS, Nakayama T. A functional rhodopsin-green fluorescent protein fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time-dependent pattern. J Biol Chem. 2001b;276:28242–28251. doi: 10.1074/jbc.M101476200. [DOI] [PubMed] [Google Scholar]
- Muresan V, Joshi HC, Besharse JC. Gamma-tubulin in differentiated cell types: localization in the vicinity of basal bodies in retinal photoreceptors and ciliated epithelia. J Cell Sci. 1993;104:1229–1237. doi: 10.1242/jcs.104.4.1229. [DOI] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19:3591–3598. doi: 10.1093/hmg/ddq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Mendez A, Gurevich EV, Kennedy MJ, Shestopalov VI, Vishnivetskiy SA, Chen J, Hurley JB, Gurevich VV, Slepak VZ. Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron. 2005a;46:555–567. doi: 10.1016/j.neuron.2005.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Mendez A, Blumer JB, Rosenzweig DH, Slepak VZ. The presence of a Leu-Gly-Asn repeat-enriched protein (LGN), a putative binding partner of transducin, in ROD photoreceptors. Invest Ophthalmol Vis Sci. 2005b;46:383–389. doi: 10.1167/iovs.04-1006. [DOI] [PubMed] [Google Scholar]
- Najafi M, Maza NA, Calvert PD. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci U S A. 2012;109:203–208. doi: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Tamura T, Yau KW. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991;97:413–435. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancy V, Callebaut I, El Marjou A, de Gunzburg J. The delta subunit of retinal rod cGMP phosphodiesterase regulates the membrane association of Ras and Rap GTPases. J Biol Chem. 2002;277:15076–15084. doi: 10.1074/jbc.M109983200. [DOI] [PubMed] [Google Scholar]
- Neubert TA, Johnson RS, Hurley JB, Walsh KA. The rod transducin alpha subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267:18274–18277. [PubMed] [Google Scholar]
- Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- Nilsson SE. Receptor cell outer segment development and ultrastructure of disk membranes in retina of tadpole (Rana pipiens) J Ultrastruct Res. 1964;11:581–602. doi: 10.1016/s0022-5320(64)80084-8. [DOI] [PubMed] [Google Scholar]
- Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- Norton AW, Hosier S, Terew JM, Li N, Dhingra A, Vardi N, Baehr W, Cote RH. Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem. 2005;280:1248–1256. doi: 10.1074/jbc.M410475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Day WT, Young RW. Rhythmic daily shedding of outer-segment membranes by visual cells in the goldfish. J Cell Biol. 1978;76:593–604. doi: 10.1083/jcb.76.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata S, Usukura J. Morphogenesis of the photoreceptor outer segment during postnatal development in the mouse (BALB/c) retina. Cell Tissue Res. 1992;269:39–48. doi: 10.1007/BF00384724. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisme W, Li J, Goldmann T, Bolch S, Wolfrum U, Smith WC. Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell Signal. 2010;22:447–456. doi: 10.1016/j.cellsig.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto-Bruc A, Fariss RN, Haeseleer F, Huang J, Buczylko J, Surgucheva I, Baehr W, Milam AH, Palczewski K. Localization of guanylate cyclase-activating protein 2 in mammalian retinas. Proc Natl Acad Sci U S A. 1997;94:4727–4732. doi: 10.1073/pnas.94.9.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagh-Roehl K, Wang E, Burnside B. Shortening of the calycal process actin cytoskeleton is correlated with myoid elongation in teleost rods. Exp Eye Res. 1992;55:735–746. doi: 10.1016/0014-4835(92)90178-u. [DOI] [PubMed] [Google Scholar]
- Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287:1612–1619. doi: 10.1074/jbc.R111.301150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS. The birth and death of photoreceptors: the Friedenwald Lecture. Invest Ophthalmol Vis Sci. 2002;43:1300–1309. [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26:1386–1404. [PubMed] [Google Scholar]
