Abstract
Background
The structural and functional abnormalities of the anterior cingulate cortex (ACC) have been reported in panic disorder (PD). Patients with PD have shown decreased gamma-aminobutyric acid (GABA) concentration in the ACC. The GABA concentration in the ACC was found to be associated with default mode network (DMN) activity in normal human subjects. Therefore, it was hypothesized that the DMN would show abnormal activity in PD.
Methods
We identified and compared the functional connectivity maps with seed region of interest (ROI) located in the perigenual area of ACC between the 11 patients with panic disorder and age- and sex- matched normal control subjects. Combining magnetic resonance spectroscopy (MRS) and resting fMRI, we investigated the correlation between the GABA concentration in the seed ROI and the index of functional connectivity between ACC and the area showing group differences.
Results
The patients with PD showed increased functional connectivity between ACC and precuneus compared to control subjects. The functional connectivity between the ACC and the precuneus negatively correlated with the GABA concentration of the ACC.
Limitations
The relatively small sample size and seed based analysis with the selection of a single ROI limits the generalizability of the result.
Conclusions
Increased functional connectivity in the two medial nodes of the resting-state default mode network, the ACC and the precuneus, might play an important role in the pathophysiology of panic disorder. The treatment aimed to normalize the functional connectivity between ACC and precuneus might have clinical benefits in PD.
Keywords: Default mode network, Anterior cingulate cortex, Gamma-aminobutyric acid, Panic disorder
1. Introduction
A key feature of panic disorder (PD) is sudden bodily sensations like palpitations or dyspnea, which are experienced by patients as inappropriately dangerous and harmful (Eley et al., 2004). Patients with PD have enhanced cardiac perception (Ehlers and Breuer, 1992) and anxiety sensitivity, a measure of a person’s tendency to fear physical symptoms believed to have dangerous consequences (McNally, 2002). Those are consistent with a cognitive-attentional model of PD suggesting that the patients with PD are sensitive to internal autonomic visceral cues, and panic attacks are induced by misinterpretation of the internal visceral cues (Hayward et al., 2000).
The anterior cingulate cortex (ACC) is a major cortical structure to which visceral input is delivered (Weston, 2012), which, in turn, sends modulatory output to the peripheral autonomic centers (Vogt and Derbyshire, 2009). Within the so-called ‘central autonomic network’ (Benarroch, 1997), the perigenual ACC, corresponding to Brodmann area 32, is involved inmonitoring and appraisal of the external environment, reciprocally connecting various brain regions to regulate stressor-related autonomic reactions (Gianaros and Sheu, 2009; Ryan et al., 2011). The electrical stimulation of the perigenual ACC evoked panic-like symptoms including increased heart rate accompanied by severe anxiety (Bancaud et al., 1976). Conversely, induced dyspnea elicited ACC activations, localized to Brodmann Areas (BA) 32 and 24 (von Leupoldt et al., 2009). Investigators have also observed increased ACC activity during anxiety provocation or anxiety imagery tasks in PD patients (Boshuisen et al., 2002; Bystritsky et al., 2001).
The involvement of GABA has long been suggested in the pathogenesis of PD, with early observations that panic symptoms were relieved after taking benzodiazepines agonists, while antagonists/inverse agonsists of GABAA/BZD receptors were panicogenic (Freitas et al., 2009). Several groups have reported ACC GABA abnormalities in patients with PD (Ham et al., 2007; Malizia et al., 1998). Using PET, Malizia et al. reported reduced benzodiazepine receptor binding of ACC GABAA receptors in patients with PD (Malizia et al., 1998). In an MRS study, one group noted abnormally decreased ACC GABA concentrations in patients with PD (Ham et al., 2007).
Combining fMRI and magnetic resonance spectroscopy (MRS) in healthy subjects, Northoff et al. found a significant positive correlation between gamma-aminobutyric acid (GABA) concentration and negative BOLD response in the ACC during an emotional judgment task (Northoff et al., 2007). The negative BOLD response during a cognitive task is the typical response of the default mode network (DMN). The DMN is a set of interconnected brain areas, which are active during rest and deactivated during activities requiring external attention (Raichle et al., 2001). The ACC comprises the anterior portion of the DMN, having functional connectivity with the posterior part of the DMN, the posterior cingulate cortex (PCC) and the precuneus.
