Abstract
Background
Activation of the P2X7 Receptor on nerve cells causes the formation of pannexin pores, which allows the influx of calcium across the cell membrane. Polyethylene glycol (PEG) and methylene blue (MB) have previously been shown to delay Wallerian degeneration if applied during microsuture repair of the severed nerve. Our hypothesis is that by modulating calcium influx via the P2X7 receptor pathway, we could improve PEG based axonal repair. The P2X7 receptor can be stimulated or inhibited using bzATP or Brilliant Blue (FCF), respectively.
Methods
A single incision rat sciatic nerve injury model was used. The defect was repaired using a previously described PEG, MB fusion protocol. Experimental animals were treated with 100 µL of 100 µM FCF solution (n=8) or 100 µL of a 30 µM bzATP solution (n=6). Control animals received neither FCF, bzATP, nor PEG. Compound Action Potentials (CAPs) were recorded prior to transection (baseline), immediately after repair, and 21 days post operatively. Animals underwent behavioral testing 3,7, 14, and 21 days post operatively. After sacrifice, nerves were fixed, sectioned, and immunostained to allow for counting of total axons.
Results
Rats treated with FCF showed an improvement as compared to control at all time points (n=8) (p= .047, .044, .014, and .0059 respectively). A statistical difference was also shown between FCF and bzATP at Day 7 (p<.05), but not shown with days 3, 14, and 21. (p>.05).
Conclusions
Blocking the P2X7 receptor improves functional outcomes after PEG mediated axonal fusion.
Keywords: traumatic neuropathy; hydrophilic polymers; single-incision axonal repair; methylene blue; polyethylene glycol; P2x7 receptor; Brilliant Blue FCF; 2′,3′-O-(benzoyl-4-benzoyl)-ATP
Introduction
Current strategies for peripheral nerve repair include nerve grafts, nerve growth guides, and micro-sutures. (1) These techniques rely on the proximal outgrowth at a rate of 1mm/day toward the targeted tissues. The time needed for re-innervation of proximal injuries (measured in months) as compared to distal injuries (measured in weeks) allows for muscle atrophy causing repair failure. (2) Nerve fusion is an emerging technology that could prevent this atrophy seen after months allowing for a successful re-innervation by the proximal axonal outgrowth. (3) The impact of nerve fusion has lead to a great interest in mammalian models of peripheral nerve repair. (4,5,6) Polyethylene glycol (PEG) based fusion has been shown to fuse the axolemmal membranes of crushed/severed nerves, the most common nerve injuries after trauma. (4) In vivo experiments have shown improved behavioral outcomes and increased numbers of axons in the distal, reattached segment. (4,5) The obstacles of nerve fusion include delaying and inhibiting the process of wallerian degeneration, preventing the sealing of the proximal nerve end, and reestablishing the morphology and functionality of the nerve. (7)
In an attempt to further enhance the efficiency of PEG based axonal fusion, which has been shown to be calcium dependent (8), we attempted to modulate calcium influx at the cellular level through selective activation or inhibition of calcium channels. Activation of the purinergic P2X7 receptor causes the formation of pannexin pores. (9,10) These holes allow for the efflux of ATP and the influx of calcium across the cell membrane. For injured neurons, this has been reported to play a role in the initiation of Wallerian degeneration. (8,11) PEG and MB have previously been shown to delay Wallerian degeneration through a receptor-independent manner if applied during microsuture repair of the severed nerve. (4,7,5)
Calcium modulation is a critical part of the PEG based axonal repair because once calcium enters the axon, the cut nerve ends seal preventing PEG based axonal fusion. Our hypothesis is that by modulating calcium influx via the P2X7 receptor pathway, that we could improve outcomes after PEG based axonal repair. Specifically, by blocking the p2x7 pathway with (FCF), a purinergic specific blocker of the p2x7 pathway, or activating with (bzATP), a nonspecific activator of the p2x7 pathway, in combination with PEG mediated fusion, we can modulate the progress of Wallerian degeneration in a rat model. (10)
Materials and Methods
All experimental procedures were approved by and performed in accordance with the standards set forth by the Institutional Animal Care and Use Committee at Vanderbilt University.
SURGICAL PROCEDURES
Female Sprague-Dawley rats were anesthetized with inhaled isoflourane, their left hind limb was shaved with clippers, and the surgical site was prepped aseptically. A two cm incision was made parallel, and approximately 1 cm caudally from the femur. Using sharp dissection, the biceps femoris was bisected along the plane of muscle fibers overlaying the sciatic nerve. The muscle fibers were then retracted exposing the left sciatic nerve. The exposed nerve was then dissected free of perineural tissue using sharp dissection and minimal retraction. The exposed nerve was bathed in Plasma-lyte A® (Baxter: Deerfield, IL) and electrophysiological testing was performed. Plasma-lyte A® is a calcium free solution containing the following (in mEq/L): (Na 140, K 5, Mg 3, Cl 98, Acetate 27, Gluconate 23) at pH 7.4 containing 294 mOsm/L.
The sciatic nerve was transected prior to the trifurcation followed by irrigation with Plasma-lyte A®. The proximal and distal nerve ends were then bathed in a 100 microliters of 100 microMolar FCF solution (Sigma-Aldrich; St. Louis, MO)(12) or a 100 microliters of a 30 microMolar bzATP (Sigma-Aldrich; St. Louis, MO) solution. Using standard microsurgical techniques, with careful attention paid to maintain orientation using the epineural blood vessels, the two ends were sutured in place using 9-0 Ethilon (Ethicon, Sommerville, NJ). Once the nerve was rejoined in an end-to-end fashion using an interrupted suturing technique and microscopic magnification, a hypotonic 1% solution of MB (Acros Organics; Morris Plains, NJ) in sterile water was applied to the coaptation site for one minute. A 50% by weight solution of PEG (3.35kD molecular weight, Sigma-Aldrich; St. Louis, MO) in sterile water was then applied to the coaptation sites for 1 minute in experimental animals. Additional animals received the surgical protocol with FCF or BzATP, but they did not receive the PEG treatment. Control animals received only the MB solution.
The wound was then irrigated with Lactated Ringers (Hospira; Lake Forest, IL) and electrophysiological testing was repeated. Lactated Ringers contains the following (in mEq/L): Na 130, K 4, Ca 2.7, Cl 109, and Lactate 28. This isotonic, calcium-containing solution is at pH 6.5 and has 273 mOsm/L. The retractor was removed and the muscle was sutured back together in the appropriate orientation using 5-0 monocryl suture (Ethicon, Sommerville, NJ). The skin was approximated using a running subcuticular 5-0 monocryl suture (Ethicon, Sommerville, NJ). All control and experimental animals were then given a subcutaneous injection of ketoprofen (5mg/kg) and allowed to emerge from anesthesia. Ketoprofen is almost completely excreted by 24 hours postoperatively, having minimal, if any, impact on behavioral testing occurring after that time point. (13)
At 3, 7, 14, and 21 days postoperatively, behavioral testing was performed. After behavioral testing on the 21 day postoperatively, the rats were again anesthetized with inhaled isoflourane and the left hind limb was prepared as previously described. Using the same exposure technique, the left sciatic nerve was exposed. The wound was irrigated with Plasma-lyte A® and electrophysiological testing was repeated.
