Abstract
Purpose
To determine the minimum enrollment duration for identifying incident cases of epilepsy in administrative data.
Methods
We performed a retrospective dynamic cohort study using Ohio Medicaid data from 1992–2006 to identify a total of 5,037 incident epilepsy cases who had at least 1 year of follow-up prior to epilepsy diagnosis (epilepsy-free interval). The incidence for epilepsy-free intervals from 1 to 8 years, overall and stratified by pre-existing disability status, was examined. The graphical approach between the slopes of incidence estimates and the epilepsy-free intervals was used to identify the minimum epilepsy-free interval that minimized misclassification of prevalent as incident epilepsy cases.
Results
As the length of epilepsy-free interval increased, the incidence rates decreased. A graphical plot showed that the decline in incidence of epilepsy became nearly flat beyond the third epilepsy-free interval.
Conclusion
The minimum of 3-year epilepsy-free interval is needed to differentiate incident from prevalent cases in administrative data. Shorter or longer epilepsy-free intervals could result in over- or under-estimation of epilepsy incidence.
Keywords: Incidence, Administrative data, Epilepsy
1. INTRODUCTION
There is increasing interest in the analysis of administrative claims data to assess health resource utilization, health outcomes, and cost in several disease entities including epilepsy (Kurth et al., 2010; Thurman et al., 2011; St Germaine-Smith et al., 2011). Several algorithms to identify epilepsy cases using claims data have been developed and validated (Holden et al., 2005a, 2005b; Pugh et al., 2008). These studies have shown that the use of a combination of diagnosis (e.g., epilepsy or seizure diagnosis) and pharmacy claims (e.g., antiepileptic drugs (AEDs)) can correctly classify 90% of epilepsy cases (Holden et al., 2005a, 2005b; Pugh et al., 2008). In addition, inclusion of both inpatient and outpatients claims will enhance the validity of epilepsy case ascertainment (Reid et al., 2012). However, algorithms to identify incident cases of epilepsy using claims data have never been investigated. The first occurrence of an epilepsy claim in the data is not necessarily indicative of an incident case because some people with established epilepsy could have been doing well and sought medical care very infrequently. In addition, some people might have received health care services through a different payer, and therefore not captured in the dataset.
Accurate identification of incident epilepsy cases in administrative data is dependent upon the amount of time an individual has contributed prior to epilepsy diagnosis. In the case of claims data, including Medicare, Medicaid, and commercial insurance, this duration extends from the time of enrollment to the time of first appearance of epilepsy claim, or “epilepsy-free interval”. A very short epilepsy-free interval will likely include prevalent cases, and a very long interval will likely exclude some incident cases with a disease-free interval occurring in between. Currently, the minimum duration of epilepsy-free interval to accurately identify incident cases of epilepsy in administrative database is unknown. The commonly used 1 or 2 years of epilepsy-free intervals are probably inadequate to distinguish between prevalent and incident cases (Thurman et al., 2011).
In this study, we assessed the minimum epilepsy-free interval by estimating and comparing incidence rates of epilepsy obtained from different lengths of epilepsy-free interval using the Ohio Medicaid claims data.
2. MATERIAL AND METHODS
2.1 Data source
We used the Ohio Medicaid enrollment and claims data between January 1, 1992 and December 31, 2006, which contained approximately 2 million participants each year (Kaiser Commission on Medicaid and the Uninsured, 2009). Medicaid is a state and federally funded health insurance program for low-income and medically vulnerable groups of people in the U.S. including children, pregnant women, adults with dependent children, people with severe disabilities, and the elderly. The Medicaid program has extensive rules for determining eligibility with considerable variation across the states (Schneider & Elias, 2002). In general, a person must belong to one of the Medicaid’s categorically eligible groups and also meet financial criteria to qualify for Medicaid.
