Abstract
Endogenous pain-inhibition is often deficient in adults with chronic pain conditions including irritable bowel syndrome (IBS). It is unclear whether deficiencies in pain-inhibition are present in young children with IBS. The present study compared endogenous pain-inhibition, somatic pain threshold, and psychosocial distress in young girls with IBS versus controls. Girls with IBS did not show significant endogenous pain-inhibition of heat pain-threshold during a cold-pressor task in contrast to controls who had significant pain-inhibition. Girls with IBS did not differ from peers on measures of somatic pain but had more symptoms of depression, somatization, and anxiety than controls. When psychological variables were included as covariates the difference in pain-inhibition was no longer significant, although poor achieved power limits interpretation of these results. Higher-order cognitive processes including psychological variables may be contributing to observed pain-inhibition. In girls with IBS, pain-inhibition was positively related to the number of days without a bowel movement. To our knowledge, this is the first study to demonstrate deficiencies of endogenous pain-inhibition in young children with IBS. Findings have implications for better understanding of onset and maintenance of IBS and other chronic pain conditions.
Keywords: Diffuse Noxious Inhibitory Controls, Conditioned Pain Modulation, Endogenous Pain Modulation, Irritable Bowel Syndrome, Children
Introduction
Nociception and subjective pain can be both facilitated and inhibited by endogenous mechanisms (via descending pathways and/or cognitive processes).14 Alterations in endogenous pain modulation may contribute to development and/or maintenance of pain conditions.10,16 For example decreased inhibitory processes and/or enhanced facilitatory processes could lead to an overall increase in nociception and pain experience. A common method of assessing endogenous pain modulation involves measuring the extent of pain inhibition of a focal-pain stimulus during a noxious heterotopic counter-stimulus.26 In animals this mechanism of pain inhibition is termed diffuse noxious inhibitory controls (DNIC).26,55 In humans a similar effect is observed however disagreement exists regarding the appropriate terminology since the mechanism of action cannot be isolated to DNIC pathways.31,64 Some have suggested the term conditioned pain modulation (CPM)64 but this has been contested as well.31 To avoid confusion this paper uses the term endogenous pain-inhibition to refer to pain inhibition during a noxious counter-stimulus (which may include several mechanisms of pain modulation). The term DNIC-like inhibition is used to refer specifically to inhibition presumed to stem from the descending pathway identified in DNIC animal models. Research has demonstrated healthy adults10,22,24,26,36,38,48,50,51,56,62 and children18 have significant endogenous pain-inhibition. However, endogenous pain-inhibition is often impaired in individuals with chronic pain.24,29,38,48
Irritable bowel syndrome (IBS) is a chronic pain condition with symptoms of recurrent abdominal pain associated with stooling, and/or changes in stool form or frequency, most commonly seen in women, that is not explained by organic disease. Like other chronic pain conditions,24,29,38,48 IBS in adults is associated with deficient endogenous pain-inhibition.51,62 As a group, adults with IBS have deficient endogenous pain-inhibition of visceral (rectal-distention) pain during cold-immersion of their foot.22,24,36,51,62 Concurrent imaging identified alterations in brain activity in areas associated with pain processing and modulation compared to controls.51,62 Not only is inhibition of visceral pain deficient in some adults with IBS,12,33,37,51,54,62 but research also indicates deficient endogenous pain-inhibition of somatic pain.22,24,36
In addition to abnormal pain-inhibition, increased visceral12,33,37,51,54,62 and somatic33,47,54 pain perception (hyperalgesia) has been described in adults with IBS. Both peripheral and central mechanisms (including psychological factors) are implicated in hyperalgesia and altered pain modulation in IBS patients,39 and psychological variables accounted for group (IBS versus control) differences in endogenous pain-inhibition in one study.36
There is limited data regarding whether alterations in pain perception and modulation observed in adults with IBS are also present in children with IBS. IBS often begins in childhood and many children with IBS or functional abdominal pain develop IBS-type symptoms in adulthood.23,61 IBS is the most frequent cause of medically unexplained recurrent abdominal pain in children11 affecting 10–46 percent of school-age children.1,27,49,53,66 To the best of our knowledge there are no studies assessing whether alterations in pain modulation are present in children with IBS. As in adults, some children with IBS have rectal hyperalgesia compared to controls.8,10,13,17 Studies in children with recurrent abdominal pain report conflicting results regarding somatic hyperalgesia.9,21,65 However, we could not identify any published studies assessing somatic hyperalgesia specifically in children with IBS. Because many children with IBS continue to have symptoms as adults, assessment of endogenous pain-inhibition and somatic hyperalgesia in children with IBS can provide insight into pain inhibitory processes early in the course of IBS.
The present study sought to determine if deficient endogenous pain-inhibition and somatic hypersensitivity are present in girls with IBS compared to healthy girls (given increased prevalence in women). We hypothesized girls with IBS would have deficient endogenous pain-inhibition and somatic hyperalgesia compared to controls. Given findings of greater psychological distress in children with IBS,5,7,34,46,59,60 we also assessed the influence of psychological variables on endogenous pain-inhibition.
Materials and Methods
Participants
Forty-three premenarchal girls ages 7–12 years (and their mothers) were recruited from primary and tertiary care pediatric clinics via chart reviews and community advertisements. Twenty-two of the participants were diagnosed with IBS and 21 were healthy controls. Parents of identified children were contacted by mail by their physician. Interested families were screened by phone for inclusion/exclusion criteria. Charts also were reviewed by a pediatric gastroenterologist (RJS) to ensure that no known medical condition accounted for the pain or remained in the differential.
