Abstract
Thiamine deficiency (TD) causes mild impairment of oxidative metabolism and region-selective neuronal loss in the brain, which may be mediated by neuronal oxidative stress, endoplasmic reticulum stress, and neuroinflammation. TD-induced brain damage is used to model neurodegenerative disorders, and the mechanism for the neuronal death is still unclear. We hypothesized that autophagy might be activated in the TD brain and play a protective role in TD induced neuronal death. Our results demonstrated that TD induced the accumulation of autophagosomes in neurons of the thalamus measured by transmission electron microscopy, and the upregulation of autophagic markers: LC3-II, Atg5 and Beclin1 as measured with western blotting. TD also increased the expression of autophagic markers and induced LC3 puncta in SH-SY5Y neuroblastoma cells. TD-induced expression of autophagic markers was reversed once thiamine was re-administered. Both inhibition of autophagy by wortmannin and Beclin1 siRNA potentiated TD-induced death of SH-SY5Y cells. In contrast, activation of autophagy by rapamycin alleviated cell death induced by TD. Intraperitoneal injection of rapamycin stimulated neuronal autophagy and attenuated TD-induced neuronal death and microglia activation in the submedial thalamus nucleus (SmTN). TD inhibited the phosphorylation of p70S6 kinase, suggesting mTOR/p70S6 kinase pathway was involved the TD-induced autophagy. These results suggest that autophagy is neuroprotective in response to TD-induced neuronal death in the central nervous system. This opens a potential therapeutic avenue for neurodegenerative diseases caused by mild impairment of oxidative metabolism.
Keywords: Autophagy, oxidative stress, neurodegeneration, thalamus, vitamin B1
Introduction
Autophagy is a conserved process that sequesters long-lived proteins and dysfunctional organelles, and transports them to lysosomes for degradation to maintain cellular homeostasis. There are three types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) (Klionsky 2007, Yang & Klionsky 2010). The autophagy described in this study refers to macroautophagy. Autophagy dysfunction has been indicated in some age-related neurodegenerative diseases, such as Alzheimer’s disease (AD) (Yu et al. 2005), Huntington’s disease (HD) (Ravikumar et al. 2004), Parkinson’s disease (PD) (Lin et al. 2012), and amyotrophic lateral sclerosis (ALS) (Chen et al. 2012b). In these diseases, the accumulation of mis-folded or aggregated proteins over time is frequently observed. Autophagic response is declining during the aging process (Martinez-Vicente et al. 2005). Deletion of the key autophagy genes in neurons induces neurodegeneration, and accumulates inclusion bodies in mice (Hara et al. 2006, Komatsu et al. 2006).
Thiamine-dependent enzymes, such as pyruvate dehydrogenase complex (PDHC, EC 1.2.4.1, EC 2.3.1.12, EC 1.6.4.3), and α-ketoglutarate dehydrogenase complex (KGDHC, EC 1.2.4.2, EC 2.3.1.61, EC 1.6.4.3), play important roles in glucose metabolism. Thiamine (vitamin B1) deficiency (TD) causes damage to the thiamine-dependent enzymes and the normal function of the mitochondria, and induces energy shortage, and chronic oxidative stress (Ke & Gibson 2004, Zhang et al. 2011). Wernicke's encephalopathy (WE) is a neuropsychiatric syndrome associated with TD in humans which is characterized by opthalmoplegia, ataxia, and memory loss (Ke et al. 2003, Rao et al. 1996, Todd & Butterworth 1999). Brain tissue from WE patients indicates gliosis, vascular damage, and neuronal loss in focal regions of the brain, such as the mammillary bodies, inferior olive, and thalamus (Gibson et al. 1999, Kril 1996, Pannunzio et al. 2000, Troncoso et al. 1981). TD induces chronic oxidative stress, inflammatory responses, selective neuronal death, and glial activation in the submedial thalamic nucleus (SmTN) in mice (Gibson et al. 1999, Ke & Gibson 2004, Yang et al. 2011). TD in animals has been extensively used to study the mechanisms of neurodegeneration since it shares common pathological features, such as selective neuronal death and microglial activation, with some aging-associated degenerative diseases, such as AD, PD, and progressive supranuclear palsy (Gibson & Zhang 2001, Park et al. 2001, Schwab et al. 1996).
In this study, we demonstrated that TD up-regulated the expression of protein markers for autophagy and increased the accumulation of autophagic vacuoles in vitro and in vivo; it also caused the accumulation of autophagosomes in neurons in the thalamus which was measured by transmission electron microscopy. Down-regulation of autophagy by wortmannin or the treatment of a siRNA targeting Beclin1 exacerbated TD-induced cell death, while activation of autophagy by rapamycin reduced TD-induced neurodegeneration. These results suggested that autophagy was a neuroprotective mechanism in response to mild impairment of oxidative metabolism.
Materials and Methods
Materials
Annexin V-FITC Apoptosis Detection Kit (K203-400) was obtained from Biovision Inc. (Mountain View, CA, USA). Anti-p70S6K antibody (sc230) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GAPDH antibody (kc-5G5) was obtained from Kangcheng Bio-tech (Shanghai, China). Anti-NeuN antibody (MAB377) was obtained from Chemicon (Temecula, CA, USA). Anti-LC3 (2775S), Beclin1 (3495s), Atg5 (2630), and p-P70S6K (9204s) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Anti-IbaI antibody (019-19741) was obtained from Wako Chemicals (Cambridge, MA, USA). Pyrithiamine hydrobromide (P0256), leupeptin (L9783), pepstatin A (P5318), rapamycin (R0395), wortmannin (W3144), 3, 3’-diaminobenzidine (DAB, D5637), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, m2128) were obtained from Sigma Chemical (St. Louis, MO, USA). All culture dishes, plates, and flasks were obtained from Corning (Corning, NY, USA).
Cell cultures and treatment
Human neuroblastoma SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, 12100-046, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Hyclone, SV30087.02), 100 U/ml streptomycin and 100 U/ml penicillin G (Invitrogen, GB15140-122). Thiamine deficiency (TD) was induced as previously described (Wang et al. 2007, Yang et al. 2011, Zhang et al. 2011). Briefly, SH-SY5Y cells were maintained in a thiamine deficient DMEM (JRH Biosciences Inc. 12100046, Lenexa, Kansas, USA) with the same supplements above and exposed to a thiamine antagonist pyrithiamine hydrobromide (300 nmol/L). In some experiments, thiamine was re-administrated after 6 days of TD treatment (6R). Leupeptin (20 µM) and pepstatin A (20 µM) were used to block lysosome-mediated degradation. Cells were incubated with leupeptin and pepstatin A for 24 hours before harvesting. Rapamycin (10 nM) and wortmannin (50 nM) were used to manipulate autophagic pathways. Rapamycin or wortmannin were added into the culture medium at the same time with pyrithiamine for 5 days.
