Abstract
Background
The many attempts to identify genes for bipolar disorder (BD) have met with limited success, which has generally been attributed to genetic heterogeneity and small gene effects. However, it is also possible that the categorical phenotypes used in genetic studies of BD are not the most informative or biologically relevant. We have explored aspects of temperament as quantitative phenotypes for BD through the use of the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Auto-questionnaire (TEMPS-A), which is designed to assess lifelong, milder aspects of bipolar symptomatology and defines five temperaments: hyperthymic, dysthymic, cyclothymic, irritable, and anxious.
Methods
We compared temperament scores between diagnostic groups and assessed heritability in a sample of 101 families collected for genetic studies of BD. A genome-wide SNP linkage study was then performed in the subset of 51 families for which genetic data was available.
Results
Significant group differences were observed between BD subjects, their first-degree relatives, and independent controls, and all five temperaments were found to be significantly heritable, with heritabilities ranging from 21% for the hyperthymic to 52% for the irritable temperaments. Suggestive evidence for linkage was observed for the hyperthymic (chromosomes 1q44, 2p16, 6q16, and 14q23), dysthymic (chromosomes 3p21 and 13q34), and irritable (chromosome 6q24) temperaments.
Limitations
The relatively small size of our linkage sample likely limited our ability to reach genome-wide significance in this study.
Conclusions
While not genome-wide significant, these results suggest that aspects of temperament may prove useful in the identification of genes underlying BD susceptibility.
Keywords: bipolar disorder, temperament, TEMPS-A, heritability, genetic linkage
INTRODUCTION
Many attempts have been made over the last few decades to resolve the genetic architecture of bipolar disorder (BD) and to identify genetic variants contributing to susceptibility. Linkage and association studies have implicated numerous chromosomal regions and candidate genes with significant evidence for an involvement in BD, yet the causal variants have remained elusive (Serretti and Mandelli, 2008). These difficulties in identifying genes for BD have generally been attributed to genetic heterogeneity and small gene effects. Recent genome-wide association (GWA) studies of BD and subsequent large meta-analyses encompassing several thousand cases and controls have produced significant evidence for association to some interesting new candidates, yet these loci explain only 1–2% of disease liability (Baum et al., 2007; Ferreira et al., 2008; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011; Scott et al., 2009; Sklar et al., 2008; Smith et al., 2009; Wellcome Trust Case Control Consortium, 2007).
Another possible explanation for the difficulties encountered in gene mapping is that the diagnostic systems used in genetic studies of BD may not be optimal. While various affective traits and disorders with a range of severity are often observed in the families of BD probands (Gershon et al., 1982; Kelsoe, 2003; Price et al., 1985), the current categorical diagnostic systems are limited in their ability to adequately define this phenotypic variation. Some have suggested that BD may be better conceptualized as part of a continuous distribution of affective phenotypes ranging from very mild, subclinical affective traits to severe affective psychoses. This is consistent with a polygenic trait for which interactions between many genes of small effect produce a continuous variation in phenotype (Akiskal, 1983; Akiskal et al., 1977; Akiskal and Pinto, 2000; Kelsoe, 2003).
Temperament is a heritable personality factor that remains stable over time and establishes a person's baseline level of mood, reactivity, and energy (Goldsmith et al., 1987). While normal variations in temperament exist within the population, it has been suggested that a dysregulation of temperament is the fundamental abnormality that predisposes to the development of bipolar spectrum disorders, with more extreme variation in temperament conferring greater risk and increased severity (Akiskal, 1995; Akiskal, 1996; Akiskal and Akiskal, 1992; Akiskal et al., 1977). In this model, temperament is influenced by numerous genes of small effect, resulting in a continuous distribution of mood regulation and reactivity, consistent with the observation of milder forms of the bipolar phenotype in family members of probands with BD and with a polygenic mode of transmission. Temperament may thus be more sensitive and closer to the underlying biological abnormalities and, as such, may be a powerful tool for identifying the genetic underpinnings of BD
Temperament can be assessed along several dimensions that define qualitatively different aspects of affective regulation, each of which may be associated with different sets of genes. The Temperament Evaluation of Memphis, Pisa, Paris, and San Diego Auto-questionnaire (TEMPS-A) is a self-report questionnaire designed to quantify temperament and assess lifelong, milder aspects of bipolar symptomatology (Akiskal et al., 2005a; Akiskal et al., 2005b). Five temperaments are defined by this scale: hyperthymic, dysthymic, cyclothymic, irritable, and anxious. The reliability and internal consistency of the TEMPS-A is well documented, and the temperaments are stable over time, with one study reporting stability up to six years (Akiskal et al., 1998; Kawamura et al., 2010; Perugi et al., 2012; Placidi et al., 1998a; Placidi et al., 1998b). Several studies have demonstrated the ability of temperament measures to predict risk for bipolar spectrum disorders and to discriminate between affected subjects, healthy relatives of affected subjects, and normal controls (Akiskal et al., 1977; Akiskal et al., 1985; Cassano et al., 1992; Evans et al., 2005; Horwath et al., 1992; Kesebir et al., 2005; Kovacs et al., 1994; Mendlowicz et al., 2005; Vazquez et al., 2007). Different temperaments within BD have also been associated with different clinical courses, rates of relapse, and response to antidepressants (Cassano et al., 1989; Koukopoulos et al., 1983; Perugi et al., 2012; Rihmer et al., 2010).
Here we have used the TEMPS-A to assess temperament as a quantitative phenotype for BD and to explore the underlying genetic architecture of BD and other affective disorders. We evaluated temperament scores for subjects with BD, major depressive disorder (MDD), clinically unaffected relatives, and independent control subjects. We have previously reported evidence for the familiality of temperament in BD (Evans et al., 2005). We now report evidence for heritability of all five temperaments in 101 BD families and the results of a subsequent genetic linkage analysis of 51 families genotyped for a single nucleotide polymorphism (SNP) linkage panel. While investigations of BD have recently moved toward GWA, which has greater power to detect weak associations to common variants, linkage remains a valuable tool in genetic studies with the ability to detect the aggregate effects of multiple rare and common variants within a gene or region, even with different mutations conferring risk in different families.
METHODS
Subjects
Subjects from BD families were selected from three different data sets collected for genetic studies of BD. The primary dataset (University of California San Diego, UCSD) was recruited at one of three sites (San Diego, Vancouver, and Cincinnati) as part of a collaborative genetic linkage study of BD (Kelsoe et al., 2001). The other two data sets were recruited at UCSD as part of the National Institute of Mental Health (NIMH) Genetics Initiative for Bipolar Disorder Waves 3 and 4 (Dick et al., 2003). Since the TEMPS-A was only administered at the San Diego site of the NIMH consortium, data was not available for the rest of the NIMH collection. Families were first identified through a proband diagnosed with bipolar I disorder or bipolar II disorder (UCSD sample) or a bipolar I sibling pair (Waves 3 and 4) (Dick et al., 2003). Each subject was interviewed and diagnosed using either a modified version of the Structured Clinical Interview for DSM-III-R (SCID; Spitzer et al., 1992) or the Diagnostic Interview for Genetics Studies (DIGS; Nurnberger et al., 1994). Interviewers were extensively trained, and reliability was regularly tested and shown to be high. A panel of clinicians reviewed the interview, medical records, and information from family informants in order to make a final DSM-IV diagnosis. Control subjects were ascertained through advertising by the UCSD Mental Health Clinical Research Center and screened using the SCID for the absence of psychiatric illness. Blood was drawn on all subjects for the establishment of lymphoblastoid cell lines. All subjects provided written informed consent according to procedures approved by the local Institutional Review Board of each university.
The TEMPS-A was administered at the time of interview and includes a total of 109 self-rated true/false questions (110 for women) designed to assess the hyperthymic, dysthymic, cyclothymic, irritable, and anxious temperaments with 21 questions (26 for anxious) specific to each temperament subscale (Akiskal et al., 2005a; Akiskal et al., 2005b). Question 84 from the irritable subscale was excluded from our analyses because it applies only to women. The TEMPS-A has been shown to have very good reliability and internal consistency with the temperaments showing good stability over time (Akiskal et al., 1998; Kawamura et al., 2010; Placidi et al., 1998a; Placidi et al., 1998b).
