Abstract
Reducing calorie intake extends the lifespan of a variety of experimental models and delays progression of age-related hearing loss (AHL). AHL is a common feature of aging and is characterized by age-related decline of hearing associated with loss of sensory hair cells, spiral ganglion neurons, and/or stria vascularis degeneration in the cochlea. Sirtuins are a family of NAD+-dependent enzymes that regulate lifespan in lower organisms and have emerged as broad regulators of cellular fate. Our recent study indicated that mitochondrial Sirt3, a member of the sirtuin family, mediates the anti-aging effects of calorie restriction (CR) on AHL in mice. Interestingly, we also found that weight loss alone may not be sufficient for maintaining normal hearing. How does CR slow the progression of AHL through regulation of Sirt3? Here we review the evidence that during CR, Sirt3 slows the progression of AHL by promoting the glutathione-mediated mitochondrial antioxidant defense system in mice. A significant reduction in food consumption in one’s daily life may not be a desirable and realistic option for most people. Therefore, identification/discovery of compounds that induce the activation of SIRT3 or glutathione reductase, or that increase mitochondrial glutathione levels has potential for maintaining good hearing through mimicking the anti-aging effects of CR in human inner ear cells.
Keywords: Sirtuin, Oxidative stress, ROS, Glutathione
1. Introduction
1.1. CR and aging
Calorie restriction (CR) is the only intervention to slow the rate of aging and to extend lifespan in diverse species (Fontana et al., 2010; Weindruch & Walford, 1988). CR also delays the onset of age-related diseases such as diabetes, cancer, cardiovascular disease, Parkinson’s disease, Alzheimer’s disease, and age-related hearing loss in rodents and humans (Fontana et al., 2010; Someya et al., 2010; Weindruch & Walford, 1988). In monkeys, two studies found that CR reduces the incidence of obesity, diabetes, and tumor (Colman et al., 2009; Mattison et al., 2012). Importantly, CR reduced age-related mortality in these long-lived primates (Colman et al., 2009). In mice, CR results in significant reduction of the levels of oxidative protein damage in aged brains, hearts, and livers (Balaban et al., 2005; Weindruch & Walford, 1988). Although research on the anti-aging effects of CR in humans is still at an early stage, CR reduces obesity incidence and levels of cholesterol, blood pressure, oxidative stress, and inflammation, and increases insulin resistance in humans (Fontana et al., 2010). How does CR slow the rate of aging? The Mitochondrial Free Radical Theory of Aging postulates that aging and age-related diseases result from accumulated oxidative damage caused by reactive oxygen species (ROS), originating from the mitochondria (Balaban et al., 2005). In agreement with this theory, overexpression of the mitochondrial antioxidant enzyme Sod2 (superoxide dismutase 2) significantly increases the lifespan of Drosophila (Sun et al., 2002), while overexpression of a mitochondrially-targeted catalase transgene results in reduced age-related pathology in the heart and increases lifespan in mice (Schriner et al., 2005). There are two major antioxidant defense systems in mitochondria: the glutathione and thioredoxin systems (Halliwell & Gutteridge, 2007; Mari et al., 2009). Glutathione acts as the major antioxidant in cells and is found mostly in the reduced form in healthy mitochondria from young mice (Mari et al., 2009). Consistent with these reports, aging results in a decreased ratio of reduced glutathione (GSH):oxidized glutathione (GSSG) in the mitochondria of brain, heart, eye, and testis from aged mice, while CR prevents the decline (Rebrin & Sohal, 2008). Yet, whether the anti-aging action of CR in the sensory organs such as inner ear is a regulated process and/or is associated with the mitochondrial antioxidant defense pathways still remains unclear.