Immunoblotting
The procedure for immunoblotting has been previously described (Wang et al. 2007, Yang et al. 2011, Zhang et al. 2009). Briefly, aliquots of the proteins (40 µg) were loaded into the lanes of a sodium dodecyl sulfate polyacrylamide gel. The proteins were separated by electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in 0.01M phosphate buffer saline (PBS, pH 7.4) and 0.05% Tween-20 at room temperature for 1 h. Subsequently, the membranes were incubated with primary antibodies directed against target proteins overnight at 4°C, followed by the appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham, Arlington Heights, IL, USA). The expression was detected by the enhanced chemiluminescence method (Amersham, GRPN2106). The density of immunoblotting was determined by the software of Quantity One (Bio-Rad Laboratories, Hercules, CA, USA).
Cell transfection
The GFP-LC3 plasmid was kindly provided by Dr. Isidoro (Trincheri et al. 2008). Plasmid transfection was performed using Lipofectamine 2000 (Invitrogen, 11668019) according to the manufacturer’s instructions. SiRNA oligonucleotides were purchased from GenePharma (Shanghai, China) and their sequences are as follows:
siBeclin1 sense, 5’- GAGCUGCCGUUAUACUGUUCUGGTT-3’
siBeclin1 antisense, 5’-CCAGAACAGUAUAACGGCAGCUCTT-3’
siControl sense, 5’-UUCUCCGAACGUGUCACGUTT-3’
siControl antisense, 5’-ACGUGACACGUUCGGAGAATT -3’
siRNAs transfection was performed using Oligofectamine (Invitrogen, 12252-011) according to the manufacturer’s instructions. SH-SY5Y cells were transfected with GFP-LC3 plasmid or siRNA for 12 hours, and then treated with pyrithiamine for 5 days.
Quantification of LC3-GFP puncta
GFP-LC3 puncta per cell were quantified as previously described (Wu et al. 2010). Briefly, SH-SY5Y cells were transfected with GFP-LC3 plasmid for 12 hours. Then, cells were cultured on coverslips and received various treatments. Cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2). The coverslips were examined under a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY, USA). The GFP-LC3 puncta within a single cell were manually counted. For each treatment group, GFP-LC3 puncta were counted in 40 cells that were randomly selected, and the mean of the number was presented.
Apoptosis and cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl- tetrazolium bromide (MTT) method as previously described (Wang et al. 2007, Zhang et al. 2011). Apoptotic cell death was determined using an Annexin-V FITC apoptosis detection kit as previously described (Wang et al. 2007, Yang et al. 2011, Zhang et al. 2011). Briefly, 2.0×105 cells were resuspended in 0.5 ml binding buffer and incubated with Annexin-V FITC and propidium iodide (PI) for 5 min in the dark at room temperature, and then detected in FACScan cytometer (BD Biosciences, San Jose, CA, USA). A FITC signal detector FL1 (excitation 488 nm, green) was used to analyze the PI signal, and a phycoerythrin emission signal detector FL2 (excitation 585 nm, red) was used to analyze the Annexin-V signal. The relative amount of apoptotic cells was determined from 2×104 using the Cellquest program (BD Biosciences).
Induction of TD in animals
Adult male C57BL/6J mice (20–25 g) were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. The procedure for animal surgery was performed in accordance with the Guidelines of Animal Care and Use Committee of the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences (CAS). Every effort was made to minimize the discomfort of animals. TD was induced in animals as previously described (Ke et al. 2003, Wang et al. 2007, Yang et al. 2011). Briefly, mice were housed in a controlled environment (one mouse/cage at 23°C and 53% humidity). The animals were fed with either a control diet (MP Biomedicals, 960420, Santa Ana, CA, USA) or a thiamine deficient diet (MP Biomedicals, 960165) ad libitum. TD animals also received a daily intraperitoneal injection of a thiamine antagonist pyrithiamine hydrobromide (5 µg/10g body weight) while control animals were injected with saline. Rapamycin was dissolved in DMSO (10 mg/ml), and diluted immediately before injections with normal saline (1:200). To activate autophagy, some animals received intraperitoneal injection of rapamycin (0.5 mg/kg body weight) on the day 4 and day 7 of TD as previously described (Erlich et al. 2007), while other animals injected with DMSO diluted with normal saline (1:200, 0.5 mg/kg body weight). There were five animals for each group.
Sample collection
For immunoblotting analysis, brain tissues were isolated, frozen in liquid nitrogen and stored at −80 °C for later analysis. Proteins were extracted with a previously described method (Yang et al. 2011, Zhang et al. 2011). Briefly, brain tissues were homogenized in an ice-cold lysis buffer containing 5 mM EDTA, 0.5% NP-40, 0.1% triton-100, 0.1% SDS, 10 mg/ml PMSF, 10 µg/ml leupeptin and 100 mM sodium orthovanadate in PBS. The supernatant fraction was collected after centrifugation. For immunohistochemical analysis, brains were extracted with a previously described method (Yang et al. 2011). Briefly, mice were injected with chloral hydrate (500 mg/kg) and perfused with 10 ml of saline, followed by 100 ml of 4% paraformaldehyde in 0.1 M PB, pH 7.2 (Ke et al. 2003, Yang et al. 2011). The brains were removed and post-fixed overnight, and then transferred to 30% sucrose for 24 hours. The brain block containing thalamus was dissected on a Rodent Brain Matrix (ASI Instruments, Warren, MI, USA) and sectioned with a sliding microtome (Microm Laborgerate GmbH, Welldorf, Germany) at the thickness of 40 µm (Yang et al. 2011). Sections were collected from Bregma level 0.70 to −2.06 (Ke et al. 2005b, Yang et al. 2011). A total of 25 sections containing SmTN were obtained per mouse.
Transmission electron microscopy
The procedure for Transmission electron microscopy has been previously described (Wang et al. 2007). Briefly, the sections of the thalamus were fixed with 2% glutaraldehyde in PBS for 2 hours and then with 2% OsO4 in PBS for 2 hours. After fixation, they were dehydrated in a graded series of alcohol, and then flat-embedded in Epon 812. Thin sections were prepared using a LKB Ultrotome Nova ultramicrotome, doubly stained with uranyl acetate and lead citrate, and analyzed with a JEOL JEM1230 electron microscope.