The final sample included 670 subjects from 101 families with TEMPS-A data available for 428 subjects with the following diagnoses: 128 subjects (29.9%) with bipolar I (BDI), 40 (9.3%) with bipolar II (BDII), 9 (2.1%) with schizoaffective disorder, bipolar-type (SA-BP), 100 (23.4%) with recurrent major depression (MDD-R), 18 (4.2%) with a single episode of major depression (MDD-SE), 148 (28.5%) with no history of mood disorders, and 11 (2.6%) of unknown diagnoses. These data, along with the number of informative relative pairs, are summarized in Table 1. We also included an independent sample of 53 control subjects with TEMPS-A data and no personal or family history of mental illness. This sample is approximately 62% female with an average age at interview of 45 (±17). All subjects were Caucasians of European ancestry.
Table 1.
Summary of the subjects and families included in the heritability and linkage analyses.
| Family Information | Heritability | Linkage |
|---|---|---|
| Number of families | 101 | 51 |
| Average size | 6.6 | 7.0 |
| Average generations | 2.5 | 2.5 |
|
Relative Pairs | ||
| Sibling | 310 | 200 |
| Half-sibling | 13 | 9 |
| Parent-child | 764 | 424 |
| Grandparent-grandchild | 302 | 148 |
| Avuncular | 249 | 123 |
| Cousin | 75 | 25 |
|
Subjects | ||
| Total Subjects | 670 (428) | 356 (236) |
| Bipolar Disorder (BD) | 200 (177) | 127 (111) |
| Major Depressive Disorder (MDD) | 133 (118) | 74 (66) |
| No history of mood disorders | 148 (122) | 72 (56) |
The numbers of subjects with TEMPS-A data in all subjects and across diagnostic categories are indicated in parentheses. The BD group includes BDI, BDII, and SA-BP, and the MDD group includes MDD-R and MDD-SE.
Genotyping
Genotyping was performed by the Center for Inherited Disease Research (CIDR) using the Illumina Infinium HumanLinkage-12 panel containing 6,090 SNP markers across the genome. A subset of the families and were genotyped as part of a larger study of 972 European ancestry families that were evaluated for linkage to BD (Badner et al., 2012). The 5,670 autosomal and X-linked SNPs that passed the initial quality control assessments by CIDR were evaluated for missingness, allele frequency, and Hardy–Weinberg equilibrium, and families were further assessed for relatedness, Mendelian errors, unlikely genotypes, and ancestry, as described in detail in elsewhere (Badner et al., 2012). Cleaned genotypes were available for 261 subjects from 51 of our 101 UCSD and NIMH families (see Table 1). The remaining 5,642 SNPs were ordered on the physical map according to Genome Build 36, and the deCODE genetic map was used to estimate genetic map distances (Kong et al., 2002). The final SNPs had an average physical spacing of 490 kb and an average genetic spacing of 0.62 cM.
Statistics
Subjects diagnosed as BDI, BDII, or SA-BP had similar scores across the temperaments and were combined into a BD group for analysis. Subjects diagnosed with MDD-R or MDD-SE also had similar temperament scores and were combined into an MDD group. Unaffected relatives and independent controls subjects were placed into the REL and CTL groups, respectively. Group differences were evaluated using oneway ANOVA with diagnosis as the grouping factor, and the Tukey HSD for unequal n's was utilized as a post-hoc test to compare specific groups.
Heritability (h2) estimates were computed using the variance component method implemented in SOLAR v.4.3.1 (Almasy and Blangero, 1998). Prior to analysis, all temperaments were transformed using the inverse normal function, which corrected the slight deviations from normality that were observed in the distributions of the raw data. Age at interview and gender were explored as potential covariates and retained in the analysis when significant (p<0.05).
Linkage analysis was performed using the inverse normal transformed and covariate-adjusted residual temperament scores. Two-point and multipoint log of the odds ratio (LOD) scores were computed using MERLIN v.1.1.2 (Abecasis et al., 2002). Although several different methodologies are available to assess linkage for quantitative traits, the pedigree-wide regression method in MERLIN was selected because it has been shown to be more robust to issues involving incomplete marker informativity and is appropriate for selected samples, allowing for the specification of population based parameters (Cordell, 2004; Schork and Greenwood, 2004a, b; Sham et al., 2002). Variance component models were instead used for analyses of the X chromosome, since the regression algorithm only supports the analysis of autosomes. For comparison, nonparametric linkage analysis of BD was performed using the SPairs statistic in MERLIN under two diagnostic models: a narrow model that included BD only and a broad model that included both BD and MDD. Multipoint identity-by-descent matrices were generated at a 1 cM resolution, slightly larger than the average spacing between SNPs. Since linkage analysis of tightly linked loci can inflate LOD scores, we required that the r2 value between markers be less than 0.1. As a correction for multiple testing, genome-wide suggestive and significant thresholds were estimated for this sample using gene-dropping simulations in MERLIN with 1,000 permutations for each temperament and diagnostic category. The suggestive and significant thresholds for each temperament and diagnosis are as follows: 2.32 and 3.18 for hyperthymic, 2.36 and 4.17 for dysthymic, 2.19 and 4.04 for cyclothymic, 2.32 and 4.45 for irritable, 1.81 and 3.07 for anxious, 1.92 and 3.17 for BD, and 1.88 and 3.1 for BD+MDD, respectively. This sample has 80% power to detect a locus explaining 45% of the trait variance across the temperaments with a LOD of 2.2.
RESULTS
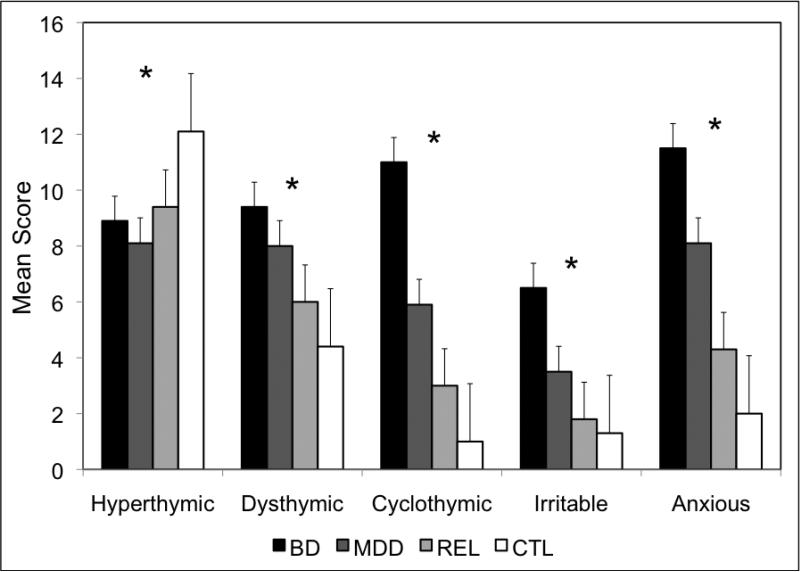
The average temperament scores in subjects with BD and MDD, along with their unaffected relatives (REL) and independent controls (CTL), are shown in Figure 1. Significant group differences (p<0.001) were observed for all five temperaments as follows: hyperthymic F=10.0, dysthymic F=37.6, cyclothymic F=97.3, irritable F=55.0, and anxious F=58.0. As shown in Table 2, post hoc analyses of the dysthymic, cyclothymic, irritable, and anxious temperaments all revealed significantly higher scores for the BD and MDD groups compared with all other groups. The dysthymic temperament also discriminated the REL and CTL groups with trends observed for the cyclothymic and anxious temperaments. For the hyperthymic temperament, the CTL group scored significantly higher, with no significant differences among the BD, MDD, and REL groups observed. Among the 177 BD subjects, females scored significantly higher than males for the dysthymic (mean 10.4 vs. 7.8, p<0.001) and anxious (mean 12.7 vs. 9.2, p=0.001) temperaments and significantly lower for the hyperthymic temperament (mean 8.1 vs. 10.4, p=0.002). Age at onset of BD had a mean of 21.3 (±10.2) years in this sample and was found to have negative correlations with all temperaments except hyperthymic (r=-0.31 to -0.39, p<0.01). Data regarding current state was available for only 99 of the 177 subjects with BD and only 48 of the 118 subjects with MDD. Of those with data, 44% of BD and 13% of MDD subjects met full criteria or were symptomatic at the time of interview. We compared the temperament scores of these subjects with those that were in remission at the time of interview and found no significant differences (p>0.05). Finally, neither disease severity nor lifetime course (with vs. without full interepisode recovery) was significantly correlated with any of the temperaments (p>0.05).
Figure 1.