The rat was then sacrificed via intracardiac injection of Fatal-Plus Solution (Vortech, Dearborn, MI). The injured nerve was harvested immediately after sacrifice and placed into 10% neutral buffered formalin. For electrophysiological testing and behavioral testing, 4 rats were in the FCF experimental group, 4 rats were in the BzATP experimental group, and 12 rats were in the baseline group. For histological testing, 8 rats were used in the FCF experimental group, 6 rats were used in the BzATP experimental group, and 7 rats were used in the control group.
ELECTROPHYSIOLOGY TESTING
Compound Action Potentials (CAPs) are a measure of axonal continuity and all were obtained using a Powerlab Data Acquisition System (ADInstruments; Colorado Springs, CO) interfaced with Scope™ 4 (ADInstruments; Colorado Springs, CO). One dual-terminal hook electrode was placed under both the proximal and distal end of the exposed nerve. The proximal electrode was used to deliver an electrical stimulus and the distal electrode was used to record the stimulus and CAPs. CAPs were recorded prior to nerve transection (baseline), immediately after repair with solution therapy, and at 21 days postoperatively.
BEHAVIORAL TESTING
Behavioral assessments were performed at 3, 7, 14, and 21 days postoperatively. Animals were not tested earlier to allow for adequate recovery from anesthesia and to minimize any confounding effects of ketoprofen administration at the time of surgery.
Foot Fall Asymmetry Score
Animals were allowed to roam freely on a mesh grid measuring 45 cm × 30 cm, with square openings measuring 2.5 cm × 2.5 cm. The grid was elevated 2 cm above a solid base. Trials for each animal were recorded for 50 total steps per hindlimb. A foot fault was scored when the stepping hind-limb fell through an opening in the grid, touching the floor. If the hindlimb was retracted after falling through the grid opening, but prior to touching the floor, the step was scored as a partial fault. A composite score was calculated using the previously reported methods and the following equation (4,14):
Composite Foot Fault score = (# Partial Faults × 1) + (# Full Faults × 2)
% Foot Fault = (Composite Foot Fault score/total number of steps) × 100%
Foot Fault Asymmetry Score = % Foot Fault (normal hindlimb) − % Foot Fault (surgical hindlimb)
HISTOLOGY
Immunohistochemistry
Immunohistochemical staining was performed using commercial antibodies specifically directed against Myelin Basic Protein (MBP) (Abcam, Cambridge, MA) and S100 alpha (S100a) (Abcam, Cambridge, MA). Formalin-fixed paraffin embedded tissues were sectioned at 5 µm, placed on slides and warmed overnight at 60°C. Slides were deparaffinized and rehydrated with graded alcohols ending in Tris buffered saline (TBS-T Wash Buffer, LabVision, Freemont, CA). For both, heat mediated target retrieval was performed in 1X Target Retrieval Solution (pH 6.0, DAKO, Carpenteria, CA). Endogenous peroxidases and non-specific background were blocked by subsequent incubations in 3% H2O2 (Fisher, Suwanee, GA) in TBS-T and serum-free Protein Block (RTU, DAKO). Primary antibodies to MBP and S100A were used at 1:800 and 1:1,600, respectively for 1 hour, followed by incubation in EnVision+ HRP Labelled Polymer (RTU, DAKO). Slides were rinsed with TBS-T between each reagent treatment and all steps were carried out at room temperature unless otherwise noted. Visualization was achieved with DAB+ chromogen (DAKO). Slides were counterstained with Mayer’s hematoxylin, dehydrated through a series of alcohols and xylenes, and then coverslipped with Acrytol Mounting Media (Surgipath, Richmond, IL).
Light Microscopy
All stained slides were examined using an Olympus Vanox-T AH-2 light microscope (Olympus, Center Valley, PA) interfaced to Pixera Pro 600 HS digital camera (Pixera Corporation; Santa Clara, CA). Multiple digital photomicrographs were captured through a 10× objective using Viewfinder V3.0.1 (Pixera Corporation; Santa Clara CA). For each nerve processed, representative cross sections were photographed distal to the site of injury and distally from the sciatic nerve. To count axons the number of stained axons on each photomicrograph, ImageJ v1.45 software combined with the Wright Cell Imaging Facility plug-in package was used in a method that has been previously reported. (15, 16) Equivalent detection thresholds were used for counting axons in each photomicrograph. The total number of axons per cross section was determined by adding the axon totals from all representative photomicrographs or a given cross section; care was taken to avoid duplicate counting of axons.
STATISTICAL ANALYSES
All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software; San Deigo, CA). To compare CAPs from all groups a Kruskal-Wallis test with Dunn’s multiple comparison test was performed. Specific CAPs comparisons were completed with an unpaired student’s t-test, where appropriate. With regard to foot fault asymmetry scores, a two way ANOVA with Bonferroni’s multiple comparison test was employed to specifically compare treatment and control groups. For comparison of axon counts Student’s t-test was used to compare specific groups. All p values were 2-tailed and significance was determined at p < .05.
IN VITRO SEALING
B104 cells
B104 cells derived from a CNS neuroblastoma of hippocampal origin (17) were used as a model system to study neuronal function in vitro (17). B104 cells have easily identifiable cell bodies and axon-like neurites, allowing each cell to be precisely transected and uniquely and individually identified (17). Data on sealing of B104 cells are consistent with data on plasmalemmal sealing from at least 20 other preparations from many phyla and different cell types in vitro and in vivo.
Cell culture
As previously reported (17), B104 cells were grown in 75 cm2 vented cap flasks (BD-Falcon, Franklin Lakes, NJ) in a humidified incubator at 37°C in 5% CO2 in 4 mL of “cell-growth media,” which consists of a 1:1 mixture of Dulbecco’s Modified Eagle’s Media and Ham’s F12 (DMEM:F12, HyClone, Logan, UT), supplemented for growth with 10% heat inactivated fetal bovine serum (FBS, Hyclone, Logan, UT), and containing 1% antibiotics (10,000 Units of Penicillin/mL and 10 mg/mL of streptomycin, Sigma-Aldrich, St. Louis, MO). Cell-growth media was changed every 2 days and cultures passaged at 80% confluency. Cells were then either sub-cultured in a vented cap flask or seeded at approximately 2000 cells/cm2 in cell-growth media on Petri dishes coated with Poly-D-lysine (Sigma-Aldrich, St. Louis, MO) to prevent cells from detaching during solution changes and/or neurite transections. After 24 hours, the cell-growth media was replaced with serum-free DMEM:F12 (Hyclone, Logan, UT) in which B104 cells then differentiate. B104 neurites were typically transected 24–48 hours after replacing the cell-growth media with serum-free DMEM:F12.