In the present investigation, we hypothesized that the perigenual ACC has an abnormal functional connectivity in the DMN of the patients with PD, and this abnormality is associated by decreased concentrations of GABA in the ACC. Therefore, we evaluated the resting state BOLD activity in PD patients and healthy comparison subjects to explore the functional connectivity of the ACC with a seed point located at the perigenual ACC, and also measured the GABA concentration of the ACC, allowing us to investigate its modulating effects on the functional connectivity of the ACC.
2. Materials and methods
2.1. Subjects
Eleven patients with PD and 11 age- and sex-matched, healthy control subjects participated in the study. The patients met DSM-IV criteria for the diagnosis of panic disorder, with or without agoraphobia. At screening, 5 patients were taking SSRI medications including paroxetine, escitalopram, venlafaxine and sertraline. Four patients were taking benzodiazepines on an as needed basis, while two patients were drug-naïve. Patients were medication free for at least 2 weeks prior to imaging. Patients with any lifetime history of major depression, psychotic disorder, bipolar disorder, eating disorder, personality disorder and other anxiety disorders including obsessive-compulsive disorder or post-traumatic stress disorder were excluded. To rule out comorbid depression, the patients were required not to have a lifetime episode of major depressive disorder, and had to have a screening visit Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) total score < 12. Subjects were also excluded if they had a substance or alcohol abuse disorder within 6 months of the diagnostic interview. Patients were also excluded from the study if they presented with any serious or unstable medical or neurologic illness. Patients and controls were recruited at the Indiana University (IU) Anxiety Disorders Clinic, located within the Adult Psychiatry Clinic at IU Hospital in Indianapolis. Patients were diagnosed using the Structured Clinical Interview for Psychiatric Disorders (SCID)(DSM-IV edition)(First, 1997). The healthy comparison group consisted of individuals with no anxiety problems or other major psychiatric diagnoses, who were screened by the non-patient edition of the SCID.
In addition, we administered, the panic disorder severity scale (PDSS) (Shear et al., 2001) to the patient group. We assessed resting anxiety, by means of a subject-rated visual analog scale (VAS) (scoring range 0 to 100 mm (most severe)) score, as well as cognitive performance on the trail making B test, immediately before each imaging session. All participants gave their written informed consent in accordance with Institutional Review Board of Indiana University School of Medicine. The demographic and clinical information of the study population is summarized in table 1.
Table 1.
Clinical characteristics of the study participants (mean±SD reported for continuous measures)
| Panic Disorder (N=11) | Control (N=11) | |
|---|---|---|
| Age (yrs) | 38.18 ± 12.76 | 38.18 ±12.40 |
| Sex | ||
| Male | 6 | 6 |
| Female | 7 | 7 |
| Duration of Illness (yrs) | 7.92 ± 11.49 | n/a |
| PDSS Total Score | 9.36 ± 2.50 | n/a |
| Baseline VAS anxiety Score | 45.82 ± 28.79 | 4.09 ± 8.01 |
| Trails B Task Score | 10.27 ± 2.57 | 12.27 ± 3.66 |
| GABA/Cr | 0.14 ± 0.08 | 0.19 ± 0.13 |
| GABA/NAA | 0.12 ± 0.06 | 0.15 ± 0.07 |
2.2 Imaging Acquisition
Imaging was performed with a 32-channel head coil array on a 3T Siemens scanner (Siemens Healthcare, Erlangen, Germany) located at the IU Center for Neuroimaging. All participants underwent a resting-state scan prior to the MRS. During resting state scan, subjects were instructed to keep their eyes closed and rest without any specific thoughts. A high resolution anatomic image was acquired using a 3D magnetization prepared rapid gradient echo (MPRAGE) MRI sequence; 160 sagittal slices, 1.0×1.0×1.2 mm3 voxels, field of view (FOV) 256×240 mm, repetition time (TR) 2300 ms, echo time (TE) 2.91 ms, flip angle 9°. The scan duration of 5:03 min was achieved by applying generalized auto-calibrating partially parallel acquisition (GRAPPA) mode, with the acceleration factor of 2.
Whole-brain blood oxygenation level dependent (BOLD) functional imaging was acquired during 333 s, using an echo-planar gradient echo pulse sequence; TR 2250 ms, TE 29 ms, FA 79°, FOV 220×220 mm, 39 slices, 2.5×2.5×3.0 mm3 voxels, GRAPPA acceleration factor of 2.