Mean scores for each temperament are indicated by diagnostic group. An * indicates that a significant difference was found across the four diagnostic groups for the respective temperament.
Table 2.
Post hoc analyses of group differences across the temperaments.
| Hyperthymic | Dysthymic | Cyclothymic | Irritable | Anxious | |
|---|---|---|---|---|---|
| BD vs. MDD | ns | 0.005 | <0.001 | <0.001 | <0.001 |
| BD vs. REL | ns | <0.001 | <0.001 | <0.001 | <0.001 |
| BD vs. CTL | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| MDD vs. REL | ns | <0.001 | <0.001 | 0.002 | <0.001 |
| MDD vs. CTL | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| REL vs. CTL | 0.002 | 0.037 | 0.068 | ns | 0.062 |
All significant and trend (p<0.10) p values are indicated with nonsignificant p values (p>0.10) indicated by “ns”.
All five temperaments were found to be significantly heritable in this sample, as shown in Table 3, with heritabilities ranging from 21% for hyperthymic to 52% for irritable. Gender was found to be a significant covariate for all but the cyclothymic temperament. Several groups have shown correlations between age at interview and the various temperaments, and age was found to be a significant covariate for all but the hyperthymic temperament in our analyses as well (Benazzi, 2009; Di Florio et al., 2010; Karam et al., 2010). Prior to conducting the linkage analyses, we confirmed the heritability of these temperaments in the subset of 51 families with available genotype data. The dysthymic, cyclothymic, irritable, and anxious temperaments produced similar heritability estimates in the genotyped sample, whereas the heritability of hyperthymic was substantially higher in the 51 genotyped families than in the complete sample of 101 families (41% vs. 21%).
Table 3.
Heritability estimates observed for the five temperaments in the 101 families.
| Informative Pairs | Covariate P Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperament | N | Sib | P-O | Other | h2r ± SE | P value | Age | Gender | Variance |
| Hyperthymic | 417 | 231 | 282 | 269 | 0.21 ± 0.10 | 0.011 | ns | <0.0001 | 0.05 |
| Dysthymic | 406 | 230 | 284 | 274 | 0.29 ± 0.11 | 0.0009 | 0.012 | <0.0001 | 0.06 |
| Cyclothymic | 408 | 229 | 292 | 277 | 0.46 ± 0.11 | <0.0001 | <0.0001 | ns | 0.12 |
| Irritable | 406 | 226 | 289 | 257 | 0.52 ± 0.11 | <0.0001 | <0.0001 | 0.013 | 0.13 |
| Anxious | 347 | 174 | 238 | 186 | 0.36 ± 0.13 | 0.0007 | <0.0001 | <0.0001 | 0.14 |
Key: Sib = sibling pairs; P-O = parent-offspring pairs; Other = half-sibling, grandparent-grandchild, avuncular, and cousin pairs; h2r=residual heritability after adjustment for significant covariates; SE=standard error; Variance = the proportion of the trait variance explained by all significant covariates. All nonsignificant p values (p>0.05) are indicated by “ns”.
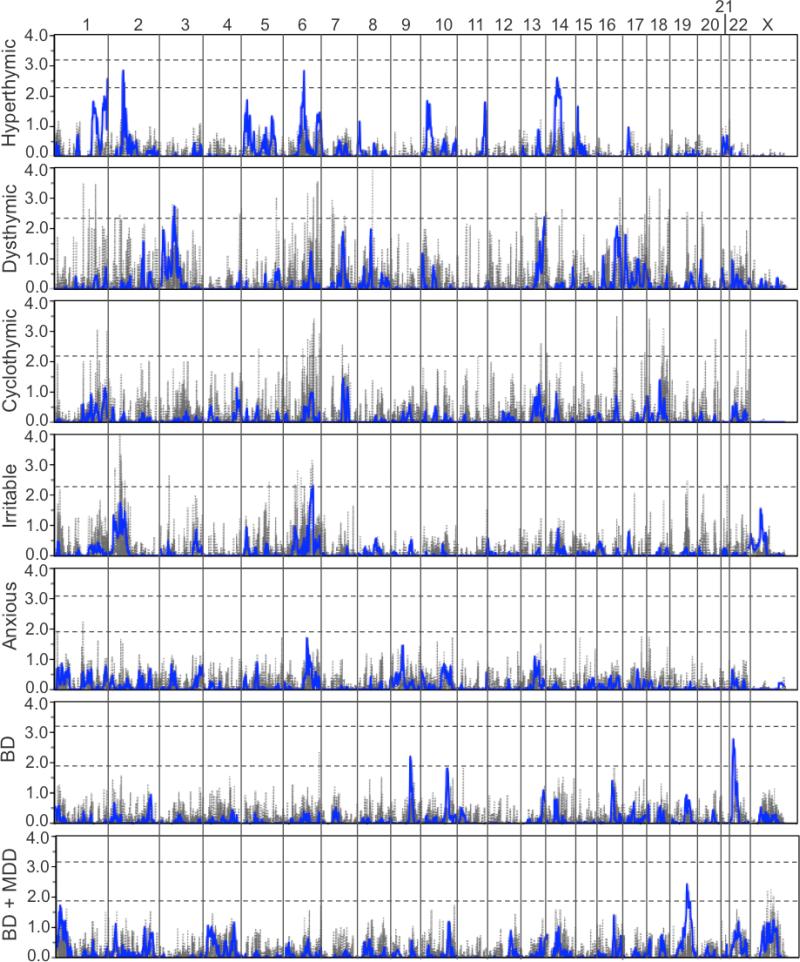
As shown in Figure 2 and summarized in Table 4, linkage analyses collectively identified several interesting regions across the temperaments, though none reached the threshold of genome-wide significance. Summaries of all multipoint and two-point LOD scores >1.0 are provided in Tables S1 and S2, respectively. Suggestive evidence of linkage was observed for hyperthymic on chromosomes 1q44, 2p16, 6q16, and 14q23, for dysthymic on chromosomes 3p21 and 13q34, and for irritable on chromosome 6q24. The cyclothymic and anxious temperaments produced several peaks with multipoint LOD scores >1.0, but none met genome-wide suggestive criteria. Numerous two-point LOD scores meeting genome-wide suggestive criteria were also observed for all temperaments but hyperthymic (see Figure 2 and Table S2), many of which occurred in regions that produced only modest multipoint LOD scores at best. No significant overlap of linked regions was observed between the temperaments.
Figure 2.
Results of the genome-wide SNP linkage scan in the 51 families for each of the five temperaments. The results from the analysis of BD diagnosis as a narrow (BD) and broad (BD + MDD) phenotype in these families are provided for comparison. Multipoint results are shown in blue with two-point results shown in gray. LOD scores are indicated on the y-axis, along with the name of the corresponding temperament or diagnostic category, and chromosomes are aligned along the x-axis end to end with the p-terminus on the left and locations indicated at the top of the figure. Dashed horizontal lines indicate significant and suggestive LOD score thresholds for each phenotype based on simulation. Note that LOD score significance thresholds for dysthymic, cyclothymic, and irritable were >4.0 and are not shown.
Table 4.
Summary of all chromosomal regions with multipoint LOD scores reaching suggestive evidence for linkage.
| Temperament or Diagnosis | Chr | Location (cM / Mb) | Peak LOD | 1-LOD Interval (cM / Mb) | Nearest Genes | Nearby Genes of Interest |
|---|---|---|---|---|---|---|
| Hyperthymic | 1q44 | 272 / 245.0 | 2.53 | 269-274 / 243.5-246.1 | SCCPDH, AHCTF1 | |
| 2p16 | 77 / 51.8 | 2.84 | 74-82 / 48.1-59.1 | |||
| 6q16 | 103 / 98.4 | 2.83 | 98-105 / 91.1-101.4 | NRXN1 C6orf167, POU3F2, FBXL4 | EPHA7, GRIK2 | |
| 14q23 | 61 / 61.0 | 2.61 | 50-80 / 52.3-78.8 | SYT16 | SYNE2, ESR2, PSEN1, NRXN3 | |
| Dysthymic | 3p21 | 71 / 47.0 | 2.74 | 62-71 / 36.5-51.7 | NBEAL2, SETD2 | CTNNB1 |
| 13q34 | 124 / 111.8 | 2.39 | 116-129 / 109.0-113.5 | SOX1 | ||
| Irritable | 6q24 | 147 / 145.0 | 2.33 | 132-149 / 133.3-147.7 | UTRN, GRM1 | STXBP5, SYNE1 |
| BD | 9q31 | 103 / 102.5 | 2.20 | 101-109 / 100.0-108.2 | LPPR1 | GRIN3A |
| 22q11 | 6 / 17.0 | 2.77 | 3-15 / 16.3-21.0 | DGCR6, PRODH | COMT | |
| BD + MDD | 19q13 | 67 / 45.2 | 2.42 | 63-84 / 41.6-55.7 | PSMC4, ZNF546, ZNF780A | ZDHHC8, MAPK1 |
Key: 1-LOD interval = genetic and physical boundaries of LOD scores within one unit of the maximum; Nearest genes = genes immediately flanking the peak LOD score, with those of particular interest based on function or previous studies underlined; Nearby Genes of Interest = genes within the 1-LOD interval that are expressed in brain or have been implicated in previous studies of psychiatric illness.