Immunohistochemical and immunofluorescent staining
Immunohistochemical and immunofluorescent staining on the brain sections was performed as previously reported (Yang et al. 2011, Zhang et al. 2009). Every fifth section was used for immunohistochemical or immunofluorescent staining of LC3, Beclin1, NeuN or IbaI staining. As in our previous studies (Yang et al. 2011, Zhang et al. 2009), sections were incubated in PBS containing 3% H2O2 and 40% methanol for 30 min at room temperature and then treated with 0.1% Triton X-100 in PBS for 10 min. The sections were washed with 0.05 M KPBS three times, blocked with 1%BSA in PBS (pH 7.2) for 60 min, and then incubated with mouse anti-NeuN (1:1,000), rabbit anti-IbaI (1:1000) or mouse anti-GFAP (1:1000) in PBS containing 1% BSA overnight at 4°C. After rinsing in PBS, sections were treated with biotinylated anti-mouse or anti-rabbit IgG respectively (Invitrogen, 1:1,000 in PBS containing 1% BSA) for 2 hours. Sections were then incubated with avidin-biotin-peroxidase complex for 2 hours (Invitrogen, 1:1,000 in PBS containing 1% BSA), rinsed in PBS, and developed in 0.05% DAB with 0.003% H2O2 in KPBS.
For double immunofluorescence staining, sections were pre-treated with 0.05 M KPBS, pH 7.2 containing 1% BSA for 60 min, and then incubated overnight with rabbit anti-LC3 polyclonal antibody (1:200) or rabbit anti-Beclin1 polyclonal antibody (1:500) at 4°C. After rinsing in KPBS, sections were incubated with Alexa488-conjugated goat anti-rabbit-IgG (1:1,000, Invitrogen, A-11034) for 2 hours, rinsed in KPBS, and incubated with anti-NeuN monoclonal antibody (1:1,000) overnight at 4°C. After rinsing in KPBS, sections were treated with Alexa555-conjugated goat anti-mouse (1:1,000, Invitrogen, A-11029) for 2 hours. Immunofluorescence images were captured and recorded using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss MicroImaging Inc.).
Quantification of neuron number and IbaI immunoreactivity
The number of neurons in the SmTN was quantified by stereological analysis as previously described (Ke et al. 2005a, Yang et al. 2011). Olympus BX61 microscope with a monitored x-y-z stage linked to stereo Investigator software (Version 8, MicroBrightField, San Diego, CA, USA) was used for the quantification of neuron numbers. Briefly, five sections across the entire SmTN were used for neuron counting. The perimeter of the SmTN was outlined, and the thickness of sections was determined before counting. The NeuN-positive cells were counted using a 40× objective with a counting frame when they appeared in focus beneath the reference optical plane. The total numbers were calculated and taken as the product of the neuron density and volume of the SmTN.
Quantitative analysis of IbaI and GFAP immunoreactivity was performed using NIH Image J software. Images of SmTN were taken, and converted into an 8-bit format. Quantifications were performed by manual threshold adjustment. The sum of the values of the pixels in the selected region was measured and averaged by the area of selected region to generate the immunoreactivity value. The average immunoreactivity of five sections across the entire SmTN of each animal was calculated and normalized with control mice. Each group included five animals for the quantification of neuron number and IbaI immunoreactivity.
Statistical analysis
Data were expressed as the mean ± SEM. Differences among treatment groups were tested using analysis of variance (ANOVA). Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post-hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests. Statistical analysis was performed using the software Prism (GraphPad software).
Results
Thiamine deficiency (TD) induced autophagy in the thalamus
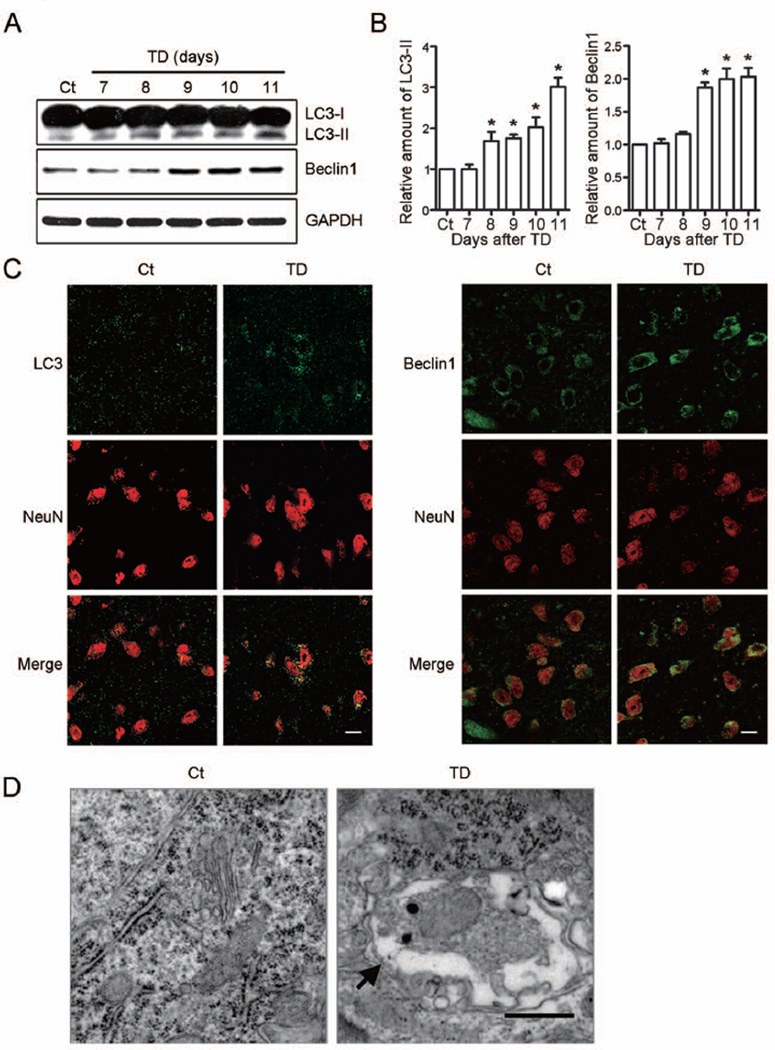
To determine whether TD activated autophagy, we evaluated the expression of LC3 and Beclin1 in the thalamus of mice after TD. LC3, a mammalian homolog of yeast Atg8, has two molecular forms: LC3-I (18 kDa) and LC3-II (16 kDa). The cytoplasmic LC3-I is processed and recruited to the autophagosomes, converting to LC3-II during autophagic activation. This characteristic conversion of LC3 is a common marker for monitoring autophagic activity (Kabeya et al. 2000, Klionsky et al. 2012, Xie et al. 2008). Beclin1, the homolog of yeast Vps30 (also known as Atg6), is a critical regulator of autophagosome maturation and endocytic trafficking (Liang et al. 2006, Liang et al. 2008, Takahashi et al. 2007). The accumulation of LC3-II increased after TD for 8 days, the increase reached to three times to the control after TD for 11 days (Figs. 1A and B). The expression of Beclin1 sharply increased after TD for 9 days, the protein of Beclin1 was doubled after TD for 10 days (Figs. 1A and B).
Fig. 1.