1.2. CR and AHL
It is well documented that hearing gradually declines during aging in laboratory animals as well as humans (Gates & Mills, 2005), and as such, age-related hearing loss (AHL) is a robust marker and common feature of mammalian aging. AHL or presbycusis is generally classified into three types based on the relationship between cochlear pathology and hearing levels: sensory hearing loss (loss of sensory hair cells), neuronal hearing loss (loss of spiral ganglion neurons), and metabolic hearing loss (stria atrophy) (Gates & Mills, 2005; Schuknecht, 1955), and most cases of AHL exhibit a mixture of these pathological changes (Gates & Mills, 2005). Evidence indicates that cochlea from aged C57BL/6 and CBA/J mice displays loss of spiral ganglion neurons and hair cells, and increased oxidative damage in the cochlea (Jiang et al., 2007; Someya et al., 2009), while overexpression of catalase in mitochondria reduces loss of hair cells and spiral ganglion neurons and oxidative DNA damage in the cochlea, and slows the progression of AHL in mice (Someya et al., 2009). Therefore, AHL is thought to result from accumulated oxidative damage in cochlear cells caused by ROS, originating from the mitochondria (Fig. 1) (Someya & Prolla, 2010). Our recent study indicated that mitochondrial Sirt3, a member of the sirtuin family, mediates the anti-aging effects of CR on AHL by promoting the glutathione-mediated mitochondrial antioxidant defense system in mice (Someya et al., 2010). How does CR slow the progression of AHL through the regulation of Sirt3? Can we maintain our good hearing by simply reducing calorie intake? To answer these questions, we review the roles of mitochondrial Sirt3 in regulating mitochondrial metabolism, the roles of mitochondrial glutathione in protecting cells against oxidative stress, and the evidence that during CR, Sirt3 slows the progression of AHL by promoting the glutathione-mediated mitochondrial antioxidant defense system in mice.
Fig. 1.
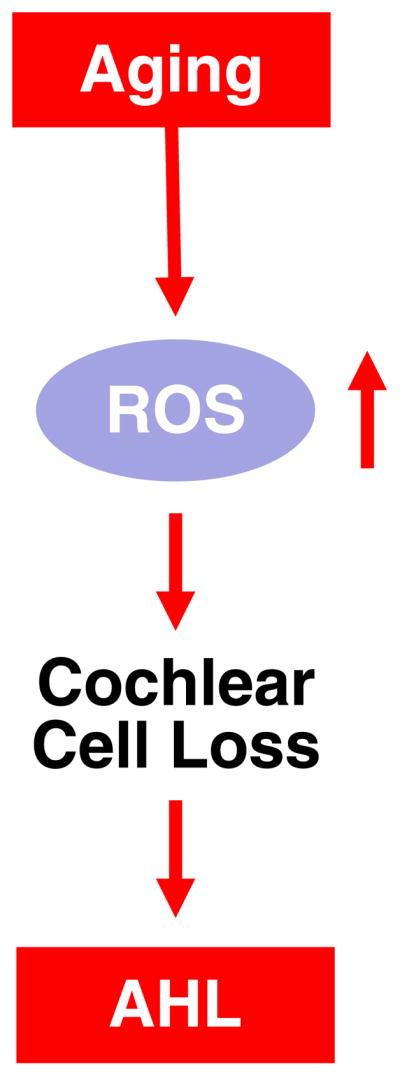
A model for AHL.
2. Mitochondrial sirtuin: Sirt3
2.1. Mitochondria and sirtuins
Lin et al. uncovered Sir2, a member of the sirtuin family and NAD+-dependent deacetylases, is essential for the extension of lifespan by CR in yeasts (Lin et al., 2004), implicating a link between sirtuins and the anti-aging action of CR. Subsequent studies in Caenorhabditis elegans and Drosophila also identified Sir2 homologues as a determinant of lifespan, and showed that CR increases Sir2 levels in these organisms (Finkel et al., 2009). So far, seven sirtuins have been identified in humans, and of these, three sirtuin proteins, SIRT3, SIRT4, and SIRT5, are found to be mitochondrial (Finkel et al., 2009). In mice, both Sirt3 and Sirt5 function as a deacetylase, while Sirt4 acts as an ADP-ribosyltransferase (Finkel et al., 2009; Someya et al., 2010). These sirtuins modify mitochondrial proteins and are thought to regulate mitochondrial metabolic pathways.