Several genes of potential interest were identified beneath the multipoint linkage peaks. The neurexin 1 gene (NRXN1), which encodes a neuronal cell surface protein involved in cell adhesion and synaptogenesis, is located under the hyperthymic peak on 2p16. Deletions within this gene have been associated with schizophrenia and autism (Kim et al., 2008; Kirov et al., 2008; Kirov et al., 2009; Szatmari et al., 2007; Walsh et al., 2008). The SET domain containing 2 gene (SETD2), which encodes a protein belonging to a class of huntingtin interacting proteins characterized by WW motifs, is located beneath the dysthymic peak on 3p21 (Faber et al., 1998). The metabotropic glutamate receptor 1 gene (GRM1), which plays a critical role in synaptic plasticity, learning, and memory, is located directly beneath the irritable peak on 6q24 (Conquet et al., 1994; Gil-Sanz et al., 2008). Coding variants in this gene have recently been implicated in both schizophrenia and BD (Frank et al., 2012). The synaptic nuclear envelope protein 1 gene (SYNE1), which has recently been implicated as a susceptibility gene for BD in a large, collaborative genome-wide association study, is also located within the 1-LOD interval of the irritable peak (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011).
Suggestive evidence for linkage was also observed for BD on chromosomes 9q31 and 22q11 and BD+MDD on chromosome 19q13. Genes of potential interest were also identified beneath these peaks, such as the lipid phosphate phosphatase-related protein type 1 gene (LPPR1) under the peak on 9q31, which is involved in neuronal plasticity (Savaskan et al., 2004). The 22q11 region has featured prominently in linkage studies of both BD and schizophrenia (Blouin et al., 1998; Edenberg et al., 1997; Gill et al., 1996; Kelsoe et al., 2001; Lachman et al., 1997; Pulver et al., 1994; Shaw et al., 1998). Deletions and microdeletions of this region are associated with schizophrenia and the “chromosome 22q11.2 deletion syndrome”, which encompasses a wide range of phenotypic variability, including the DiGeorge and velocardiofacial syndromes, and often presents with higher rates of schizophrenia and BD (Driscoll et al., 1993; Karayiorgou et al., 1995; Murphy et al., 1999; Papolos et al., 1996). Flanking the peak LOD score on 22q11 are the DiGeorge syndrome critical region gene 6 (DGCR6), which may play a role in neural crest cell migration, and the proline dehydrogenase (oxidase) 1 gene (PRODH), which encodes a mitochondrial protein that catalyzes the first step in proline degradation. Both have been implicated as candidate genes for schizophrenia (Guilmatre et al., 2009; Lang et al., 2007; Liu et al., 2002a; Liu et al., 2002b). These regions did not overlap with any of those observed for the temperaments.
DISCUSSION
Since BD patients display significant clinical variability, which may reflect underlying genetic heterogeneity, the use of the DSM diagnosis of BD as a phenotype may not have the best power to detect causal genes. We have explored the use of temperament as a quantitative genetic trait influencing susceptibility to BD. In this study, significant group differences were observed across all five temperaments with the dysthymic, cyclothymic, irritable, and anxious scales in particular showing the expected trend of more pathological scores for BD subjects, followed by MDD, then unaffected relatives, and finally controls. These results are consistent with our previous study that reported evidence of familiality for the five TEMPS-A scales in a sample of 28 overlapping families (Evans et al., 2005). The hyperthymic scale revealed a different pattern wherein controls scored significantly higher than all other groups, and BD subjects did not differ from their affected or unaffected relatives. This pattern has been observed in several other studies as well and may reflect the ability of the hyperthymic temperament to detect a latent genetic vulnerability to developing BD (Evans et al., 2005; Mendlowicz et al., 2005; Vazquez et al., 2007). It should be noted, however, that mixed results have been found for this temperament, with BD subjects scoring highest in some studies (Chiaroni et al., 2005; Kesebir et al., 2005). We also observed significant gender differences for the temperaments with females scoring higher for dysthymic and anxious and males scoring higher for hyperthymic. While a similar pattern has been observed in healthy subjects (Vazquez et al., 2012), as well as in other studies of BD (Evans et al., 2005; Greenwood et al., 2012a), a recent study of lithium response in BD failed to find gender differences for any of the temperaments, although the sample was quite small (Rybakowski et al. 2013).
Temperament is considered a heritable trait, yet few studies have directly assessed the heritability of the TEMPS-A scales in families with BD and other affective disorders. In addition to demonstrating the familiality of temperament in this sample, we have further shown that all five temperaments are significantly heritable and thus suitable quantitative phenotypes for genetic analyses of BD susceptibility. In another study of temperament in 241 subjects from 31 extended Caucasian South African families, heritabilities were estimated as 51% for hyperthymic, 29% for dysthymic, and 75% for irritable (Savitz et al., 2008). Anxious was not evaluated in this study, and cyclothymic was not found to be heritable. The heritability estimate for dysthymic from this previous study is identical to what we have found here, and both estimates for irritable are very high. The heritability of hyperthymic was significantly higher in this previous study than in ours, although we note that the heritability of this temperament in the linkage families was substantially higher at 41%. Conversely, we found cyclothymic to be quite heritable in our study at 46%. The discrepancies in these heritability estimates are likely due not only to differences in the two samples, both in terms of size and composition, but are also likely attributable to the different programs used for analysis. While the Savitz et al. study used QTDT to estimate heritability as part of a genetic association analysis, we have used the variance components method in SOLAR for this purpose (Abecasis et al., 2000; Almasy and Blangero, 1998). Despite these differences, there is consistent evidence for the familiality and heritability of temperament across studies.
Few studies investigating temperament in BD through genetic analyses have been published to date. One study of selected candidate genes found suggestive associations for the BDNF val66met and COMT val158met polymorphisms with the hyperthymic and irritable temperaments, respectively (Savitz et al., 2008). The genomic regions including these genes, 11p13 and 22q11, respectively, did not show any evidence of linkage to the temperaments in this study, although the 22q11 region did show suggestive evidence of linkage to BD, and the COMT gene is located within the 1-LOD interval. A linkage study of the cyclothymic temperament in 28 BD families identified a genome-wide suggestive peak on chromosome 18p11 with a LOD of 2.7. While we did not find any evidence for linkage of cyclothymic to this region in the current study, we note that this previous study utilized microsatellite markers with an average spacing of 8 cM, compared with the SNP linkage panel used here with 0.6 cM spacing, and included far fewer families than the present study. A more recent GWA study of 1,263 independent BDI subjects identified significant associations for the hyperthymic temperament on chromosomes 12q15 and 22q13 in the MDM1 and FBLN1 genes, respectively, and for irritable on chromosome 1q32 in the INST7 and DTL genes (Greenwood et al., 2012a). While we did not find evidence for linkage to 12q15 or 22q13 in this study, there was some evidence to support the 1q32 region with a maximum multipoint LOD of 1.83 observed for hyperthymic and suggestive two-point LOD scores of 3.45 and 2.62 observed for dysthymic (see Tables S1 and S2). Two other regions of interest from this GWA study, 13q31 for cyclothymic and 13q33 for irritable, also produced suggestive two-point LODs in this study. For the 13q31 region, a LOD of 2.36 was observed for dysthymic, and LODs of 2.58 and 2.51 were observed for cyclothymic and irritable, respectively, in the 13q33 region (see Table S2). No other regions of overlap were observed between these two studies. Of course, one might not expect an association study of unrelated BDI subjects to produce the same findings as a linkage study of BD that includes both affected and unaffected family members and a broader definition of BD (BDI, BDII, and SA-BP). This is especially true considering that association studies are most appropriate for the detection of common variation, while linkage studies are more suited to the detection of rare variants or regions of allelic heterogeneity (Ott et al., 2011).