Thiamine deficiency (TD)-induced autophagy in the thalamus. (A) TD increased the expression of LC3-II and Beclin1 in the thalamus as determined by immunoblotting. (B) The relative amount of LC3-II and Beclin1 was quantified and normalized to the expression of GAPDH. The results were mean ± SEM (n = 5, *p < 0.05, compared with control group). (C) TD increases the immunoreactivity of LC3 and Beclin1 (green) in NeuN-positive neurons (red) in SmTN after TD for 9 days. Scale bar = 10 µm. (D) The autophagosomes within neurons in SmTN were revealed by electron microscopy (arrows) after TD for 10 days. Scale bar = 500 nm.
Double-immunofluorescence staining for LC3 and Beclin1 with neuronal marker NeuN showed that the expression of LC3 and Beclin1 increased in NeuN-positive neurons (Fig. 1C). Autophagosomes occurred in the neurons of SmTN after TD for 10 days under transmission electron microscopy (Fig. 1D). These data indicated that TD activated autophagy in neurons.
mTOR pathway was involved in TD-induced autophagic response
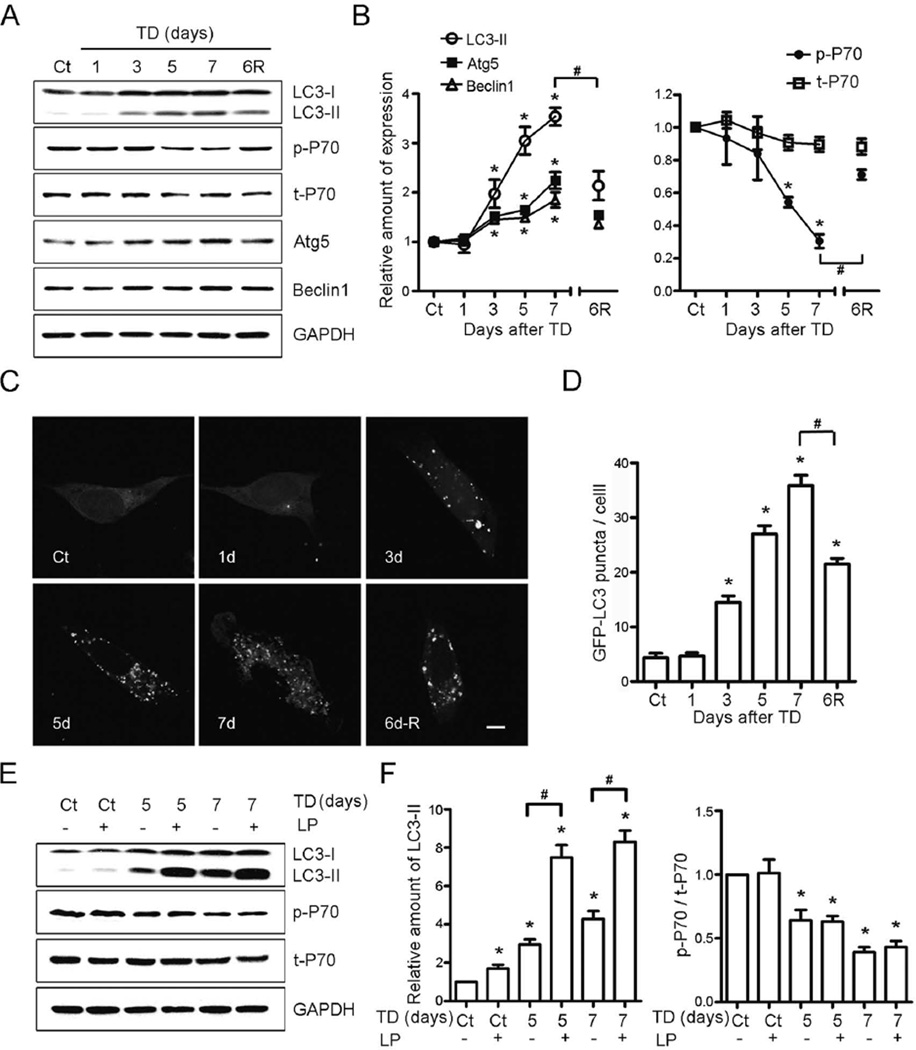
To explore the mechanism for TD-induced autophagy, we measured autophagic markers in a well-established in vitro model for TD (Yang et al. 2011). The expression of LC3-II, Beclin1 and Atg5 increased in neuroblastoma cells SH-SY5Y after TD for 3 days in vitro. The increase of the markers could be rescued when thiamine was re-administered after TD for 6 days (Figs. 2A and B). Autophagosome formation was measured with SH-SY5Y cells transfected with GFP-LC3 plasmid. The GFP-LC3-positive puncta occurred in SH-SY5Y cells after TD for 3 days, and the number of GFP-LC3-positive puncta increased seven times after TD for 7 days. The GFP-LC3-positive puncta were partly reversible by re-administration of thiamine after TD for 6 days (Figs. 2C and D).
Fig. 2.
TD-induced autophagy in SH-SY5Y neuroblastoma cells. (A) The expression of LC3, phosphorylated p70S6K (p-P70), p70S6K (t-P70), Atg5 and Beclin1 increased after TD for the indicated times as determined by immunoblotting; TD-induced alterations were reversed by re-administration of thiamine. (B) The relative amount of LC3-II, Atg5 and Beclin1 was quantified and normalized to the expression of GAPDH. p-P70 was quantified and normalized to the expression of t-P70. The results were means ± SEM of three replicates (*p < 0.05, compared with Ct; #p < 0.05, compared with TD7). (C) GFP-LC3 puncta were revealed by confocal microscopy for the indicated times. Scale bar = 5 µm. (D) GFP-LC3 puncta per cell were determined as described under the Materials and Methods. The results were means ± SEM of three replicates (*p < 0.05, compared with Ct; #p < 0.05, compared with TD7). (E) Leupeptin and pepstatin A (LP) increased the level of LC3-II, but had no effect on p-P70. (F) The relative amount of LC3 II was quantified and normalized to GAPDH. p-P70 was quantified and normalized to t-P70. The results were means ± SEM of three replicates (*p < 0.05, compared with controls; #p < 0.05, compared with TD5 or TD7 without LP).
