Abstract
Background
The bronchodilator response (BDR) reflects the reversibility of airflow obstruction and is recommended as an adjunctive test to diagnose asthma. The validity of the commonly used definition of BDR, a 12% or greater change in FEV1 from baseline, has been questioned in childhood.
Objectives
We sought to examine the diagnostic accuracy of the BDR test by using 3 large pediatric cohorts.
Methods
Cases include 1041 children with mild-to-moderate asthma from the Childhood Asthma Management Program.
Control subjects (nonasthmatic and nonwheezing) were chosen from Project Viva and Home Allergens, 2 population-based pediatric cohorts. Receiver operating characteristic curves were constructed, and areas under the curve were calculated for different BDR cutoffs.
Results
A total of 1041 cases (59.7% male; mean age, 8.9 ± 2.1 years) and 250 control subjects (46.8% male; mean age, 8.7 ± 1.7 years) were analyzed, with mean BDRs of 10.7% ± 10.2% and 2.7% ± 8.4%, respectively. The BDR test differentiated asthmatic patients from nonasthmatic patients with a moderate accuracy (area under the curve, 73.3%).
Despite good specificity, a cutoff of 12% was associated with poor sensitivity (35.6%). A cutoff of less than 8% performed significantly better than a cutoff of 12% (P = .03, 8% vs 12%).
Conclusions
Our findings highlight the poor sensitivity associated with the commonly used 12% cutoff for BDR. Although our data show that a threshold of less than 8% performs better than 12%, given the variability of this test in children, we conclude that it might be not be appropriate to choose a specific BDR cutoff as a criterion for the diagnosis of asthma.
Keywords: Asthma, bronchodilator response, diagnosis
In asthmatic patients the response to bronchodilators reflects the reversibility of airway airflow obstruction. High bronchodilator response (BDR) in asthmatic patients has been associated with poor clinical outcomes,1 increased airway inflammation,2 and increased response to inhaled corticosteroids,1,3 supporting that BDR has prognostic and therapeutic relevance. The BDR test provides additional information to the clinical history and is recommended by international guidelines as an adjunct to the clinical history in the diagnosis of asthma.4
BDR can be expressed by using different methods, with the 3 most common being the percentage of FEV1, the percentage of the initial predicted value for FEV1, and absolute change in FEV1 after administration of a short-acting bronchodilator. A significant BDR is commonly defined as a 12% or greater and 200 mL or greater change in FEV1 from baseline.5 This 12% criterion, which was recently reaffirmed in a large international study,6 approximates the 95th percentile for percentage change in FEV1 after bronchodilator administration in general population studies, which mainly consist of adults.7 The forced oscillation technique, which provides information on airway resistance and reactance, has also been used to measure response to bronchodilators and to differentiate between asthmatic and nonasthmatic patients,8,9 especially in younger children who are unable to cooperate during spirometric testing. However, this technique is not widely used because standardized guidelines are lacking.
Although the National Asthma Education and Prevention Program’s Expert Panel Report 3 uses the 12% cutoff as evidence of airway reversibility in establishing the diagnosis of asthma4 and this cutoff is used to include subjects in several childhood asthma trials,10,11 the validity of the 12% cutoff has been questioned in the pediatric population.12 BDR tends to increase with decreasing baseline FEV1.13 In subjects with low baseline FEV1, small changes in absolute FEV1 in response to a bronchodilator translate into large percentage changes in FEV1. Because most children with asthma have baseline FEV1 within the normal reference range, the increase in FEV1 after bronchodilator administration is limited. Dundas et al14 suggest that a 9% or greater increase in FEV1 provided the most acceptable sensitivity (50%) and specificity (86%) to detect previous wheeze in 5- to 10-year-old children. These results were corroborated by a more recent study showing that a BDR of 9% or greater was optimal at differentiating asthmatic from nonasthmatic children with similar sensitivity and specificity.12 This study was performed in a predominantly Hispanic cohort and thus might have limited generalizability to children of other ethnicities.
In this study we determined the BDR cutoff that best differentiates between children with mild-to-moderate asthma and children without asthma in a large and predominantly white population. We hypothesized that a BDR cutoff of 12% would be specific but not sufficiently sensitive for diagnosing asthma in children.
METHODS
Subjects
Participants from 3 pediatric cohorts were included in this study. Asthma cases consisted of children enrolled in the Childhood Asthma Management Program (CAMP). The demographics of the CAMP subjects and the study design have been previously reported.15 Briefly, this multicenter trial randomized 1041 children with mild-to-moderate asthma aged 5 to 13 years to budesonide, nedocromil, or placebo. Children were included if they had asthma symptoms 2 or more times per week, used an asthma medication daily, or used an inhaled bronchodilator twice per week for 6 or more months and had a positive methacholine challenge test result. The long-term effects of these treatments on lung growth were evaluated.15 Follow-up visits occurred at 2 and 4 months after randomization and every 4 months thereafter for an average of 4.3 years.
The control patients for this study were selected from 2 pediatric general population cohorts. Project Viva is a prospective cohort study examining the effect of prenatal and perinatal factors on maternal and child health. Details of the study design have been described previously.16 This prebirth cohort consisted of 2128 singleton infants, the mothers of whom were recruited from Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts. Of these children, 1116 attended Project Viva’s 7-year in-person visit, 819 of whom attempted the BDR test and 468 had valid results. The Home Allergens cohort is a birth cohort of children with a parental history of allergy or asthma. Details of the study design have been published.17 Five hundred five infants were recruited within 48 hours of birth in the metropolitan Boston area. Telephone questionnaires about symptoms and diagnoses of atopic disease were administered every 2 months for the first 2 years of life and then every 6 months. A total of 284 children were followed until age 12 years, when 250 of them performed the bronchodilator test. Subjects from these 2 population-based cohorts were selected as control subjects if they had never been given a diagnosis of asthma or had never wheezed, both assessed through a questionnaire or interview (n = 197 in Project Viva and n = 53 in Home Allergens).
