Abstract
Objective
To determine if men with azoospermia are at an elevated risk of developing cancer in the years following an infertility evaluation.
Design
Cohort Study
Setting
United States andrology clinic
Patients
2,238 men with complete records were evaluated for infertility at a single andrology clinic in Texas from 1989 to 2009.
Interventions
None
Main Outcome Measures
Cancer incidence was determined by linkage to the Texas Cancer Registry.
Results
In all, 451 men had azoospermia and 1,787 were not azoospermic with a mean age at infertility evaluation of 35.7 years. Compared to the general population, infertile men had a higher risk of cancer with 29 cases observed compared with 16.7 expected (SIR 1.7, 95% CI 1.2–2.5). When stratifying by azoospermia status, azoospermic men had an elevated risk of cancer (SIR 2.9, 95% CI 1.4–5.4). Infertile men without azoospermia had a trend towards a higher rate of cancer (SIR 1.4, 95% CI 0.9–2.2). The Cox regression model revealed that azoospermic men had 2.2-fold higher cancer risk compared to not azoospermic men (HR 2.2, 95% CI 1.0–4.8).
Conclusions
Men with azoospermia have an increased risk of subsequently developing cancer, suggesting a possible common etiology between azoospermia and cancer development. Additional follow-up of azoospermic men after reproductive efforts end may be warranted.
Keywords: azoospermia, male infertility, neoplasms
Introduction
Azoospermia, the absence of sperm in the ejaculate, is estimated to affect 1% of all men and up to 15% of men with infertility.(1) An estimated 4 million US men (ages 15–45) have reported infertility. This data suggests that up to 600,000 reproductive aged U.S. men may have azoospermia with most of these men having nonobstructive azoospermia (NOA).(2) Nonobstructive azoospermia is due to defects in spermatogenesis, and investigators now suspect that the majority of NOA has an underlying genetic basis. However, the etiology of most patients’ testicular dysfunction remains unknown.(3, 4) Of importance, defects in DNA repair mechanisms and abnormalities in cell cycle control have been demonstrated at high rates in men with NOA.(5, 6)
Since the genetic basis for male infertility has become increasingly studied, researchers have now identified many DNA repair genes previously identified in cancer syndromes that regulate key processes in gamete production.(3) For example, mutations in the Lynch Syndrome gene MLH1 have been identified in azoospermic men.(7, 8) Moreover, mice genetically engineered to be deficient in DNA repair genes ERCC1 (excision repair cross-complementing gene 1) or MSH2 (MutS homolog 2) are azoospermic with severe testicular germ cell loss and subsequently develop tumors early in life.(9, 10) Emerging data has also demonstrated that azoospermic men have severely impaired recombination frequencies which can impact genomic health.(5)
As defects in genomic regulation can also lead to carcinogenesis, infertile men may also be at an elevated risk of cancer development. (11) A multi-institutional study of infertile couples in California IVF centers noted increased rates of testis and prostate cancers in men identified as having male factor infertility.(12, 13) However, azoospermic men could not be identified. Similarly, investigators linked semen data from the Copenhagen Sperm Analysis Laboratory to the Danish cancer registry and identified an increased risk of germ cell tumors in men without azoospermia but with abnormal semen characteristics.(14) Importantly, in the Danish cohort, no other cancers were found to have higher rates in subfertile men. Moreover, other studies have not identified higher risks for non-germ cell cancers in infertile men.(15–18) In all studies, however, azoospermic men could not be separately examined. Given our emerging knowledge about the overlap between DNA repair pathways and azoospermia, we sought to determine if men with azoospermia are at an elevated risk of developing cancer in the years following an infertility evaluation.
Methods
Study Population
After Institutional Review Board approval, an initial study cohort was identified with available data from 1989 to 2009 contained in the andrology database at the Baylor College of Medicine Special Procedures Laboratory in the Scott Department of Urology. The laboratory performs a high volume of semen analyses for fertility evaluations, sperm preparation for cryopreservation or intrauterine insemination, and evaluation after vasectomy or vasectomy reversal, as well as other andrology laboratory testing. For men with multiple semen analyses, only the first test was used in the present study. For men with azoospermia, a repeat semen analysis confirmed azoospermia in 89% of cases. The methods used for analysis of semen (sperm concentration, motility, and volume) have been previously described.(19)
In total, 22,089 men had semen data available. As the Texas Cancer Registry only reliably captures diagnoses of state residents, men with out of state/country or missing addresses were excluded from the final analysis. (n=14,607) Men with a history of vasectomy were excluded. (n=1,196) Men who were not seen for infertility or for uncertain reasons were excluded. (n=3,999) Finally, men with a cancer diagnosis within 6 months of the initial semen analysis were excluded. (n=49) Thus, the final patient population included 2,238 men evaluated for infertility. We then stratified the cohort based on the presence of sperm in the ejaculate.