Lysosomal protease inhibitors, leupeptin and pepstatin A, were used to block autophagic flux. Lysosomal protease inhibitors can inhibit the degradation of LC3-II (Kovacs et al. 1982, Takeshige et al. 1992, Tanida et al. 2005, Mizushima & Yoshimori 2007). The expression of LC3-II doubled when SH-SY5Y cells were treated with TD together with leupeptin and pepstatin A, suggesting that TD enhanced the formation of autophagosomes rather than inhibited LC3-II degradation (Figs. 2E and F). As mammalian target of rapamycin (mTOR) is a negative regulator of autophagy suppression of mTOR activates autophagy (Hosokawa et al. 2009, Jung et al. 2009). P70S6 kinase (p70S6K) is the substrate of mTOR, and its phosphorylation reflects the activity of mTOR (Wullschleger et al. 2006). TD decreased phosphorylation of p70S6K, but had no effect on the total p70S6K (Figs. 2A and B). The reduction of p70S6K phosphorylation caused by TD was almost totally rescued when thiamine was re-added to the culture media after TD for 6 days (Figs. 2A and B). Leupeptin and pepstatin A neither affected the basal phosphorylation of p70S6K nor TD-induced alterations of p70S6K phosphorylation (Figs. 2E and F). These data indicated that mTOR/p70S6K pathway was involved in autophagic response induced by TD.
Inhibition of autophagy potentiated TD-induced cell death
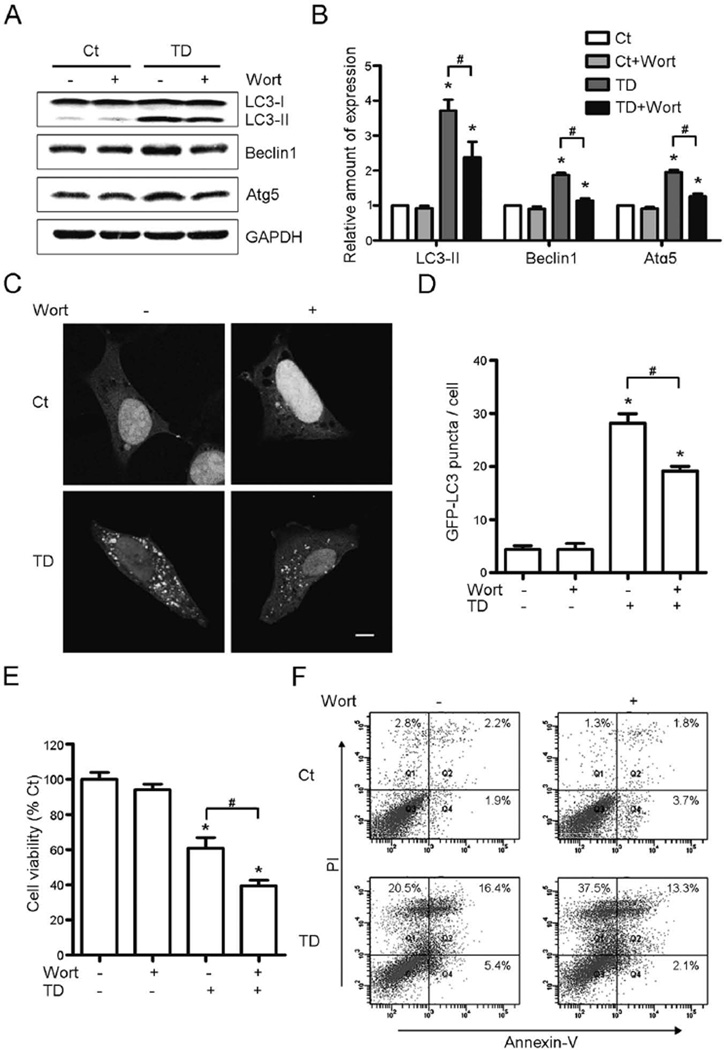
To detect the effect of autophagy on TD-induced cell death, we blocked autophagy with wortaminnin, a PI3-kinase inhibitor (Blommaart et al. 1997). Wortmannin blocked TD-induced up-regulation of markers for autophagy, Atg5, Beclin1, and LC3-II (Figs. 3A and B), and also reduced TD-enhanced formation of LC3 puncta (Figs. 3C and D). More importantly, TD-induced SH-SY5Y cell death increased 21.51% when wortmannin was added as measured by cell viability (Fig. 3E). The cell death (total percentage of Annexin-V positive and PI positive cells) increased from 37.33% to 51.83% (Fig. 3F).
Fig. 3.
Effect of wortmannin on TD-induced autophagy and cell death. SH-SY5Y cells were treated with pyrithiamine (0 or 300 nmol/L) and wortmannin (Wort, 0 or 50 nM) for 5 days. (A) Wortmannin decreased TD-induced upregulation of LC3-II, Beclin1 and Atg5. (B) The expression of LC3-II, Beclin1 and Atg5 was quantified and normalized to GAPDH. (C) Wortmannin decreased TD-induced upregulation of GFP-LC3 puncta. Scale bar = 5 µm. (D) The GFP-LC3 puncta per cell were counted. (E) Inhibition of autophagy by wortmannin promoted TD-induced cell death was determined by MTT assay. (F) The cell death was measured by Annexin-V/PI staining. All of the results were means ± SEM of three replicates (*p < 0.05, compared with Ct without wortmannin; #p < 0.05, compared with TD without wortmannin).
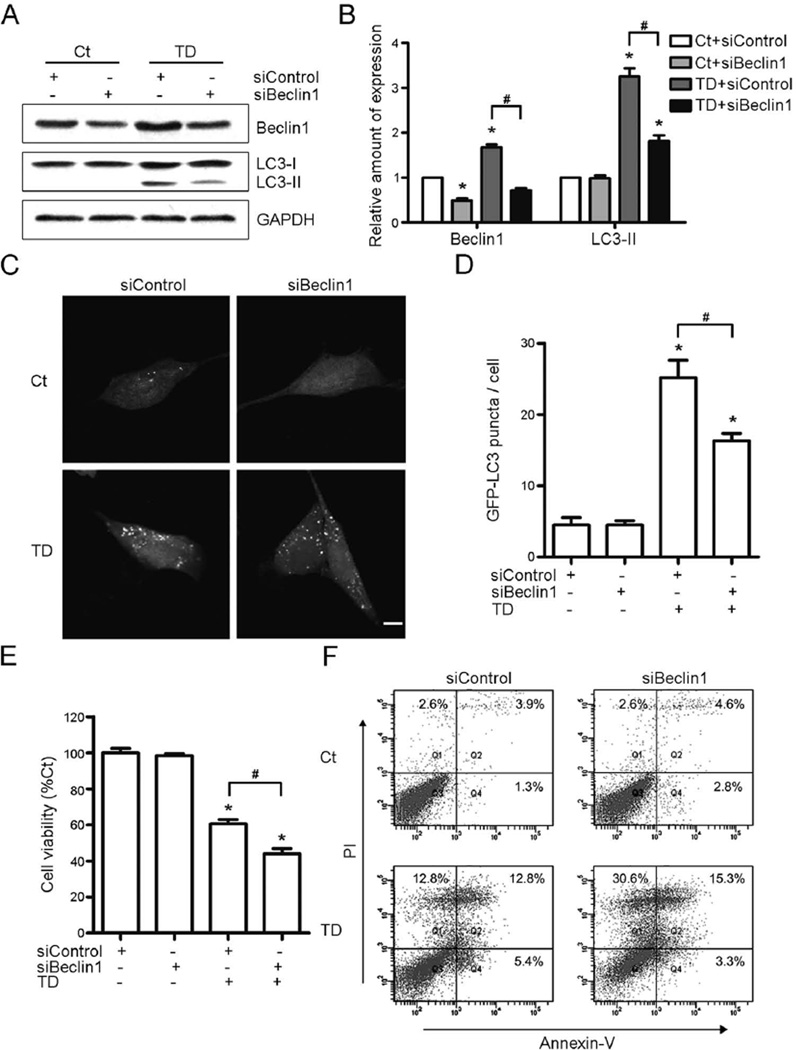
Beclin1 regulates autophagosome maturation and endocytic trafficking; down-regulation of Beclin1 decreases the activity of autophagy (Klionsky et al. 2012, Qu et al. 2003, Yue et al. 2003). Expression of Beclin1 was down-regulated by a small interfering RNA (siRNA) specifically targeting Beclin1 (Fig. 4A). Beclin1 siRNA not only down-regulated the expression of Beclin1, but also blocked TD-induced up-regulation of Beclin1 in cultured SH-SY5Y cells (Figs. 4A and B). TD-induced accumulation of LC3-II (Figs. 4A and B) and the LC3 puncta (Figs. 4C and D) could also be inhibited by the Beclin1 siRNA. TD-induced cell death was exacerbated when the SH-SY5Y cells were pretreated with Beclin1 siRNA (Figs. 4E and F). The cell death increased from 32.42% to 49.45% (Fig. 4F). These data demonstrated that inhibition of autophagy exacerbated TD-induced cell death.