To identify persons with disabilities for categorical eligibility under Medicaid, states are required to use the definition of disability defined under the Supplemental Security Income program (Schneider et al., 2000). However, not every person with physical and mental impairment, regardless of the severity, qualifies for SSI benefits on the basis of disability. Furthermore, some states including Ohio have the option to use a more restrictive definition of disability (Meyer & Zeller, 1999). Consequently, Medicaid individuals with disability are a diverse group of people with a wide variety of conditions that cause physical and/or psychological impairments and limitations (Schneider & Elias, 2002). Some of these conditions such as autism, and mental retardation are closely related to epilepsy (Fisher et al., 2012). Moreover, many of the disabled Medicaid individuals have several forms of disabilities (Meyer & Zeller, 1999). Ultimately, Medicaid serves a disproportionate share of the most vulnerable and severely limited group of persons with disabilities.
Medicaid claims data consists of enrollment and claims files. The enrollment files contained data on periods of enrollment, eligibility category, dates of enrollment, and demographic characteristics of each enrollee. The claims files contained data on office visits, emergency room visits, hospital admissions, procedures performed, date of service, and filled prescriptions including drug name, dose, and number of days supplied. The diagnoses were coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) while the procedures performed were coded using the Current Procedural Terminology, 4th Edition (CPT-4).
The research protocol was approved by the Institutional Review Boards at Case Western Reserve University and by the Ohio Department of Job and Family Services.
2.2 Identification of study cohort
Subjects entered our retrospective dynamic cohort if they enrolled in Medicaid for at least 12 months, and were at least 18 years of age at the time of enrollment. Individuals who had enrollment gaps between the first and the last date of enrollment of more than 20%, who enrolled in managed care program, Medicare program, or spend-down program were excluded from analyses as essential data might be missing.
2.3 Epilepsy case ascertainment
We used standard criteria for epilepsy case ascertainment consistent with previous studies using claims data (Thurman et al., 2011; Paradis et al., 2010; Manjunath et al., 2011). Individuals were identified as having epilepsy if they met all of the following criteria:
At least 1 visit with an epilepsy diagnosis (ICD-9-CM: 345.xx); or at least 2 visits, on different dates, with a diagnosis of non-febrile convulsions (ICD-9-CM: 780.3 or 780.39). The epilepsy onset or epilepsy index date was determined as the date of the first diagnosis of epilepsy or the second diagnosis of non-febrile convulsion.
At a minimum of 30 days after epilepsy index date, there was at least 1 more visits related to epilepsy or non-febrile convulsions (ICD-9-CM: 345.xx, 780.3, or 780.39).
A minimum of 2 pharmacy dispensing claims, at least 30 days apart subsequent to the epilepsy index date, for any of the following AEDs: carbamazepine, ethosuximide, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, pregabalin, primidone, tiagabine, topiramate, valproic acid, or zonisamide.
2.4 Definition of incident case of epilepsy
Cohort members meeting the epilepsy case definition but had less than 1 year of enrollment in Medicaid were identified as prevalent cases. Individuals were considered incident cases when they enrolled in Medicaid for at least 1 year and met the epilepsy case definition for the first time without any preceding seizure or epilepsy diagnosis. The duration of epilepsy-free interval was then lengthened by adding 1 year at a time, up to a maximum of 8 years, the greatest interval for which we could obtain stable incidence estimates. As the number of epilepsy-free interval increased, some of the incident cases with shorter epilepsy-free intervals would become prevalent cases, and therefore, were excluded from the analysis of incidence estimates for the longer epilepsy-free intervals. For example, subjects with 2-year epilepsy-free interval would be classified as prevalent cases and were not included in the incidence estimate of the 3-year epilepsy-free interval. The epilepsy incidence of each epilepsy-free interval was then examined.
2.5 Statistical analysis
For each epilepsy-free interval, we calculated the incidence rate of epilepsy per 1,000 person-years by dividing the number of incident cases of epilepsy by the number of accrued person years across the study period and multiplying by 1,000. Assuming Poisson distribution of the observed epilepsy cases, we computed 95% confidence intervals of the incidence rates based on the exact midpoint method (Rothman & Boice, 1979) using an online calculator program available at http://www.sph.emory.edu/~cdckms/exact-rate.html.