2.2. CR and Sirt3
Bellizzi et al. (2005) found that a polymorphism associated with reduced levels of SIRT3 mRNA expression was absent in males older than 90 years of age. Sedentary old human individuals displayed lower SIRT3 protein levels in the mitochondria of the skeletal muscle, while endurance-trained individuals displayed elevated SIRT3 protein levels in the mitochondria regardless of age (Lanza et al., 2008). In mice, Sirt3 regulates electron transport chain complex I activity and ATP levels (Ahn et al., 2008), while Hirschey et al. revealed that Sirt3 modulates mitochondrial fatty acid oxidation (Hirschey et al., 2010). Cardiac hypertrophy is a common age-associated disease, caused by hypertension (Pillai et al., 2010). Previous studies have shown that Sirt3-deficient mice display mild cardiac hypertrophy and interstitial fibrosis by 8 weeks of age, while Sirt3 blocks hypertrophy by deacetylating the FoxO3A forkhead transcription factor, which in turn induces FoxO3A nuclear localization and increases expression of the antioxidant enzymes such as catalase and Sod2 (Pillai et al., 2010). Moreover, cardiac hypertrophy was associated with a decrease in intracellular levels of NAD+, while exogenous addition of NAD+ blocked cardiac hypertrophy in vitro as well as in vivo (Pillai et al., 2010), suggesting an important role of Sirt3 in preventing age-associated cardiac disorder. A recent report has shown that fasting or CR upregulated Sirt3 proteins in the skeletal muscle of mice (Pillai et al., 2010). CR also increased expression of Sirt3 proteins in primary mouse cardiomyocytes, whereas overexpression of Sirt3 protected these cells from oxidative stress-induced cell death (Pillai et al., 2010). Furthermore, Qiu et al. found that Sirt3 reduces oxidative stress by activating mitochondrial Sod2 under CR conditions (Qiu et al., 2010). Together, these findings provide evidence linking Sirt3, CR, and antioxidant defenses.
3. Glutathione-mediated antioxidant defense system
3.1. Mitochondrial glutathione and ROS
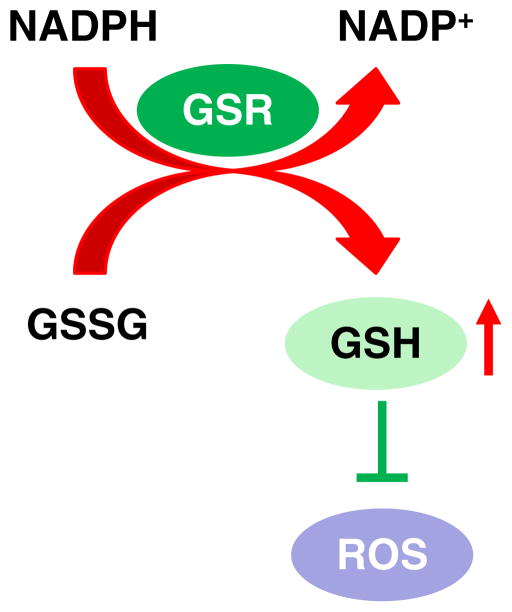
Mitochondria are the major source of reactive oxygen species (ROS) (Balaban et al., 2005). An elaborate antioxidant system has evolved to control the damaging effects of ROS. The system includes the antioxidant enzymes SOD, catalase, GPX (glutathione peroxidase), and GSR (glutathione reductase), and a variety of small-molecule antioxidants such as glutathione and thioredoxin (Halliwell & Gutteridge, 2007). In mitochondria, glutathione acts as the major antioxidant protecting key mitochondrial components such as mitochondrial DNA, enzymes, and membranes from ROS-induced damage (Halliwell & Gutteridge, 2007; Mari et al., 2009). The majority of intracellular ROS are continuously generated as a by-product of electron transport respiration metabolism (Balaban et al., 2005; Halliwell & Gutteridge, 2007). These ROS include superoxide ( ) and hydroxyl radical (•OH) and hydrogen peroxide (H2O2). In the mitochondrial matrix, SOD2 converts superoxide into hydrogen peroxide, which is then decomposed to water by glutathione peroxidase using GSH as a substrate (Halliwell & Gutteridge, 2007; Mari et al., 2009). GSSG is then regenerated to GSH by GSR (Halliwell & Gutteridge, 2007; Mari et al., 2009) (Fig. 2). Thus, GSR plays a critical role in maintaining the mitochondrial GSH/GSSG redox state and reducing mitochondrial ROS levels in cells (Fig. 2).
Fig. 2.
Control of ROS by GSR. NADPH-dependent glutathione reductase (GSR) regenerates reduced glutathione (GSH) from oxidized glutathione (GSSG). This leads to reduced levels of ROS.