Transection of neurites of B104 cells
Prior to transection, the differentiation medium was washed out of the dishes twice with Ca2+-free phosphate buffered saline (referred to as “Ca2+-free saline”, PBS −/−, HyClone, Logan, UT) and replaced with Ca2+-free saline. Unless stated otherwise, all neurites were transected in Ca2+-free saline using a sharpened, pulled-glass micro-capillary tube ("micro-knife"), which was placed on a micro-manipulator (Narishige Instruments, East Meadow, NY) and quickly drawn across the surface of the Petri dish, etching a score line that showed the path of the knife. We were able to uniquely and individually identify each transected cell by the relation of the transected neurite to its soma and to the score mark on the plate (17). Cells were exposed to Ca2+-free saline for a maximum of ten minutes. The Ca2+-free saline was then replaced with a phosphate buffered saline containing 1 mM Ca2+ (“Ca2+-saline”, PBS+/+, HyClone, Logan, UT) to initiate the sealing process (17). Cells were exposed to Ca2+-saline for five minutes prior to assessment of sealing.
Assessment of plasmalemmal sealing
Dye exclusion is the most reliable and reproducible measure of plasmalemmal sealing (17). Other methods of evaluating sealing often have ambiguous or un-interpretable results (17). Membrane bound structures are often damaged during fixation for electron microscopy, thereby preventing accurate observations of vesicle accumulation at the damage site (17).
We added a fluorescent dye, 3 kDa Texas Red dextran (Molecular Probes, Eugene, OR), to the Ca2+-free saline as an indicator of rapid plasmalemmal sealing. For all experiments, the dye was thoroughly washed out with Ca2+-saline after a 10 min exposure to Texas Red dextran and sealing was assessed. Transected cells that excluded dye were counted as “sealed”. Cells that did not exclude dye were counted as “not sealed”. We consistently used 3 kDa Texas Red dextran to assess sealing in all other experiments reported herein to avoid any variation in sealing time due to differences in dye molecular weight (17).
B104 cells were observed under an inverted Zeiss Axio Vert A1 fluorescent microscope with a 40×, long focal length lens and illuminated by a Lumen Dynamics X-Cite series 120Q light source. We transected 10–30 uniquely identifiable cells within 5–10 min in a Petri dish containing the Ca2+-free saline, Texas Red Dextran and, in some, bzATP. Sealing of individually identified cells transected nearer to (< 50 µm), and farther from (> 50 µm), the soma were typically observed on the same Petri dish. For this paper, we only counted cells with neurites transected farther from the soma. To insure that transections “farther from the soma” were greater than 50 µm, other cells were purposefully cut as far from the cell body as possible (typically 80–100 µm). At least 44 cells total were cut using at least two Petri dishes with an average of 64. Cells with transection distances not obviously “farther from” the soma were not included in dye exclusion counts.
Pharmacological Reagents and Toxins
All pharmacological agents were dissolved in distilled water, unless otherwise noted.: [2’(3’)-O-(4-Benzoylbenzoyl)adenosine 5’-triphosphate triethylammonium salt : BzATP ,Sigma B6396), Texas Red Dextran, 3000 MW, Neutral (Invitrogen D3329).
Statistical analyses
For each experimental treatment group, at a given PC time, the data were pooled for all cells (n) from all Petri dishes (N). “Sealing probability” was defined as the percent of a set of individually-transected and uniquely identified cells that excluded 3 kDa Texas Red dextran (sealed) at a given PC time. This value was obtained by dividing the total number of sealed cells by the total number of transected cells. Therefore, a “sealing probability” of 30% means that 30% of transected cells completely excluded dye at a given PC time.
The Cochran-Mantel-Haenszel χ2 (CMH Χ2) test for independence was used as the most appropriate statistical test to determine whether differences between sealing probabilities at a given PC time for different experimental treatments were statistically significant (p < 0.05), as previously described (17). Briefly, the CMH Χ2 test for independence is used to compare data separated into two by two contingency tables (sealed or not sealed, and control vs. experimental data sets). Furthermore, the CMH Χ2 test requires binary outputs, which applies to our sealing assay (sealing is treated as a “yes” or “no” event at a given PC time). Since sealing of a given cell and, but not each Petri dish (sometimes containing over 100 transected cells), are independent events, measures of variance (such as SE or SD) are not applicable.
Results
ELECTROPHYSIOLOGY DATA
Electrophysiological testing immediately post-repair revealed a difference between baseline (mean 4.965 n = 12) and FCF (mean 0.8225, n = 4) (p=0.0091) (Figure 1). A difference was also seen between BzATP (mean 5.045 n = 4) and FCF (mean 0.8225, n = 4) (p = 0.0208), but no difference was seen between Baseline and BzATP (Figure 1). Baseline CAPs were present in all animals (mean 4.965 ± 2.032 mV, minimum 1.360 mV, maximum 8.240 mV; n= 12) and the combined results are shown (Figure 1). Individually, no statistically significant differences were detected between baseline CAPs in the experimental control groups (Use One-way Anova): FCF (mean 5.230 ± 2.608 mV, min 1.940 mV, max 8.240 mV), experimental BzATP (mean 5.032 ± 2.631 mV, min 1.360 mV, max 7.500 mV), experimental oATP (mean 4.633 ± 1.081 mV, min 3.490 mV, max 5.600 mV), or control groups (data not shown) prior to neurotomy. After nerve transection and repair CAPs were obtained for most animals within the experimental groups. Controls for PEG fusion (n = 6), FCF without PEG (n = 6), and BzATP without PEG (n = 6) had no CAPs immediately after repair or 21 days post-repair. By contrast, 21 day post-repair CAPs were found in FCF-treated animals (n = 6) and not in the BzATP (n = 6) treated animals. Values for the FCF treated animals were statistically smaller than baseline values (p = 0.0012). (Figure 2) 3 week FCF CAP values were mean 1.105 ± 1.713, min 0.000, max 3.400, n = 6. Thus, the FCF treated group was the only group that exhibited CAPs at 21 days, as compared to control and BzATP. While there was no statistically significant difference between baseline and immediate BzATP-treated CAPs (p = 0.9567), CAPs could not be obtained in the BzATP with PeG, BzATP without PEG, FCF without PEG, or the Control groups at 21 days. (Figure 2)
Figure 1. P2x7 Modulation Affects Compound Action Potential Amplitudes of Rat Sciatic Nerves Immediately after a PEG-Fused Microsurgical Repair.