To determine the duration of epilepsy-free period that minimizes misclassifying prevalent epilepsy cases as incident cases, we first obtained absolute values of the slopes of incidence estimates between adjacent epilepsy-free intervals. We then plotted these absolute values of the slopes against the epilepsy-free intervals and visually identified the interval where the graph stopped changing from its original steep decline to a less steep decline or a plateau. Because of the over-representation of disabling physical and/or mental ailments in the Medicaid population, several of which were risk factors for epilepsy, we stratified the analysis on pre-existing disability status.
2.7 Sensitivity analysis
To test the robustness of study findings, we performed the analysis in a sub-cohort of 28,646 individuals who continuously enrolled in the Medicaid from 1992 to 2000. This fixed sub-cohort represented approximately 10% of the entire study population. Similar to the primary analysis, we calculated the incidence estimates of 1- to 8-year epilepsy-free intervals. The graphical approach was used to determine the minimum epilepsy-free interval that is necessary for identifying incident epilepsy cases using claims data.
3. RESULTS
Of the 318,123 Medicaid enrollees who met the inclusion criteria, 178,206 (56.0%) were female and 64,243 (20.2%) were African-American. The mean age at cohort entry was 31.8 years (range, 18–108 years). The mean observation period was 3.8 years (range, 1–14 years). Among individuals in the study cohort, 9,661 people met the epilepsy case definition. Of those, 4,624 individuals had less than 1 year of Medicaid enrollment and therefore were classified as prevalent cases. The remaining 5,037 individuals, who met the epilepsy case definition for the first time at 1 or more years after Medicaid enrollment, were identified as incident cases. As the duration of epilepsy-free interval increased, the number of subjects who met the criteria for incident cases decreased. The accrued person-years, epilepsy cases, and incidence rate for the entire cohort and within categories of pre-existing disability status by epilepsy-free period are shown in Table 1. The incidence of epilepsy decreased with increasing duration of the epilepsy-free interval. Incidence rates in subjects with 1- and 8-year epilepsy-free interval were 5.50/1,000 and 2.65/1,000 person-years, respectively. Within each interval, the incidence of epilepsy among people without pre-existing disability was lower than that of people with pre-existing disability.
Table 1.
Incidence rates of epilepsy among Medicaid beneficiaries according to the number of preceding epilepsy-free interval: 1992–2006
| Epilepsy -free interval (years) | Number of person-years | Incident cases of epilepsy | Incidense (per 1,000 person-years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Disability | Without disability | All | Disability | Without disability | All | Disability | Without disability | ||||
| IR | 95%CI | IR | 95%CI | IR | 95%CI | |||||||
| 1 | 916221.4 | 367685.8 | 548535.6 | 5037 | 4356 | 681 | 5.50 | 5.35, 5.65 | 11.85 | 11.50, 12.20 | 1.24 | 1.15, 1.34 |
| 2 | 764621.3 | 343382.7 | 421238.6 | 3468 | 3125 | 343 | 4.54 | 4.39, 4.69 | 9.10 | 8.79, 9.42 | 0.81 | 0.73, 0.90 |
| 3 | 646984.3 | 321082.5 | 325901.8 | 2621 | 2422 | 199 | 4.05 | 3.90, 4.21 | 7.54 | 7.25, 7.85 | 0.61 | 0.53, 0.70 |
| 4 | 545883.9 | 300024.4 | 245859.5 | 2098 | 1963 | 135 | 3.84 | 3.68, 4.01 | 6.54 | 6.26, 6.84 | 0.55 | 0.46, 0.65 |
| 5 | 471023.1 | 279522.3 | 191500.8 | 1688 | 1596 | 92 | 3.58 | 3.42, 3.76 | 5.71 | 5.43, 6.00 | 0.48 | 0.39, 0.59 |
| 6 | 413878.8 | 258817.0 | 155061.8 | 1379 | 1310 | 69 | 3.33 | 3.16, 3.51 | 5.06 | 4.79, 5.34 | 0.44 | 0.35, 0.56 |
| 7 | 370819.7 | 238818.9 | 132000.8 | 1123 | 1066 | 57 | 3.03 | 2.86, 3.21 | 4.46 | 4.20, 4.74 | 0.43 | 0.33, 0.56 |
| 8 | 332814.3 | 217930.4 | 114883.9 | 883 | 841 | 42 | 2.65 | 2.48, 2.83 | 3.86 | 3.60, 4.13 | 0.37 | 0.27, 0.49 |
Abbreviations: All, all subjects; Disability, subjects with pre-existing disability; IR, incidence rate; Without disability, subjects with no pre-existing disability; 95% CI, 95% confidence interval.