In all 3 cohorts the children’s parents or guardians provided informed consent, and the study was approved by the respective local institutional review board.
Spirometry and BDR
In CAMP the BDR test was performed at randomization and at subsequent visits during which a methacholine challenge was not administered.18 Spirometry was performed at least 4 hours after the last use of a short-acting bronchodilator and at least 24 hours after the last use of a long-acting bronchodilator. For the purpose of this analysis, we used the BDR test at randomization, at which point subjects had been off their regular asthma medications for at least 28 days but were allowed to use a rescue bronchodilator and prednisone, if needed. The BDR test was performed around age 7 years for Project Viva (range, 6.6-10.9 years) and at age 12 years for Home Allergens (range, 11.1-12.7 years). In Project Viva spirometry was performed with the EasyOne Spirometer (NDD Medical Technologies, Andover, Mass). In Home Allergens spirometry was performed by using the Eagle+ spirometer (Collins Medical, Louisville, Colo). In all 3 cohorts postbronchodilator spirometric measures were obtained at least 15 minutes after administration of 2 puffs (90 μg per puff) of albuterol. Spirometric performance was required to meet American Thoracic Society criteria for acceptability and reproducibility, with each subject producing at least 3 acceptable spirograms, 2 of which must have been reproducible.19
In this study we defined BDR as a percentage change in absolute FEV1 after albuterol administration, as follows:
Given the lack of consensus in the definition of BDR, we have also calculated BDR as a percentage of the initial predicted value for FEV1 in CAMP, as follows:
and compared it with the former definition.
Statistical analysis
A descriptive analysis of baseline characteristics was performed. A Pearson correlation test was used to evaluate the association between the 2 definitions of BDR in CAMP. The study population was divided into a training set, consisting of a random selection of 50 cases and 50 control subjects, and a validation set, consisting of the remaining subjects, to evaluate the diagnostic accuracy of the BDR test. This allows for validation of results within our cohorts. Receiver operating characteristic curves were constructed, and areas under the curve (AUCs) were calculated for different BDR thresholds (R package pROC).20 The AUCs for different thresholds were compared by using the DeLong test. A sensitivity analysis was performed to assess how the diagnostic accuracy of the BDR test varies across different severities of asthma. AUCs were examined for the CAMP subjects with and without evidence of baseline airflow obstruction (FEV1 percent predicted <80% and ≥80%, respectively). P values are 2-sided. All analyses were performed with R software, version 2.12.1 (www.r-project.org).
RESULTS
Patient demographics
A total of 1041 children with mild-to-moderate asthma were included from the CAMP cohort (cases). Control subjects consisted of 250 children from the Project Viva and Home Allergens cohorts who had no history of wheezing and asthma at the time of the BDR test. Baseline characteristics of the study population by asthma status are presented in Table I. Although their baseline FEV1 percent predicted values were similar and within normal limits (93.7% ± 14.3% in cases and 98.4% ± 12.2% in control subjects), the mean BDR differed between the 2 groups, as expected. Both groups consisted of predominantly white subjects. The control subjects from Project Viva were younger than those from Home Allergens (7.9 ± 0.8 vs 11.7 ± 0.5 years, respectively), but other baseline characteristics were similar, including race/ethnicity, baseline FEV1 percent predicted, and BDR (see Table E1 in this article’s Online Repository at www.jacionline.org). Baseline characteristics of subjects who performed a BDR test and those who did not are shown in Table E2 in this article’s Online Repository at www.jacionline.org. In both Project Viva and Home Allergens subjects who underwent the BDR test had a higher prevalence of asthma and wheezing, whereas other measures of atopy were similar to those of the subjects who did not perform the test. However, the control subjects selected for this analysis were not different from the control subjects who did not undergo the BDR test in terms of age, anthropometric measures, and race (see Table E3 in this article’s Online Repository at www.jacionline.org).
TABLE I.
Baseline characteristics
| Cases (n = 1041) | Control subjects (n = 250) |
|
|---|---|---|
| Male sex, no. (%) | 621 (59.7) | 117 (46.8) |
| Age (y), mean (SD) | 8.9 (2.1) | 8.7 (1.7) |
| Height (cm), mean (SD) | 133.6 (13.8) | 133.0 (11.5) |
| Weight (kg), mean (SD) | 33.4 (12.1) | 31.0 (8.9) |
| BMI (%), median (IQR) | 68.0 (41.3-89.2) | 62.4 (32.1-82.8) |
| Race, no. (%) | ||
| White | 711 (68.3) | 190 (76.0) |
| Black | 138 (13.3) | 20 (8.0) |
| Hispanic | 98 (9.4) | 7 (2.8) |
| Other | 94 (9.0) | 33 (13.2) |
| FEV1 (L), mean (SD) | 1.65 (0.48) | 1.72 (0.49) |
| FEV1 (% predicted), mean (SD) | 93.7 (14.3) | 98.4 (12.2) |
| BDR (%), mean (SD) | 10.7 (10.2) | 2.7 (8.4) |
BMI, Body mass index.
Comparison of different definitions of BDR
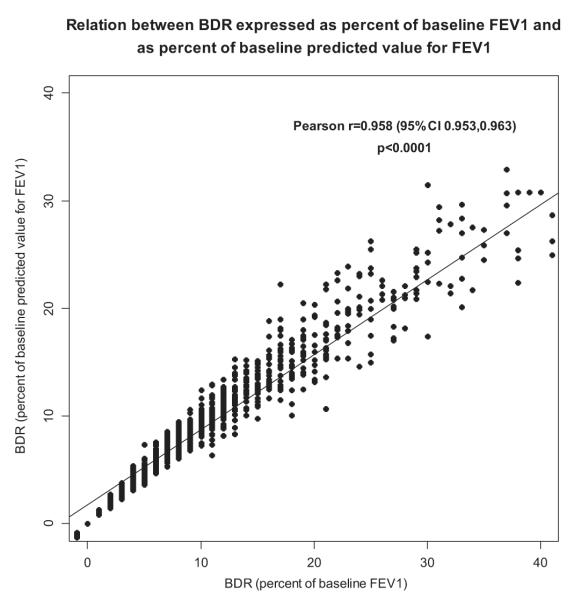
Given the lack of consensus on how to define BDR, we compared BDR defined as (1) percentage of initial FEV1 and (2) percentage of initial predicted value for FEV1. In CAMP asthmatic patients these 2 definitions were almost perfectly correlated (Pearson correlation, r = 0.958; Fig 1). Therefore we used BDR as a percentage of initial FEV1 in the subsequent analyses because the commonly accepted 12% cutoff is based on this definition.