- Patil H, Tserentsoodol N, Saha A, Hao Y, Webb M, Ferreira PA. Selective loss of RPGRIP1-dependent ciliary targeting of NPHP4, RPGR and SDCCAG8 underlies the degeneration of photoreceptor neurons. Cell Death Dis. 2012;3:e355. doi: 10.1038/cddis.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet JA, Bragin A, Calvert PD, Nikonov SS, Mani S, Zhao X, Besharse JC, Pierce EA, Knox BE, Pugh EN., Jr Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J Cell Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- Peitzsch RM, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Peshenko IV, Olshevskaya EV, Lim S, Ames JB, Dizhoor AM. Calcium-myristoyl tug is a new mechanism for intramolecular tuning of calcium sensitivity and target enzyme interaction for guanylyl cyclase-activating protein 1: Dynamic connection between N-fatty acyl group and EF-hand controls calcium sensitivity. J Biol Chem. 2012;287:13972–13984. doi: 10.1074/jbc.M112.341883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol. 1983;96:265–276. doi: 10.1083/jcb.96.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JJ, Orisme W, Fellows J, McDowell JH, Shelamer CL, Dugger DR, Smith WC. A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:3988–3998. doi: 10.1167/iovs.05-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preininger AM, Kaya AI, Gilbert JA, 3rd, Busenlehner LS, Armstrong RN, Hamm HE. Myristoylation exerts direct and allosteric effects on Galpha conformation and dynamics in solution. Biochemistry. 2012;51:1911–1924. doi: 10.1021/bi201472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Phototransduction in vertebrate rods and cones: Molecular mechanisms of amplification, recovery and light adaptation. In: Stavenga DG, DeGrip WJ, Pugh EN Jr, editors. Molecular mechanisms in visual transduction. Elsevier; Amsterdam: 2000. pp. 183–255. [Google Scholar]
- Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Xiong W, Cepko CL. Loss of daylight vision in retinal degeneration: are oxidative stress and metabolic dysregulation to blame? J Biol Chem. 2012;287:1642–1648. doi: 10.1074/jbc.R111.304428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Diener DR, Geimer S, Cole DG, Rosenbaum JL. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querubin A, Lee HR, Provis JM, O’Brien KM. Photoreceptor and ganglion cell topographies correlate with information convergence and high acuity regions in the adult pigeon (Columba livia) retina. The Journal of comparative neurology. 2009;517:711–722. doi: 10.1002/cne.22178. [DOI] [PubMed] [Google Scholar]
- Rachel RA, May-Simera HL, Veleri S, Gotoh N, Choi BY, Murga-Zamalloa C, McIntyre JC, Marek J, Lopez I, Hackett AN, Brooks M, den Hollander AI, Beales PL, Li T, Jacobson SG, Sood R, Martens JR, Liu P, Friedman TB, Khanna H, Koenekoop RK, Kelley MW, Swaroop A. Combining Cep290 and Mkks ciliopathy alleles in mice rescues sensory defects and restores ciliogenesis. J Clin Invest. 2012;122:1233–1245. doi: 10.1172/JCI60981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala A, Daly RJ, Tanito M, Allen DT, Holt LJ, Lobanova ES, Arshavsky VY, Rajala RV. Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation. Biochemistry. 2009;48:5563–5572. doi: 10.1021/bi9000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala RV, Anderson RE. Rhodopsin-regulated insulin receptor signaling pathway in rod photoreceptor neurons. Mol Neurobiol. 2010;42:39–47. doi: 10.1007/s12035-010-8130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas L, Disher RM, Wensel TG. Nucleotide exchange and cGMP phosphodiesterase activation by pertussis toxin inactivated transducin. Biochemistry. 1991;30:11637–11645. doi: 10.1021/bi00114a005. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Rattner A, Chen J, Nathans J. Proteolytic shedding of the extracellular domain of photoreceptor cadherin. Implications for outer segment assembly. J Biol Chem. 2004;279:42202–42210. doi: 10.1074/jbc.M407928200. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Williams J, Cooke C, Savchenko A, Lyubarsky A, Pugh EN, Nathans J. A photoreceptor-specific cadherin is essential for the structural integrity of the outer segment and for photoreceptor survival. Neuron. 2001;32:775–786. doi: 10.1016/s0896-6273(01)00531-1. [DOI] [PubMed] [Google Scholar]
- Regus-Leidig H, Tom Dieck S, Specht D, Meyer L, Brandstatter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol. 2009;512:814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- Reid DM, Friedel U, Molday RS, Cook NJ. Identification of the sodium-calcium exchanger as the major ricin-binding glycoprotein of bovine rod outer segments and its localization to the plasma membrane. Biochemistry. 1990;29:1601–1607. doi: 10.1021/bi00458a035. [DOI] [PubMed] [Google Scholar]