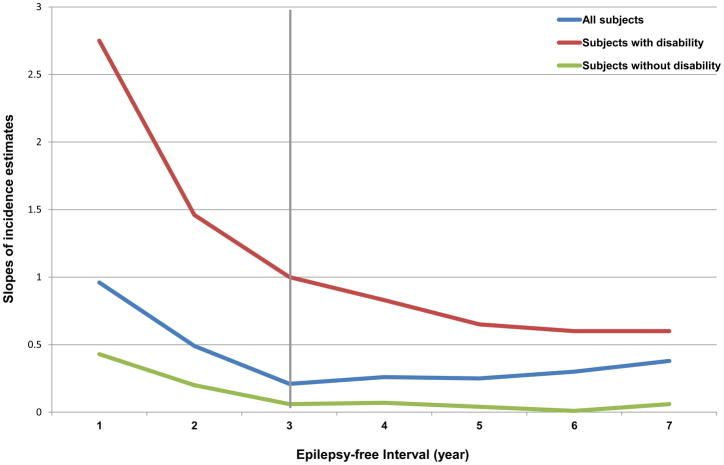
Figure 1 shows a sharp decline in the slopes of incidence estimates during 1- and 2-year epilepsy-free intervals. The slopes became nearly flat after 3-year epilepsy-free interval. Similar pattern was also noted among people with pre-existing disability and best seen among those without pre-existing disability.
Figure 1.
Changes of the incidence of epilepsy by different length of epilepsy-free intervals. An absolute value of the slope for each epilepsy-free interval was calculated by dividing the difference of the incidence estimates between the adjacent epilepsy-free intervals with the difference of the epilepsy-free intervals.
The analysis of the sub-cohort of Medicaid individuals who continuously enrolled in the program showed that 2,051 individuals developed epilepsy with a total follow-up of 259,968 person-years. Similar to the primary analysis, an inverse relationship between the incidence estimates and the duration of epilepsy-free intervals was noted. A graphical plot of the slopes of incidence estimates and epilepsy-free intervals also showed a minimal decline after 3-year epilepsy-free interval.
4. DISCUSSION
We demonstrate that identification of an incident case of epilepsy in administrative data requires at least 3 years of enrollment prior to the first occurrence of epilepsy claim. An overlapping of incidence estimates between 3- and 7-year epilepsy-free interval among people without disability lends some support to this observation. Shorter epilepsy-free intervals, either 1 or 2 years, appear inadequate to distinguish between prevalent and incident cases, possibly resulting in overestimation of incidence rates.
The minimum length of a disease-free interval necessary for identifying incidence cases using administrative data is dependent on the clinical course of the disease and the frequency of health service utilization (Abbas et al., 2012). When the disease is inactive and/or does not affect daily activities, persons with chronic disease may seek medical care very infrequently, resulting in sporadic documentation of the disease in the dataset. The first appearance of diagnosis and/or pharmacy claims, therefore, cannot be assumed to represent a new diagnosis of the disease, especially when the duration of a disease-free interval is not long enough. For diabetes, colorectal cancer, and heart failure, the disease-free interval that is adequate for incidence estimates is found to be 39, 51, and 48 months, respectively (Abbas et al., 2012). Our data show that the minimum epilepsy-free interval needed to identify persons with newly diagnosed epilepsy in Medicaid population is 36 months. This duration seems reasonable given that on average Medicaid beneficiaries with epilepsy make approximately 2 office visits and receive 12 AED refills each year (Halpern et al., 2011). The epilepsy-free intervals that are shorter or longer than 3 years might be necessary if the population of interest have different pattern of medical service utilization. For example, longer than 36-month epilepsy-free interval is probably needed for estimating incidence of epilepsy in elderly population, as older adults with epilepsy often respond well to AEDs (Arain & Abou-Khalil, 2009), and therefore are likely to have sporadic visits with epilepsy diagnosis (Halpern et al., 2011). Knowing the pattern of health service utilization of the population of interest is very important for determining the duration of disease-free interval that is adequate to distinguish incident from prevalent cases.