In order to confirm the accuracy of diagnosis based on semen analysis data, 101 of the 451 men with azoospermia (22%) had their charts reviewed. The population was chosen based on availability as the remaining charts have been archived and were unavailable for review. 91% were classified as nonobstructive azoospermia with 9% classified as obstructive azoospermia. Given that this was only a sample of the azoospermic population, no further exclusions were made based upon chart review.
Cancer Registry Linkage
All men in the andrology database were linked to the Texas Cancer Registry (TCR). The TCR is a statewide population-based registry that serves as the foundation for measuring the Texas cancer burden and comprehensive cancer control efforts. The TCR contains information on all cases of histologically confirmed cancer from January 1, 1995 until December 31, 2009 (the last year with complete data available when linkage was performed – October 2011). The TCR provides data on date of diagnosis, age at diagnosis, site of cancer (ICDO-3 codes), tumor behavior (International Statistical Classification of Diseases for Oncology, Second Edition [ICDO-3] coding), and histologic type (ICDO-3 coding).
Automated, probabilistic matching was performed using social security number, first name, middle name, last name, date of birth, and address. All matches were reviewed by PB and ME. Only the first cancer diagnosis was included in the analysis.
Statistical analysis
Cancer cases prior to 1995 could not be identified, thus our analysis was truncated to begin on January 1, 1995 for the men with a prior semen analysis. Men accrued at risk time from their initial semen analysis until cancer diagnosis or December 31, 2009 (the final year that complete cancer data was available). The rate of cancer in our cohort was compared to the general Texas population. We calculated the expected number of cases by multiplying the number of years at risk by the 5-year age strata cancer rates from the Texas Cancer Registry for the study period. Standardized Incidence Rates (SIRs) were calculated by dividing the observed number of cancer cases by the expected number of cases. Analyses were performed on the entire cohort as well as subgroups of infertile men with and without azoospermia.
We also analyzed the risk of cancer in infertile men with and without azoospermia using a Cox proportional hazards regression model while adjusting for age and year of evaluation. Comparison between Kaplan Meier curves was performed using log rank function. All p values were two sided with p<0.05 considered statistically significant. Analyses were performed using Stata 10 (StataCorp LP, College Station, Texas).
Results
In all, 2,238 infertile men were identified of whom 451 had azoospermia and 1,787 did not have azoospermia. Table 1 presents the characteristics of the infertile men in the study. There were no significant differences between men with and without azoospermia. Mean age of men at evaluation was 35.7 years of age. Follow up duration was 6.7 years and was similar between men with and without azoospermia.
Table 1.
Characteristics of men evaluated for infertility azoospermia or Not Azoospermia.
| Characteristic | Azoospermia | Not Azoospermia | p value* |
|---|---|---|---|
| n | 451 | 1,787 | |
| Age at SA, mean (SD) | 35.5 (8.3) | 35.8 (6.9) | 0.5 |
| Age at SA, n (%) | |||
| 20–29 | 313 (17.6) | 110 (24.6) | |
| 30–39 | 1,065 (59.8) | 242 (54.1) | |
| 40–49 | 356 (20) | 74 (16.6) | |
| 50–59 | 39 (2.2) | 15 (3.4) | |
| 60+ | 8 (0.5) | 6 (1.3) | |
| Age at last follow up or CA diagnosis, mean (SD) | 42.0 (9.2) | 42.6 (7.7) | 0.15 |
| Follow-up time, mean (SD) | 6.5 (3.6) | 6.8 (3.7) | 0.1 |
| 1985–1990 | 0 (0) | 4 (0.2) | 0.06 |
| 1991–1995 | 12 (2.7) | 82 (4.6) | |
| Year of evaluation | |||
| 1996–2000 | 116 (25.7) | 433 (24.2) | |
| 2001–2005 | 191 (42.4) | 829 (46.4) | |
| 2006–present | 132 (29.3) | 439 (24.6) | |
| Total Motile Sperm Count, mean (SD), millions | 0 | 54.6 (88.5) | <0.01 |
Comparisons made using ANOVA for all except year of evaluation which employed Chi-squared test.