- Reidel B, Orisme W, Goldmann T, Smith WC, Wolfrum U. Photoreceptor vitality in organotypic cultures of mature vertebrate retinas validated by light-dependent molecular movements. Vision Res. 2006;46:4464–4471. doi: 10.1016/j.visres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Reidel B, Thompson JW, Farsiu S, Moseley MA, Skiba NP, Arshavsky VY. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M1110.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault L, Kuhlmann J, Henkel A, Wittinghofer A. Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1) Cell. 2001;105:245–255. doi: 10.1016/s0092-8674(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Rohlich P. The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell Tissue Res. 1975;161:421–430. doi: 10.1007/BF00220009. [DOI] [PubMed] [Google Scholar]
- Roof D, Adamian M, Jacobs D, Hayes A. Cytoskeletal specializations at the rod photoreceptor distal tip. J Comp Neurol. 1991;305:289–303. doi: 10.1002/cne.903050210. [DOI] [PubMed] [Google Scholar]
- Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig DH, Nair KS, Wei J, Wang Q, Garwin G, Saari JC, Chen CK, Smrcka AV, Swaroop A, Lem J, Hurley JB, Slepak VZ. Subunit dissociation and diffusion determine the subcellular localization of rod and cone transducins. J Neurosci. 2007;27:5484–5494. doi: 10.1523/JNEUROSCI.1421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc Natl Acad Sci U S A. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I, Pepermans E, Estivalet A, Carette D, Aghaie A, Ebermann I, Lelli A, Iribarne M, Hardelin JP, Weil D, Sahel JA, El-Amraoui A, Petit C. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. The Journal of cell biology. 2012;199:381–399. doi: 10.1083/jcb.201202012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale WS, Besharse JC, Piperno G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil Cytoskeleton. 1988;9:243–253. doi: 10.1002/cm.970090306. [DOI] [PubMed] [Google Scholar]
- Salinas RY, Baker SA, Gospe SM, 3rd, Arshavsky VY. A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS ONE. 2013;8:e54292. doi: 10.1371/journal.pone.0054292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameshima M, Uehara F, Ohba N. Specialization of the interphotoreceptor matrices around cone and rod photoreceptor cells in the monkey retina, as revealed by lectin cytochemistry. Exp Eye Res. 1987;45:845–863. doi: 10.1016/s0014-4835(87)80101-x. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Dees C, Zeilmaker GH. Development and degeneration of retina in rds mutant mice: observations in chimaeras of heterozygous mutant and normal genotype. J Embryol Exp Morphol. 1986;98:111–121. [PubMed] [Google Scholar]
- Sanyal S, Deruiter A, Hawkins RK. Development and Degeneration of Retina in Rds Mutant Mice - Light-Microscopy. J Comp Neurol. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Konigstorfer A, Sudhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS. ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol. 2006;168:1288–1298. doi: 10.2353/ajpath.2006.050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Hirsch JA, Gurevich VV, Engelman DM, Sigler PB, Fleming KG. Visual arrestin activity may be regulated by self-association. J Biol Chem. 1999;274:21186–21190. doi: 10.1074/jbc.274.30.21186. [DOI] [PubMed] [Google Scholar]
- Schwahn U, Lenzner S, Dong J, Feil S, Hinzmann B, van Duijnhoven G, Kirschner R, Hemberger M, Bergen AA, Rosenberg T, Pinckers AJ, Fundele R, Rosenthal A, Cremers FP, Ropers HH, Berger W. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet. 1998;19:327–332. doi: 10.1038/1214. [DOI] [PubMed] [Google Scholar]
- Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak T, Wolfrum U. Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol Cell. 2011;103:449–466. doi: 10.1042/BC20110034. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Shu X, Black GC, Rice JM, Hart-Holden N, Jones A, O’Grady A, Ramsden S, Wright AF. RPGR mutation analysis and disease: an update. Hum Mutat. 2007;28:322–328. doi: 10.1002/humu.20461. [DOI] [PubMed] [Google Scholar]
- Sinha S, Majumder A, Belcastro M, Sokolov M, Artemyev NO. Expression and subcellular distribution of UNC119a, a protein partner of transducin alpha subunit in rod photoreceptors. Cell Signal. 2013;25:341–348. doi: 10.1016/j.cellsig.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrand FS. The ultrastructure of the outer segments of rods and cones of the eye as revealed by the electron microscope. J Cell Physiol. 1953;42:15–44. doi: 10.1002/jcp.1030420103. [DOI] [PubMed] [Google Scholar]