Linkage studies of BD over the last two decades have revealed a variety of chromosomal regions that may harbor susceptibility loci, including chromosomal regions 4p16, 5p15, 6q21, 8q24, 10q26, 11p15, 12q24, 13q32, 17q25, 18p11, 18q22-23, 20q13, 21q22, 22q12-13, and Xq26 (Serretti and Mandelli, 2008), with one meta-analysis emphasizing the 13q32 and 22q13 regions (Badner and Gershon, 2002), another implicating the 9p22, 10q11, and 14q24-32 regions (Segurado et al., 2003), and a combined analysis emphasizing the 6q21 and 8q24 regions (McQueen et al., 2005). A recent study of 972 bipolar families using this same SNP linkage panel indicated the strongest findings for chromosomes 6q21 and 9q21 (Badner et al., 2012). Our previous linkage analyses of 20 extended pedigrees for BD revealed strong evidence of linkage to chromosome 22q11, as well as suggestive evidence for linkage to 3p21, 3q27, 5p15, 10q, 13q31-q34, and 21q22 (Kelsoe et al., 1996; Kelsoe et al., 2001; Lachman et al., 1997). Further analysis of an additional 34 pedigrees found strong evidence for linkage to 6q25 and suggestive evidence for 13q32 and 17p12 (Greenwood et al., 2012b; Shaw et al., 2003). Of the 51 linkage families resented here, 28 overlapped with these two previous studies. With the notable exceptions of 3p21 and 13q34, for which suggestive evidence for linkage to dysthymic was observed, and 22q11, which showed suggestive evidence for BD, none of these regions featured prominently in our analyses. However, suggestive two-point LOD scores were observed for the 6q21, 13q31, 13q33, 17q25, 18q11, and 22q13 regions across several temperaments (see Table S2). The 6q16 linkage observed for the hyperthymic temperament in this sample was also observed for BD in both our previous study of 34 families and in the NIMH wave 3 sample, both of which overlap in part with the sample presented here (Dick et al., 2003; Greenwood et al., 2012b).
One might expect that the temperaments with the highest heritabilities would produce the strongest genetic signals, yet, as our results demonstrate, this is not necessarily the case. Irritable had one of the highest heritabilities in this study at 52% and yet only produced one multipoint linkage signal meeting genome-wide suggestive criteria. Conversely, hyperthymic produced at total four suggestive linkage peaks despite having the lowest heritability in the study at 21%, although we note that the heritability of this temperament in the linkage subset was substantially higher (41%). Both cyclothymic and anxious, with heritabilities of 46% and 36%, respectively, failed to produce a single linkage peak meeting genome-wide suggestive criteria, while dysthymic produced two suggestive linkage peaks with a lower heritability of 29%. These results illustrate that heritability estimates are not perfect predictors of the potential ‘mapability’ of the underlying genetic variants. A phenotype with a relatively low heritability may exhibit large effects of a small number of genes, which would facilitate mapping. Alternatively, a phenotype may be highly heritable but also highly polygenic, similar to BD itself, which would significantly complicate gene mapping by producing low-level signals across a multitude of genomic regions. It is also possible that the hyperthymic and dysthymic temperaments are particularly associated with a genetic vulnerability for BD and thus produced the strongest findings in our study. This idea is supported by the findings of a prospective study on the offspring and siblings of BD patients, which showed that the hyperthymic and dysthymic temperaments were present before a superimposed mood episode developed (Akiskal et al., 1985). Other temperaments may instead be the result of modifying factors that have little impact on BD susceptibility.
There are several limitations to this study. Though the TEMPS-A is designed to assess temperament as a lifelong characteristic, with the subscales demonstrating good stability over time (Kawamura et al., 2010; Placidi et al., 1998), it is possible that the subject's self-assessment might be influenced by state at the time of testing. Since data regarding current state was only available for a subset of subjects with BD or MDD, adjusting the temperament scores for state would effectively eliminate a majority of subjects with mood disorders. However, a comparison of subjects who met full criteria or were symptomatic with those who were in remission at the time of interview and found no significant group differences in temperament scores. Another study of 1,263 BD subjects observed only very minimal differences in temperament scores between subjects who were euthymic vs. depressed or manic at the time of testing (Greenwood et al. 2012). It is also possible that other comorbid conditions, particularly drug and alcohol abuse, may impact temperament scores, although directionality would be difficult to determine, since one's temperament may just as likely predispose to such conditions. In any case, we were unable to adjust the scores for other clinical factors, since complete information was not available across the sample. Additionally, with a linkage study of five temperaments, multiple comparisons are an issue. Several studies of healthy controls and/or subjects with BD and MDD have shown a pattern of strong correlations between the dysthymic, cyclothymic, irritable, and anxious temperaments with the hyperthymic temperament showing minimal, if any, correlations with the other temperaments, a pattern we have also observed here among the BD subjects and all subjects combined (data not presented) (Bloink et al., 2005; Greenwood et al., 2012a; Matsumoto et al., 2005; Perugi et al., 2012). It is thus difficult to determine the appropriate correction in this case, since four of the five temperaments are highly correlated with one another. Finally, our modest sample of 51 families lacks sufficient power to reliably detect loci with smaller effects in a linkage analysis. This may also explain why no suggestive peaks for the cyclothymic and anxious temperaments were observed in this study. Still, we have identified several regions of potential interest for three of the temperaments meeting genome-wide suggestive criteria, which were verified through simulation.
If more pathological temperament scores are associated with a greater risk of developing BD, affective temperaments may represent the most prevalent phenotypic expression of the genes underlying BD (Akiskal, 2002). The results of our analyses suggest that measures of temperament may have utility in illuminating the genetic architecture of BD. While the absence of significant or suggestive linkage signals for all temperaments may be an issue of power deficiencies in our sample, it is also likely a reflection of the genetic complexity of the temperaments. Despite these complexities, we have identified several regions meeting genome-wide suggestive criteria that provide support for several interesting genes and chromosomal regions. The extent to which these regions harbor specific mutations that are involved in BD susceptibility, or the temperaments themselves, remains a topic for future discussion. Aside from genomic considerations, the relatively high heritabilities of the temperaments reported here are consistent with their putative evolutionary significance for our species, which, for its survival, depends on leadership, exploration, avoidance of danger, and emotions that signal anger and threat (Akiskal and Akiskal, 2005).
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all of the participants and support staff who made this study possible.
Role of the Funding Source
This work was supported by Novartis Pharma AG and by grants to JRK from the Department of Veterans Affairs and the National Institute of Mental Health (NIMH) (MH47612, MH59567, MH68503, and MH078151), the UCSD Mental Health Clinical Research Center (MH30914), and the UCSD General Clinical Research Center (M01 RR00827). Support for genotyping was provided to JAB and WB by research grant MH077314 from the NIMH, JAB was supported by MH078151 from the NIMH, and TAG was supported by grant K01-MH087889 from the NIMH. The Department of Veterans Affairs and NIMH had no further roles in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS
Dr. Greenwood is responsible for the conception and design of the study, data analysis and interpretation, and drafting and critically revising the article. Drs. Badner and Byerley provided cleaned genotype data for the sample, and Dr. Badner provided statistical support. Drs. Keck, McElroy, Remick, and Sadovnick participated in subject recruitment. Dr. Akiskal participated in the conception and design of the study. Dr. Kelsoe participated in the conception and design of the study, subject recruitment, data interpretation, and critical revision of the article. All authors approved the final manuscript.
CONFLICT OF INTEREST
Drs. Akiskal, Badner, Byerley, Greenwood, Kelsoe, Remick, and Sadovnick have no competing financial interests to report. Dr. McElroy is a consultant to or member of the scientific advisory board of Alkermes, Eli Lilly, Shire, and Teva and has received unrelated research support from the Agency for Healthcare Research & Quality (AHRQ), Alkermes, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest Labs, GalaxoSmith Kline, Jazz Pharmaceuticals, Marriott Foundation, Orexigen Therapeutics, Shire, and Takeda Pharmaceutical Company. Dr. McElroy is also an inventor on United States Patent No. 6,323,236 B2 for the use of sulfamate derivatives for treating impulse control disorders and has received payments from Johnson & Johnson Pharmaceutical Research and Development, which has exclusive rights under the patent. Dr. Keck is a co-inventor on a United States Patent (No. 6,387,956) for treating obsessive-compulsive spectrum disorder through tramadol and has received no financial gain.