Of the 101 azoospermic patients in whom charts were reviewed, 92 (91%) were classified as nonobstructive azoospermia. Etiologies were diagnosed in 59 (64%) of the nonobstructive azoospermic men including idiopathic (n=33), Y chromosome deletion or abnormal karyotype (n=16), varicocele (n=16), or cryptorchidism (n=9). Of the 9 patients with obstructive azoospermia, 3 had a prior vasectomy, 4 had congenital absence of the vas deferens, and 2 had ejaculatory duct dysfunction.
A total of 29 men in the cohort developed cancer of whom 2.2% had azoospermia and 1.1% did not (Supplementary Table 1). The mean time from semen analysis to cancer diagnosis was 5.8 years (SD 3.6).
Compared to the general Texas population, infertile men had a higher risk of overall cancer with 29 cases observed with only 16.7 expected (SIR 1.7, 95% CI 1.2–2.5; Table 2). However, when stratifying by azoospermia status, the azoospermic men had a significantly elevated risk of cancer (SIR 2.9, 95% CI 1.4–5.4). In contrast, infertile men without azoospermia had a similar rate of cancer to the general Texas population (SIR 1.4, 95% CI 0.9–2.2) although a trend towards an elevated risk existed.
Table 2.
Age-aggregated Standardized Incidence Ratios (SIRs) and 95% CIs for cancer in men evaluated for infertility (stratified by azoospermia status).
| n | Age-adjusted cancer incidence per 1000 person-years | Observed | Expected | SIR (95%) | |
|---|---|---|---|---|---|
| All infertility | 2,238 | 2.1 (1.6, 2.7) | 29 | 16.7 | 1.7 (1.2, 2.5)* |
| Azoospermia | 451 | 3.3 (1.7, 4.8) | 10 | 3.4 | 2.9 (1.4, 5.4)* |
| Not Azoospermia | 1,787 | 1.6 (1.0, 2.1) | 19 | 13.3 | 1.4 (0.9, 2.2) |
<0.05
The increased cancer risk of azoospermic men was also examined after stratifying by time from semen analysis. Excluding men with a diagnosis of cancer within one year (SIR 2.6, 95% CI 1.2, 5.0), two years (SIR 2.7, 95% CI 1.2, 5.1), or three years (SIR 2.2, 95% CI 0.9, 4.5) following the infertility evaluation, did not meaningfully impact the cancer incidence in azoospermic men.
On subanalysis, we limited the cohort to men evaluated for infertility under age 50, when most reproductive efforts are likely to occur (Table 3). Again, infertile men as a group had a higher risk of cancer than the general Texas population (SIR 2.0, 95% CI 1.3–2.9). Younger azoospermic men had a significantly higher risk (SIR 3.7, 95% CI 1.7–7.0) while not azoospermic men had a trend toward a higher risk compared to the general Texas male population (SIR 1.6, 95% CI 0.9–2.5). As the age of initial semen analysis decreased, the SIR increased for azoospermic men. The highest risk existed for men who had a semen analysis prior to age 30 (SIR 8.1, 95% CI 1.0–29.3). In contrast, there was no effect of age of semen analysis and risk of cancer for not azoospermic men.
Table 3.
Standardized Incidence Ratios (SIRs) and 95% CIs for cancer in men evaluated for infertility stratified by age at the time of semen analysis.