FIG 1.
Correlation between BDR expressed as percentage of baseline FEV1 and as percentage of baseline predicted value for FEV1 in CAMP (n = 1041).
Diagnostic accuracy of the BDR test
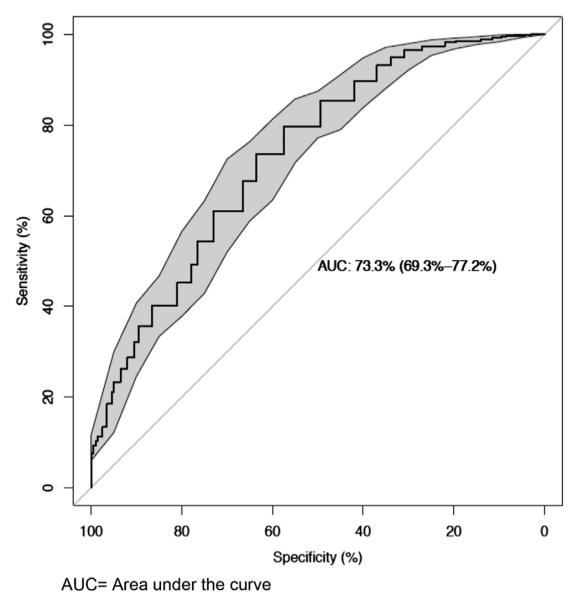
For the purposes of this analysis, the study population was divided into a training set and a validation set. In the training set (n = 100) the BDR test differentiated children with and without asthma with a moderate accuracy (AUC, 74.5%; 95% CI, 69.0% to 80.0%). The AUC was replicated in the validation set at 73.3% (95% CI, 69.2% to 77.2%; Fig 2). Similar AUCs were obtained when the analysis was stratified by sex (AUC, 72.5% in male and 74.0% in female subjects). Table II shows the sensitivity and specificity for different BDR cutoff values. Despite good specificity (89.5%), a cutoff of 12% offered low sensitivity (35.6%). A BDR cutoff of 8% (sensitivity, 54.4%; specificity, 76.5%) or less performed significantly better than a cutoff of 12% (comparing AUCs: P = .031, 8% vs 12%). A cutoff of 5% offered the highest AUC (68.6%), with a sensitivity of 73.6% and specificity of 63.5% (Table II).
FIG 2.
Receiver operating characteristic curve in validation set comparing 991 patients with mild-to-moderate asthma from CAMP and 200 control subjects from Project Viva and Home Allergens.
TABLE II.
A BDR cutoff of 8% or less performed significantly better than a cutoff of 12%
| Sensitivity | Specificity | AUC (95% CI) |
P value (comparing with AUC for 12%) |
|
|---|---|---|---|---|
| BDR cutoff (%) | ||||
| 12 | 35.6 | 89.5 | 62.5 (59.9-65.1) | — |
| 11 | 40.1 | 86.5 | 63.3 (60.5-66.1) | .261 |
| 10 | 45.2 | 81.0 | 63.1 (60.0-66.2) | .613 |
| 9 | 49.1 | 78.0 | 63.6 (60.3-66.8) | .414 |
| 8 | 54.4 | 76.5 | 65.4 (62.1-68.8) | .031 |
| 7 | 61.1 | 73.0 | 67.0 (63.6-70.5) | .003 |
| 6 | 67.6 | 66.5 | 67.1 (63.5-70.7) | .007 |
| 5 | 73.6 | 63.5 | 68.6 (64.9-72.2) | .001 |
Sensitivity analysis
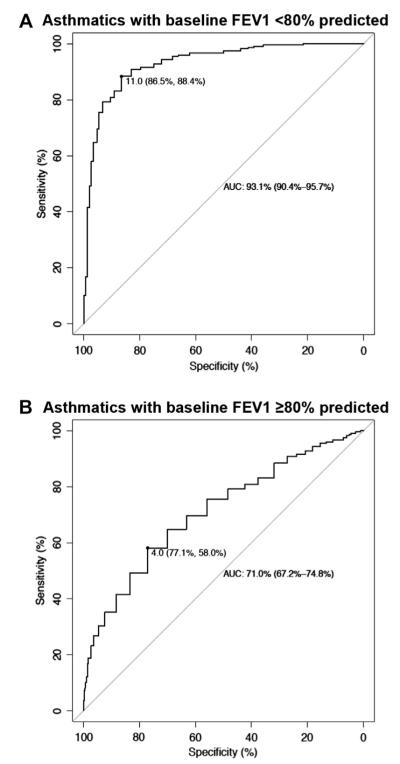
A total of 148 CAMP subjects had an initial FEV1 percent predicted value of less than 80%. Their mean BDR was 23.6% ± 14.6%. The BDR test differentiated these subjects from control subjects with a high accuracy (AUC, 93.1%; 95% CI, 90.4% to 95.7%; Fig 3, A). In contrast, the diagnostic accuracy of the BDR test was lower for CAMP subjects with an initial FEV1 percent predicted value of 80% or greater (n = 881; AUC, 71.0%; 95% CI, 67.2% to 74.8%; Fig 3, B). This subset of children also had a lower BDR (mean, 8.6 ± 7.4).
FIG 3.