Fig. 4.
Effect of Beclin1 siRNA on TD-induced autophagy and cell death. (A) Beclin1 siRNA decreased TD-induced increase of LC3-II and Beclin1. (B) The relative amount of LC3-II and Beclin1 was quantified and normalized to GAPDH. (C) Beclin1 siRNA decreased TD-induced increase of GFP-LC3 puncta. Scale bar = 5 µm. (D) The GFP-LC3 puncta per cell were quantified. (E and F) Beclin1 siRNA exacerbated TD-induced cell death was determined by MTT assay (E) and Annexin-V/PI staining (F). These results were means ± SEM of three replicates (*p < 0.05, compared with Ct with siControl; #p < 0.05, compared with TD with siControl).
Rapamycin alleviated cell death induced by TD
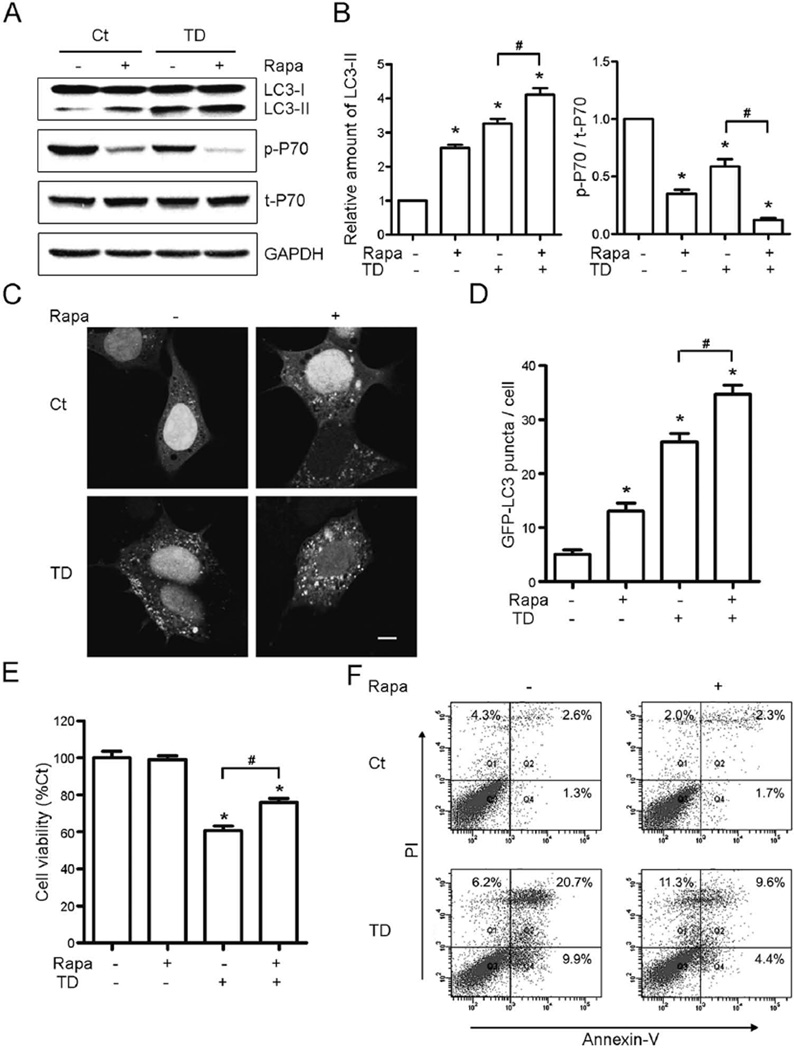
To confirm autophagy involved TD-induced cell death, rapamycin, an inhibitor of mTOR, was used to activate autophagy in vitro and in vivo. Rapamycin (10 nM) decreased the basal level of p-p70S6K and increased the expression of LC3-II, and it also exacerbated TD-induced inhibition of p70S6K phosphorylation and upregulation of LC3-II in SH-SY5Y cells (Figs. 5A and B). Rapamycin also increased both basal and TD-induced formation of GFP-LC3 puncta (Figs. 5C and D). Rapamycin alone did not affect cell viability, but decreased TD-induced cell death by 16.50% (Fig. 5E). It was confirmed by the flow cytometry that TD-induced apoptotic cell death decreased from 39.40% to 23.07% (Fig. 5F).
Fig. 5.
Effect of rapamycin on TD-induced autophagy and cell death. SH-SY5Y cells were treated with pyrithiamine (0 or 300 nmol/L) and rapamycin (Rapa, 0 or 10 nM) for 5 days. (A) The expression of LC3, p-P70, and t-P70 was determined by immunoblotting. (B) The relative amount of LC3-II was quantified and normalized to GAPDH. p-P70 was quantified and normalized to t-P70. (C) Rapamycin increases TD-induced upregulation of GFP-LC3 puncta in SH-SY5Y cells. Scale bar = 5 µm. (D) The GFP-LC3 puncta per cell were quantified. (E and F) Rapamycin protected TD-induced cell death measured by the cell viability (E) and Annexin-V/PI staining (F). All the results were means ± SEM of three replicates (*p < 0.05, compared with Ct without rapamycin; #p < 0.05, compared with TD without rapamycin).