Up to approximately 66% of persons with epilepsy (PWE), who enter long-term remission, have seizure relapse after discontinuation of AEDs (Specchio & Beghi, 2004; Schmidt & Loscher, 2005). This particular group of PWE poses significant challenges to the identification of incident cases in administrative data, since they can be misclassified as newly diagnosed cases when epilepsy diagnosis and/or AED claims re-emerge in the dataset for the first time after a period of disappearance. Several studies have shown that seizure relapse usually occurs during the first 12-24 months subsequent to AED withdrawal (Berg & Shinnar, 1994; Specchio & Beghi, 2004; Lossius et al., 2008; Bonnett et al., 2011). For PWE who are seizure-free after epilepsy surgery, seizures normally recur within 36 months after cessation of AEDs (Schmidt et al., 2004). Based on these findings, a 3-year epilepsy-free interval is sufficiently long enough to exclude a majority of people with recurrent seizures following AED discontinuation. Epilepsy-free intervals that are less than 3 years are likely inadequate to exclude these “pseudo” newly diagnosed epilepsy cases.
The annual incidence of epilepsy in the U.S. ranges between 15 and 71 per 100,000 person-years, depending on the population of interest, method of case ascertainment, and diagnostic accuracy (Hauser et al., 1993; Annegers et al., 1999; Holden et al., 2005b; Benn et al., 2008; Kroner et al., 2012). In general, children and elderly individuals have higher incidence of epilepsy than young and middle-aged adults (Hauser et al., 1993; Annegers et al., 1999; Benn et al., 2008). The Rochester epidemiology project reports that the incidence of epilepsy in adults aged 25–64 is around 24 to 55 per 100,000 person-years (Hauser et al., 1993). The incidence of epilepsy among members of health maintenance organizations in the same age group is much lower, ranging between 12.4 and 20.9 per 100,000 person-years (Annegers et al., 1999). A low incidence of epilepsy in managed care population is likely due to a healthy-worker effect as members of the health maintenance organizations are employed individuals who are likely to be healthy (Annegers et al., 1999). In contrast, Medicaid population is full of people with multiple chronic medical conditions and/or mental illnesses. Many of the adult Medicaid beneficiaries also have some types of disabilities (Schneider & Elias, 2002). Several of these comorbid conditions, especially those that cause physical and/or functional disabilities such as brain tumor, traumatic brain injury, and stroke are well known risk factors for epilepsy. Moreover, several studies have shown that people in the low socioeconomic class have high risk of developing epilepsy (Heaney et al., 2002; Hesdorffer et al., 2005; Benn et al., 2008). The high incidence of epilepsy among Medicaid population, therefore, is expected. When beneficiaries with pre-existing disabilities are excluded from the analysis, the incidence estimate decreases dramatically, but remains notably elevated compared to the general population. The incidence of epilepsy among Medicaid individuals without disability was 61 per 100,000 person-years when a 3-year epilepsy-free interval was used. In general, the non-disabled adults without any medical condition are ineligible for Medicaid unless they are low-income parents of minor children (Schneider & Elias, 2002). A high incidence estimate in non-disabled adult Medicaid population could be due to the fact that a majority of non-disabled Medicaid individuals are likely to have some types of chronic medical and/or psychiatric illnesses.