2.3. Image Analysis
2.3.1. Preprocessing and Functional Connectivity Analysis
Analysis of Functional NeuroImages (AFNI) (Cox, 1996) was used to perform the initial preprocessing steps of despiking, slice timing correction for interleaved acquisition, and head motion correction by rigid-body aligning each volume to the first scan image. The noise and nuisance physiological signals associated with white matter and ventricles, cardiac and respiratory signals, as well as possible head coil/hardware artifacts were regressed out using the ANATICOR method (Jo et al., 2010). Due to recent concern regarding head movement effects on resting-state fMRI (Power et al., 2012), we assessed the degrees of head motion using two criteria that are the frame-wise displacement (FD) and AFNI’s Euclidean norms of motion parameters (||d||L2). There was no significant motion frame at both threshold of FD > 0.5 and/or ||d||L2 > 0.3 that have been recommended for estimating motion effects and no group difference in the parameters for head motion. The ANATICOR model is known to be less sensitive to the head motion effects than other preprocessing models.
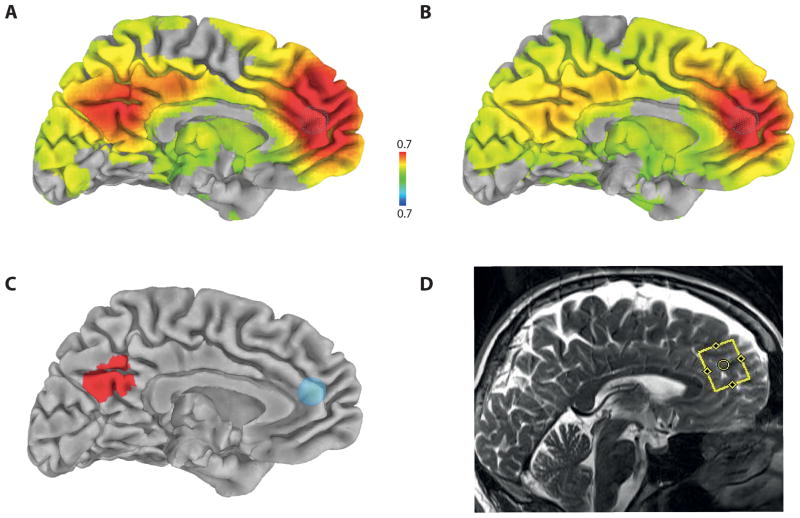
The preprocessed functional images were converted to Talairach space using the N27 template. The correlation coefficient map of whole intracranial brain was calculated with the reference time series of the MRS region of interest (ROI) using AFNI’s 3dfim+. The 7 mm radius sphere seed ROI was selected at the approximate center of MRS ROI in the perigenual ACC area projected on the N27 template (x=0, y=41, z=16, Brodmann area 32) (Figure 1-C). Correlation values were normalized with Fisher’s Z-transformation and then smoothed with a 7-mm Gaussian filter for group analysis.
FIG. 1.
Functional connectivity maps for the patients with panic disorder (A) and control healthy subjects (B) as measured by Fisher-transformed inter-voxel correlation coefficient projected on brain surface in Talairach space at p<0.01 (family-wise error corrected using Monte Carlo simulation), using seed region at the ACC (x=0, y=41, z=16, r=7mm) represented by a sky-blue-colored sphere in (C). The red color areas in the precuneus represent significant group difference at p<0.01 (family-wise error corrected using Monte Carlo simulation) (C). The region of interest positioned at the ACC (yellow line rectangular box) for the measurement of GABA concentration in a representative subject (D).
To determine the mean resting-state functional connectivity map for each group of the PD patients and control subjects, the Fisher’s Z-transformed correlation coefficients were tested to determine whether they were significantly different from zero using a one-sample t-test. A two-sample t-test was used to identify group differences between the two groups of panic versus control group. All resulting t-maps were family-wise error corrected at p < 0.01 for whole brain areas via Monte Carlo simulations with 3dClustSim module within AFNI.
2.3.2. MRS for measurement of GABA concentration
GABA detection was performed in each subject from the ROI in the ACC using the MEGA-PRESS J-editing sequence (TR/TE=1500/68ms, 196 averages with the editing pulse centered at 1.9 ppm and 196 averages with the pulse centered at 7.5 ppm in an interleaved fashion). Data processing and quantification were performed with LCModel (Provencher, 1993), fitting each spectrum as a weighted linear combination of basis spectra from individual metabolites. Basis sets were generated from density matrix simulations of the sequence using published values for chemical shifts and J-couplings, with an exact treatment of metabolite evolution during the two frequency-selective MEGA inversion pulses. Difference basis spectra were obtained by subtracting the simulated metabolite response to selective inversion at 7.5 ppm from that at 1.9 ppm.