3.2. Glutathione and aging
In healthy mitochondria from young mice, glutathione is found mostly in the reduced form (Mari et al., 2009) and during aging, the ratio of GSH:GSSG, or the glutathione redox state, declines in the mitochondria of brain, heart, eye, and testis of mice (Rebrin & Sohal, 2008). In contrast, CR is known to increase the ratio of GSH:GSSG in the mitochondria of these tissues (Rebrin & Sohal, 2008), revealing that GSH may play a role in the CR-mediated pathways. Glutathione also plays a role in mitochondrial apoptosis that is regulated by Bcl-2 family members (Mari et al., 2009). The evidence indicates that mitochondrial GSH depletion triggers mitochondrial permeabilization and the activation of caspases, leading to apoptosis (Mari et al., 2009). Because the abundance of the GSH/GSSG redox couple (a redox couple is a reducing species (GSH) and its corresponding oxidized form (GSSG) interconvertible by means of an oxidation-reduction reaction) is three to four orders of magnitude higher than the other redox couples such as NADPH/NADP+, NADH/NAD+, and reduced thioredoxin/oxidized thioredoxin, it is thought that the GSH/GSSH couple is the primary cellular determinant of the cellular redox state, and that a decline in the GSH/GSSG redox status during aging may affect the overall intracellular redox environment as well as cell viability (Rebrin & Sohal, 2008). In agreement with this hypothesis, one of the earliest biochemical symptoms in Parkinson’s disease is total GSH depletion (Perry and Yong 1986), while brain GSH elevation by treatment of GSH-ethyl ester protected neuronal cells against dopamine loss in the Parkinson’s disease rat model (Zeevalk et al., 2005). Collectively, these findings indicate that a strategy to increase mitochondrial GSH levels could be beneficial in slowing the development of age-related neurodegenerative diseases.
4. Link between CR, Sirt3, and GSH
4.1. Sirt3 slows the development of AHL under CR
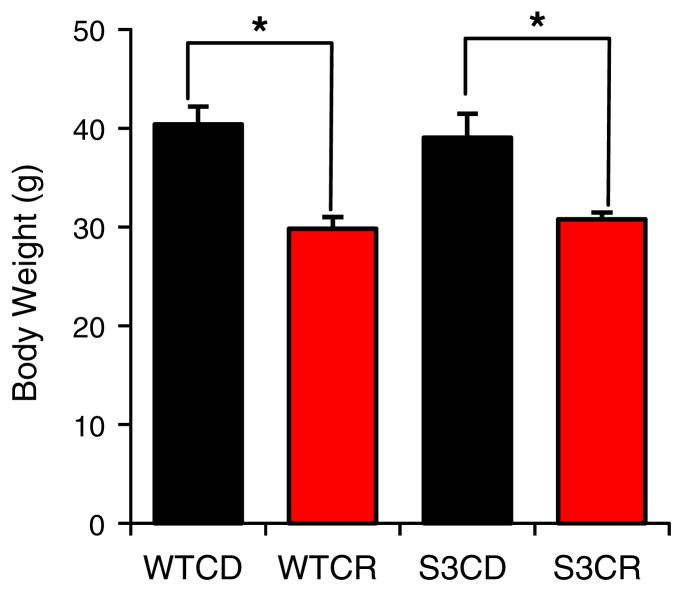
We have recently shown that during CR, mitochondrial Sirt3 slows the progression of AHL by promoting the glutathione-mediated mitochondrial antioxidant defense system (Someya et al., 2010): using Sirt3 knockout (KO) and WT mice, we conducted a 10 month CR dietary study (a 25% CR) and measured hearing levels in these animals by the auditory brainstem response (ABR) analysis. As expected, aging resulted in increased ABR hearing thresholds in WT mice on control diets. Surprisingly, CR did not delay the progression of AHL in Sirt3 KO mice, even though CR delayed the onset of AHL in WT mice, indicating that Sirt3 is required for the CR-mediated prevention of AHL. Another interesting finding was that CR failed to slow the development of AHL in Sirt3 KO mice despite the fact that these animals displayed significant reduction of body weight: there was no difference in body weight between calorie restricted WT and Sirt3 KO mice (Fig. 3). The hearing test results were confirmed by histological observations that CR reduced loss of hair cells and spiral ganglion neurons in the cochlea of WT mice, but not in Sirt3 KO mice. We then found that CR reduced oxidative DNA damage, increased the ratios of mGSH:GSSG, and decreased mGSSG levels in cochlea of 5-month-old WT mice, but not in age-matched Sirt3 KO mice. Therefore, during CR, Sirt3 can protect cochlear cells from oxidative stress and maintains hearing by enhancing the GSH-mediated mitochondrial antioxidant defense system.