IBS was defined according to the pediatric Rome III criteria as abdominal pain (without inflammatory, metabolic, anatomic, or neoplastic explanation) occurring at least once a week with a duration of 2 months or more and meeting at least 2 of the following criteria 25% of the time; improved with defecation, onset associated with change in stool frequency, or onset associated with change in stool form.40 IBS participants had at least two physician visits in the past year for abdominal pain or IBS symptoms to ensure that their symptoms were current. Children were excluded due to the presence of another chronic pain condition, organic GI illness, other significant chronic health condition (requiring daily medication or specialty follow-up care), decreased growth velocity, GI blood loss, unexplained fever, vomiting, chronic severe diarrhea, weight loss ≥5% of their body weight within a 3-month period, current use of anti-inflammatory medications, or previous use of GI medication that provided complete relief of symptoms. Healthy control children were excluded for similar reasons or if they had report of abdominal pain. Participants also were excluded if they were not proficient in English, or if developmental disabilities would interfere with questionnaire completion. All participants were screened on the study day to ensure participants did not have current acute pain ≥3 (0–10 scale) and had not ingested narcotic pain medications within the past week or over the counter pain medications within the past 24 hours.
Participants were part of a larger study assessing physiological and psychological factors contributing to IBS in young girls (data from this larger study is not yet published or submitted for publication). All procedures were approved by the Baylor College of Medicine Institutional Review Board. Informed consent and assent were obtained from the parent and child, respectively, prior to participation.
Procedure
Girls and their mothers came to the Children’s Nutrition Research Center to participate in the study. As part of the larger study, girls had blood drawn and swallowed a PillCam (Given Imaging Inc., Duluth, GA). Data from blood draws and the PillCam are not presented in the present study. Children and parents independently completed questionnaires. Children under 8 years of age had questionnaires read to them. After questionnaire completion, girls participated in the endogenous pain-inhibition procedure while their mothers waited in a nearby room. Children and their mothers then were instructed on completion of an IBS symptom diary that was completed at home during the subsequent 14-day period.15,25,57 Trained research personnel administered all procedures.
Psychological Questionnaires
Psychological variables influence supraspinal modulation of pain44,63 and have been found to account for the lack of endogenous pain-inhibition in adult patients with IBS. One study postulated that common underlying mechanisms may contribute to psychological symptoms and alterations in pain sensitivity and modulation; they also suggested that descending modulation (i.e. endogenous pain-inhibition) and psychological factors have independent effects on impaired pain inhibition in persons with IBS.36 This is consistent with research indicating that a noxious counter-stimulus and psychological variables influence descending pain modulation via two distinct neurological pathways,4,14,43 and both pathways likely play a role in alterations of pain perception and modulation in IBS. Therefore, the following questionnaires were used to assess relevant psychological variables.
Children’s Somatization Inventory-35 (CSI)
The CSI assesses children’s somatization symptoms and has both a parent and child report form. The questionnaire was originally designed for use with children with recurrent abdominal pain.60 It is recommended for use in children ages 7–18 years of age.30 The original child- and parent-report versions (35 items each) were used. Each item lists a symptom and the child, or her parent, indicated on a 5-point Likert scale (0=“not at all,” 1= “a little,” 2= “some,” 3= “a lot,” 4= “a whole lot”) how much they, or their child, was “bothered by each symptom” during the preceding two weeks. Scores range between 0 and 140 with increasing scores indicating greater levels of somatization. The CSI has demonstrated good test-retest reliability with a 3-month interval in a functional abdominal pain population (r = .66) and good internal consistency (α = .92); and is correlated with the CBCL Somatic Complaints scale (r = .42) suggesting good criterion-related validity.58
Behavior Assessment System for Children – Second Edition (BASC-2)
The BASC-2 is a questionnaire that assesses behavioral and emotional problems in children. The questionnaire has both parent and child report forms consisting of true/false and Likert scale items.41 The BASC-2 is well validated and in widespread use. Parent report forms used in this study are normed for children as young as 6 years of age. Child-report forms are normed for ages 8 years and above, however, we used this form for 7-year-olds in this study and did not have any invalid protocols (as assessed by BASC-2 validity scales). Questions were read aloud to children under eight years of age. Anxiety and depression subscales from both parent and child report forms were used in the present study. Scores are reported as t-scores.
DNIC assessment
Experimental stimulus
The experimental stimulus consisted of contact heat pain produced by an FDA approved Thermal Sensory Analyzer (PATHWAY ATS) system (Medoc, Ramat-Yishai, Israel) with a 30 × 30 mm Peltier surface stimulator (contact thermode) applied to the volar surface of the right forearm. The method of limits was used to determine heat pain threshold and participants were instructed to press a button to stop the stimulus “as soon as the heat becomes painful.” Stimuli began at 32.0 °C and increased at a rate of 1.5°C/s until the participant indicated the heat had become painful. Once pain threshold was reached, the temperature decreased at a rate of 8°C/s. There was a maximum temperature of 55 °C to prevent tissue damage. The temperature at which participants stopped the stimulus was used as the measure of pain threshold.
Noxious Counter-Stimulus
The noxious counter-stimulus involved immersion of the left hand up to the wrist in circulated ice water maintained at a temperature of 12°C ± 1°C. The cold-pressor apparatus was constructed out of an insulated cooler. A screen separated the ice from the participant’s hand. The water was constantly circulated to prevent localized warming around the hand. To reduce movement of the hand, participants were instructed to rest their open hand palm down on netting. Participants were asked to leave their hand in the water for 60 seconds but were told that they could remove their hand at any point if the water became “too uncomfortable.” Immediately after removing their hand from the water children rated the pain due to the cold pressor using a 0–10 numerical rating scale with 0 labeled as “no pain at all” and 10 labeled as “the worst pain you can imagine.” The numerical rating scale has evidence of validity as a method for assessment of pain intensity in children.32,35,57
Endogenous pain-inhibition assessment
Children were first provided instruction regarding the endogenous pain-inhibition assessment procedure. Heat pain threshold was practiced twice to familiarize children with the stimulation and allow them to practice terminating the stimulation when pain threshold was reached. The contact thermode was then moved distally to the adjacent area of the forearm to prevent sensitization. Next, endogenous pain-inhibition assessment began with a baseline pain threshold assessment. Heat pain threshold was assessed four times with 15-second interstimulus-intervals (ISI). The contact thermode was again moved distally to the adjacent area of the forearm. The child was then asked to place her hand in the cold pressor and leave it there until “told to take it out” which is about “one minute.” After the hand was in the water for 20 seconds, three more heat pain threshold assessments were completed with an ISI of 15 seconds. After pain threshold was reached for the third stimulus the child was told to remove her hand from the water and was given a towel to dry her hand. The child then verbally rated the pain due to the cold pressor with a 0–10 numerical rating scale (described above). The first pain threshold rating was excluded from the baseline phase. Pain thresholds during baseline and during the counter-stimulus were calculated by averaging pain threshold temperatures for the 3 heat stimulations during each phase.