Separate receiver operating characteristic curves comparing 148 asthmatic patients with a baseline FEV1 of less than 80% of predicted value and 250 control subjects (A) and 881 asthmatic patients with a baseline FEV1 of 80% or greater and 250 control subjects (B). The best threshold based on the AUC, along with its specificity and sensitivity, are shown.
DISCUSSION
In this analysis of the diagnostic accuracy of the BDR test, we found that the commonly used BDR cutoff of 12% was associated with a poor sensitivity and that a BDR cutoff of 8% or lower performed better than 12% at differentiating between children with and without asthma using 3 predominantly white populations.
The BDR test is recommended as an adjunct in the diagnosis of asthma, along with the clinical history and signs of inflammation, such as eosinophilia and increased IgE levels. This test assesses the degree of reversible airway obstruction with the administration of a bronchodilator and has been shown to provide independent information in the assessment of asthma.21 The widely accepted BDR cutoff of 12% is based on general population and asthmatic patient studies consisting of mostly adults, who might have a higher interindividual variability in baseline FEV1. A recent international study examined the BDR test in more than 3900 healthy adult never smokers participating in the population-based Burden of Obstructive Lung Disease study.6 The authors reaffirmed that the 95th percentile for BDR was 12%, with BDR expressed as a percentage of absolute baseline FEV1. Although this study provides confirmation to current guidelines in adults, our findings suggest that this cutoff might not be appropriate for the diagnosis of asthma in children given its poor sensitivity, which could result in missed cases of asthma. Studies have shown that the BDR tends to increase with decreasing baseline lung function,13 and therefore BDR might be higher in asthmatic adults. In the Burden of Obstructive Lung Disease study cohort, the mean percent predicted FEV1 values in the entire cohort were generally lower than in our study. In contrast, in asthmatic children baseline lung function tends to remain normal,22,23 even in a subset of patients with severe asthma24; hence a 12% change from baseline with bronchodilator might be difficult to reach. This inference is in accordance with our sensitivity analysis, in which only a minority of CAMP subjects had a baseline FEV1 of less than 80% of predicted value.
The choice of an optimal BDR cutoff is complex. Maximizing sensitivity and specificity is key in the evaluation of any diagnostic test. In the case of asthma, there is no clear consensus on what is an acceptable tradeoff between these 2 test properties because this decision depends on the treatment threshold. The treatment threshold is in turn determined by the costs and benefits of the treatment and consideration of the harm caused by the treatment of false-positive cases and the nontreatment of false-negative cases. Furthermore, the positive and negative predictive values will depend on the prevalence of asthma, and these differ from a general pediatrician’s office to a pulmonologist’s office, where the BDR test is commonly performed. In patients with mild-to-moderate asthma, as with this study population, treatment can consist of a bronchodilator on an as-needed basis or a daily inhaled corticosteroid, both with different pharmacologic profiles. Hence choosing an optimal cutoff based on prespecified acceptable values of sensitivity and specificity is difficult. Rather, we have used the area under the receiver operating characteristic curve to guide our selection of an appropriate cutoff.
Although a BDR cutoff of 12% provided good specificity (89.5%), it offered poor sensitivity (35.6%). A cutoff of 8%, which had a significantly better AUC than 12%, was associated with a sensitivity of 54.4% and a specificity of 76.5%. The higher performance of a BDR cutoff of less than 12% is in accordance with a previous study of the BDR test in a mainly Hispanic pediatric population, which identified a BDR cutoff of 9% as the optimal value, with a sensitivity of 42.5% and a specificity of 86.3%.12 This suggests that the BDR test is robust enough to withstand variations in ethnicity.
Although our findings identified that a BDR cutoff of 8% performed significantly better than a cutoff of 12%, it is important to note that changes in FEV1 of less than 8% are generally considered to be within measurement variability.5,25 This variability is likely higher in children given their imperfect technique. Thus using a cutoff of 8% as a criterion for the diagnosis of asthma might not be appropriate either. For these reasons, we suggest using caution when picking a BDR cutoff to aid in the diagnosis of asthma. Overall, the BDR test performs poorly in diagnosing asthma in children and might be a better reflection of changes in bronchomotor tone in response to bronchodilators, which in turn might reflect the frequency of asthma symptoms or exacerbations.
Based solely on the AUC, our data suggest a BDR cutoff of 5% as the optimal cutoff (Table II) with a sensitivity of 73.6% and specificity of 63.5%. This threshold would not be clinically useful given that it is within the range of measurement variability. Interestingly, our sensitivity analysis showed that the diagnostic accuracy of the BDR test was higher for subjects with lower baseline lung function. The optimal BDR cutoff for children with an FEV1 of 80% of predicted value or less based on the AUC was 11%, reflecting the high mean BDR in these persons compared with control subjects. In contrast, the “optimal” 5% cutoff derived from the overall data likely reflects that the majority of the cases have mild asthma, with a significant overlap of the BDR between cases and control subjects (Table I).
Although not the focus of this study, it is interesting to consider the consistency with which children with asthma meet the criterion for a BDR cutoff of less than 12% over time. Using the CAMP cohort, Sharma et al26 reported that, similarly to a cutoff of 12%, a consistent BDR of 10% or greater over 4 years predicted nighttime awakenings, oral steroid bursts, and emergency department visits or hospitalizations. Future studies should explore whether a consistent BDR response at lower thresholds would also predict future clinical outcomes and diagnoses of asthma.