This version of the MEGA-PRESS technique results in difference spectra containing not only a GABA peak, but also a small amount of homocarnosine that exists in all GABA-edited spectra and co-edited macromolecules (“MM30”) at 3.0 ppm. Therefore, an extra Gaussian peak at 3.0 ppm was added to the LCModel calculation to explicitly fit MM30 and a soft constraint was applied to the ratio of MM30 and a resonance at 0.9 ppm from macromolecules (MM09) (MM30/MM09=0.667±0.1), in order to eliminate the MM30 contribution to the GABA signal. The ratios of GABA to total creatine (GABA/Cr) and to N-acetyl aspartate (GABA/NAA) are reported, to cancel out variations in signal due to coil loading or partial volume effects.
2.3.3. GABA Concentration and Functional Connectivity
The mean Z-transformed correlation coefficient in the area that showed the group difference of the correlation between ACC and the precuneus was correlated with the parameters representing GABA concentration as measured by GABA/NAA ratio of the ACC. The mean Z-transformed correlation coefficient was also correlated with the scores of the behavioral data including the VAS anxiety, trail making B test, and PDSS scores.
3. Results
The resting state functional connectivity maps in each group and t-statistics map of the group differences were projected on C. Holmes’ pial brain surface in Talairach space (http://afni.nimh.nih.gov/afni/suma) (Fig. 1). As shown in Figure 1, while the functional connectivity in the healthy control subjects is widely distributed, the patient group showed a rather distinct increase of the functional connectivity centered on the precuneus. This increased ACC-precuneus connectivity was significantly different between the patients with PD and control subjects (family-wise error corrected at p<0.01).
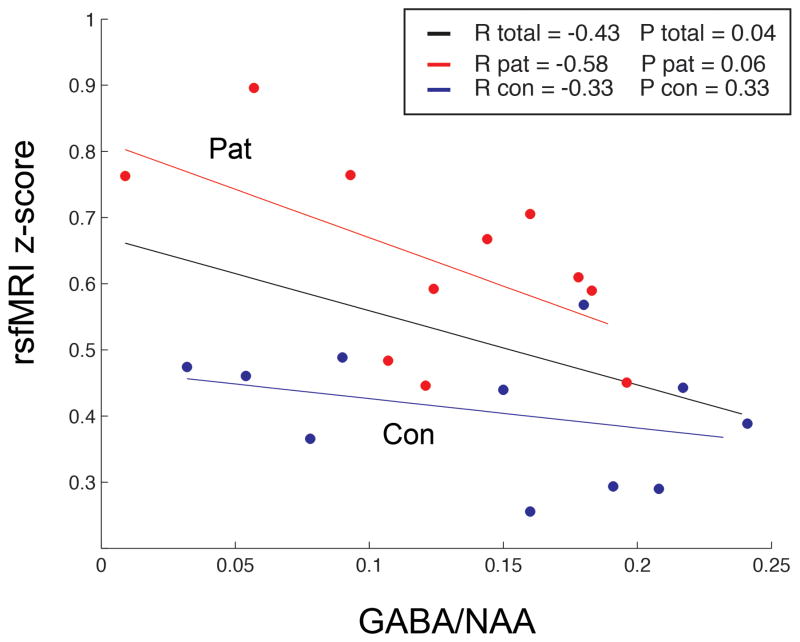
Figure 2 shows significant negative correlations between ACC-precuneus functional connectivity and GABA/NAA concentration ratios of ACC in the complete (n=22) sample (R =−0.43, p < 0.05). There was a trend of correlation between ACC-precuneus functional coupling and GABA/NAA ratio of ACC within the PD patient group (R =−0.58, p =0.06). In the control group alone, GABA concentration was not correlated with ACC-precuneus functional coupling. No correlations were observed between ACC-precuneus functional coupling and the scores of VAS anxiety, the trail B task, and the PDSS, either in total sample or within each group.
FIG. 2.
The Fisher-transformed correlation coefficient between the ACC and the precuneus had a negative correlation with the GABA/NAA ratio in the ACC in the patients with PD (R=−0.43, p=0.04).