Endogenous pain-inhibition was interpreted as a change score that was computed by subtracting heat pain threshold (in °C) during the noxious counter-stimulus from heat pain threshold during baseline heat stimuli. Thus, a positive change score represents an increase in pain threshold or pain inhibition and a negative change score represents a decrease in pain threshold or pain facilitation. A zero change score represents no change in pain threshold.
IBS Symptom Diary
To assess current symptoms of IBS, a validated 14-day paper diary of abdominal pain and stooling frequency and form was completed by participants at home following the above procedures.15,25,57 Children completed the diary on their own; however, parents were asked to prompt children to complete the diary. Parents called and submitted pain/stooling ratings once a day via an automated phone survey. Three pain and interference ratings were made each day (upon awakening, after lunch, bedtime) to reflect morning, afternoon, and evening symptoms. Pain intensity ratings were made on a 0 to 10 numerical rating scale (NRS) with anchors of “no pain at all” and “worst pain you can imagine.” NRS scales have been shown to be valid in assessment of pain intensity in children seven years and older.32,35,57 Children also noted whether their pain interfered with their functioning and whether or not they had a bowel movement during each diary interval.
IBS symptoms of pain (pain frequency, average pain rating, and interference with activities due to pain) and stooling patterns (number of bowel movements per day, number of days without a bowel movement) were computed to reflect current symptoms. Pain frequency was defined as the number of episodes of abdominal pain reported (pain greater than or equal to one on a 0–10 scale). Average pain rating was computed by averaging the pain intensity of ratings given during a pain episode and dividing by the number of pain episodes. Interference with activities was defined as the number of times a child reported that her pain interfered with functioning.
Data Analysis
Independent samples t-tests were conducted to assess for group differences between IBS and healthy controls on psychological variables, somatic pain, and endogenous pain-inhibition. Psychological variables that were found to be significantly different between groups were used as covariates in follow-up analyses in order to control for non-specific effects of psychological distress on pain modulation. Univariate ANCOVAs were conducted with endogenous pain-inhibition as the dependent variable, group membership (IBS/control) as the fixed factor, and psychological variables as covariates. Since percent change cannot be conducted on Celsius temperatures (an interval scale) temperatures were converted to Kelvin (an absolute scale) and then percent change was calculated by subtracting baseline heat pain threshold from heat pain threshold during the counter-stimulus and dividing by baseline heat pain threshold. Separate ANCOVAs were conducted for parent and child report of psychological variables. Pearson’s r correlations assessed the relation between endogenous pain-inhibition and IBS symptoms (average pain ratings, pain frequency, pain-related interference with activities, mean bowel movements per day, and number of days without a bowel movement) within each group separately. Cohen’s d effect sizes are presented for t-tests and partial eta-squared (η2) is presented for ANCOVAs. Cohen6 provides guidelines for interpreting d (small = .2, medium = .5, large = .8) and η2 (small = .01, medium = .06, large = .14). Effects were considered significant at p < 0.05. Data are shown as mean ± standard deviation.
Results
Forty-three girls participated in the study (IBS = 22, healthy controls = 21). Four healthy controls rated pain due to the cold-pressor as zero out of ten. Given that endogenous pain-inhibition assessed in this study by definition necessitates a noxious/painful conditioning stimulus, endogenous pain-inhibition would not be expected to occur in participants who did not experience pain during the noxious counter-stimulus. Thus, these four participants were excluded from the analyses. Visual inspection of the data identified an outlier in the pain outcome data and experimental records indicated that this control participant had an unusually stressful experience with the experimental protocols that immediately preceded endogenous pain-inhibition testing. Specifically, this participant underwent two attempts to draw blood and fainted. She then attempted and failed to swallow the PillCam multiple times. As a result, data from this participant were excluded from the analyses. Therefore, final analyses included 22 girls with IBS and 16 healthy controls (n = 38 total). Results for the final sample are presented in the text and in Table 1 (psychological variables). Data for all 43 subjects (i.e., including those excluded) are provided in the Supplemental Table for comparison.
Table 1.
Psychological Outcome Variables
| Psychological Variable | IBS | Healthy Controls |
n (IBS, HC) |
|---|---|---|---|
| Parent-report child anxiety | 60.6 ± 13.9* | 48.0 ± 9.4* | 22, 16 |
| Parent-report child depression | 49.4 ± 10.0* | 43.9 ± 5.1* | 22, 16 |
| Parent-report child somatization | 18.8 ± 11.4* | 2.5 ± 2.6* | 18, 16 |
| Parent somatization | 51.1 ± 9.3 | 49.8 ± 10.3 | 18, 15 |
| Parent depression | 54.2 ± 11.2 | 48.9 ± 8.5 | 17, 15 |
| Parent anxiety | 50.1 ± 11.2 | 47.4 ± 10.0 | 17, 16 |
| Child-report anxiety | 53.0 ± 11.9* | 43.6 ± 7.3* | 22, 16 |
| Child-report depression | 48.3 ± 9.1 | 43.9 ± 4.9 | 22, 16 |
| Child-report somatization | 29.9 ± 17.1* | 13.9 ± 13.4* | 14, 15 |
Note.
p <.05; Data are presented as Mean ± Standard Deviation; IBS = Irritable bowel syndrome; HC = Healthy Control; Data are in t-scores with the exception of somatization which is a raw score.
Power analyses indicated that given this sample size and an alpha of .05, power to detect a medium effect for the t-tests was good (power = .92). Power to detect a medium effect for covariate analyses was not as high (child-report covariate power = .25; parent-report covariate power = .29). The covariates were included in the paper as exploratory analyses.