REFERENCES
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Akiskal HS. The bipolar spectrum--the shaping of a new paradigm in psychiatry. Curr Psychiatry Rep. 2002;4:1–3. doi: 10.1007/s11920-002-0001-1. [DOI] [PubMed] [Google Scholar]
- Akiskal KK, Akiskal HS. The theoretical underpinnings of affective temperaments: implications for evolutionary foundations of bipolar disorder and human nature. J Affect Disord. 2005;85:231–239. doi: 10.1016/j.jad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Akiskal KK, Haykal RF, Manning JS, Connor PD. TEMPS-A: progress towards validation of a self-rated clinical version of the Temperament Evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J Affect Disord. 2005a;85:3–16. doi: 10.1016/j.jad.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Djenderedjian AM, Rosenthal RH, Khani MK. Cyclothymic disorder: validating criteria for inclusion in the bipolar affective group. Am J Psychiatry. 1977;134:1227–1233. doi: 10.1176/ajp.134.11.1227. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Downs J, Jordan P, Watson S, Daugherty D, Pruitt DB. Affective disorders in referred children and younger siblings of manic-depressives. Mode of onset and prospective course. Arch Gen Psychiatry. 1985;42:996–1003. doi: 10.1001/archpsyc.1985.01790330076009. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Mendlowicz MV, Jean-Louis G, Rapaport MH, Kelsoe JR, Gillin JC, Smith TL. TEMPS-A: validation of a short version of a self-rated instrument designed to measure variations in temperament. J Affect Disord. 2005b;85:45–52. doi: 10.1016/j.jad.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Placidi GF, Maremmani I, Signoretta S, Liguori A, Gervasi R, Mallya G, Puzantian VR. TEMPS-I: delineating the most discriminant traits of the cyclothymic, depressive, hyperthymic and irritable temperaments in a nonpatient population. J Affect Disord. 1998;51:7–19. doi: 10.1016/s0165-0327(98)00152-9. [DOI] [PubMed] [Google Scholar]
- Akiskal HS. The bipolar spectrum: new concepts in classification and diagnosis. In: Grinspoon L, editor. Psychiatry Update: The American Psychiatric Association Annual Review. American Psychiatric Press; Washington, DC: 1983. pp. 271–292. [Google Scholar]
- Akiskal HS, Pinto O. Soft bipolar spectrum: footnotes to Kraepelin on the interface of hypomania, temperament and depression. In: Marneros A, Angst J, editors. Bipolar Disorders: 100 Years after Manic-depressive Insanity. Kluwer Academic; Dordrecht: 2000. pp. 37–62. [Google Scholar]
- Akiskal HS. Toward a temperament-based approach to depression: implications for neurobiologic research. Adv Biochem Psychopharmacol. 1995;49:99–112. [PubMed] [Google Scholar]
- Akiskal HS. The temperamental foundations of affective disorders. In: Mundt C, Hahlweg K, Fiedler P, editors. Interpersonal Factors in the Origin and Course of Affective Disorders. Gaskell; London: 1996. pp. 3–30. [Google Scholar]
- Akiskal HS, Akiskal K. Cyclothymic, hyperthymic and depressive temperaments as subaffective variants of mood disorders. In: Tasman A, Riba MB, editors. Annual Review. American Psychiatric Press; Washington, DC: 1992. pp. 43–62. [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Badner JA, Koller D, Foroud T, Edenberg H, Nurnberger JI, Jr., Zandi PP, Willour VL, McMahon FJ, Potash JB, Hamshere M, Grozeva D, Green E, Kirov G, Jones I, Jones L, Craddock N, Morris D, Segurado R, Gill M, Sadovnick D, Remick R, Keck P, Kelsoe J, Ayub M, Maclean A, Blackwood D, Liu CY, Gershon ES, McMahon W, Lyon GJ, Robinson R, Ross J, Byerley W. Genome-wide linkage analysis of 972 bipolar pedigrees using single-nucleotide polymorphisms. Mol Psychiatry. 2012;17:818–826. doi: 10.1038/mp.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, Georgi A, Schumacher J, Schwarz M, Abou Jamra R, Hofels S, Propping P, Satagopan J, Detera-Wadleigh SD, Hardy J, McMahon FJ. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi F. Cyclothymic temperament: the impact of age. Psychopathology. 2009;42:165–169. doi: 10.1159/000207458. [DOI] [PubMed] [Google Scholar]
- Bloink R, Brieger P, Akiskal HS, Marneros A. Factorial structure and internal consistency of the German TEMPS-A scale: validation against the NEO-FFI questionnaire. J Affect Disord. 2005;85:77–83. doi: 10.1016/S0165-0327(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ullrich G, McGrath J, Kasch L, Lamacz M, Thomas MG, Gehrig C, Radhakrishna U, Snyder SE, Balk KG, Neufeld K, Swartz KL, DeMarchi N, Papadimitriou GN, Dikeos DG, Stefanis CN, Chakravarti A, Childs B, Housman DE, Kazazian HH, Antonarakis S, Pulver AE. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet. 1998;20:70–73. doi: 10.1038/1734. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of bipolar II and related disorders: with hypomanic episodes (or cyclothymia) and with hyperthymic temperament. J Affect Disord. 1992;26:127–140. doi: 10.1016/0165-0327(92)90044-7. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Akiskal HS, Musetti L, Perugi G, Soriani A, Mignani V. Psychopathology, temperament, and past course in primary major depressions. 2. Toward a redefinition of bipolarity with a new semistructured interview for depression. Psychopathology. 1989;22:278–288. doi: 10.1159/000284608. [DOI] [PubMed] [Google Scholar]
- Chiaroni P, Hantouche EG, Gouvernet J, Azorin JM, Akiskal HS. The cyclothymic temperament in healthy controls and familially at risk individuals for mood disorder: endophenotype for genetic studies? J Affect Disord. 2005;85:135–145. doi: 10.1016/j.jad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Cordell HJ. Bias toward the null hypothesis in model-free linkage analysis is highly dependent on the test statistic used. Am J Hum Genet. 2004;74:1294–1302. doi: 10.1086/421476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Florio A, Hamshere M, Forty L, Green EK, Grozeva D, Jones I, Caesar S, Fraser C, Gordon-Smith K, Jones L, Craddock N, Smith DJ. Affective temperaments across the bipolar-unipolar spectrum: examination of the TEMPS-A in 927 patients and controls. J Affect Disord. 2010;123:42–51. doi: 10.1016/j.jad.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr. Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the national institute of mental health genetics initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll DA, Salvin J, Sellinger B, Budarf ML, McDonald-McGinn DM, Zackai EH, Emanuel BS. Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counselling and prenatal diagnosis. J Med Genet. 1993;30:813–817. doi: 10.1136/jmg.30.10.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, Mayeda A, Rau NL, Smiley C, Rice JP, Goate A, Reich T, Stine OC, McMahon F, DePaulo JR, Meyers D, Detera-Wadleigh SD, Goldin LR, Gershon ES, Blehar MC, Nurnberger JI., Jr. Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22. Am J Med Genet. 1997;74:238–246. [PubMed] [Google Scholar]
- Evans L, Akiskal HS, Keck PE, Jr., McElroy SL, Sadovnick AD, Remick RA, Kelsoe JR. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affect Disord. 2005;85:153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Faber PW, Barnes GT, Srinidhi J, Chen J, Gusella JF, MacDonald ME. Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RA, McRae AF, Pocklington AJ, van de Lagemaat LN, Navarro P, Croning MD, Komiyama NH, Bradley SJ, Challiss RA, Armstrong JD, Finn RD, Malloy MP, MacLean AW, Harris SE, Starr JM, Bhaskar SS, Howard EK, Hunt SE, Coffey AJ, Ranganath V, Deloukas P, Rogers J, Muir WJ, Deary IJ, Blackwood DH, Visscher PM, Grant SG. Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder. PLoS One. 2012;6:e19011. doi: 10.1371/journal.pone.0019011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI, Jr., Goldin LR, Bunney WE., Jr. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry. 1982;39:1157–1167. doi: 10.1001/archpsyc.1982.04290100031006. [DOI] [PubMed] [Google Scholar]