- Skiba NP, Hopp JA, Arshavsky VY. The effector enzyme regulates the duration of G protein signaling in vertebrate photoreceptors by increasing the affinity between transducin and RGS protein. J Biol Chem. 2000;275:32716–32720. doi: 10.1074/jbc.C000413200. [DOI] [PubMed] [Google Scholar]
- Slepak VZ, Artemyev NO, Zhu Y, Dumke CL, Sabacan L, Sondek J, Hamm HE, Bownds MD, Arshavsky VY. An effector site that stimulates G-protein GTPase in photoreceptors. J Biol Chem. 1995;270:14319–14324. doi: 10.1074/jbc.270.24.14319. [DOI] [PubMed] [Google Scholar]
- Slepak VZ, Hurley JB. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life. 2008;60:2–9. doi: 10.1002/iub.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Bolch S, Dugger DR, Li J, Esquenazi I, Arendt A, Benzenhafer D, McDowell JH. Interaction of arrestin with enolase1 in photoreceptors. Invest Ophthalmol Vis Sci. 2011;52:1832–1840. doi: 10.1167/iovs.10-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Sandstrom MM, Leung IY, Zucker CL, Neuringer M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci. 2002;43:2815–2818. [PubMed] [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 2004;6:1109–1113. doi: 10.1038/ncb1186. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY. Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem. 2004;279:19149–19156. doi: 10.1074/jbc.M311058200. [DOI] [PubMed] [Google Scholar]
- Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- Song JH, Song H, Wensel TG, Sokolov M, Martemyanov KA. Localization and differential interaction of R7 RGS proteins with their membrane anchors R7BP and R9AP in neurons of vertebrate retina. Mol Cell Neurosci. 2007;35:311–319. doi: 10.1016/j.mcn.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Song X, Vishnivetskiy SA, Seo J, Chen J, Gurevich EV, Gurevich VV. Arrestin-1 expression level in rods: balancing functional performance and photoreceptor health. Neuroscience. 2011;174:37–49. doi: 10.1016/j.neuroscience.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. Invest Ophthalmol Vis Sci. 1988;29:1012–1020. [PubMed] [Google Scholar]
- Spira AW, Milman GE. The structure and distribution of the cross-striated fibril and associated membranes in guinea pig photoreceptors. Am J Anat. 1979;155:319–337. doi: 10.1002/aja.1001550304. [DOI] [PubMed] [Google Scholar]
- Spiwoks-Becker I, Glas M, Lasarzik I, Vollrath L. Mouse photoreceptor synaptic ribbons lose and regain material in response to illumination changes. Eur J Neurosci. 2004;19:1559–1571. doi: 10.1111/j.1460-9568.2004.03198.x. [DOI] [PubMed] [Google Scholar]
- Steinberg RH. Phagocytosis by pigment epithelium of human retinal cones. Nature. 1974;252:305–307. doi: 10.1038/252305a0. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, Anderson DH. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Wood I. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Vol. 187. Royal Society; 1974. Pigment epithelial cell ensheathment of cone outer segments in the retina of the domestic cat; pp. 461–478. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Wood I. Clefts and microtubules of photoreceptor outer segments in the retina of the domestic cat. J Ultrastruct Res. 1975;51:307–403. doi: 10.1016/s0022-5320(75)80102-x. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Landers RA, al-Ubaidi MR, Rayborn ME, Hollyfield JG. Photoreceptor outer segment development in Xenopus laevis: influence of the pigment epithelium. Dev Biol. 1994;162:169–180. doi: 10.1006/dbio.1994.1076. [DOI] [PubMed] [Google Scholar]
- Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY. Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem. 2005;280:29250–29255. doi: 10.1074/jbc.M501789200. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Sokolov M, Trieu LH, Arshavsky VY. Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci. 2006;26:1146–1153. doi: 10.1523/JNEUROSCI.4289-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 2009;49:479–489. doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K, Howells AJ, Pyliotis NA. Biology of eye pigmentation in insects. Advances in insect physiology. 1982;16:119–166. [Google Scholar]
- Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190:953–963. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci. 1994;14:5818–5833. doi: 10.1523/JNEUROSCI.14-10-05818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15:2027–2037. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83:S12–22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara A, Varner HH, Hollyfield JG. Proteoglycans in the mouse interphotoreceptor matrix. II. Origin and development of proteoglycans. Exp Eye Res. 1989;48:815–839. doi: 10.1016/0014-4835(89)90066-3. [DOI] [PubMed] [Google Scholar]
- Tian M, Xu CS, Montpetit R, Kramer RH. Rab3A mediates vesicle delivery at photoreceptor ribbon synapses. J Neurosci. 2012;32:6931–6936. doi: 10.1523/JNEUROSCI.0265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol. 2005;168:825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Brandstatter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326:339–346. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E. Intersegmental fusion in vertebrate rod photoreceptors. Rod cell structure revisited. Invest Ophthalmol Vis Sci. 1995;36:1918–1933. [PubMed] [Google Scholar]
- Travis GH, Brennan MB, Danielson PE, Kozak CA, Sutcliffe JG. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Travis GH, Groshan KR, Lloyd M, Bok D. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- Trivedi D, Colin E, Louie CM, Williams DS. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric kinesin-2. J Neurosci. 2012;32:10587–10593. doi: 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt LL, Wang E, Pagh-Roehl K, Burnside B. Microtubule nucleation and organization in teleost photoreceptors: microtubule recovery after elimination by cold. J Neurocytol. 1990;19:213–223. doi: 10.1007/BF01217299. [DOI] [PubMed] [Google Scholar]
- Tsang SH, Burns ME, Calvert PD, Gouras P, Baylor DA, Goff SP, Arshavsky VY. Role for the target enzyme in deactivation of photoreceptor G protein in vivo. Science. 1998;282:117–121. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- Tsang SH, Gouras P, Yamashita CK, Kjeldbye H, Fisher J, Farber DB, Goff SP. Retinal degeneration in mice lacking the gamma subunit of the rod cGMP phosphodiesterase. Science. 1996;272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Usukura J, Bok D. Changes in the localization and content of opsin during retinal development in the rds mutant mouse: immunocytochemistry and immunoassay. Exp Eye Res. 1987;45:501–515. doi: 10.1016/s0014-4835(87)80061-1. [DOI] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Bertini E, Dallapiccola B, Gleeson JG. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Fisher SK, Bernstein SA, Hale IL, Linberg KA, Matsumoto B. Evidence that microtubules do not mediate opsin vesicle transport in photoreceptors. J Cell Biol. 1989;109:3053–3062. doi: 10.1083/jcb.109.6.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol. 2008a;15:373–380. doi: 10.1038/nsmb.1396. [DOI] [PubMed] [Google Scholar]
- Veltel S, Kravchenko A, Ismail S, Wittinghofer A. Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Lett. 2008b;582:2501–2507. doi: 10.1016/j.febslet.2008.05.053. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31:4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH, 2nd, Deretic D, Wandinger-Ness A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell. 2011;22:3289–3305. doi: 10.1091/mbc.E11-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RL, Goodenough DA, Goodenough UW. Membrane particle arrays associated with the basal body and with contractile vacuole secretion in Chlamydomonas. J Cell Biol. 1977;72:133–143. doi: 10.1083/jcb.72.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XH, Shen L, Brush RS, Michaud N, Al-Ubaidi MR, Gurevich VV, Hamm HE, Lem J, Dibenedetto E, Anderson RE, Makino CL. Overexpression of rhodopsin alters the structure and photoresponse of rod photoreceptors. Biophys J. 2009;96:939–950. doi: 10.1016/j.bpj.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RW, Dowling JE. Anatomical evidence for cone and rod-like receptors in the gray squirrel, ground squirrel, and prairie dog retinas. J Comp Neurol. 1975;159:439–460. doi: 10.1002/cne.901590402. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley HE, Young S. Cilia in the fetal and neonatal canine retina. Tissue Cell. 1985;17:335–340. doi: 10.1016/0040-8166(85)90052-7. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Gabriel R. Functional anatomy of the photoreceptor and second-order cell mosaics in the retina of Xenopus laevis. Cell Tissue Res. 1999;297:35–46. doi: 10.1007/s004410051331. [DOI] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, Yoder BK, Leroux MR. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res. 2002;42:455–462. doi: 10.1016/s0042-6989(01)00228-0. [DOI] [PubMed] [Google Scholar]