IBS participants had an average body mass index of 19.3 ± 5.4 vs. 18.2 ± 3.1 in control participants (p = .49). Mean age of IBS participants was 9.8 ± 1.5 years vs. 9.4 ± 1.4 years in control participants (p = .49). Fourteen of the IBS participants were classified as constipation predominant, one as diarrhea predominant, one mixed/alternating and the remaining were unsubtyped (i.e. according to Rome criteria: “insufficient abnormality of stool consistency to meet criteria for” constipation-predominant, diarrhea-predominant or mixed subtypes28). Fifty-four percent of IBS participants were Caucasian, 22% African American, 18% Hispanic, and 5% Asian. Control participants were 50% Caucasian, 38% African American, and 13% Hispanic. Nine percent of IBS participants were in second grade, 23% in third, 18% in fourth, 27% in fifth, 18% in sixth, and 5% in seventh. Thirteen percent of IBS participants were in second grade, 25% in third, 25% in fourth, 31% in fifth, and 6% in sixth.
Endogenous Pain Inhibition
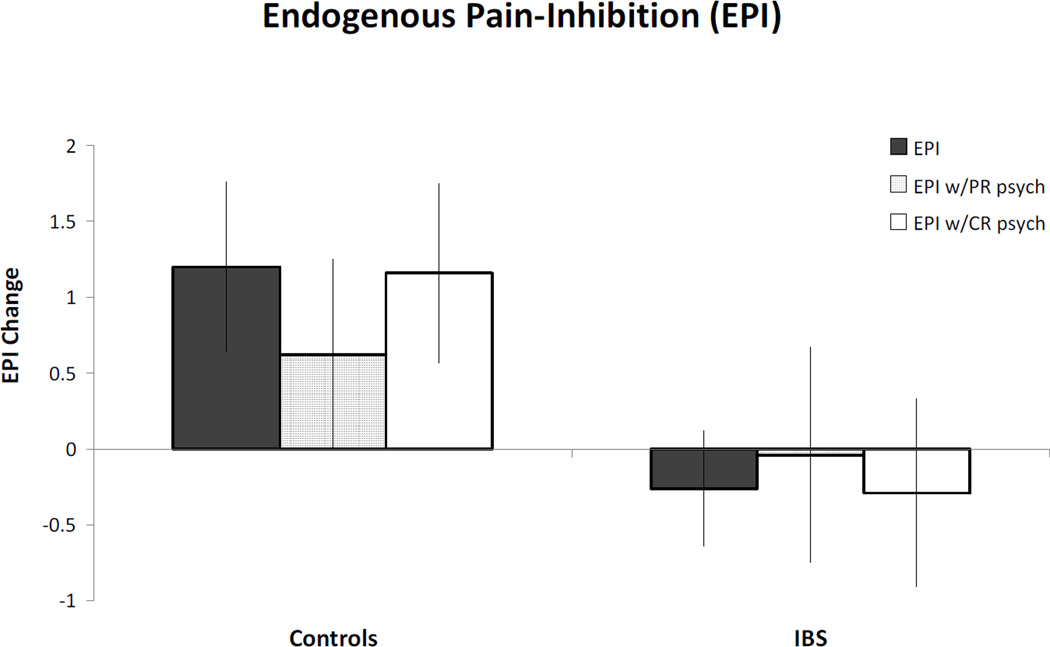
Figure 1 presents data regarding group differences in endogenous pain-inhibition. There was a significant difference between groups in endogenous pain-inhibition (p = .03, d = .71). The mean change score for IBS participants was −0.26°C ± 1.79 representing a slight facilitation of pain during the noxious counter-stimulus, which represents a percent change in Kelvin of −.08%. In contrast, the mean change score for the healthy children was 1.20°C ± 2.30 representing inhibition of pain during the noxious counter-stimulus, which represents a percent change in Kelvin of .38%. Thus, there was a lack of endogenous pain-inhibition in IBS both absolutely and compared to healthy children.
Figure 1. Endogenous Pain-Inhibition.
Positive number represents pain inhibition and negative number represents pain facilitation. IBS is irritable bowel syndrome. Solid grey bars represent endogenous pain-inhibition effect for each group without any covariates. Middle (lined) bars represent endogenous pain-inhibition effect with significant parent-report (PR) psychological variables (child anxiety, child depression, and child somatization) included as covariates. White bars represent endogenous pain-inhibition effect with significant child-report (CR) psychological variables (anxiety & somatization) included as covariates. * indicates significance at p<.05. Although results are no longer significant after covariates are included, a similar pattern of results is present.
Somatic Pain
There was no significant difference in baseline heat pain thresholds (p = .70, d = .13) between IBS (40.04°C ± 4.94) and healthy children (39.47°C ± 3.88). Similarly, there was no significant difference in numerical rating scores (0–10) for the cold-pressor conditioning stimulus (p = .34, d = .32) between IBS (7.23 ± 2.62) and healthy children (6.41 ± 2.55).
Psychological Variables
IBS participants had greater child-reported anxiety (p = .01, d = .95) and somatization symptoms (p = .01, d = 1.04) compared to healthy children. There was no significant difference between groups on child-reported depressive symptoms (p = .06, d = .61). IBS participants had higher scores on parent reports of child anxiety (p < .01, d = 1.06), depressive symptoms (p = .03, d = .70), and somatization symptoms (p < .01, d = 1.97) compared to healthy children. ds < .37). Despite group differences, the majority of individual scores for anxiety and depressive symptoms for both groups were within the normal range (68–95% of participants) and did not reflect clinically significant symptoms. Only 3–13% of participants were in the at-risk range and 0–18% had clinically significant scores, with parent report of child anxiety having the most persons with clinically significant scores.
Because psychological variables differed between IBS and control children, two exploratory univariate ANCOVAs were conducted with significant psychological variables included as covariates. Questionnaire data were missing for some participants and thus sample size decreased for these analyses. Participants with complete child report data included 14 IBS and 15 controls, and participants with complete parent report data included 18 IBS and 16 controls. One ANCOVA included child report variables that differed significantly between groups (anxiety and somatization) and a second ANCOVA included significant parent report variables (child anxiety, depression, and somatization). For both analyses group differences in endogenous pain-inhibition were no longer significant (ps > .13) with psychological variables as covariates and with the reduced sample size. Child report of anxiety and somatization accounted for 1% and 2% of the variance in pain-inhibition respectively. In comparison, parent report of child psychological variables accounted for more variance in pain inhibition with anxiety, somatization, and depression accounting for 15%, <1%, and 12% of the variance, respectively. However, effect sizes for the group difference in pain-inhibition remained about medium in size (η2 = .09 for child-report analysis; η2 = .04 for parent-report analysis) indicating the difference in endogenous pain-inhibition may remain relevant even after controlling for psychological variables (see Figure 1).