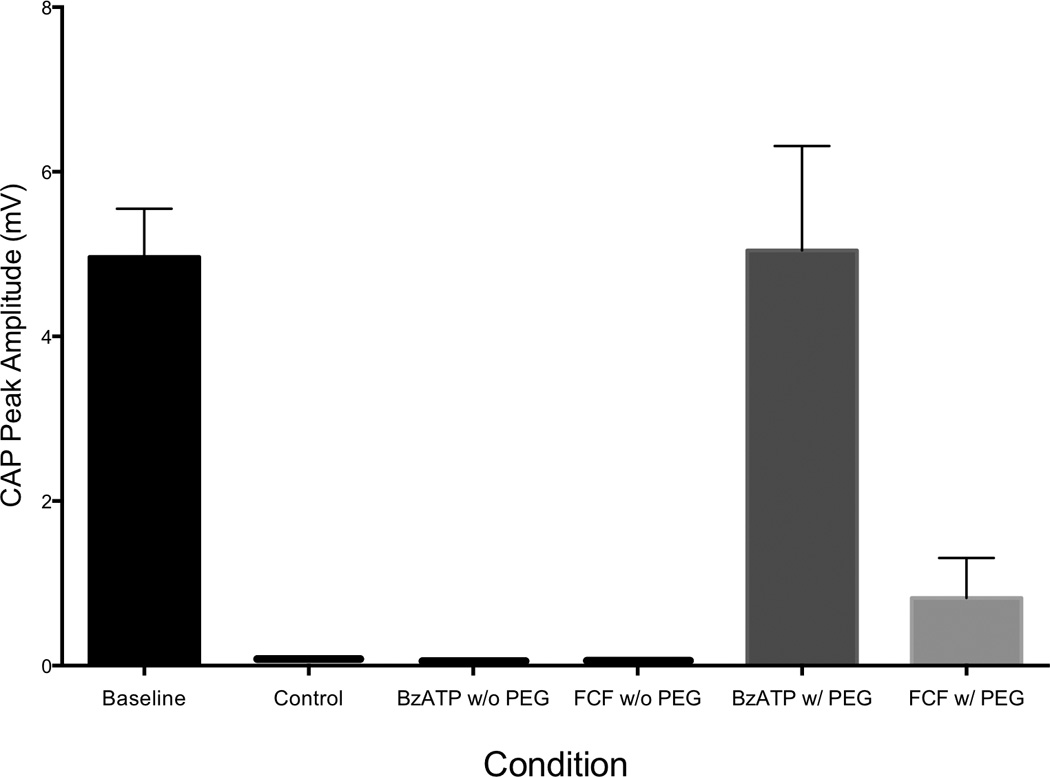
FCF treatment groups demonstrated a statistically significant decrease in Compound Action Potential Amplitudes as compared to Baseline and BzATP treatment groups immediately after PEG-based Nerve Fusion (50% PEG 3.35kD). (p < 0.0091 and p < 0.0208). Baseline n=12, BzATP n=4, FCF n=4.
Figure 2. Blocking the P2x7 Receptor Preserves the Compound Action Potential of Rat Sciatic Nerves 21 Days after a PEG-Fused Microsurgical Repair.
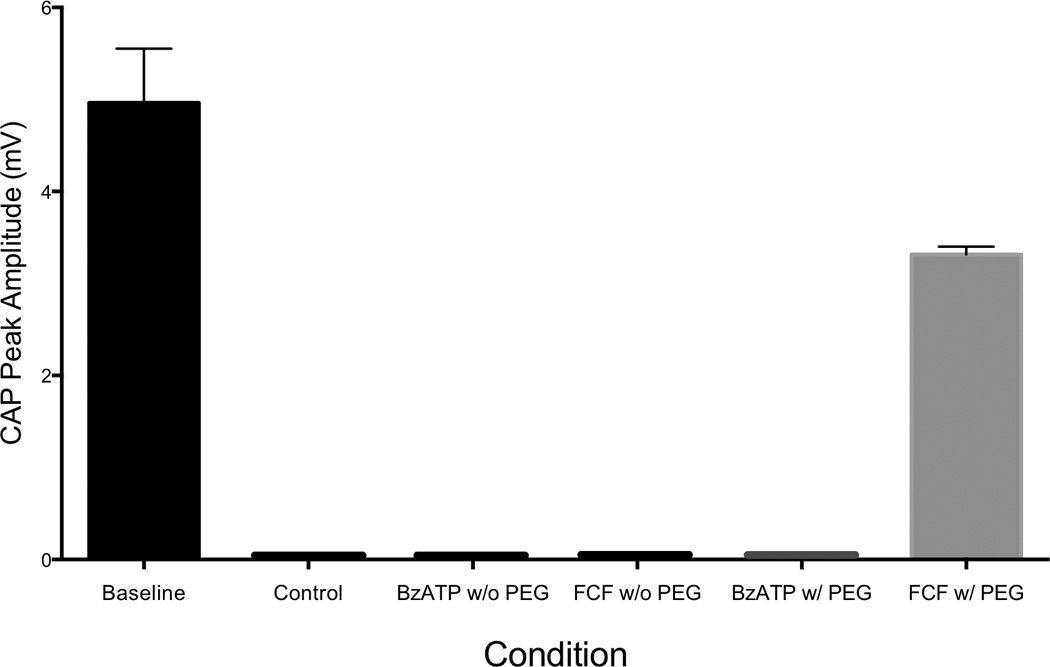
FCF treatment groups maintained a Compound Action potential at 21 days while the BzATP and Control Treatments groups did not. The CAP recorded for the FCF treatment group was statistically less than baseline. (p < 0.0012) FCF n=6, Baseline n=12
BEHAVIORAL DATA
Foot Fall Asymmetry Score
Footfall asymmetry scores were significantly improved for FCF treatment group compared to FCF without PEG at 7, 14, and 21 days postoperatively (p<.05). (Figure 3). The FCF treatment group was improved compared to control at 3, 7, 14, and 21 days (p<.05)There was a difference seen on Day 7 between FCF with PEG and BzATP with PEG (p<.05), but that trend was not seen on day 3, 14, and 21 (p>.05). On days 3, 7, and 14 BzATP with PEG was statistically better than BzATP without PEG (p<.05). (Figure 3) FCF with PEG Treated values for footfall asymmetry scores for postoperative day 3 had a mean value of −75.25 ± 21.75 (min −116, max −42, n = 8), postoperative day 7 had a mean value of −66.25 ± 5.020 (min −86, max −46, n = 8), postoperative day 14 had a mean value of −52.75 ± 3.890 (min −74, max −42, n = 8), and postoperative day 21 had a mean value of −55.50 ± 6.276 (min −80, max −22, n = 8). BzATP with PEG Treated values for footfall asymmetry scores for postoperative day 3 had a mean value of −81 ± 17.29 (min −108, max −64, n = 6), postoperative day 7 had a mean value of −90.00 ± 4.517 (min −106, max −78, n = 5), postoperative day 14 had a mean value of −68.00 ± 7.616 (min −90, max −44, n = 5), and postoperative day 21 had a mean value of −68.80± 5.499 (min −88, max −58, n = 5). FCF without PEG values for footfall asymmetry scores for postoperative day 3 had a mean value of −102.0 ± 7.155 (min −116, max −96, n = 6), postoperative day 7 had a mean value of −99.67 ± 9.416 (min −116, max −90, n = 6), postoperative day 14 had a mean value of −95.33 ± 11.84 (min −118, max −86, n = 6), and postoperative day 21 had a mean value of −69.33 ± 15.42 (min −86, max −42, n = 6). BzATP without PEG values for footfall asymmetry scores for postoperative day 3 had a mean value of −121.0 ± 8.075 (min −130, max −112, n = 6), postoperative day 7 had a mean value of −118.3 ± 10.07 (min −128, max −100, n = 6), postoperative day 14 had a mean value of −117.7 ± 12.23 (min −138, max −102, n = 6), and postoperative day 21 had a mean value of −86.00 ± 12.96 (min −102, max −66, n = 6). Control values for footfall asymmetry scores for postoperative day 3 had a mean value of −98.4 ± 8.877 (min −114, max −92, n = 5), postoperative day 7 had a mean value of −88 ± 4 (min −102, max −72, n = 8), postoperative day 14 had a mean value of −72.75 ± 3.228 (min −92, max −62, n = 8), and postoperative day 21 had a mean value of −80.29 ± 3.637 (min −94, max −66, n = 7).