4. Discussion
By combining fMRI and MRS techniques to assess the functional connectivity of the ACC mediated by its GABA concentration during resting state, we found increased functional connectivity between the ACC and the precuneus in the patients with PD compared to control subjects. Since the two structures, the ACC and precuneus, are two core medial components of the DMN (Deco et al., 2011), the increased functional connectivity between those structures can be considered in the context of the function of the DMN. The DMN is known to be involved in the processing of internally-generated thoughts (Buckner et al., 2008). The subjects are isolated from the external stimuli, watching a black screen during resting state. Therefore, they are sensitive to their own internal signals, which might be mediated by the ACC. As mentioned, the ACC is known to be bi-directionally involved in viscera-motor and cardiovascular responses (Vogt and Derbyshire, 2009). In the DMN, areas of the perigenual ACC in our ROI were reported to show peak correlations with stress-evoked cardiovascular reactivity (x=−2, y=44, z=16) and reactivity to a conflict situation (x=−6, y=44, z=16) (Ryan et al., 2011).
Unlike the ACC, the precuneus is not directly connected to visceral autonomic functions (Vogt and Derbyshire, 2009). Instead, the precuneus functions as a hub to organize attention where information from various areas of the brain is integrated (Lin et al., 2011). While the ACC was involved specifically in self-referential processing (Qin and Northoff, 2011), the posterior part of the DMN including the precuneus/PCC is involved in a wide spectrum of attentional processes like monitoring the environment, and assessing the context of stimuli (Vogt and Derbyshire, 2009; Wagner et al., 2005). Considering the function of the precuneus to modulate and integrate attention (Lin et al., 2011), we tentatively suggest that the increased functional connectivity of the ACC with the precuneus could be a functional combination of each structure, which might be translated into a subject’s tendency to have too much attention directed to internal visceral signals, one of the key clinical features of PD. The treatment aimed to normalize the functional connectivity between ACC and precuneus using transcranial magnetic stimulation (TMS), a promising method to manipulate cortico–cortical connectivity (Fox et al., 2012), might have clinical benefits in PD.
Despite the rise in the number of resting-state fMRI studies, studies of the underlying neurochemical mechanisms of the DMN are scarce. In healthy subjects, Northoff et al. found a positive correlation between the GABA concentration in the ACC and the negative BOLD response in the same area during emotional stimulation (Northoff et al., 2007). Although they implicated GABA in decreasing neuronal activity of the DMN while observing the negative BOLD response of the ACC during an emotional task, they did not directly measure the resting-state brain activity. Muthukumaraswamy et al. measured resting state BOLD response and GABA concentration of the visual cortex simultaneously; however they did not explore the functional connectivity between brain areas (Muthukumaraswamy et al., 2009). Previously, it has been unclear whether GABA could modulate the functional connectivity of DMN of human brain. The negative correlations of GABA concentration of the ACC with the functional connectivity between the ACC and the precuneus found in the total subject sample and the PD patient group in the present study is, thus, the first direct evidence that the functional connectivityof the DMN is modulated by GABA concentrations.
4.1. Limitations
There are several limitations in the study including the small sample size and seed-based analysis with the selection of single ROI. A recent study, recruiting 11 patients with PD selected seed ROI in the posterior cingulate cortex, reported no difference of DMN activity in comparison to control subjects (Pannekoek et al., 2012). The different location of the seed point, data acquisition at the end of task-related scans, and the pooling of the data from different centers might have contributed to the negative group difference of DMN in that study (Pannekoek et al., 2012). The small sample size of our study might also have accounted for the lack of correlations between clinical symptoms and the functional connectivity in the PD patients. In a hypothesis-driven approach, we explored resting-state functional connectivity associated with only the ACC, which could ignore other brain areas having group differences. In a previous study recruiting 10 patients with anxiety disorder among which 3 patients had PD, the functional connectivity of the amygdala with the posterior cingulate cortex and the precuneus was decreased (Hahn et al., 2011). Further studies with larger sample size and different methods of analysis are needed to confirm our current findings.
5. Conclusion
This is the first study to report a DMN abnormality in the patients with PD. We found increased functional connectivity between the ACC and the precuneus in the patients with PD. Although it remains hypothetical, the increased functional connectivity might be associated with the patients’ catastrophic misinterpretations of the internal autonomic visceral cues. We also found that the GABA concentration of the ACC was associated with the functional connectivity between ACC and precuneus, suggesting a role of GABA in the pathophysiology of panic disorder via abnormal functional connectivity of the ACC with the precuneus.