Relation between Endogenous Pain Inhibition and Pain and Stooling Symptoms
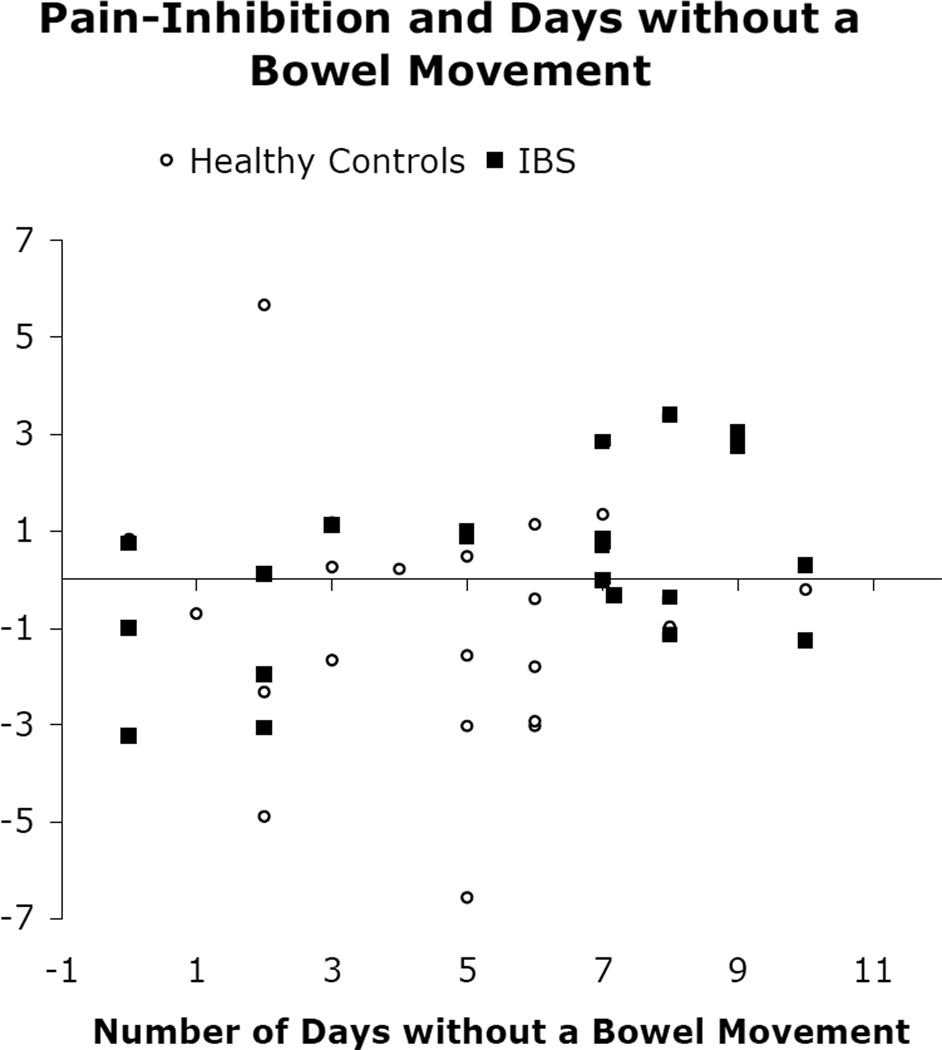
Relations between IBS symptom variables and endogenous pain-inhibition were assessed in IBS participants and healthy participants separately. In girls with IBS, there was a significant correlation (r = .47, p = .03) between endogenous pain-inhibition and the number of days (out of 14) without a bowel movement, suggesting that endogenous pain-inhibition is greater in IBS participants who had more days without a bowel movement (Figure 2). This correlation was not significant in healthy girls (r = .11, p = .64). There was a trend for endogenous pain-inhibition to correlate with mean number of bowel movements per day in girls with IBS (r = −.37, p = .10) but not in healthy girls (r = −.21, p = .36). Endogenous pain-inhibition was not correlated with average pain ratings (IBS r = −.07, p = .77; HC r = .13, p = .37), pain frequency (r = .08, IBS p = .74; HC r = .40, p = .51), or pain-related interference with activities (IBS r = −.03, p = .91; HC r = −.21, p = .25) assessed via diary.
Figure 2. Pain Inhibition and Days without a Bowel Movement.
Positive change score represents pain inhibition and negative change score represents pain facilitation. IBS is irritable bowel syndrome.
Discussion
We observed that young girls with irritable bowel syndrome (IBS) had deficient endogenous pain-inhibition compared to healthy peers. Consistent with previous research18 the noxious counter-stimulus (cold-pressor) effectively engaged endogenous pain-inhibition of contralateral heat pain perception (increased pain threshold) in healthy girls. In contrast, girls with IBS did not evidence pain-inhibition during the noxious counter-stimulus. The difference in endogenous pain-inhibition between groups had a medium (approaching large) effect size. In contrast to findings of somatic hyperalgesia in adults with IBS33,47,54 and children with recurrent abdominal pain,9 we did not find somatic hyperalgesia (baseline heat pain thresholds or rated pain intensity during cold-pressor task).