Figure 3. Blocking the P2X7 Receptor Improves Behavioral Recovery After Axonal Fusion.
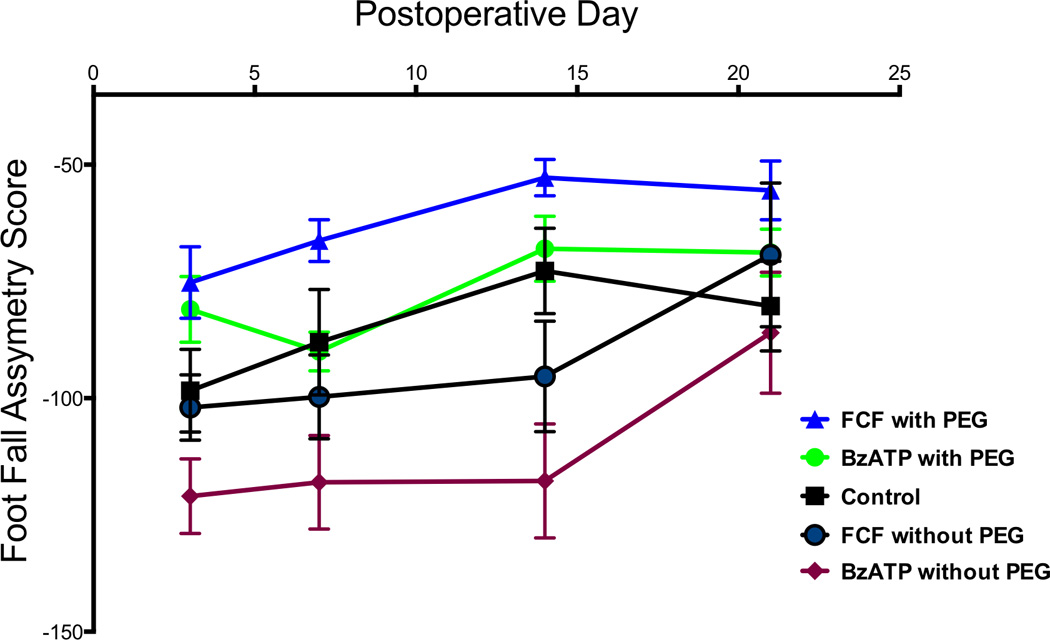
The P2x7 receptor is activated by BzATP, an ATP analog, and competitively antagonized by FCF. At 7 days and 14 days FCF with PEG was statistically improved compared to FCF without PEG (p<.05). On day 7, FCF with PEG was improved compared to BzATP with PEG (p<.05), however, on days 3, 14, and 21 there were no differences seen statistically. On day 3, day 7, day 14 BzATP with PEG was statistically better than BzATP without PEG (p<.05). FCF was statistically better than Control at all time points.
IN VITRO SEALING
Figure 4 shows the ratios of sealed to unsealed neurons where different concentrations of BzATP were used. Neurons treated with 0.3 µM, 3 µM, 30 µM, and 300 µM had a sealed to unsealed ratio of 0.2558±0.4415, 0.4833±0.5039, 0.4714±0.5028, and 0.3205±0.4697 respectively. There was a statistical difference between 3 µM and 0.3 µM (p=.0245) as well as 30 µM and 0.3 µM (p=.0289). There was so difference between 3 µM, 30 µM, and 300 µM concentrations.
Figure 4. The Ideal Concentration of BzATP for Fusion is Between 3 and 30 MicroMolar.
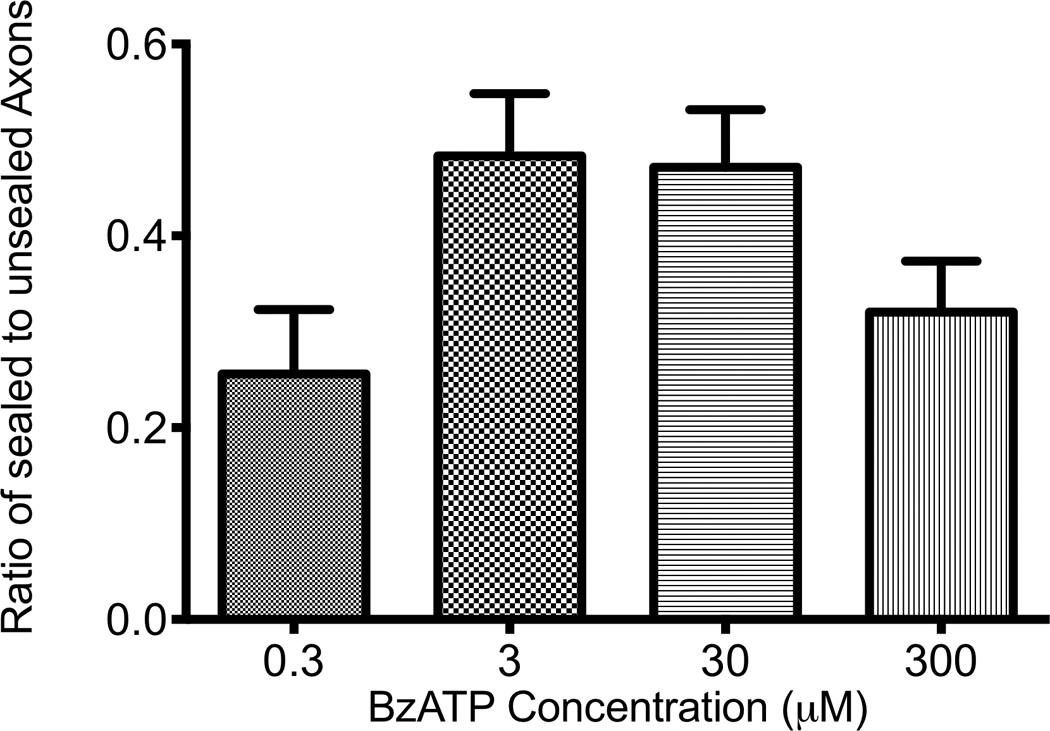
Ideal concentrations were determined to be between 3 and 30 µM. There a statistical difference 3 µM and 0.3 µM as well as 30 µM and 0.3 µM (p<.05). No statistical difference was shown between 300 µM and the other groups.