There is no clear consensus on how the BDR should be expressed. Some authors suggest an advantage of using BDR as a percentage of the initial predicted value for FEV1 over a percentage of initial FEV1 because this definition is less dependent on baseline lung function.25,27 In our population of children with mild-to-moderate asthma, these 2 definitions of BDR were highly correlated (r = 0.958). This agreement is in accordance with the fact that most of these children had normal baseline FEV1 percent predicted values. On the basis of these results, the use of either definition of BDR might be acceptable in children with mild-to-moderate asthma, whereas BDR as a percentage of predicted FEV1 should be considered in populations with a high heterogeneity in baseline lung function, such as children with severe asthma.24
Our findings can have varied and wide-ranging implications, depending on the clinical situation. Clinically, the BDR test is an imperfect diagnostic test, as suggested by the AUC of less than 70% for any cutoff, and it should be used only as an adjunct for the diagnosis of asthma. Using a cutoff of 12% or greater as “significant” bronchodilation might also result in underdiagnosis and undertreatment of asthma, given the poor associated sensitivity. In asthma research several studies use the 12% BDR cutoff to select patients for inclusion in the study. In children this criterion will lead to a subset of patients selected for a higher degree of airway airflow obstruction, which might limit the generalizability of the results. In addition, it might be more difficult to recruit the number of patients required given the restriction by this criterion. This consideration is especially important in asthma genetic and pharmacogenetic studies, in which large sample sizes are often required. On the other hand, in intervention studies a higher cutoff could select for children who have a higher chance of showing an intervention effect.
Several limitations of our study need to be addressed. This study was designed to examine how well the BDR test differentiates children with mild-to-moderate asthma from children without asthma. This is also a predominantly white population. The diagnostic accuracy of the BDR test was derived from these populations and might not be generalizable to other situations, such as more severe asthma. However, previous studies found similar results,12 including one comparing subjects with mild intermittent wheeze with control subjects.14 This supports the use of the BDR test as an adjunct in patients with different degrees of symptom severity. Asthma is a heterogeneous disease, and in addition to pharmacogenetic predisposition in the response to bronchodilators,28-30 there is evidence that patients with the allergic asthma phenotype have higher BDR,31 whereas others have a lower response.32 Thus the diagnostic value of the BDR test might depend on the asthma phenotype. Finally, a minority of subjects in CAMP received rescue prednisone in the weeks before randomization. Although these are short courses of corticosteroids, this could have affected the participants’ responsiveness to a bronchodilator and made cases more similar to control subjects, which would lead to a bias toward a decreased accuracy of the BDR test in this study.
In conclusion, this study demonstrated that the commonly used BDR cutoff of 12% in adults offers poor sensitivity as an adjunct test in the diagnosis of asthma in children. Our results from populations of children with mild-to-moderate asthma suggest that a BDR cutoff of 8% or less performed significantly better than a cutoff of 12%. However, given the variability of this test in children, it might not be appropriate to choose 8% as the optimal cutoff. Thus we advise against choosing a specific BDR cutoff for the diagnosis of asthma but rather to use the BDR test as a guide in the treatment and diagnosis of asthma because the persistence of a positive response might be associated with worse clinical outcomes in asthmatic patients over time.
Supplementary Material
TABLE E1. Baseline characteristics of control subjects
TABLE E2. Characteristics of subjects who did and did not perform BDR in Project Viva and Home Allergens
TABLE E3. Characteristics of control subjects who did and did not perform the BDR test in Project Viva and Home Allergens
Clinical implications.
The BDR reflects the reversibility of airflow obstruction. The commonly used cutoff of 12% is associated with poor sensitivity in the diagnosis of asthma in children and might result in missed cases of asthma.
Acknowledgments
CAMP CREDIT ROSTER
Source of funding The CAMP trial and CAMP Continuation Study were supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute (NHLBI) and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources. The CAMP Continuation Study Phases 2 and 3 were supported by grants U01HL075232, U01HL075407, U01HL075408, U01HL075409, U01HL075415, U01HL075416, U01HL075417, U01HL075419, U01HL075420, and U01HL075408 from the NHLBI. The National Jewish Health site was also supported in part by Colorado CTSA grant UL1RR025780 from the National Center for Research Resources/National Institutes of Health (NIH) and UL1TR000154 from the National Center for Advancing Transitional Sciences/NIH.
The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources. Additional support for this research came from grants P50 HL67664, U01 HL65899, and T32 HL07427 from the National Institutes of Health and the National Heart, Lung, and Blood Institute. Home Allergens is funded by National Institute of Allergy and Infectious Diseases/R01 grant AI035786. Project Viva is funded by R01 HL064925 and R01 HD034568.
S. M. Tse, M. W. Gillman, and K. G. Tantisira have been supported by/have received one or more grants from or have one or more grants pending with the National Institutes of Health (NIH). D. R. Gold has been supported by one or more grants from the National Institute of Allergy and Infectious Diseases. A. L. Fuhlbrigge has been supported by one or more grants from and has received support for travel from the National Heart, Lung, and Blood Institute (NHLBI); is a Board member for Merck; has consultancy arrangements with Merck, GlaxoSmithKline, ICON Medical Imaging, Sunovion, the Lovelace Respiratory Research Institute, and Dmagi; and has received one or more grants from or has one or more grants pending with the NHLBI and the Agency for Healthcare Research and Quality. A. A. Litonjua has been supported by one or more grants from the NIH and has received royalties from UpToDate.
Abbreviations used
- AUC
Area under the curve
- BDR
Bronchodilator response
- CAMP
Childhood Asthma Management Program
Footnotes
MEMBERS OF THE CAMP RESEARCH GROUP
Clinical centers
ASTHMA, Inc, Seattle, Wash: Paul Williams, MD (Principal Investigator); Mary V. Lasley, MD (Co-Director); Tamara Chinn, MSN, ARNP (Coordinator). Michele Hinatsu, MSN, ARNP; Clifton T. Furukawa, MD; Leonard C. Altman, MD; Frank S. Virant, MD; Michael S. Kennedy, MD; Stephen Tilles, MD. Jonathan W. Becker, MD (1995-2010); C. Warren Bierman, MD (1992-1997); Dan Crawford, RN (1996-2002); Thomas DuHamel (1991-2004); Heather Eliassen, BA (1996-1999); Babi Hammond (1996-1999); Miranda MacLaren (2008-2011); Dominick A. Minotti, MD (1992-2003); Chris Reagan (1992-2003); Gail Shapiro (1991-2006, Principal Investigator); Marian Sharpe, RN (1992-1994); Ashley Tatum, MD (2004-2007); Grace White (1991-2007). Timothy G. Wighton, PhD (1994-1998).