Results are consistent with adult studies reporting that as a group, women with IBS have reduced endogenous pain-inhibition compared to healthy peers.22,24,36,51 However, to our knowledge this is the first study to document this deficiency of endogenous pain-inhibition is also present in young girls with IBS. This suggests deficits in pain inhibitory processes are present at an early stage of IBS illness. A potential explanation is that impaired pain-inhibition develops very early in the course of a chronic or recurrent pain condition such as IBS. Alternatively, deficiencies in endogenous pain-inhibition may be present prior to IBS onset and predispose or even cause a person to develop IBS. Research has found that pre-term infants with repeated pain experiences had deficient endogenous pain-inhibition during school-age,18 suggesting that frequent exposure to pain may alter development or functioning of pain modulation systems. It is unknown whether girls with IBS in this study had numerous pain experiences as infants but they have had frequent pain associated with IBS. Further research should assess whether children with IBS have pre-existing deficits in endogenous pain-inhibition, whether this develops in the course of their IBS, whether deficits in pain-inhibition are predictive of onset or severity of IBS, or progression from an acute to chronic condition.
Also consistent with research in adults with IBS,22 extent of endogenous pain-inhibition in girls with IBS (or in healthy girls) was not correlated with most IBS symptoms including pain intensity, pain frequency, interference with activities, and frequency of bowel movements (BM) per day. There was, however, a significant correlation in girls with IBS (but not those without) between number of days without a BM and pain-inhibition. Thus, girls with IBS who have more significant constipation symptoms may have less impairment of endogenous pain-inhibition. Although not statistically significant, there was a trend for girls with IBS who had more frequent BMs on the average day to have greater deficits in pain-inhibition. Together these findings suggest girls with constipation-predominant IBS may be less likely to have deficiencies of endogenous pain-inhibition. It is possible these girls have fewer pain/stooling episodes which in turn leads to less insult to pain modulation systems (and thus fewer alterations in modulation processes) compared to peers with diarrhea-predominant IBS. Further research should seek to replicate these results and evaluate these relations more thoroughly.
Children with recurrent abdominal pain and IBS have been found to have greater levels of psychological distress compared to healthy children.5,7,34,46,59,60 This pattern was replicated in the present study finding children with IBS, compared to controls, had higher scores (although most were not clinically elevated) on child and parent reports of child anxiety and somatization, and on parent reports of child depressive symptoms. A bidirectional relationship between pain and psychological distress seems a most likely explanation. Given the known influence of psychological state on pain perception (i.e., negative mood/anxiety lead to hyperalgesia44,63) children who experience negative emotions or mental health concerns also are likely to experience increased pain perception and have more associated distress. Further, children who experience chronic or recurrent pain may be more likely than healthy children to develop symptoms of a mental health condition.2,52
A recent study in adults found impairments of endogenous pain-inhibition in IBS persist after controlling for group differences in psychological variables,22 while another study did not replicate this finding.36 We found that after controlling for psychological variables group differences in endogenous pain-inhibition were no longer significant. However, the present study was powered to detect potential differences in pain-inhibition and was underpowered to detect a medium effect in covariate analyses conducted, thus, results should be interpreted with caution.
Regardless, as suggested by Piche, et.al.,36 psychological variables and DNIC-like pain inhibition likely have independent (and concurrent) contributions to alterations in pain modulation in persons with IBS that may reflect a common underlying mechanism. Psychological variables and emotional state modulate pain with negative emotions (of low to moderate intensity) facilitating pain and positive emotions (and high intensity negative emotions) inhibiting pain.42,43,45 Research indicates psychological variables modulate pain through the periaqueductal gray (PAG) and rostroventromedial medulla (RVM)14 and DNIC-like pain inhibition modulates pain through a spinoreticular-spinal loop26,55,56 and is distinct from the PAG and RVM.3 Although distinct neurologically these processes do not act in complete isolation from each other. DNIC-like pain inhibition has been shown to partially mediate the relation between catastrophizing and pain;20 and cognitive expectations about pain have been shown to eliminate or block normal endogenous pain-inhibition effects.19 Thus, these are likely independent processes acting concurrently that may alter, negate, or enhance the others impact leading to the observed net effect on pain. The present study does not enable delineation of the specific mechanisms of the pain-inhibition effect and power was poor for analyses including psychological variables. However, results suggest psychological variables contributed at least in part to group differences in pain-inhibition and should be considered along with DNIC-like inhibition. Indeed, parent report of child anxiety and depression were respectively large and medium effects but did not account for all the variance in endogenous pain-inhibition. Further research should attempt to better delineate the roles of both mechanisms of endogenous pain-inhibition.
Somatic pain perception (cold pressor pain ratings and baseline heat pain threshold) did not differ between IBS and control groups. The literature has shown conflicting results regarding whether differences in somatic pain exist between children with functional gastrointestinal disorders and healthy children.9,21,65 Given known individual variability in pain perception a group difference in pain perception that is small in magnitude (i.e., small effect size) may be difficult to detect in a small sample. A study with 100 children with chronic/recurrent abdominal pain found somatic hyperalgesia compared to controls,9 whereas studies with smaller sample sizes (group n = 14–23) have not found significant group differences in pain perception.21,65 Nonetheless, it is possible that large samples will find significant effects that are so small in magnitude they are not clinically meaningful. In comparison to research in children, several studies in adults with IBS have found significant somatic hyperalgesia compared to controls with relatively small sample sizes (group n = 9–17).33,47,54 It is possible somatic hyperalgesia develops over the course of a chronic pain condition and therefore is less apparent, or of smaller magnitude, in children. Further, the poorer ability of children, compared to adults, to provide accurate self-report of pain could cause greater random error in pain outcomes masking group differences. However, this latter theory is not supported by one study that failed to find group differences between children with abdominal pain and controls in the more objective measure of somatosensory evoked potentials in response to painful stimuli.21
The present study has some limitations. The relatively small sample size may have limited power to detect potentially significant effects with exploratory ANCOVAs. Also inclusion of only girls in this study limits generalizability to boys. The inclusion of 7-year-old girls poses some concern for interpretation of the BASC-2 child self-report questionnaire which is normed for ages 8 and above. We attempted to control for this by reading questions aloud to 7-year-olds and carefully scrutinizing the validity indices, however, results should be repeated with a questionnaire normed in this age group. In addition, several participants in the control group had to be excluded because they did not rate the cold-pressor task as painful (which is necessary to evoke a DNIC-like pain-inhibition). It is unclear why these participants did not report the cold-pressor to be painful. Finally, a nonpainful counter-stimulus procedure (such as immersing the hand in room temperature water) to control for nonspecific changes in pain should be considered in future studies. An investigation published during the course of this study included a nonpainful counter-stimulus,22 and found that controlling for pain-inhibition during the nonpainful stimulus increased the difference in endogenous pain-inhibition between healthy women and women with IBS.22
In summary, we are the first to report that young girls with IBS have deficient endogenous pain-inhibition compared to healthy controls. Further, we replicated limited data indicating endogenous pain-inhibition is present in young children.18 Findings contribute to research and clinical understandings of the onset and development of IBS in childhood, suggesting deficient pain-inhibition is an early manifestation of IBS. Research should further investigate the contributions of impaired endogenous pain-inhibition and psychological distress to IBS and other chronic pain conditions. Our findings should be confirmed in larger, and younger, samples which would help to better define potential risk factors leading to onset of IBS and/or development of chronic pain symptoms.