An epilepsy-free interval that is optimal for identifying incident epilepsy cases may vary across studies, depending on the objectives of the studies and the sample size of study population. However, a minimum of 3-year epilepsy-free interval is absolutely required to identify incident epilepsy cases using claims data. Shorter epilepsy-free intervals, either 1 or 2 years, appear inadequate to distinguish between prevalent and incident cases. In our recent analysis, we chose a 5-year epilepsy-free interval to estimate the incidence of epilepsy (Kaiboriboon et al., 2013). In that study, we felt that highly specific criteria to identify truly newly diagnosed epilepsy cases were necessary since we did not have access to the clinical information. The epilepsy-free intervals that are longer than 5 years would be too restrictive as persons with truly newly diagnosed epilepsy who recently enrolled into the program will not be included. Epilepsy is a disease with significant burden to the individuals, especially within the first few years after diagnosis when the disease is quite active (Pugh et al., 2005). It, therefore, is reasonable to assume that those who are eligible for Medicaid are more than likely to enroll into the program not long after the diagnosis. In addition, an epilepsy-free interval that is too long may introduce selection bias toward an older population and a large sample size is probably needed to obtain a stable incidence estimate. More importantly, people who continuously enroll in the program for an extended period of time are likely to be different from those with lower enrollment duration (e.g., having less severe diseases and/or better compliance). How long is too long remains unspecified. Given a high prevalence of chronic diseases in Medicaid population and low quality of care that they receive, which could lead to high mortality (Liu et al., 2006; LaPar et al., 2010; Bisgaier & Rhodes, 2011; Koroukian et al., 2012), requiring 7 or 8 years of continuous enrollment is probably too selective. If the specificity of case ascertainment is the utmost concern, a 5- or 6-year epilepsy-free interval should suffice to distinguish incident from prevalent cases for most purposes.
Our study has limitations that are common to studies using administrative data. The accuracy of case ascertainment in this study was mainly based on diagnosis codes without any clinical information. We, however, used very stringent criteria to identify subjects with epilepsy: at least 2 claims of 345.xx, or 3 or more claims of 780.3 or 780.39 that appeared at least 30 days apart. Moreover, subjects had to have at least 2 AED claims, one at the time of epilepsy diagnosis and the other at least 30 days after the diagnosis. These criteria are very conservative, and therefore highly specific to identify subjects with epilepsy, compared to previous studies (Holden et al., 2005b; Faught et al., 2012). However, without clinical information, we cannot confirm the accuracy of epilepsy case ascertainment with absolute certainty. In addition, subjects who in fact had epilepsy but were not actively taking AEDs would not be included in the analysis. As most, if not all, people with epilepsy, especially those who are publicly or privately insured, are on at least 1 AED (Halpern et al., 2011), only a very small number of subjects with epilepsy are left untreated. Exclusion of these subjects is unlikely to affect our findings.
It is debatable whether our findings are generalizable to other administrative dataset. Nonetheless, our data suggest that classifying persons with epilepsy who have short period of enrollment (either 1 or 2 years) as incident cases should be performed with extreme caution.
5. CONCLUSIONS
Defining the minimum length of epilepsy-free interval is critical for identification of incident epilepsy cases using claims data. A minimum enrollment of 3 years prior to epilepsy diagnosis is required to differentiate incident from prevalent cases. A 1- or 2-year epilepsy-free interval is inadequate and likely results in misclassification of incident epilepsy cases.
HIGHLIGHTS.
Identifying incident cases in claims data depends on the amount of time an individual has contributed prior to diagnosis.
The minimum of 3-year epilepsy-free interval is required to distinguish between prevalent and incident cases.
Shorter or longer epilepsy-free intervals might lead to over- or under-estimation of epilepsy incidence.