| Age at SA | Male Infertility Category | Total population(n) | Observed | Expected | SIR (95%) |
|---|---|---|---|---|---|
| <50 | All | 2,129 | 26 | 13.3 | 2.0 (1.3, 2.9)* |
| <45 | All | 1,973 | 21 | 10.5 | 2.0 (1.2, 3.1)* |
| <40 | All | 1,632 | 17 | 7.1 | 2.4 (1.4, 3.8)* |
| <35 | All | 977 | 6 | 3.5 | 1.7 (0.6, 3.8) |
| <30 | All | 312 | 2 | 0.9 | 0.9 (0.3, 8.1) |
| <50 | Azoospermia | 419 | 9 | 2.5 | 3.7 (1.7, 7.0)* |
| <45 | Azoospermia | 389 | 8 | 1.8 | 4.4 (1.9, 8.7)* |
| <40 | Azoospermia | 334 | 7 | 1.3 | 5.4 (2.2, 11.0)* |
| <35 | Azoospermia | 223 | 4 | 0.7 | 5.6 (1.5, 14.3)* |
| <30 | Azoospermia | 86 | 2 | 0.2 | 8.1 (1.0, 29.3) |
| <50 | Not Azoospermia | 1,710 | 17 | 10.9 | 1.6 (0.9, 2.5) |
| <45 | Not Azoospermia | 1,584 | 13 | 8.6 | 1.5 (0.8, 2.6) |
| <40 | Not Azoospermia | 1,298 | 10 | 5.8 | 1.7 (0.8, 3.2) |
| <35 | Not Azoospermia | 754 | 2 | 2.8 | 0.7 (0.1, 2.6) |
| <30 | Not Azoospermia | 389 | 0 | 0.6 | 0 (0, 5.7) |
<0.05
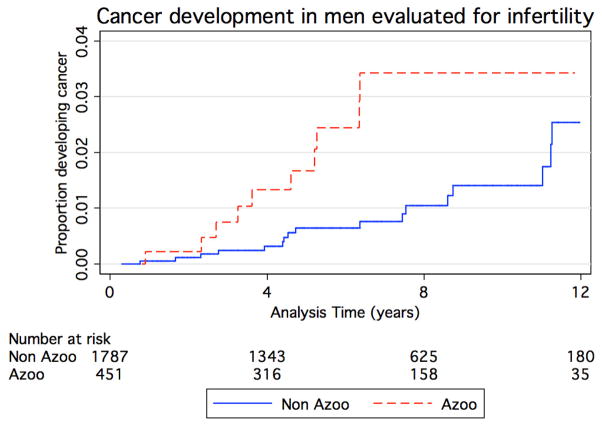
Figure 1 demonstrates the unadjusted Kaplan Meier curve estimating the occurrence of cancer stratified by azoospermia status (azoospermia versus not azoospermia). After adjusting for age and year of evaluation, the Cox regression model revealed that azoospermic men had 2.2 fold higher risk compared to not azoospermic men (HR 2.2, 95% CI 1.0–4.8)
Figure 1.
Kaplan-Meier curves comparing proportion of infertile men developing cancer with (red dashed line) and without (blue solid line) azoospermia (p=0.02).
Discussion
The current study found that infertile men were 1.7 times more likely to develop cancer than the general Texas population. Among men evaluated for infertility, men with azoospermia had a 2.9 fold increased risk. Infertile men who produced sperm in the ejaculate (i.e. not azoospermic) had a trend toward an increased risk of cancer compared to the general population. To our knowledge, the current study demonstrates the first report of an increased cancer risk in azoospermic men.
The link between male infertility and a later cancer diagnoses has been explored. Cohort studies in both Europe and the United States suggest that infertility is a risk factor for testicular cancer.(12, 14) The risk of non germ cell cancers in infertile men remains uncertain. Walsh et al reported a 2.6 fold increased risk of high grade prostate cancer in men with male factor infertility.(13) A case control study in the U.S., reported a trend toward an association between male infertility and breast cancer.(20) In contrast, other groups have not identified an increased risk of non germ cell cancers. A Danish group did not identify an increased risk of any other cancers in their cohort of men (n=32,442).(14) Moreover, a case control study in Sweden found infertility to be protective against prostate cancer (OR 0.45, 95% CI 0.25–0.83).(18) Thus, it remains uncertain if infertile men, as a unique identifiable group, are at an increased risk of non germ cell tumors.