To determine whether the activation of autophagy protected against TD-induced neuronal death in vivo, mice were injected intraperitoneally with rapamycin (0.5 mg/kg body weight) during TD day 4 to day 7. Rapamycin increased LC3 immunoreactivity in neurons in SmTN; rapamycin together with TD further increased LC3 immunoreactivity (Fig. 6A). To quantify the neuron loss, NeuN-positive neurons in the SmTN were measured by stereological analysis (Fig. 6B). NeuN-positive neurons in the SmTN lost 61.26% after TD for 9 days; however, NeuN-positive neurons only lost 39.17% in rapamycin-treated group following TD (Fig. 6B). In control mice, IbaI-positive microglia in the SmTN showed small cell bodies and thin branches (Fig. 6C). After TD for 9 days, the IbaI-positive microglia in the SmTN displayed larger cell bodies and thicker branches indicative of the activation of microglia. Rapamycin reduced the number of active microglia (Fig. 6C). TD also induced astrocyte activation which was revealed by GFAP immunostaining. Rapamycin apparently inhibited TD-induced astrocyte activation (Fig. 6D). Together, these results suggested that rapamycin activated autophagy and alleviated TD-induced damage in SmTN.
Fig. 6.
Effect of rapamycin on TD-induced neuronal death and microglia activation in SmTN. (A) Rapamycin induced LC3-positive (green) puncta increased in NeuN-positive (red) cells in SmTN. Scale bar = 10µm. (B) TD-induced loss of NeuN-positive cells in SmTN was reduced by the treatment with rapamycin. Left panel: NeuN immunohistochemistry (IHC) was performed to identify neurons in SmTN. Scale bar = 50 µm. Right panel: NeuN-positive cells in SmTN were quantified by stereological analysis. (C) TD-induced IbaI-positive microglia activation in SmTN was reduced by the treatment with rapamycin. Left panel: IbaI IHC was performed to identify active microglia in SmTN. Scale bar = 50 µm. Right panel: The IbaI immunoreactivity in SmTN was quantified. (D) TD-induced astrocyte activation in SmTN was reduced by the treatment with rapamycin. Left panel: GFAP IHC was performed to identify astrocytes in SmTN. Scale bar = 50 µm. Right panel: The GFAP immunoreactivity in SmTN was quantified. All the results were mean ± SEM of five animals (*p < 0.05, compared with Ct without rapamycin; #p < 0.05, compared with TD without rapamycin).
Discussion
Thiamine deficiency (TD) produces WKS in humans, and induces selective neuronal death which is a common feature of several neurodegenerative diseases (Sechi & Serra 2007). Sub-clinical TD is a problem in the elderly population (Isenberg-Grzeda et al. 2012, O'Keeffe 2000, Zhang et al. 2013). TD induces selective neurodegeneration in the SmTN of mice brain (Ke et al. 2003, Ke & Gibson 2004). The mechanisms underlying TD-induced neuronal damage are complex and may be involved in neuroinflammation (Yang et al. 2011, Ke et al. 2005a, Ke et al. 2005b, Karuppagounder et al. 2007), ER stress (Wang et al. 2007), oxidative stress (Kruse et al. 2004, Zhang et al. 2011), and calcium-related RNA editing (Lee et al. 2010). This study for the first time showed that TD caused the activation of autophagy which played a protective role during TD. Inhibition of autophagy exacerbated TD-induced neuronal death whereas activation of autophagy protected neurons in the SmTN against TD-induced cell death.
Autophagy maintains cellular homeostasis and plays a significant role in the protein quality control in neurons (Pan et al. 2008a). An increase of autophagic vacuoles and related structures of autophagy has been found in several neurodegenerative diseases, including Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD) (Anglade et al. 1997, Kegel et al. 2000, Nixon et al. 2005). Suppression of autophagy by deficiency for Atg5 or Atg7 in neural cells causes the accumulation of cytoplasmic inclusion bodies and neurodegeneration disease (Hara et al. 2006, Komatsu et al. 2006). Beclin1 deficiency in APP mice reduces neuronal autophagy, disrupts lysosomes, promotes Aβ accumulation, and results in neurodegeneration; correspondingly, an increase in Beclin1 expression reduces amyloid pathology in APP mice (Pickford et al. 2008). In α-Synuclein Models of PD, overexpression of Beclin1 activates autophagy, reduces the accumulation of α-synuclein, and ameliorates the associated neuritic alterations (Spencer et al. 2009). We have recently shown that activation of autophagy protects neurons against ethanol-induced neurodegeneration (Chen et al. 2012a). These studies suggest that activation of autophagy promotes the degradation of misfolded and aggregated proteins, and alleviates neurodegeneration. Consistent with the role in these neurodegenerative disorders, autophagy appears to be a neuroprotective mechanism in our TD model. Therefore, enhancement of autophagy may be a new therapeutic approach for neurodegenerative disorders. For example, rapamycin or its analogues have been used to enhance autophagy in animals (Pan et al. 2008a). In animal models of PD, rapamycin attenuates SNpc dopaminergic cell death and striatal dopaminergic loss in mice (Dehay et al. 2010, Pan et al. 2008b). The food supplement containing rapamycin improves learning and memory deficits and reduces amyloid-β and tau pathology in a mouse model of AD (Caccamo et al. 2010). We showed that rapamycin activated autophagy and decreased TD-induced neuronal death as well as microglia/astrocyte activation in mice (Fig. 6).
As a catabolic process, autophagy sequesters long-lived proteins and dysfunctional organelles, and then transports them to lysosomes for degradation to recycle cellular components. For example, depletion of total amino acids or serum starvation activates autophagy (Mizushima 2007). Autophagy can also be triggered by protein aggregates, oxidative stress, and damage cytoplasmic organelles such as the mitochondria (Madeo et al. 2009). Several molecular pathways are involved in the activation of autophagy: (1) unc-51–like kinase and negative regulator-mTOR; (2) Vps34/class III phosphatidylinositol 3-phosphate kinase complex I (PI3K complex I); (3) the Atg9 cycling complex; and (4) the Atg5-Atg12 and LC3 conjugation systems (Simonsen & Tooze 2009). We demonstrated that TD decreased the activity of mTOR by inhibition of p70S6K phosphorylation, and increased the Atg12-Atg5 conjugation and the expression of LC3-II. Beclin1, an important component of PI3K complex I, regulates autophagosome maturation and endocytic trafficking (Liang et al. 2006, Liang et al. 2008, Takahashi et al. 2007). TD increased the expression of Beclin1 (Figs. 1 and 2), and down-regulation of Beclin1 by siRNA decreased the formation of autophagosomes (Fig. 4). Re-administration of thiamine effectively rescued the TD-induced decrease of phosphorylation of p70S6K and TD-induced accumulation of LC3-II, Atg5 and Beclin1 (Fig. 2). Although TD inhibits mTOR activity, we cannot exclude the involvement of other signaling pathways. The effects of TD are complex; it induces damages to mitochondria, ER stress, and oxidative stress which all may potentially activate autophagy. This possibility is supported by the finding that TD and rapamycin (a mTOR inhibitor) have an additive effect.