Fig. 3.
Body weight. The body weight of the mice was measured in control diet WT (WTCD) and Sirt3 KO (S3CD), and calorie-restricted WT (WTCR) and Sirt3 KO (S3CR) mice at 12 months of age. *Significantly different from control diet mice (P<0.05) (Someya et al., 2010).
NADP+-dependent Idh2 from mitochondria converts NADP+ to NADPH, thereby promoting regeneration of GSH by supplying NADPH to NADPH-dependent GSR, which converts GSSG to GSH, the active form of glutathione (Halliwell & Gutteridge, 2007; Yu et al., 2012) (Fig. 2). Our study using Western blotting, followed by immunoprecipitation revealed that Sirt3 regulated Idh2 activity through deacetylation (Someya et al., 2010). CR also increased Idh2 activity and NADPH levels in mitochondria of the cochlea from WT mice, but not from Sirt3 KO mice, suggesting that during CR, Sirt3 induces deacetylation and activation of Idh2 in the mitochondria of cochlea (Fig. 4). Together, these findings provide evidence that the maintenance of normal hearing by CR requires Sirt3 in mammals.
4.2. Cdh23 mutation and AHL
We note that these CR studies were conducted using Sirt3 KO mouse in the background of the C57BL/6J strain, which carries a specific mutation in the cadherin 23 gene (Cdh23753A) that encodes a component of the hair cell tip link (Someya & Prolla, 2010). Because this mutation is known to promote early onset of AHL in C57BL/6J mice, it is thought that hair cells in C57BL/6J mice may be more susceptible to oxidative stress during aging. However, oxidative damage increases with age in the cochlea of both the C57BL/6J and CBA/J strains (Jiang et al., 2007; Someya et al., 2009), which does not carry the Cdh23753A mutation (Jiang et al., 2007). We therefore speculate that the Cdh23753A allele affects age of onset of AHL, but the basic mechanisms of cochlear aging are similar in both C57BL/6J and CBA/J strains, as far as the basic roles of oxidative stress in AHL.
5. Conclusions
5.1. Do ROS play an essential role in the CR-mediated prevention of AHL?
It is widely accepted that ROS plays a role in aging and age-related disease and that the Free Radical Theory of Aging is a popular explanation of how we lose our hearing during aging. Yet, recent studies have shown that overexpression of the antioxidant enzymes such as cytosolic Sod1, mitochondrial Sod2, or catalase does not extend the lifespan of mice (Pérez et al., 2009), conflicting with the Free Radical Theory of Aging and our previous finding that overexpression of Cat in mitochondria slowed the progression of AHL in mice (Someya et al., 2009). A possible explanation for these contradicting results is that ROS may not play a role in the aging process, but in the pathogenesis of age-related disorders, including AHL. Most of the antioxidant enzyme studies discussed in this review support the idea that alterations in ROS levels contribute to the development of AHL. Yet, the question still remains to what degree the age-related increase in ROS production and/or the age-related decline in antioxidant defenses in the cochlea contribute to the progression of AHL in mammals.
5.2. Does CR extend lifespan and/or healthspan in primates?
A new study on calorie restricted rhesus monkeys at the National Institute on Aging revealed that a simple decrease in calorie intake has no significant effect on the monkey’s longevity (Mattison et al., 2012). This finding contradicts the previous report from the Wisconsin National Primate Research Center (WNPRC)/University of Wisconsin that CR reduced age-related mortality in rhesus monkeys; 13% of the CR group died from age-related diseases compared to with 37% of the control diet group (Colman et al., 2009). However, the two long-lived primate studies found remarkably similar health benefits of CR. Both studies indicated that calorie restricted rhesus monkeys show reduced incidence of age-related obesity, diabetes, and tumor (Colman et al., 2009; Mattison et al., 2012), suggesting that CR does improve healthspan (the period when a person or animal is fully fit and healthy), but may not extend lifespan in long-lived primates such as monkeys and humans. It is therefore likely that CR can reduce incidence of AHL, a common age-related disorder, in monkeys and humans. We postulate that aging and age-related disease research will start focusing more on healthspan in the next decade.