Supplementary Material
Perspective.
This study found that young girls with irritable bowel syndrome (IBS) have deficient endogenous pain inhibition compared to healthy girls, which is consistent with the adult literature. This information can facilitate clinicians in identification of risk factors for onset/maintenance of IBS and other chronic pain conditions.
Acknowledgments
This research was supported by Grant Number RC2 NR011959 to MH and RJS and R01 NR05337 to RJS from the National Institutes of Health, the Daffy’s Foundation, the USDA/ARS under Cooperative Agreement No. 6250-51000-043, and P30-DK56338 which funds the Texas Medical Center Digestive Disease Center. This work is in collaboration with the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX and does not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors had a financial or commercial interest in any company or organization sponsoring the research.
References
- 1.Apley J. The Child with Abdominal Pains. London: Blackwell Scientific; 1975. [Google Scholar]
- 2.Barnes D, Gatchel RJ, Mayer TG, Barnett J. Changes in MMPI profiles of chronic low back pain patients following successful treatment. J Spinal Disord. 1990;3:353–355. [PubMed] [Google Scholar]
- 3.Bouhassira D, Bing Z, Le Bars D. Studies of the brain structures involved in diffuse noxious inhibitory controls The mesencephalon. J Neurophysiol. 1990;64:1712–1723. doi: 10.1152/jn.1990.64.6.1712. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res. 1992;595:353–357. doi: 10.1016/0006-8993(92)91071-l. [DOI] [PubMed] [Google Scholar]
- 5.Campo JV, Bridge J, Ehmann M, Altman S, Lucas A, Birmaher B, Di LC, Iyengar S, Brent DA. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113(4):817–824. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. Statistical power analysis for the behavioral sciences (rev ed) Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1977. [Google Scholar]
- 7.Czyzewski DI, Eakin MN, Lane MM, Jarrett M, Shulman RJ. Recurrent Abdominal Pain in Primary and Tertiary Care: Differences and Similarities. Children's Health Care. 2007;36:137–153. doi: 10.1080/02739610701334970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiLorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr. 2001;139(6):838–843. doi: 10.1067/mpd.2001.118883. [DOI] [PubMed] [Google Scholar]
- 9.Duarte MA, Goulart EM, Penna F. Pressure pain threshold in children with recurrent abdominal pain. J Pediatr Gastroentero Nutr. 2000;31(3):280–285. doi: 10.1097/00005176-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls(DNIC): association with clinical variables. Pain. 2003;106(3):427–437. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.El-Matary W, Spray C, Sandhu B. Irritable bowel syndrome: the commonest cause of recurrent abdominal pain in children. Eur J Pediatr. 2004;163(10):584–588. doi: 10.1007/s00431-004-1503-0. [DOI] [PubMed] [Google Scholar]
- 12.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59(4):489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 13.Faure C, Wieckowska A. Somatic referral of visceral sensations and rectal sensory threshold for pain in children with functional gastrointestinal disorders. J Pediatr. 2007;150(1):66–71. doi: 10.1016/j.jpeds.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Textbook of Pain. Philadelphia, PA: Elsevier/Churchill Livingstone; 2006. pp. 125–142. [Google Scholar]
- 15.Gaylord N, Carson S. Assessing recurrent abdominal pain in children. Nurse Pract. 1983;8:19–24. [PubMed] [Google Scholar]
- 16.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27(8):729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Ginkel RV, Voskuijl WP, Benninga MA, Taminiau JAJM, Boeckxstaens GE. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology. 2001;120:31–38. doi: 10.1053/gast.2001.20898. [DOI] [PubMed] [Google Scholar]
- 18.Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Perterm births: Can neonatal pain alter the development of endogenous gating systems? Eur J Pain. 2008;12:945–951. doi: 10.1016/j.ejpain.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Goffaux P, Redmond JW, Rainville P, Marchand S. Descending analgesia - When the spine echoes what the brain expects. Pain. 2007;130:137–143. doi: 10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Goodin BR, McGuire L, Allshouse M, Stapleton L, Haythornthwaite JA, Burns N, Mayes LA, Edwards RR. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain. 2009;10(2):180–190. doi: 10.1016/j.jpain.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Hermann C, Zohsel K, Hohmeister J, Flor H. Cortical correlates of an attentional bias to painful and innocuous somatic stimuli in children with recurrent abdominal pain. Pain. 2008;136(3):397–406. doi: 10.1016/j.pain.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain. 2010;26(2):104–109. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrett M, Heitkemper M, Czyzewski DI, Shulman RJ. Recurrent abdominal pain in children: forerunner to adult irritable bowel syndrome? J Soc Pediatr Nurs. 2003;8(3):81–89. doi: 10.1111/j.1088-145x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 24.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143(3):172–178. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol Stool Form Scale for Children. J Pediatr. 2011;159(3):437–441. doi: 10.1016/j.jpeds.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Bars D, Villanueva L. Electrophysiological evidence for the activation of descending inhibitory controls by nociceptive afferent pathways. Prog Brain Res. 1988;77:275–299. doi: 10.1016/s0079-6123(08)62795-8. [DOI] [PubMed] [Google Scholar]
- 27.Levine M. Recurrent abdominal pain in school children: the loneliness of the long-distance physician. Pediatr Clin North Am. 1978;31:969–991. doi: 10.1016/s0031-3955(16)34680-6. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth GF, Thompsoon WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional Bowel Disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63(3):341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 30.Meesters C, Muris P, Ghys A, Reumerman T, Rooijmans M. The Children's Somatication Inventory: Further evidence for its reliability and validity in a pediatric and a community sample of Dutch children and adolescents. J Ped Psychol. 2003;28(6):413–422. doi: 10.1093/jpepsy/jsg031. [DOI] [PubMed] [Google Scholar]