Acknowledgments
This work was supported by the Epilepsy Foundation (to P.B. and K.K.). This publication was also made possible by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439, from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors appreciate the review and comments by Mr. James Gearheart of the Ohio Department of Job and Family Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas S, Ihle P, Koster I, Schubert I. Estimation of disease incidence in claims data dependent on the length of follow-up: a methodological approach. Health Serv Res. 2012;47:746–755. doi: 10.1111/j.1475-6773.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia. 1999;40:502–506. doi: 10.1111/j.1528-1157.1999.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Arain AM, Abou-Khalil BW. Management of new-onset epilepsy in the elderly. Nat Rev Neurol. 2009;5:363–371. doi: 10.1038/nrneurol.2009.74. [DOI] [PubMed] [Google Scholar]
- Benn EK, Hauser WA, Shih T, Leary L, Bagiella E, Dayan P, Green R, Andrews H, Thurman DJ, Hesdorffer DC. Estimating the incidence of first unprovoked seizure and newly diagnosed epilepsy in the low-income urban community of Northern Manhattan, New York City. Epilepsia. 2008;49:1431–1439. doi: 10.1111/j.1528-1167.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S. Relapse following discontinuation of antiepileptic drugs: a meta-analysis. Neurology. 1994;44:601–608. doi: 10.1212/wnl.44.4.601. [DOI] [PubMed] [Google Scholar]
- Bisgaier J, Rhodes KV. Auditing access to specialty care for children with public insurance. N Engl J Med. 2011;364:2324–2333. doi: 10.1056/NEJMsa1013285. [DOI] [PubMed] [Google Scholar]
- Bonnett LJ, Shukralla A, Tudur-Smith C, Williamson PR, Marson AG. Seizure recurrence after antiepileptic drug withdrawal and the implications for driving: further results from the MRC Antiepileptic Drug Withdrawal Study and a systematic review. J Neurol Neurosurg Psychiatry. 2011;82:1328–1333. doi: 10.1136/jnnp.2010.222885. [DOI] [PubMed] [Google Scholar]
- Faught E, Richman J, Martin R, Funkhouser E, Foushee R, Kratt P, Kim Y, Clements K, Cohen N, Adoboe D, Knowlton R, Pisu M. Incidence and prevalence of epilepsy among older US Medicare beneficiaries. Neurology. 2012;78:448–453. doi: 10.1212/WNL.0b013e3182477edc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Dezort C, Nordli DR, Berg AT. Routine developmental and autism screening in an epilepsy care setting. Epilepsy Behav. 2012;24:488–492. doi: 10.1016/j.yebeh.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Renaud JM, Vickrey BG. Impact of insurance status on access to care and out-of-pocket costs for U.S. individuals with epilepsy. Epilepsy Behav. 2011;22:483–489. doi: 10.1016/j.yebeh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Heaney DC, MacDonald BK, Everitt A, Stevenson S, Leonardi GS, Wilkinson P, Sander JW. Socioeconomic variation in incidence of epilepsy: prospective community based study in south east England. BMJ. 2002;325:1013–1016. doi: 10.1136/bmj.325.7371.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Tian H, Anand K, Hauser WA, Ludvigsson P, Olafsson E, Kjartansson O. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia. 2005;46:1297–1303. doi: 10.1111/j.1528-1167.2005.10705.x. [DOI] [PubMed] [Google Scholar]
- Holden EW, Grossman E, Nguyen HT, Gunter MJ, Grebosky B, Von Worley A, Nelson L, Robinson S, Thurman DJ. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag. 2005a;8:1–14. doi: 10.1089/dis.2005.8.1. [DOI] [PubMed] [Google Scholar]
- Holden EW, Thanh Nguyen H, Grossman E, Robinson S, Nelson LS, Gunter MJ, Von Worley A, Thurman DJ. Estimating prevalence, incidence, and disease-related mortality for patients with epilepsy in managed care organizations. Epilepsia. 2005b;46:311–319. doi: 10.1111/j.0013-9580.2005.30604.x. [DOI] [PubMed] [Google Scholar]
- Kaiboriboon K, Bakaki PM, Lhatoo SD, Koroukian SM. Incidence and prevalence of treated epilepsy among poor health & low income Americans. Neurology. 2013 doi: 10.1212/WNL.0b013e318293e1b4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Commission on Medicaid and the Uninsured. Medicaid enrollment in 50 states, June 2008 data update. 2009 Retrieved May 4, 2012, from http://www.kff.org/medicaid/upload/7606-04.pdf.
- Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118:4271–4279. doi: 10.1002/cncr.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner BL, Fahimi M, Kenyon A, Thurman DJ, Gaillard WD. Racial and socioeconomic disparities in epilepsy in the District of Columbia. Epilepsy Res. 2013;103:279–287. doi: 10.1016/j.eplepsyres.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T, Lewis BE, Walker AM. Health care resource utilization in patients with active epilepsy. Epilepsia. 2010;51:874–882. doi: 10.1111/j.1528-1167.2009.02404.x. [DOI] [PubMed] [Google Scholar]
- LaPar DJ, Bhamidipati CM, Mery CM, Stukenborg GJ, Jones DR, Schirmer BD, Kron IL, Ailawadi G. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–550. doi: 10.1097/SLA.0b013e3181e8fd75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, Ko CY. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- Lossius MI, Hessen E, Mowinckel P, Stavem K, Erikssen J, Gulbrandsen P, Gjerstad L. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus Study) Epilepsia. 2008;49:455–463. doi: 10.1111/j.1528-1167.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- Manjunath R, Paradis PE, Duh MS, Parise H, Lafeuille MH, Lefebvre P, Faught RE. Burden of uncontrolled epilepsy in a privately-insured population. Neurology. 2011;76:A614. doi: 10.1212/WNL.0b013e318271f77e. [DOI] [PubMed] [Google Scholar]
- Meyer JA, Zeller PJ. Profiles of disability: employment and health coverage. 1999 Retrieved January 12, 2013, from http://www.kff.org/medicaid/loader.cfm?url=/commonspot/security/getfile.cfm&PageID=13325.
- Paradis PE, Manjunath R, Duh MS, Lafeuille MH, Mishagina N, Parise H, LR, Lefebvre P, Faught E. Uncontrolled epilepsy in a Medicaid population. Epilepsy Current. 2010;11 (Suppl 1):538. [Google Scholar]
- Pugh MJ, Copeland LA, Zeber JE, Cramer JA, Amuan ME, Cavazos JE, Kazis LE. The impact of epilepsy on health status among younger and older adults. Epilepsia. 2005;46:1820–1827. doi: 10.1111/j.1528-1167.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Pugh MJ, Van Cott AC, Cramer JA, Knoefel JE, Amuan ME, Tabares J, Ramsay RE, Berlowitz DR. Trends in antiepileptic drug prescribing for older patients with new-onset epilepsy: 2000-2004. Neurology. 2008;70:2171–2178. doi: 10.1212/01.wnl.0000313157.15089.e6. [DOI] [PubMed] [Google Scholar]
- Reid AY, St Germaine-Smith C, Liu M, Sadiq S, Quan H, Wiebe S, Faris P, Dean S, Jette N. Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res. 2012;102:173–179. doi: 10.1016/j.eplepsyres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Boice JD. Epidemiologic analysis with a programmable calculator. Bethesda, MD: National Institutes of Health; 1979. [Google Scholar]
- Schmidt D, Baumgartner C, Loscher W. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure-free patients after epilepsy surgery: a review of current clinical experience. Epilepsia. 2004;45:179–186. doi: 10.1111/j.0013-9580.2004.37803.x. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Loscher W. Uncontrolled epilepsy following discontinuation of antiepileptic drugs in seizure-free patients: a review of current clinical experience. Acta Neurol Scand. 2005;111:291–300. doi: 10.1111/j.1600-0404.2005.00408.x. [DOI] [PubMed] [Google Scholar]
- Schneider A, Elias R. The Medicaid resource book. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002. [Google Scholar]
- Schneider A, Strohmeyer V, Ellberger R. Medicaids eligibility for individuals with disabilities. 2000 Retrieved December 11, 2012, from http://www.kff.org/medicaid/loader.cfm?url=/commonspot/security/getfile.cfm&PageID=13323.
- Specchio LM, Beghi E. Should antiepileptic drugs be withdrawn in seizure-free patients? CNS Drugs. 2004;18:201–212. doi: 10.2165/00023210-200418040-00001. [DOI] [PubMed] [Google Scholar]
- St Germaine-Smith C, Liu M, Quan H, Wiebe S, Jette N. Development of an epilepsy-specific risk adjustment comorbidity index. Epilepsia. 2011;52:2161–2167. doi: 10.1111/j.1528-1167.2011.03292.x. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R, Kroner B, Labiner D, Liow K, Logroscino G, Medina MT, Newton CR, Parko K, Paschal A, Preux PM, Sander JW, Selassie A, Theodore W, Tomson T, Wiebe S. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52 (Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]