5.3. Does the anti-aging action of CR require significant reduction of body weight?
As discussed above, recent works have demonstrated that reducing calorie intake and/or staying lean can substantially increase healthspan of experimental animals as well as humans (Fontana et al., 2010; Weindruch & Walford, 1988). It is therefore thought that the anti-aging effects of CR require significant reduction of body weight through reducing food consumption. This hypothesis is supported by a number of reports that obesity promotes a variety of age-related diseases, such as diabetes and high blood pressure (Paeratakul et al., 2002). Is significant reduction of body weight sufficient to maintain normal hearing? Surprisingly, our Sirt3 study revealed that CR failed to slow the development of AHL in Sirt3 KO mice despite the fact that these animals displayed significant reduction of body weight (Someya et al., 2010) (Fig. 3). Therefore, weight loss alone may not be sufficient for maintaining normal hearing. We speculate that the maintenance of normal hearing by CR is a regulated process and requires specific regulatory proteins such as SIRT3 in humans.
5.4. Future perspectives in AHL research
If maintaining normal hearing by CR requires specific regulatory proteins such as SIRT3, it is then likely that a pharmaceutical compound that induces SIRT3 activation has potential for maintaining normal hearing in humans. This idea is supported by findings that Sirt3 is required for the CR-mediated prevention of AHL in mice (Someya et al., 2010) and that a SIRT3 polymorphism has been associated with human longevity (Bellizzi et al., 2005). Glutathione is a critical factor in the control of cell survival as a number of studies show that mitochondrial glutathione depletion results in increased cellular damage and cell death (Mari et al., 2009). In agreement with these reports, centenarians have higher glutathione reductase activity in red blood cells in comparison with young healthy adults (Andersen et al., 1998; Klapcinska et al., 2000), while mitochondrial glutathione levels decline in multiple tissues of mice during aging (Rebrin & Sohal, 2008). Our recent findings indicate that CR slows the development of AHL by promoting mitochondrial antioxidant defense through the regulation of Gsr (Someya et al., 2010). This could also explain our previous finding that overexpression of catalase in mitochondria slowed the progression of AHL, as the activity of mitochondrial catalase would prevent the consumption of GSH otherwise required by glutathione peroxidase in the reduction of mitochondrial hydrogen peroxide (Someya et al., 2009). We therefore postulate that delaying the onset of AHL by targeting a single gene or protein in a single molecular pathway is possible, and that identification/discovery of compounds that activate SIRT3 or GSR, or that increase mitochondrial GSH levels has the potential to maintain normal hearing through mimicking the anti-aging effects of CR in the human inner ear cells.
Acknowledgments
S.S. is supported by the NIH/NIDCD (1R03DC011840-01) and the American Federation for Aging Research Foundation.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Jeune B, Nybo H, Nielsen JB, Andersen-Ranberg K, Grandjean P. Low activity of superoxide dismutase and high activity of glutathione reductase in erythrocytes from centenarians. Age Ageing. 1998;27:643–648. doi: 10.1093/ageing/27.5.643. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford; New York: 2007. [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapcinska B, Derejczyk J, Wieczorowska-Tobis K, Sobczak A, Sadowska-Krepa E, Danch A. Antioxidant defense in centenarians (a preliminary study) Acta Biochim Pol. 2000;47:281–292. [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012 doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord. 2002;26:1205–1210. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Jeevanandam V, Gupta MP. Mitochondrial SIRT3 and heart disease. Cardiovasc Res. 2010;88:250–256. doi: 10.1093/cvr/cvq250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Sohal RS. Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev. 2008;60:1545–1552. doi: 10.1016/j.addr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Presbycusis. Laryngoscope. 1955;65:402–419. [Google Scholar]
- Someya S, Prolla TA. Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev. 2010;131:480–486. doi: 10.1016/j.mad.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. C.C. Thomas; Springfield, Ill., U.S.A: 1988. [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J. Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal. 2005;7:1117–1139. doi: 10.1089/ars.2005.7.1117. [DOI] [PubMed] [Google Scholar]