- 31.Michaux G. "Recommendations on terminology and practice of psychophysical DNIC testing". Eur J Pain. 2010;14:1068–1069. doi: 10.1016/j.ejpain.2010.05.014. Comments on by Yarnitsky et al., 14(4) 339. [DOI] [PubMed] [Google Scholar]
- 32.Miro J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. Eur J Pain. 2009;13(10):1089–1095. doi: 10.1016/j.ejpain.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin J Pain. 2007;23(4):323–330. doi: 10.1097/AJP.0b013e318032e496. [DOI] [PubMed] [Google Scholar]
- 34.Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002;91(1):45–50. doi: 10.1080/080352502753457932. [DOI] [PubMed] [Google Scholar]
- 35.Pagé MG, Katz J, Stinson J, Isaac L, Martin-Pichora AL, Campbell F. Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: Sensitivity to change over time. J Pain. 2012;13(4):359–369. doi: 10.1016/j.jpain.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148(1):49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133(4):1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Potvin S, Larouche A, Normand E, de Souza J, Gaumond I, Marchand S, Grignon S. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. Eur J Pain. 2010;14(7):742–746. doi: 10.1016/j.ejpain.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7(8):529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Rasquin A, Di LC, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds CR, Kamphaus RW. Manual for the behavior assessment system for children. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- 42.Rhudy JL, Meagher MW. Fear and anxiety: Divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- 43.Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. [Google Scholar]
- 44.Rhudy JL, Williams AE, McCabe KM, Nguyen MV, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. doi: 10.1111/j.1469-8986.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 45.Rhudy JL, Williams AE, McCabe KM, Russell JL, Maynard LJ. Emotional control of nociceptive reactions (ECON): Do affective valence and arousal play a role? Pain. 2008;136:250–261. doi: 10.1016/j.pain.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Robins PM, Schoff KM, Glutting JJ, Abelkop AS. Discriminative validity of the Behavioral Assessment System for Children-parent rating scales in children with recurrent abdominal pain and matched controls, in Anonymous. Psychology in the Schools. 2003:145–154. [Google Scholar]
- 47.Rodrigues AC, Nicholas Verne G, Schmidt S, Mauderli AP. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain. 2005;115(1–2):5–11. doi: 10.1016/j.pain.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP, Nappi G. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia. 2006;26(7):782–789. doi: 10.1111/j.1468-2982.2006.01130.x. 26 7:782–789, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Saps M, Sztainberg M, Di LC. A prospective community-based study of gastroenterological symptoms in school-age children. J Pediatr Gastroenterol Nutr. 2006;43(4):477–482. doi: 10.1097/01.mpg.0000235979.41947.f6. [DOI] [PubMed] [Google Scholar]
- 50.Serrao M, Rossi P, Sandrini G, Parisi L, Amabile GA, Nappi G, Pierelli F. Effects of diffuse noxious inhibitory controls on temporal summation of the RIII reflex in humans. Pain. 2004;112(3):353–360. doi: 10.1016/j.pain.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126(1–3):79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Sternbach RA, Wolf SR, Murphy RW, Akeson WH. Traits of pain patients: The low-back "loser". Psychosomatics. 1973;14:226–229. doi: 10.1016/S0033-3182(73)71337-2. [DOI] [PubMed] [Google Scholar]
- 53.Uc A, Hyman PE, Walker LS. Functional gastrointestinal disorders in African American children in primary care. J Pediatr Gastroentero Nutr. 2006;42(3):270–274. doi: 10.1097/01.mpg.0000189371.29911.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93(1):7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 55.Villanueva L, de Pommery J, Menetrey D, Le Bars D. Spinal afferent projections to subnucleus reticularis dorsalis in the rat. Neurosci Letters. 1991;134:98–102. doi: 10.1016/0304-3940(91)90517-w. [DOI] [PubMed] [Google Scholar]
- 56.Villanueva L, Le Bars D. The activation of bulbo-spinal controls by peripheral nociceptive inputs: diffuse noxious inhibitory controls. Biol Res. 1995;28(1):113–125. [PubMed] [Google Scholar]
- 57.Von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Walker LS, Garber J. Children's Somatization Inventory: Preliminary manual. Nashville, TN: Vanderbilt University Medical Center; 1993. [Google Scholar]
- 59.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm child Psychol. 1991;19(4):379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 60.Walker LS, Garber J, Greene JW. Psychosocial correlates of recurrent childhood pain: a comparison of pediatric patients with recurrent abdominal pain, organic illness, and psychiatric disorders. J Abnorm Psychol. 1993;102:248–258. doi: 10.1037//0021-843x.102.2.248. [DOI] [PubMed] [Google Scholar]
- 61.Walker LS, Guite JW, Duke M, Barnard JA, Greene JW. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. J Pediatr. 1998;132:1010–1015. doi: 10.1016/s0022-3476(98)70400-7. [DOI] [PubMed] [Google Scholar]
- 62.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53(11):1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams AE, Rhudy JL. Supraspinal modulation of trigeminal nociception and pain. Headache. 2009;49(5):704–720. doi: 10.1111/j.1526-4610.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 64.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14(4):339. doi: 10.1016/j.ejpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Zohsel K, Hohmeister J, Flor H, Hermann C. Somatic pain sensitivity in children with recurrent abdominal pain. Am J Gastroenterol. 2008;103(6):1517–1523. doi: 10.1111/j.1572-0241.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 66.Zuckerman B, Stevenson J, Bailey V. Stomachaches and headaches in a community sample of preschool children. Pediatrics. 1987;79:677–682. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.