Brigham & Women’s Hospital and Harvard Vanguard Medical Associates, Boston, Mass: Anne Fuhlbrigge, MD (Principal Investigator); Anne Plunkett, NP, MS (Coordinator). Nancy Madden, RN, BSN; Susan Anderson; Mark Boehnert, MD; Anita Feins, MD; Amanda Gentile; Natalia Kandror, MD; Kelly Mac-Aulay, MD; Ernestina Sampong; Scott Weiss MD. Walter Torda, MD (Co-Investigator Director, 1993-2003); Martha Tata, RN (1993-2002); Sally Babigian, RN (1997-1999); Peter Barrant, MD (2004-2007); Linda Benson (1998-2004); Jose Caicedo (1998-1999); Tatum Calder (1998-2001); Christine Darcy (2001-2008); Anthony DeFilippo (1994-2000); Cindy Dorsainvil (1998-2001); Julie Erickson (1998-1999); Phoebe Fulton (1997); Mary Grace, RN (1994-1996); Jennifer Gilbert (1997-1998); Dirk Greineder, MD (1993-2000); Stephanie Haynes (1993-1998); Margaret Higham, MD (1996-1998); Deborah Jakubowski (1999); Susan Kelleher (1993-1997); Jay Koslof, PhD (1993-1995); Dana Mandel (1996-1998); Patricia Martin (2001-2003); Agnes Martinez (1994-1997); Jean McAuliffe (1994-1995); Erika Nakamoto (2002-2004); Paola Pacella (1993-1998); Paula Parks (1993-1995); Johanna Sagarin (1998-1999); Kay Seligsohn, PhD (1995-2004); Susan Swords (2003-2005); Meghan Syring (1998-2001); June Traylor, MSN, RN (1996-1998); Melissa Van Horn, PhD (1996-1999); Carolyn Wells, RN (1993-1995); Ann Whitman, RN (1994-1996).
The Hospital for Sick Children, Toronto, Ontario, Canada: Hartmut Grasemann, MD (Principal Investigator); Melody Miki, RN, BSN (Coordinator); Melinda Solomon, MD; Padmaja Subbarao, MD. Ian MacLusky, MD, FRCP (Director 1999-2007); Joe Reisman, MD, FRCP(C), MBA (Director, 1996-1999); Henry Levison, MD, FRCP(C) (Director, 1992-1996); Anita Hall, RN (Coordinator, 1993-2007). Yola Benedet (1994-1999); Susan Carpenter, RN (1998-2001); Jennifer Chay (2004); Michelle Collinson, RN (1994-1998); Jane Finlayson-Kulchin, RN (1994-1998); Kenneth Gore, MA (1993-1999); Nina Hipolito, RN (2003-2004); Noreen Holmes, RRT (1998-1999); Erica Hoorntje, RN (2002-2003); Sharon Klassen, MA (1999-2000); Joseé Quenneville, MSc (1993-1995); Renée Sananes, PhD (1993-2004); Christine Wasson, PhD (1999); Margaret Wilson, RN (2001-2002).
Johns Hopkins Asthma & Allergy Center, Baltimore, Md: N. Franklin Adkinson, Jr, MD (Director); Deborah Bull, LPN (Coordinator); Stephanie Philips, RN. Peyton Eggleston, MD (Co-Director, 1991-2004); Karen Huss, DNSc (Co-Investigator, 1991-2004); Leslie Plotnick, MD (Co-Investigator, 1991-1999); Margaret Pulsifer, PhD (Co-Investigator, 1993-2004); Cynthia Rand, PhD (Co-Investigator, 1991-2004). Elizabeth Aylward, PhD (1991-2004), Nancy Bollers, RN (Coordinator, 1993-2004); Kathy Pessaro (2004-2007); Barbara Wheeler, RN, BSN (Coordinator, 1991-1999).
National Jewish Health, Denver, Colo: Stanley Szefler, MD (Director); Ronina Covar, MD (Medical Director); Harold S. Nelson, MD (Co-Director 1991-2000, Co-Investigator 2000-present); Bruce Bender, PhD (Co-Investigator); Andrew Liu, MD (Co-Investigator); D. Sundström (Coordinator); Melanie Phillips; Michael P. White; Melanie Gleason, PA-C. Kristin Brelsford (1997-1999); Jessyca Bridges (1995-1997); Jody Ciacco (1993-1996); Michael Eltz (1994-1995); Jeryl Feeley, MA (Coordinator, 1992-1995); Michael Flynn (1995-1996); Tara Junk-Blanchard (1997-2000); Joseph Hassell (1992-1998); Marcia Hefner (1992-1994); Caroline Hendrickson, RN (1995-1998; Coordinator, 1995-1997); Daniel Hettleman, MA (1995-1996); Charles G. Irvin, PhD (1992-1998); Alan Kamada, PharmD (1994-1997); Marzena Krawiec, MD (2008-2010); Gary Larsen, MD (Co-Investigator, 2000-2010); Sai Nimmagadda, MD (1993-1996); Kendra Sandoval (1995-1997); Jessica Sheridan (1994-1995); Joseph Spahn, MD (Co-Investigator 1993-2010); Gayle Spears, PA-C (2003-2007); Trella Washington (1993-1997); Eric Willcutt, MA (1996-1997). We also thank the pediatric allergy/immunology and pulmonary fellows for their participation: Ivan Cardona, MD; Kirstin Carel, MD; Jayna Doshi, MD; Rich Hendershot, MD; Jeffrey Jacobs, MD; Neal Jain, MD; June-ku Brian Kang, MD; Tracy Kruzick, MD; Harvey Leo, MD; Beth Macomber, MD; Jonathan Malka, MD; Chris Mjaanes, MD; John Prpich, MD; Lora Stewart, MD; Ben Song, MD; Grace Tamesis, MD.