HISTOLOGICAL DATA
Figure 5 shows representative nerve sections staining for axons using antibodies specific for myelin basic protein 21 days after injury. Nerves treated with FCF did show a trend of increased myelinated axonal counts (1830±351.1, n=8) compared to BzATP (1690±166.5, n=6), or control (1614±237.4, n=5), but this trend did not reach statistical significance (p=.3853, p=.2971), however, this could be confounded by axonal outgrowth because axons could have grown over 2cm from the neurorrhaphy site in this timeframe.
Figure 5.
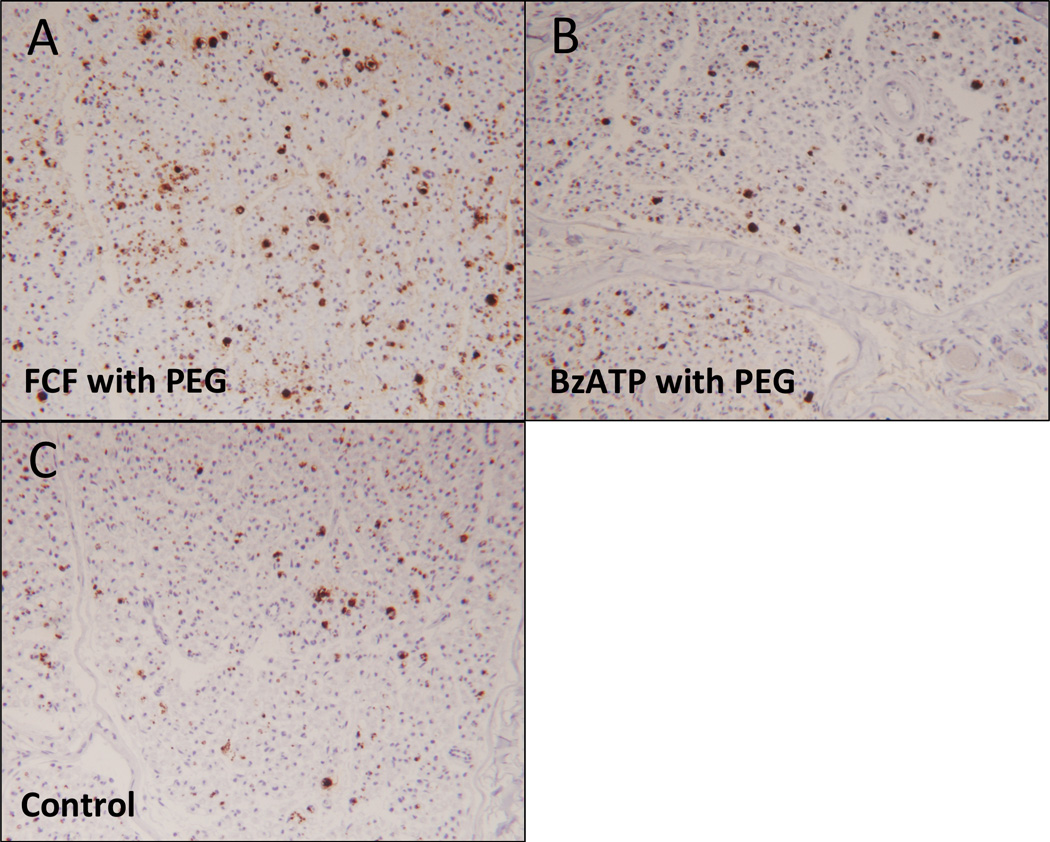
Representative photomicrographs of myelin basic protein staining, 21 days postoperatively, distal nerve cross section comparing:
A. FCF with PEG treated nerve
B. BzATP with PEG treated nerve
C. Control nerve
Discussion
Peripheral nerve injury is common, occurring in over 17,500 cases annually in the United State. (18,19) Techniques such as nerve grafts, tissue matrices, and nerve growth guides have been designed to enhance the number of axons regenerating by outgrowths from surviving distal stumps. (3) Regardless of repair strategy, regenerating axons may take months to reach the denervated target tissues when injuries are proximal which results in poor outcomes. (3, 20) Electrophysiological studies of distal nerve segments after cut severance in rats revealed that distal stumps, when stimulated directly, most will fail to conduct CAPs by 24 hours and all failed to conduct CAPs at 36 hours even if repaired. (21) In our prior reports on PEG fusion, we demonstrated that PEG fusion of severed nerves can delay this process and behavioral studies also showed that PEG fused nerves improved functional outcomes after nerve transection. (7,5)
Calcium modulation is a critical part of PEG based axonal repair because once calcium enters the axon, the cut nerve ends seal preventing PEG based axonal fusion. (7) In an attempt to further enhance the efficiency of PEG based axonal fusion (7), we attempted to modulate calcium influx after injury at the cellular level through selective activation or inhibition of channels that allow calcium influx after injury. P2X receptors are a family of ligand-gated ion channels consisting of proteins with intracellular amino and carboxy termini and a large extracellular loop between 2 hydrophobic segments that bind extracellular ATP. (10) Seven different subtypes of this receptor have been cloned, the first isolated from smooth muscle and the remaining from nervous tissue. (22) Activation of this receptor has been shown to increase calcium influx by causing the formation of pannexin pores after nerve injury. (23,24,25). These holes allow for the influx of calcium across the cell membrane. Figure 6 and Figure 7 demonstrate the proposed effects of P2X7 on intracellular calcium. For injured neurons, this is believed to play a role in the initiation of Wallerian degeneration. (8,11) Blocking the P2X7 pathway with Brilliant Blue (FCF), a purinergic specific blocker of the P2X7 pathway, or activating with (bzATP), a nonspecific activator of the P2X7 pathway, (25) in combination with PEG mediated fusion, we can enhance, with FCF, or reduce, with bzATP, the post PEG fusion compound action potentials. We believe this smaller current of calcium ions crossing the plasmalemmal membrane is due to closed pannexin pores caused by the Antagonist effects of FCF. (Figure 1) Figure 2, however, shows that fused axons treated with FCF are more likely to maintain the ability to support CAP transmission after 3 weeks compared to either control, or BzATP treated axons. Figure 3 shows the progression of behavioral function over 21 days. Remarkably, rats treated with FCF, which blocks the P2X7 pathway, showed significant improvement compared to control points (n=8). A statistical difference was also shown between FCF and bzATP at Day 7 (p = .008), but not shown with days 3, 14, and 21. (p=.60, .0733, and .1724, respectively). This data implies that blocking the P2X7 receptor improves functional outcomes compared to controls. Footfall behavioral data is a more specific test for sciatic nerve function whereas CAPs are more sensitive for sciatic nerve function. This could potentially explain the discrepancy seen in Footfall data versus CAPs at 3 weeks.
Figure 6. Blocking the P2X7 receptor improves long term functional outcomes.