While the etiology of the association between testis cancer and infertility is not precisely known, investigators have posited convincing explanations. For example, defects in DNA repair will impair both meiosis and mitosis, thus conceivably affecting spermatogenesis and increasing the likelihood of carcinogenesis.(4–6) In addition, researchers have hypothesized that the testicular dysgenesis syndrome results from disruptions that occur during fetal life which impair normal testicular development leading to defective sperm production and higher rates of testis cancer in the adult.(21)
Indeed, as up to 25% of the male genome is involved in reproduction, it is likely that other nonprocreative processes may also be affected by aberrations in fertility.(3) In order to confirm the accuracy of diagnosis based on semen analysis data, available charts of men with azoospermia were reviewed. Based on chart review, 91% of the azoospermic men would be defined as having nonobstructive azoospermia or severely impaired testicular function. It is likely that nonobstructive azoospermic men represent a heterogeneous group with various and as yet undefined genetic etiologies. Indeed, current genetic testing for NOA men including Y chromosome microdeletions and karyotyping will identify pathology in only 20% of men.(22–24) In our cohort, 17% were diagnosed as having a genetic etiology using these two well-recognized clinical diagnostic tests. The current report suggests that severe defects in sperm production may also manifest with impaired health, namely higher rates of cancer development.
It is interesting to note that the standardized incidence rates for cancer increased as the age of the men examined declined. While the reason is uncertain, it may be that younger men may be more likely to have primary rather than secondary infertility (i.e. intrinsic testicular failure, central hypogonadotropic hypogonadism, progressive testicular damage from a varicocele) and as such represent a more homogenous group of nonobstructive azoospermic men where a genetic cause would be more likely. Lifestyle factors, which can be different based on men’s ages, may also play a role. An alternative hypothesis is that the younger men may have more severe or more readily apparent phenotypes (e.g. Klinefelter Syndrome, prior chemotherapy, markedly atrophic testes) and were more likely to present earlier having suspected their own impaired fertility. However, based on chart review, no trends in diagnoses were apparent.
One may argue that treatment intensity would differ between azoospermic and not azoospermic men and could lead to the observed findings. However, this is unlikely given that in vitro fertilization with testicular sperm extraction remains the treatment mainstay for men with azoospermia which should not affect a man’s overall cancer risk. Most likely a shared etiology between azoospermia and carcinogenesis provides the link.
Several other important limitations of this study warrant mention. A large proportion of our original database was excluded prior to analysis due to missing data, thus limiting our cohort size. It is also possible that men may have moved out of state after evaluation, thus limiting the ability to record more incident cancers. However, we would expect migration to involve azoospermic and not azoospermic in similar proportions. Therefore the misclassification would be nondifferential which would underestimate the effect of azoospermia on cancer incidence. While we attempted to limit the analysis to men evaluated for infertility, it is conceivable that some men who had undergone prior surgical sterilization were improperly classified as azoospermic. Indeed, this was confirmed by chart review. However, this misclassification would be expected to bias the results toward unity and affected only a small fraction of our cohort.
Next, the findings could represent a detection bias as men who presented for infertility evaluation may also have better access to medical care and opportunity to be diagnosed with a cancer. In such a case, however, we would expect similar increased rates of cancer in both azoospermic and not azoospermic men whereas the highest rates were seen only with azoospermic men. In addition, we excluded men diagnosed within six months of semen analysis to limit the possibility of including men who were cryopreserving sperm with a prior cancer diagnosis. Nevertheless, it is possible that some fertility preservation patients may have been included given the possible delay and imprecision in cancer diagnosis date. However, a sensitivity analysis excluding with a cancer diagnosis up to 3 years after semen analysis revealed similar standardized incidence rates arguing against this possibility.
Nevertheless, the current report is the first suggesting an increased risk of cancer in men diagnosed with azoospermia. While other research has suggested increased risk of testicular cancer in all infertile men, the current research supports a more limited population that is most at risk and broadens the malignancies that may develop. In fact, the risk of cancer for an azoospermic man is similar to that for a man 10 years older. However, for the estimated 600,000 reproductive aged men with azoospermia, while the relative risk of cancer is elevated about three fold, it is reassuring that the absolute risk remains low. Future research should focus on the identification of the genetic links between impaired spermatogenesis and cancer and well as determining if azoospermic men warrant increased cancer screening.
Supplementary Material
Cancers diagnosed in men evaluated for infertility (stratified by azoospermia status)
Acknowledgments
Cancer data have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, 1100 W. 49th Street, Austin, Texas, 78756, http://www.dshs.state.tx.us/tcr/default.shtm, or (512) 458-7523.
Funding
This study is supported in part by P01HD36289 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, National Institutes of Health (to DJL and LIL).