University of California, San Diego, and Kaiser Permanente Southern California Region, San Diego, Calif: Robert S. Zeiger, MD, PhD (Director); Noah Friedman, MD (Co-Investigator); Michael H. Mellon, MD (Co-Investigator); Michael Schatz, MD (Co-Investigator); Terrie Long, RN (Coordinator). Travis Macaraeg. Sandra Christensen, MD (2004-2007); James G. Easton, MD (Co-Director, 1993-1994); M. Feinberg (1997-1998); Linda L. Galbreath (1991-2002); Jennifer Gulczynski (1998-1999); Kathleen Harden, RN (Coordinator, 1993-2010); Ellen Hansen (1995-1997); Al Jalowayski, PhD (Co-Investigator, 1991-2005); Elaine Jenson (2004-2007); Alan Lincoln, PhD (Co-Investigator, 1991-2003); Jennie Kaufman (1994); Shirley King, MSW (1992-1999); Brian Lopez (1997-1998); Michaela Magiari-Ene, MA (1994-1998); Kathleen Mostafa, RN (1994-1995); Avraham Moscona (1994-1996); Catherine A. Nelle, RN (1991-2005); Jennifer Powers (2001-2003); Elsa Rodriguez (2003-2007); Eva Rodriguez, RRT (1994-2008); Karen Sandoval (1995-1996); Nevin W. Wilson, MD (Co-Director, 1991-1993).
University of New Mexico, Albuquerque, NM: Hengameh H. Raissy, PharmD (Director); Aaron Jacobs (Co-Investigator); H. William Kelly, PharmD (Director, 1998-2011); Mary Spicher, RN (Coordinator). Christina Batson; Michelle Harkings, MD; Katie McCallum. Robert Annett, PhD (Co-Investigator, 1993-2004); Teresa Archibeque (1994-1999); Naim Bashir, MD (Co-Investigator, 1998-2005); H. Selda Bereket (1995-1998); Marisa Braun (1996-1999); Carrie Bush (1995-1999); Shannon C. Bush (2002-2007); Michael Clayton, MD (Co-Investigator, 1999-2001); Angel Colon-Semidey, MD (Co-Investigator, 1997-2000); Sara Devault (1993-1997); Anna Esparham (2004-2007); Roni Grad, MD (Co-Investigator, 1993-1995); David Hunt, RRT (1995-2004); Jeanne Larsson, RN (1995-1996); Sandra McClelland, RN (Coordinator, 1993-1995); Bennie McWilliams, MD (Co-Investigator, Director, 1992-1998); Elisha Montoya (1997-2000); Margaret Moreshead (1996-1999); Shirley Murphy, MD (Co-Investigator, 1992-1994); Barbara Ortega, RRT (1993-1999); David Weers (1997-1998); Jose Zayas (1995-1996).
Washington University, St Louis, Mo: Robert C. Strunk, MD (Director); Leonard Bacharier, MD (Co-Investigator); Denise Rodgers, RPFT (Coordinator). Ellen Albers, RN, CNS-P, MSN (1997-1999); Gregg Belle, MA (1996-2001); Gordon R. Bloomberg, MD (Co-Investigator, 1994-2007); W. Patrick Buchanan (1998-2001); Mary Caesar, MHS (1993-1996); James M. Corry, MD (Co-Investigator, 1994-2004); Karen DeMuth (2006-2007); Marisa Dolinsky, MA (1996-2001); Edwin B. Fisher, PhD (1993-2001); Stephen J. Gaioni, PhD (1993-2001); Emily Glynn, RN, MSN, CSPNP (1993-2001); Bernadette D. Heckman, MA (1996-2001); Debra Kemp, RN, BSN (1994-2001); Lila Kertz, MSN, RN, CPNP (2005-2007); Claire Lawhon (1994-2003); Valerie Morgan, RRT (2000-2004); Cynthia Moseid (1997); Tina Oliver-Welker, CRTT (1994-2007); Diana Richardson (1994-1997); Elizabeth Ryan, PhD (1994-1997); Sharon Sagal, MD (1996-2001); Thomas F. Smith, MD (1993-1998); Susan Sylvia, PhD (1994-1997); Carl Turner (1995-1997); Deborah K. White, RPFT, RRT (1994-2007).
Resource centers
Data Coordinating Center, Johns Hopkins University, Baltimore, Md: James Tonascia, PhD (Director). Patricia Belt; Karen Collins; Betty Collison; John Dodge; Michele Donithan, MHS; Cathleen Ewing; Rosetta Jackson; Patrick May, MS; Jill Meinert; Girlie Reyes; Michael Smith; Alice L. Sternberg, ScM; Mark L. Van Natta, MHS; Annette Wagoner; Laura Wilson, ScM; Robert Wise, MD; Katherine Yates, ScM.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, Md: Virginia Taggart, MPH (Project Officer); Lois Eggers; James Kiley, PhD; Howard Moore; Gang Zheng, PhD. Paul Albert, PhD (1991-1999); Suzanne Hurd, PhD (1991-1999); Sydney Parker, PhD (1991-1994); Pamela Randall (1992-2003); Margaret Wu, PhD (1991-2001).
Committees
Data and Safety Monitoring Board: Michelle Cloutier, MD (Chair); John Connett, PhD; Leona Cuttler, MD; Frank Gilliland, MD, PhD. Clarence E. Davis, PhD (1993-2003); Howard Eigen, MD (1993-2009, Chair); David Evans, PhD (1993-2007); Meyer Kattan, MD (1993-2007); Rogelio Menendez, MD (1993-2007); F. Estelle R. Simons, MD (1993-2007); Sanford Leikin, MD (1993-1999).