- Gil-Sanz C, Delgado-Garcia JM, Fairen A, Gruart A. Involvement of the mGluR1 receptor in hippocampal synaptic plasticity and associative learning in behaving mice. Cereb Cortex. 2008;18:1653–1663. doi: 10.1093/cercor/bhm193. [DOI] [PubMed] [Google Scholar]
- Gill M, Vallada H, Collier D, Sham P, Holmans P, Murray R, McGuffin P, Nanko S, Owen M, Antonarakis S, Housman D, Kazazian H, Nestadt G, Pulver AE, Straub RE, MacLean CJ, Walsh D, Kendler KS, DeLisi L, Polymeropoulos M, Coon H, Byerley W, Lofthouse R, Gershon E, Golden L, Crow T, Byerley W, Freedman R, Laurent C, Bodeau-Pean S, d'Amato T, Jay M, Campion D, Mallet J, Wildenauer DB, Lerer B, Albus M, Ackenheil M, Ebstein RP, Hallmayer J, Maier W, Gurling H, Curtis D, Kalsi G, Brynjolfsson J, Sigmundson T, Petursson H, Blackwood D, Muir W, St. Clair D, He L, Maguire S, Moises HW, Hwu H, Yang L, Wiese C, Tao L, Liu X, Kristbjarnason H, Levinson DF, Mowry BJ, Donis-Keller H, Hayward NK, Crowe RR, Silverman JM, Nancarrow DJ, Read CM. A combined analysis of D22S278 marker alleles in affected sib-pairs: support for a susceptibility locus for schizophrenia at chromosome 22q12. Schizophrenia Collaborative Linkage Group (Chromosome 22). Am J Med Genet. 1996;67:404–405. doi: 10.1002/(SICI)1096-8628(19960216)67:1<40::AID-AJMG6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, Hinde RA, McCall RB. Roundtable: what is temperament? Four approaches. Child Dev. 1987;58:505–529. [PubMed] [Google Scholar]
- Greenwood TA, Akiskal HS, Akiskal KK, Kelsoe JR. Genome-wide association study of temperament in bipolar disorder reveals significant associations with three novel Loci. Biol Psychiatry. 2012a;72:303–310. doi: 10.1016/j.biopsych.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Nievergelt CM, Sadovnick AD, Remick RA, Keck PE, Jr., McElroy SL, Shekhtman T, McKinney R, Kelsoe JR. Further evidence for linkage of bipolar disorder to chromosomes 6 and 17 in a new independent pedigree series. Bipolar Disord. 2012b;14:71–79. doi: 10.1111/j.1399-5618.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Dubourg C, Mosca AL, Legallic S, Goldenberg A, Drouin-Garraud V, Layet V, Rosier A, Briault S, Bonnet-Brilhault F, Laumonnier F, Odent S, Le Vacon G, Joly-Helas G, David V, Bendavid C, Pinoit JM, Henry C, Impallomeni C, Germano E, Tortorella G, Di Rosa G, Barthelemy C, Andres C, Faivre L, Frebourg T, Saugier Veber P, Campion D. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009;66:947–956. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, Weissman MM. Depressive symptoms as relative and attributable risk factors for first-onset major depression. Arch Gen Psychiatry. 1992;49:817–823. doi: 10.1001/archpsyc.1992.01820100061011. [DOI] [PubMed] [Google Scholar]
- Karam EG, Salamoun MM, Yeretzian JS, Mneimneh ZN, Karam AN, Fayyad J, Hantouche E, Akiskal K, Akiskal HS. The role of anxious and hyperthymic temperaments in mental disorders: a national epidemiologic study. World Psychiatry. 2010;9:103–110. doi: 10.1002/j.2051-5545.2010.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Akiyama T, Shimada T, Minato T, Umekage T, Noda Y, Ukawa K, Hashidume C, Sakai Y, Otowa T, Sasaki T, Akiskal HS. Six-year stability of affective temperaments as measured by TEMPS-A. Psychopathology. 2010;43:240–247. doi: 10.1159/000313522. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR. Arguments for the genetic basis of the bipolar spectrum. J Affect Disord. 2003;73:183–197. doi: 10.1016/s0165-0327(02)00323-3. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Sadovnick AD, Kristbjarnarson H, Bergesch P, Mroczkowski-Parker Z, Drennan M, Rapaport MH, Flodman P, Spence MA, Remick RA. Possible locus for bipolar disorder near the dopamine transporter on chromosome 5. Am J Med Genet. 1996;67:533–540. doi: 10.1002/(SICI)1096-8628(19961122)67:6<533::AID-AJMG4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, Masser D, Ungerleider S, Rapaport MH, Wishart WL, Luebbert H. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci U S A. 2001;98:585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir S, Vahip S, Akdeniz F, Yuncu Z, Alkan M, Akiskal H. Affective temperaments as measured by TEMPS-A in patients with bipolar I disorder and their first-degree relatives: a controlled study. J Affect Disord. 2005;85:127–133. doi: 10.1016/j.jad.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Gumus D, Chen W, Norton N, Georgieva L, Sari M, O'Donovan MC, Erdogan F, Owen MJ, Ropers HH, Ullmann R. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O'Donovan MC, Owen MJ. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr Bull. 2009;35:851–854. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K. A high-resolution recombination map of the human genome. Nat Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Akiskal HS, Gatsonis C, Parrone PL. Childhood-onset dysthymic disorder. Clinical features and prospective naturalistic outcome. Arch Gen Psychiatry. 1994;51:365–374. doi: 10.1001/archpsyc.1994.03950050025003. [DOI] [PubMed] [Google Scholar]
- Koukopoulos A, Caliari B, Tundo A, Minnai G, Floris G, Reginaldi D, Tondo L. Rapid cyclers, temperament, and antidepressants. Compr. Psychiatry. 1983;24:249–258. doi: 10.1016/0010-440x(83)90076-7. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Kelsoe JR, Remick RA, Sadovnick AD, Rapaport MH, Lin M, Pazur BA, Roe AM, Saito T, Papolos DF. Linkage studies suggest a possible locus for bipolar disorder near the velo-cardio-facial syndrome region on chromosome 22. Am J Med Genet. 1997;74:121–128. doi: 10.1002/(sici)1096-8628(19970418)74:2<121::aid-ajmg2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20:687–702. doi: 10.1159/000110430. [DOI] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002a;99:16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, Lenane M, Robertson B, Wijsman EM, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002b;99:3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Akiyama T, Tsuda H, Miyake Y, Kawamura Y, Noda T, Akiskal KK, Akiskal HS. Reliability and validity of TEMPS-A in a Japanese non-clinical population: application to unipolar and bipolar depressives. J Affect Disord. 2005;85:85–92. doi: 10.1016/j.jad.2003.10.001. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, Depaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlowicz MV, Jean-Louis G, Kelsoe JR, Akiskal HS. A comparison of recovered bipolar patients, healthy relatives of bipolar probands, and normal controls using the short TEMPS-A. J Affect Disord. 2005;85:147–151. doi: 10.1016/j.jad.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Ott J, Kamatani Y, Lathrop M. Family-based designs for genome-wide association studies. Nat Rev Genet. 2011;12:465–474. doi: 10.1038/nrg2989. [DOI] [PubMed] [Google Scholar]
- Papolos DF, Faedda GL, Veit S, Goldberg R, Morrow B, Kucherlapati R, Shprintzen RJ. Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry. 1996;153:1541–1547. doi: 10.1176/ajp.153.12.1541. [DOI] [PubMed] [Google Scholar]
- Perugi G, Toni C, Maremmani I, Tusini G, Ramacciotti S, Madia A, Fornaro M, Akiskal HS. The influence of affective temperaments and psychopathological traits on the definition of bipolar disorder subtypes: a study on bipolar I Italian national sample. J Affect Disord. 2012;136:e41–49. doi: 10.1016/j.jad.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Placidi GF, Maremmani I, Signoretta S, Liguori A, Akiskal HS. A prospective study of stability and change over 2 years of affective temperaments in 14-18 year-old Italian high school students. J Affect Disord. 1998a;51:199–208. doi: 10.1016/s0165-0327(98)00182-7. [DOI] [PubMed] [Google Scholar]