Footnotes
Role of authors
ME, LIL and DL conceived the study. ME, LIL, DL, and DH collected and cleaned the data. PB performed the linkage of the institutional dataset to the cancer registries. PB and ME reviewed all matches for accuracy. All authors assisted in data analysis and interpretation. ME drafted the manuscript. All authors critically reviewed, revised, and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Committee AP. Evaluation of the azoospermic male. Fertil Steril. 2008;90:S74–7. doi: 10.1016/j.fertnstert.2008.08.092. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JE, Farr SL, Jamieson DJ, Warner L, Macaluso M. Infertility services reported by men in the United States: national survey data. Fertil Steril. 2009;91:2466–70. doi: 10.1016/j.fertnstert.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat mede. 2008;14:1197–213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee S, Ridgeway AD, Lamb DJ. DNA mismatch repair and infertility. Curr Opin Urol. 2010;20:525–32. doi: 10.1097/MOU.0b013e32833f1c21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonsalves J, Sun F, Schlegel PN, Turek PJ, Hopps CV, Greene C, et al. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875–83. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- 6.Maduro MR, Casella R, Kim E, Levy N, Niederberger C, Lipshultz LI, et al. Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell-only syndrome. Mol Hum Reprod. 2003;9:61–8. doi: 10.1093/molehr/gag013. [DOI] [PubMed] [Google Scholar]
- 7.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 8.Ji G, Long Y, Zhou Y, Huang C, Gu A, Wang X. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC. 2012;10:49. doi: 10.1186/1741-7015-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul C, Povey JE, Lawrence NJ, Selfridge J, Melton DW, Saunders PT. Deletion of genes implicated in protecting the integrity of male germ cells has differential effects on the incidence of DNA breaks and germ cell loss. PLoS One. 2007;2:e989. doi: 10.1371/journal.pone.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reitmair AH, Schmits R, Ewel A, Bapat B, Redston M, Mitri A, et al. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet. 1995;11:64–70. doi: 10.1038/ng0995-64. [DOI] [PubMed] [Google Scholar]
- 11.Jiricny J, Nystrom-Lahti M. Mismatch repair defects in cancer. Curr Opin Genet Dev. 2000;10:157–61. doi: 10.1016/s0959-437x(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 12.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Int Med. 2009;169:351–6. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer. 2010;116:2140–7. doi: 10.1002/cncr.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–92. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg ML, Park Y, Brinton LA, Hollenbeck AR, Schatzkin A. Fatherhood and incident prostate cancer in a prospective US cohort. Int J Epidem. 2011;40:480–7. doi: 10.1093/ije/dyq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giwercman A, Richiardi L, Kaijser M, Ekbom A, Akre O. Reduced risk of prostate cancer in men who are childless as compared to those who have fathered a child: a population based case-control study. Int J Can. 2005;115:994–7. doi: 10.1002/ijc.20963. [DOI] [PubMed] [Google Scholar]
- 17.Negri E, Talamini R, Bosetti C, Montella M, Franceschi S, La Vecchia C. Risk of prostate cancer in men who are childless. Int J Can. 2006;118:786–7. doi: 10.1002/ijc.21369. author reply 8. [DOI] [PubMed] [Google Scholar]
- 18.Ruhayel Y, Giwercman A, Ulmert D, Rylander L, Bjartell A, Manjer J, et al. Male infertility and prostate cancer risk: a nested case-control study. Ca Caus Con. 2010;21:1635–43. doi: 10.1007/s10552-010-9592-8. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4. Cambridge: Published on behalf of the World Health Organization by Cambridge University Press; 1999. [Google Scholar]
- 20.Thomas DB, Jimenez LM, McTiernan A, Rosenblatt K, Stalsberg H, Stemhagen A, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidem. 1992;135:734–48. doi: 10.1093/oxfordjournals.aje.a116360. [DOI] [PubMed] [Google Scholar]
- 21.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 22.Dohle GR, Halley DJ, Van Hemel JO, van den Ouwel AM, Pieters MH, Weber RF, et al. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod. 2002;17:13–6. doi: 10.1093/humrep/17.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Stahl PJ, Masson P, Mielnik A, Marean MB, Schlegel PN, Paduch DA. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril. 2010;94:1753–6. doi: 10.1016/j.fertnstert.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Oates R. Evaluation of the azoospermic male. Asian J Androl. 2012;14:82–7. doi: 10.1038/aja.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cancers diagnosed in men evaluated for infertility (stratified by azoospermia status)