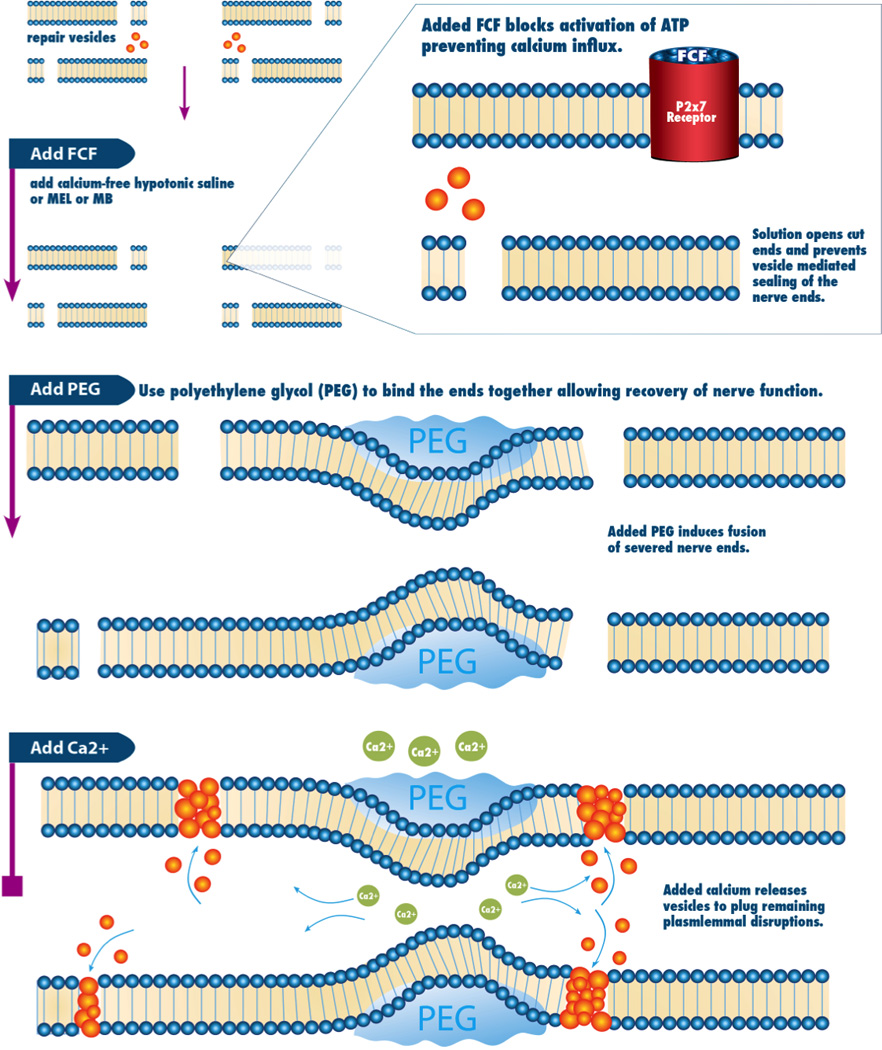
Proposed mechanism of PEG-mediated axon fusion with the role of P2x7 on intracellular calcium levels.
Figure 7. P2x7 activated after axonal injury resulting in calcium influx.
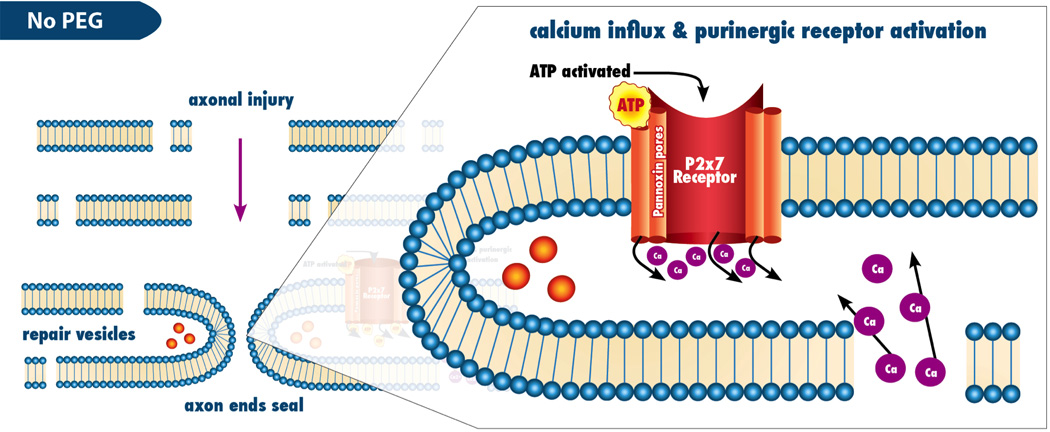
Mechanism by which P2x7 contributes to the axonal injury response
Interestingly, in-vitro studies using bzATP and our B104 neurite axonal injury model have shown that bzATP can enhance sealing in a dose dependent fashion (Fig 4). Although at high doses, bzATP becomes toxic to cells (data not shown), lower doses in the 3–30uM range statistically increase sealing of transected neurites. We believe this premature sealing prevents PEG based fusion and explains why the bzATP PEG fused animal behavior models that of no PEG fusion control (Figure 3). Interestingly, this is one of the first reports of increased sealing of transected B104 neurites in the absence of calcium. Possible explanations for this bzAPT / P2X7 mediated sealing after axon injury include alteration of intracellular calcium stores to augment fusion, or could even imply an alternative, calcium independent, mechanism of axonal sealing after injury.
We also noted a trend toward improvement in axon survival noted in immunohistochemical studies at 3 weeks after nerve transection and repair. Figure 5 Taken together, these data give evidence that receptor based modulation of calcium entry after nerve injury can enhance outcomes after PEG based axonal fusion in a rat model. We believe that this is most likely due to prevention of P2X7 mediated calcium influx after axonal injury and could have implications for this and other nerve repair strategies.
Historically, experimental models of nerve injury have shown that calcium modulation can improve nerve recovery. (26,27) These authors demonstrated that nerve outgrowth and recovery could be increased through calcium channel modulation. These elegant animal studies were followed by a remarkable case report in which calcium channel blockade with nimodipine resulted in advanced recovery after laryngeal nerve transection in a human patient. (26) Our work lends further evidence that calcium modulation may be beneficial after peripheral nerve injury. Treatment with FCF can improve early functional outcomes after PEG fusion of acutely transected nerves. (Figure 3) Fusion of transected axons after injury or resection could revolutionize nerve repair strategies. If these strategies demonstrate progress in larger animal models where the distance between end organs and nerve injury site are larger, we envision that this line of investigation will eventually find its application in human nerve injury. For patients with proximal nerve injuries or brachial plexus injuries, these advancements might mean more than just earlier recovery, but might allow for recovery of muscle function that is not possible using current nerve repair strategies. Since the rate of axonal outgrowth after standard neurorrhaphy techniques is only 1 mm per day, for proximal neuronal injuries that are over 30 cm from the motor endplate, reinnervation occurs too late for functional recovery. By avoiding the current delays in recovery associated with axonal outgrowth, patients may experience more immediate and overall improved outcomes.