- Placidi GF, Signoretta S, Liguori A, Gervasi R, Maremmani I, Akiskal HS. The semi-structured affective temperament interview (TEMPS-I). Reliability and psychometric properties in 1010 14-26-year old students. J Affect Disord. 1998b;47:1–10. doi: 10.1016/s0165-0327(97)00122-5. [DOI] [PubMed] [Google Scholar]
- Price RA, Kidd KK, Pauls DL, Gershon ES, Prusoff BA, Weissman MM, Goldin LR. Multiple threshold models for the affective disorders: the Yale-NIMH collaborative family study. J Psychiatr Res. 1985;19:533–546. doi: 10.1016/0022-3956(85)90071-8. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver AE, Karayiorgou M, Wolyniec PS, Lasseter VK, Kasch L, Nestadt G, Antonarakis S, Housman D, Kazazian HH, Meyers D, et al. Sequential strategy to identify a susceptibility gene for schizophrenia: report of potential linkage on chromosome 22q12-q13.1: Part 1. Am J Med Genet. 1994;54:36–43. doi: 10.1002/ajmg.1320540108. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Dembinska D, Kliwicki S, Akiskal KK, Akiskal HH. TEMPS-A and long-term lithium response: positive correlation with hyperthymic temperament. J Affect Disord. 2013;145:187–189. doi: 10.1016/j.jad.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Rihmer Z, Akiskal KK, Rihmer A, Akiskal HS. Current research on affective temperaments. Curr Opin Psychiatry. 2010;23:12–18. doi: 10.1097/YCO.0b013e32833299d4. [DOI] [PubMed] [Google Scholar]
- Savaskan NE, Brauer AU, Nitsch R. Molecular cloning and expression regulation of PRG-3, a new member of the plasticity-related gene family. Eur J Neurosci. 2004;19:212–220. doi: 10.1046/j.1460-9568.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- Savitz J, van der Merwe L, Ramesar R. Personality endophenotypes for bipolar affective disorder: a family-based genetic association analysis. Genes Brain Behav. 2008;7:869–876. doi: 10.1111/j.1601-183X.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- Schork NJ, Greenwood TA. Got bias? The authors reply. Am J Hum Genet. 2004a;75:723–727. [Google Scholar]
- Schork NJ, Greenwood TA. Inherent bias toward the null hypothesis in conventional multipoint nonparametric linkage analysis. Am J Hum Genet. 2004b;74:306–316. doi: 10.1086/381714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Muglia P, Kong XQ, Guan W, Flickinger M, Upmanyu R, Tozzi F, Li JZ, Burmeister M, Absher D, Thompson RC, Francks C, Meng F, Antoniades A, Southwick AM, Schatzberg AF, Bunney WE, Barchas JD, Jones EG, Day R, Matthews K, McGuffin P, Strauss JS, Kennedy JL, Middleton L, Roses AD, Watson SJ, Vincent JB, Myers RM, Farmer AE, Akil H, Burns DK, Boehnke M. Genome-wide association and meta-analysis of bipolar disorder in individuals of European ancestry. Proc Natl Acad Sci U S A. 2009;106:7501–7506. doi: 10.1073/pnas.0813386106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr., Craddock N, DePaulo JR, Baron M, Gershon ES, Ekholm J, Cichon S, Turecki G, Claes S, Kelsoe JR, Schofield PR, Badenhop RF, Morissette J, Coon H, Blackwood D, McInnes LA, Foroud T, Edenberg HJ, Reich T, Rice JP, Goate A, McInnis MG, McMahon FJ, Badner JA, Goldin LR, Bennett P, Willour VL, Zandi PP, Liu J, Gilliam C, Juo SH, Berrettini WH, Yoshikawa T, Peltonen L, Lonnqvist J, Nothen MM, Schumacher J, Windemuth C, Rietschel M, Propping P, Maier W, Alda M, Grof P, Rouleau GA, Del-Favero J, Van Broeckhoven C, Mendlewicz J, Adolfsson R, Spence MA, Luebbert H, Adams LJ, Donald JA, Mitchell PB, Barden N, Shink E, Byerley W, Muir W, Visscher PM, Macgregor S, Gurling H, Kalsi G, McQuillin A, Escamilla MA, Reus VI, Leon P, Freimer NB, Ewald H, Kruse TA, Mors O, Radhakrishna U, Blouin JL, Antonarakis SE, Akarsu N. Genome Scan Meta-Analysis of Schizophrenia and Bipolar Disorder, Part III: Bipolar Disorder. Am J Hum Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Mandelli L. The genetics of bipolar disorder: genome ‘hot regions,’ genes, new potential candidates and future directions. Mol Psychiatry. 2008;13:742–771. doi: 10.1038/mp.2008.29. [DOI] [PubMed] [Google Scholar]
- Sham PC, Purcell S, Cherny SS, Abecasis GR. Powerful regression-based quantitative-trait linkage analysis of general pedigrees. Am J Hum Genet. 2002;71:238–253. doi: 10.1086/341560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SH, Kelly M, Smith AB, Shields G, Hopkins PJ, Loftus J, Laval SH, Vita A, De Hert M, Cardon LR, Crow TJ, Sherrington R, DeLisi LE. A genome-wide search for schizophrenia susceptibility genes. Am J Med Genet. 1998;81:364–376. doi: 10.1002/(sici)1096-8628(19980907)81:5<364::aid-ajmg4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Shaw SH, Mroczkowski-Parker Z, Shekhtman T, Alexander M, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr., Kelsoe JR. Linkage of a bipolar disorder susceptibility locus to human chromosome 13q32 in a new pedigree series. Mol Psychiatry. 2003;8:558–564. doi: 10.1038/sj.mp.4001267. [DOI] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EN, Bloss CS, Badner JA, Barrett T, Belmonte PL, Berrettini W, Byerley W, Coryell W, Craig D, Edenberg HJ, Eskin E, Foroud T, Gershon E, Greenwood TA, Hipolito M, Koller DL, Lawson WB, Liu C, Lohoff F, McInnis MG, McMahon FJ, Mirel DB, Murray SS, Nievergelt C, Nurnberger J, Nwulia EA, Paschall J, Potash JB, Rice J, Schulze TG, Scheftner W, Panganiban C, Zaitlen N, Zandi PP, Zollner S, Schork NJ, Kelsoe JR. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–763. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R, Segre A, Pericak-Vance MA, Cuccaro ML, Gilbert JR, Wright HH, Abramson RK, Betancur C, Bourgeron T, Gillberg C, Leboyer M, Buxbaum JD, Davis KL, Hollander E, Silverman JM, Hallmayer J, Lotspeich L, Sutcliffe JS, Haines JL, Folstein SE, Piven J, Wassink TH, Sheffield V, Geschwind DH, Bucan M, Brown WT, Cantor RM, Constantino JN, Gilliam TC, Herbert M, Lajonchere C, Ledbetter DH, Lese-Martin C, Miller J, Nelson S, Samango-Sprouse CA, Spence S, State M, Tanzi RE, Coon H, Dawson G, Devlin B, Estes A, Flodman P, Klei L, McMahon WM, Minshew N, Munson J, Korvatska E, Rodier PM, Schellenberg GD, Smith M, Spence MA, Stodgell C, Tepper PG, Wijsman EM, Yu CE, Roge B, Mantoulan C, Wittemeyer K, Poustka A, Felder B, Klauck SM, Schuster C, Poustka F, Bolte S, Feineis-Matthews S, Herbrecht E, Schmotzer G, Tsiantis J, Papanikolaou K, Maestrini E, Bacchelli E, Blasi F, Carone S, Toma C, Van Engeland H, de Jonge M, Kemner C, Koop F, Langemeijer M, Hijmans C, Staal WG, Baird G, Bolton PF, Rutter ML, Weisblatt E, Green J, Aldred C, Wilkinson JA, Pickles A, Le Couteur A, Berney T, McConachie H, Bailey AJ, Francis K, Honeyman G, Hutchinson A, Parr JR, Wallace S, Monaco AP, Barnby G, Kobayashi K, Lamb JA, Sousa I, Sykes N, Cook EH, Guter SJ, Leventhal BL, Salt J, Lord C, Corsello C, Hus V, Weeks DE, Volkmar F, Tauber M, Fombonne E, Shih A, Meyer KJ. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez GH, Nasetta S, Mercado B, Romero E, Tifner S, Ramon Mdel L, Garelli V, Bonifacio A, Akiskal KK, Akiskal HS. Validation of the TEMPS-A Buenos Aires: Spanish psychometric validation of affective temperaments in a population study of Argentina. J Affect Disord. 2007;100:23–29. doi: 10.1016/j.jad.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Vazquez GH, Tondo L, Mazzarini L, Gonda X. Affective temperaments in general population: a review and combined analysis from national studies. J Affect Disord. 2012;139:18–22. doi: 10.1016/j.jad.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.