Steering Committee: Robert Strunk, MD (Study Chair); N. Franklin Adkinson, MD; Robert Annett, PhD (1992-1995, 1997-1999); Bruce Bender, PhD; Mary Caesar, MHS (1994-1996); Reuben Cherniack, MD (Study Chair 1993-2007); Ronina Covar, MD; Thomas R. DuHamel, PhD (1992-1994, 1996-1999); Anne Fuhlbrigge, MD; Hartmut Grasemann, MD; H. William Kelly, PharmD; Henry Levison, MD (1992-1996); Alan Lincoln, PhD (1994-1995); Ian MacLusky, MD (1999-2006); Bennie McWilliams, MD (1992-1998); Curtis L. Meinert, PhD; Sydney Parker, PhD (1991-1994); Hengameh H. Raissy, Pharm D; Joe Reisman, MD, FRCP(C), MBA (1991-1999); Denise Rodgers; Kay Seligsohn, PhD (1996-1997); Gail G. Shapiro, MD (1991-2006); Marian Sharpe (1993-1994); D. Sundström (1998-1999); Stanley Szefler, MD; Virginia Taggart, MPH; Martha Tata, RN (1996-1998); James Tonascia, PhD; Scott Weiss, MD, MS; Barbara Wheeler, RN, BSN (1993-1994); Paul Williams, MD; Robert Wise, MD; Robert Zeiger, MD, PhD.
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006;117:1264–71. doi: 10.1016/j.jaci.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Puckett JL, Taylor RW, Leu SY, Guijon OL, Aledia AS, Galant SP, et al. An elevated bronchodilator response predicts large airway inflammation in mild asthma. Pediatr Pulmonol. 2010;45:174–81. doi: 10.1002/ppul.21172. [DOI] [PubMed] [Google Scholar]
- 3.Kerstjens HA, Brand PL, Quanjer PH, van der Bruggen-Bogaarts BA, Koeter GH, Postma DS. Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Dutch CNSLD Study Group. Thorax. 1993;48:722–9. doi: 10.1136/thx.48.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 6.Tan WC, Vollmer WM, Lamprecht B, Mannino DM, Jithoo A, Nizankowska-Mogilnicka E, et al. Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax. 2012;67:718–26. doi: 10.1136/thoraxjnl-2011-201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorber DB, Kaltenborn W, Burrows B. Responses to isoproterenol in a general population sample. Am Rev Respir Dis. 1978;118:855–61. doi: 10.1164/arrd.1978.118.5.855. [DOI] [PubMed] [Google Scholar]
- 8.Vu LT, Demoulin B, Nguyen MT, Nguyen YT, Marchal F. Respiratory impedance and response to salbutamol in asthmatic Vietnamese children. Pediatr Pulmonol. 2010;45:380–6. doi: 10.1002/ppul.21201. [DOI] [PubMed] [Google Scholar]
- 9.Shin YH, Jang SJ, Yoon JW, Jee HM, Choi SH, Yum HY, et al. Oscillometric and spirometric bronchodilator response in preschool children with and without asthma. Can Respir J. 2012;19:273–7. doi: 10.1155/2012/560323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–85. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naive asthmatic children. J Pediatr. 2007;151:457–62. e1. doi: 10.1016/j.jpeds.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Weir DC, Sherwood Burge P. Measures of reversibility in response to bronchodilators in chronic airflow obstruction: relation to airway calibre. Thorax. 1991;46:43–5. doi: 10.1136/thx.46.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60:13–6. doi: 10.1136/thx.2004.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 16.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–5. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–36. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 18.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt EW, Cook EF, Covar RA, Spahn J, Fuhlbrigge AL. Identifying the components of asthma health status in children with mild to moderate asthma. J Allergy Clin Immunol. 2008;121:1175–80. doi: 10.1016/j.jaci.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170:426–32. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 23.Paull K, Covar R, Jain N, Gelfand EW, Spahn JD. Do NHLBI lung function criteria apply to children? A cross-sectional evaluation of childhood asthma at National Jewish Medical and Research Center, 1999-2002. Pediatr Pulmonol. 2005;39:311–7. doi: 10.1002/ppul.20161. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–9. e1–13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koeter GH, Dekhuijzen PN, et al. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Thorax. 1992;47:429–36. doi: 10.1136/thx.47.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Litonjua AA, Tantisira KG, Fuhlbrigge AL, Szefler SJ, Strunk RC, et al. Clinical predictors and outcomes of consistent bronchodilator response in the childhood asthma management program. J Allergy Clin Immunol. 2008;122:921–8. e4. doi: 10.1016/j.jaci.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waalkens HJ, Merkus PJ, van Essen-Zandvliet EE, Brand PL, Gerritsen J, Duiverman EJ, et al. Assessment of bronchodilator response in children with asthma. Dutch CNSLD Study Group. Eur Respir J. 1993;6:645–51. [PubMed] [Google Scholar]
- 28.Duan QL, Gaume BR, Hawkins GA, Himes BE, Bleecker ER, Klanderman B, et al. Regulatory haplotypes in ARG1 are associated with altered bronchodilator response. Am J Respir Crit Care Med. 2011;183:449–54. doi: 10.1164/rccm.201005-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan QL, Du R, Lasky-Su J, Klanderman BJ, Partch AB, Peters SP, et al. A polymorphism in the thyroid hormone receptor gene is associated with bronchodilator response in asthmatics. Pharmacogenomics J. 2013;13:130–6. doi: 10.1038/tpj.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhry S, Que LG, Yang Z, Liu L, Eng C, Kim SO, et al. GSNO reductase and beta2-adrenergic receptor gene-gene interaction: bronchodilator responsiveness to albuterol. Pharmacogenet Genomics. 2010;20:351–8. doi: 10.1097/FPC.0b013e328337f992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benton AS, Wang Z, Lerner J, Foerster M, Teach SJ, Freishtat RJ. Overcoming heterogeneity in pediatric asthma: tobacco smoke and asthma characteristics within phenotypic clusters in an African American cohort. J Asthma. 2010;47:728–34. doi: 10.3109/02770903.2010.491142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE E1. Baseline characteristics of control subjects
TABLE E2. Characteristics of subjects who did and did not perform BDR in Project Viva and Home Allergens
TABLE E3. Characteristics of control subjects who did and did not perform the BDR test in Project Viva and Home Allergens