Future Directions
Since nerve repair is often not available immediately at the time of injury, we will expand our trials to include techniques that may increase our ability to fuse severed axons in a delayed nerve repair model. We have evidence that we can successfully delay nerve repair up to 24hrs after injury and still fuse the severed axons, but note that if the repair is delayed beyond 3 days that nerve degeneration will reduce our ability to fuse-repair severed axons. (data not shown) Interestingly, we note a trend that behavioral outcomes only improved roughly 25% in the 24 hour delayed nerve injury experiment compared over 50% improvement typically seen at similar post fusion times (data not shown) for animals that underwent nerve severance with immediate repair. As noted in this report, we have been able to demonstrate that modulation of calcium influx into the injured axons by treating the wound bed with FCF at the time of injury, and immediately before fusion repair improves outcomes (Figure 3). We plan to use FCF to see if we can improve outcomes in a delayed nerve repair model where animals have their sciatic nerves injured, then repaired 24hr later to try to bolster outcomes to the level seen when the fusion repair is performed immediately at the time of injury.
Limitations
Larger animal model should be studied in order to confirm that both the PEG fusion effect and the calcium modulation effect of FCF are maintained and are relevant to human nerve injuries. In our small animal model, 8 weeks after nerve repair axonal outgrowth in control groups that have appropriate neurorrhaphy typically improve and this may confound our ability to assess differences in treatment groups. Also, we do not know how many axonal segments are PEG-fused or the specificity of the motor or sensory proximal-distal axonal fusions. Misalignments can be compounded when using nerve grafts to repair gaps, or when mixed nerves, as opposed to purely motor or sensory nerves are repaired. If outcomes in future large animal studies are suboptimal, rotational studies may be required to evaluate functional outcomes in situations where nerve fascicles are purposely misaligned. If this is a persistent problem in our nerve repairs, fascicular based fusion or more microsutures may be required to improve alignment and minimize the effects of rotation.
In summary, we report that combination therapy of PEG fusion and calcium modulation technique can improve electrophysiologic and behavioral outcomes after nerve transection. Future studies to include the testing with larger animal models and even nerve grafts for nerve injuries with nerve gaps allografts to build on the foundational work reported in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalbermatten DF, Pettersson J, et al. New fibrin conduit for peripheral nerve repair. J Reconstr Microsurg. 2009;25(1):27–33. doi: 10.1055/s-0028-1090619. [DOI] [PubMed] [Google Scholar]
- 2.Lago N, Navarro X. Correlation between target reinnervation and distribution of motor axons in the injured rat sciatic nerve. Journal of Neurotrauma. 2006;23(2):227–240. doi: 10.1089/neu.2006.23.227. [DOI] [PubMed] [Google Scholar]
- 3.Evans PJ, Bain JR, et al. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559(2):315–321. doi: 10.1016/0006-8993(91)90018-q. [DOI] [PubMed] [Google Scholar]
- 4.Britt JM, Kane JR, et al. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104(2):695–703. doi: 10.1152/jn.01051.2009. [DOI] [PubMed] [Google Scholar]
- 5.Sexton KW, Pollins AC, et al. Hydrophilic polymers enhance early functional outcomes after nerve autografting. The Journal of surgical research. 2012 doi: 10.1016/j.jss.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson J, Shi R, et al. Polyethylene glycol rapidly restores physiological functions in damaged sciatic nerves of guinea pigs. Neurosurgery. 2002;50(1):147–156. doi: 10.1097/00006123-200201000-00023. discussion 156-147. [DOI] [PubMed] [Google Scholar]
- 7.Bittner GD, Keating CP, et al. Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. Journal of neuroscience research. 2012;90(5):967–980. doi: 10.1002/jnr.23023. [DOI] [PubMed] [Google Scholar]
- 8.Yoo S, Nguyen MP, et al. Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca(2+)-regulated proteins. J Neurosci Res. 2003;74(4):541–551. doi: 10.1002/jnr.10771. [DOI] [PubMed] [Google Scholar]
- 9.Chessell IP, Hatcher JP, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Arbeloa J, Perez-Samartin A, et al. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiology of disease. 2012;45(3):954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8(4):243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Brophy, et al. Methods and Compositions for Vein Harvest and Autografting. US2011/0190572A1. United States Patent Application Publication. 2011
- 13.Kantor TG. Ketoprofen: a review of its pharmacologic and clinical properties. Pharmacotherapy. 1986;6(3):93–103. doi: 10.1002/j.1875-9114.1986.tb03459.x. [DOI] [PubMed] [Google Scholar]
- 14.Sedy J, Urdzikova L, et al. Methods for behavioral testing of spinal cord injured rats. Neurosci Biobehav Rev. 2008;32(3):550–580. doi: 10.1016/j.neubiorev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics international. 2004;11:36–42. [Google Scholar]
- 16.Marina N, Bull ND, Martin KR. A semiautomated targeted sampling method to assess optic nerve axonal loss in a rat model of glaucoma. Nat Protoc. 2010;5:1642. doi: 10.1038/nprot.2010.128. [DOI] [PubMed] [Google Scholar]
- 17.Spaeth CS, Robison T, et al. Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neurosci Res. 2012;90(5):955–966. doi: 10.1002/jnr.23022. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH. Green's Operative Hand Surgery. Churchill Livingstone. 2011:5. [Google Scholar]
- 19.Kang JR, Zamorano DP, et al. Limb salvage with major nerve injury: current management and future directions. J Am Acad Orthop Surg. 2011;19(Suppl 1):S28–S34. doi: 10.5435/00124635-201102001-00006. [DOI] [PubMed] [Google Scholar]
- 20.Eidelberg E, Straehley D, et al. Relationship between Residual Hindlimb-Assisted Locomotion and Surviving Axons after Incomplete Spinal-Cord Injuries. Experimental Neurology. 1977;56(2):312–322. doi: 10.1016/0014-4886(77)90350-8. [DOI] [PubMed] [Google Scholar]
- 21.Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley DA, Sanger JR, et al. Identifying motor and sensory myelinated axons in rabbit peripheral nerves by histochemical staining for carbonic anhydrase and cholinesterase activities. Brain Res. 1988;453(1–2):79–88. doi: 10.1016/0006-8993(88)90145-x. [DOI] [PubMed] [Google Scholar]
- 23.Schemann M, Sann H, et al. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265(5 Pt 1):G1005–G1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- 24.Nichols CM, Myckatyn TM, et al. Choosing the correct functional assay: a comprehensive assessment of functional tests in the rat. Behav Brain Res. 2005;163(2):143–158. doi: 10.1016/j.bbr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Johnson EO, et al. Long-term evaluation of rabbit peripheral nerve repair with end-to-side neurorrhaphy in rabbits. Microsurgery. 2006;26(4):262–267. doi: 10.1002/micr.20237. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson P, Janson AM, et al. Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001;437(1):106–117. doi: 10.1002/cne.1273. [DOI] [PubMed] [Google Scholar]
- 27.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients (vol 397, pg 350, 1999) Nature. 1999;399(6731):84–84. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]