- Williams DS, Fisher SK. Prevention of rod disk shedding by detachment from the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1987;28:184–187. [PubMed] [Google Scholar]
- Wilson JE. Hexokinases. Rev Physiol Biochem Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- Wolfrum U, Giessl A, Pulvermuller A. Centrins, a novel group of Ca2+-binding proteins in vertebrate photoreceptor cells. Adv Exp Med Biol. 2002;514:155–178. doi: 10.1007/978-1-4615-0121-3_10. [DOI] [PubMed] [Google Scholar]
- Wolfrum U, Schmitt A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton. 2000;46:95–107. doi: 10.1002/1097-0169(200006)46:2<95::AID-CM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, Jackson PK. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem. 2000;275:13191–13194. doi: 10.1074/jbc.c900853199. [DOI] [PubMed] [Google Scholar]
- Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthalmol. 1969;82:151–159. doi: 10.1001/archopht.1969.00990020153002. [DOI] [PubMed] [Google Scholar]
- Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol. 2005;25:4129–4137. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen H, Zhao C, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S, Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT, Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118:2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Willardson BM, Wilkins JF, Jensen GJ, Thornton BD, Bitensky MW. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J Biol Chem. 1994;269:24050–24057. [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW. An hypothesis to account for a basic distinction between rods and cones. Vision Res. 1971a;11:1–5. doi: 10.1016/0042-6989(71)90201-x. [DOI] [PubMed] [Google Scholar]
- Young RW. Shedding of discs from rod outer segments in the rhesus monkey. J Ultrastruct Res. 1971b;34:190–203. doi: 10.1016/s0022-5320(71)90014-1. [DOI] [PubMed] [Google Scholar]
- Young RW. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res. 1977;61:172–185. doi: 10.1016/s0022-5320(77)80084-1. [DOI] [PubMed] [Google Scholar]
- Young RW. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci. 1978;17:105–116. [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–184. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchigna S, Oh H, Wilsch-Brauninger M, Missol-Kolka E, Jaszai J, Jansen S, Tanimoto N, Tonagel F, Seeliger M, Huttner WB, Corbeil D, Dewerchin M, Vinckier S, Moons L, Carmeliet P. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29:2297–2308. doi: 10.1523/JNEUROSCI.2034-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Schietroma C, Zampighi LM, Woodruff M, Wright EM, Brecha NC. Conical tomography of a ribbon synapse: structural evidence for vesicle fusion. PLoS ONE. 2011;6:e16944. doi: 10.1371/journal.pone.0016944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Constantine R, Frederick JM, Baehr W. The prenyl-binding protein PrBP/delta: A chaperone participating in intracellular trafficking. Vision Res. 2012:19–25. doi: 10.1016/j.visres.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, Inana G, Whitby FG, Jorgensen EM, Hill CP, Tong L, Baehr W. UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci. 2011;14:874–880. doi: 10.1038/nn.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Huang W, Zhang H, Zhu X, Craft CM, Baehr W, Chen CK. Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol Vis. 2003;9:231–237. [PubMed] [Google Scholar]
- Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W. Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2007;104:8857–8862. doi: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu XH, Zhang K, Chen CK, Frederick JM, Prestwich GD, Baehr W. Photoreceptor cGMP phosphodiesterase delta subunit (PDEdelta) functions as a prenyl-binding protein. J Biol Chem. 2004;279:407–413. doi: 10.1074/jbc.M306559200. [DOI] [PubMed] [Google Scholar]
- Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]