Acknowledgments
The authors gratefully acknowledge Michele Beal and Courtney Robbins for their professional and dedicated work in data acquisition (Center for Neuroimaging, Department of Radiology and Imaging Sciences, Indiana University School of Medicine). We further want to thank Dr. Anantha Shekhar and the staff of the Indiana CTSI center for their support of this project. This work was supported by a Collaborative Research Grant by the Indiana Clinical and Translational Sciences Institute, funded in part by grant # RR 02576 from the National Institutes of Health, National Center for Research Resources.
Funding Source:
This work was supported by a Collaborative Research Grant by the Indiana Clinical and Translational Sciences Institute, funded in part by grant # RR 02576 from the National Institutes of Health, National Center for Research Resources.
Footnotes
Contributors:
Yong-Wook Shin, M.D., Ph.D.a, Mario Dzemidzic, Ph.D. b,c, Hang Joon Jo, Ph.D.d, Zaiyang Long, M.S.e, Carla Medlock, M.S.f, Ulrike Dydak, Ph.D.e, Andrew W Goddard, M.D.b,f
Conflict of Interest:
Dr. Goddard discloses financial support by a Naurex industry trial and a Janssen-independent study grant. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bancaud J, Talairach J, Geier S, Bonis A, Trottier S, Manrique M. Manifestations comportementales induites par la stimulation électrique du gyrus cingulaire antérieur chez l’homme. Rev Neurol (Paris) 1976;132:705–724. [PubMed] [Google Scholar]
- Benarroch EE. Central autonomic network: functional organization and clinical correlations. Futura Pub. Co; Armonk, N.Y: 1997. [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Pontillo D, Powers M, Sabb FW, Craske MG, Bookheimer SY. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12:3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. J Abnorm Psychol. 1992;101:371–382. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stirling L, Ehlers A, Gregory AM, Clark DM. Heart-beat perception, panic/somatic symptoms and anxiety sensitivity in children. Behav Res Ther. 2004;42:439–448. doi: 10.1016/S0005-7967(03)00152-9. [DOI] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders: SCID - I: clinician version: administration booklet. American Psychiatric Press; Washington, D.C: 1997. [Google Scholar]
- Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas RL, Uribe-Marino A, Castiblanco-Urbina MA, Elias-Filho DH, Coimbra NC. GABA(A) receptor blockade in dorsomedial and ventromedial nuclei of the hypothalamus evokes panic-like elaborated defensive behaviour followed by innate fear-induced antinociception. Brain Res. 2009;1305:118–31. doi: 10.1016/j.brainres.2009.09.096. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Ham BJ, Sung Y, Kim N, Kim SJ, Kim JE, Kim DJ, Lee JY, Kim JH, Yoon SJ, Lyoo IK. Decreased GABA levels in anterior cingulate and basal ganglia in medicated subjects with panic disorder: a proton magnetic resonance spectroscopy (1H-MRS) study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:403–411. doi: 10.1016/j.pnpbp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Hayward P, Ahmad T, Wardle J. Attention to bodily sensations: a test of the cognitive-attentional model of panic. Depress Anxiety. 2000;12:203–208. doi: 10.1002/1520-6394(2000)12:4<203::AID-DA3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Hasson U, Jovicich J, Robinson S. A neuronal basis for task-negative responses in the human brain. Cereb Cortex. 2011;21:821–830. doi: 10.1093/cercor/bhq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–338. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ. Aberrant limbic and salience network resting-state functional connectivity in panic disorder without comorbidity. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JP, Sheu LK, Gianaros PJ. Resting state functional connectivity within the cingulate cortex jointly predicts agreeableness and stressor-evoked cardiovascular reactivity. Neuroimage. 2011;55:363–370. doi: 10.1016/j.neuroimage.2010.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Houck P, Wang T. Reliability and validity of the Panic Disorder Severity Scale: replication and extension. J Psychiatr Res. 2001;35:293–296. doi: 10.1016/s0022-3956(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire WG. Visceral Circuits and Cingulate-Mediated Autonomic Functions. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; Oxford ; New York: 2009. p. xxxiv.p. 829. [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Buchel C. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48:200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weston CSE. Another major function of the anterior cingulate cortex: the representation of requirements. Neuroscience and biobehavioral reviews. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]