There is a controversy about the effect of autophagy on cell death. Under some stress conditions, the activation of autophagy aggravates cell death that is called “autophagic cell death” or “type II cell death” (Baehrecke 2005, Kroemer & Jaattela 2005, Maiuri et al. 2007). A recent study demonstrates that loss of function of Atg5 decreases vacuole formation and autophagic cell death in response to interferon-γ stimulation (Pyo et al. 2005). Autophagy inhibitors and knocking down autophagy-related genes alleviates cell death induced by zVAD (Yu et al. 2006). However, more evidence supports that autophagy is cytoprotective to prevent cell death (Levine & Yuan 2005, Liang et al. 1998, Lum et al. 2005). Under the condition of nutrient depletion, inhibition of autophagy by down-regulation of atg genes increases apoptosis (Boya et al. 2005). IL-3 withdrawal induces autophagosome formation is also required for cell survival (Lum et al. 2005). TD-induced neuronal death was exacerbated when autophagy was inhibited by wortmannin or siRNA targeting Beclin1, while TD-induced neuronal death was alleviated when autophagy was activated by rapamycin. Therefore, autophagy plays a neuroprotective role during TD-induced neuronal damage.
In summary, TD induces neuronal autophagy in vitro and in vivo. Autophagy is a self-protective response to TD-induced damage. When neurons sense the damage, they activate autophagy to alleviate TD-induced neurotoxicity. These results provide a new insight into the pathophysiological mechanisms of neuroprotection and offer a potential novel therapeutic strategy for neurodegenerative diseases.
Acknowledgements
We would like to thank Jacqueline A. Frank for reading this manuscript. This research was supported by grants from the Ministry of Science and Technology of China (2010CB912000; 2007CB947100), the National Natural Science Foundation of China (31271142, 30870812), the Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS2008006), the Program of Clinical Research Center, Institute for Nutritional Sciences and Xuhui Central Hospital (CRC20100010), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-R-08). Dr. J. Luo was supported by grants from NIH/NIAAA (AA015407 and AA019693).
Abbreviations
- AD
Alzheimer’s disease
- BSA
bovine serum albumin
- DAB
3, 3’-diaminobenzidine
- DMEM
Dulbecco’s modified Eagle’s medium
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- HD
Huntington’s disease
- IHC
immunohistochemistry
- KGDHC
α-ketoglutarate dehydrogenase complex
- LP
leupeptin and pepstatin A
- mTOR
mammalian target of rapamycin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- NeuN
neuronal specific nuclear protein
- PB
phosphate buffer
- PBS
phosphate buffer saline
- PD
Parkinson’s disease
- PDHC
pyruvate dehydrogenase complex
- PI
propidium iodide
- Rapa
rapamycin
- siRNA
small interfering RNA
- SmTN
submedial thalamic nucleus
- TD
thiamine deficiency
- TEM
transmission electron microscopy
- TPBS
PBS (pH 7.4) with 0.05% Tween-20
- WE
Wernicke's Encephalopathy
- Wort
wortmannin
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histology and histopathology. 1997;12:25–31. [PubMed] [Google Scholar]
- Baehrecke EH. Autophagy: dual roles in life and death? Nature reviews. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. European journal of biochemistry / FEBS. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Molecular and cellular biology. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. The Journal of biological chemistry. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ke Z, Xu M, et al. Autophagy is a protective response to ethanol neurotoxicity. Autophagy. 2012a;8 doi: 10.4161/auto.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang X, Song L, Le W. Autophagy dysregulation in amyotrophic lateral sclerosis. Brain pathology (Zurich, Switzerland) 2012b;22:110–116. doi: 10.1111/j.1750-3639.2011.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiology of disease. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Park LC, Zhang H, Sorbi S, Calingasan NY. Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Annals of the New York Academy of Sciences. 1999;893:79–94. doi: 10.1111/j.1749-6632.1999.tb07819.x. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Zhang H. Abnormalities in oxidative processes in non-neuronal tissues from patients with Alzheimer's disease. J Alzheimers Dis. 2001;3:329–338. doi: 10.3233/jad-2001-3308. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Molecular biology of the cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53:507–516. doi: 10.1016/j.psym.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Molecular biology of the cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiology of disease. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke ZJ, Calingasan NY, DeGiorgio LA, Volpe BT, Gibson GE. CD40-CD40L interactions promote neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Neurochemistry international. 2005a;47:204–215. doi: 10.1016/j.neuint.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Calingasan NY, Karuppagounder SS, DeGiorgio LA, Volpe BT, Gibson GE. CD40L deletion delays neuronal death in a model of neurodegeneration due to mild impairment of oxidative metabolism. Journal of neuroimmunology. 2005b;164:85–92. doi: 10.1016/j.jneuroim.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, DeGiorgio LA, Volpe BT, Gibson GE. Reversal of thiamine deficiency-induced neurodegeneration. Journal of neuropathology and experimental neurology. 2003;62:195–207. doi: 10.1093/jnen/62.2.195. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Gibson GE. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochemistry international. 2004;45:361–369. doi: 10.1016/j.neuint.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 2000;20:7268–7278. doi: 10.1523/JNEUROSCI.20-19-07268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nature reviews. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kovacs AL, Reith A, Seglen PO. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin or a lysosomotropic amine. Experimental cell research. 1982;137:191–201. doi: 10.1016/0014-4827(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Kril JJ. Neuropathology of thiamine deficiency disorders. Metabolic brain disease. 1996;11:9–17. doi: 10.1007/BF02080928. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Kruse M, Navarro D, Desjardins P, Butterworth RF. Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochemistry international. 2004;45:49–56. doi: 10.1016/j.neuint.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Lee S, Yang G, Yong Y, et al. ADAR2-dependent RNA editing of GluR2 is involved in thiamine deficiency-induced alteration of calcium dynamics. Molecular neurodegeneration. 2010;5:54. doi: 10.1186/1750-1326-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nature cell biology. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- Liang C, Lee JS, Inn KS, et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nature cell biology. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. Journal of virology. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Sgobio C, et al. Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Kroemer G. Autophagy for the avoidance of neurodegeneration. Genes & development. 2009;23:2253–2259. doi: 10.1101/gad.1858009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature reviews. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M, Sovak G, Cuervo AM. Protein degradation and aging. Experimental gerontology. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes & development. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. Journal of neuropathology and experimental neurology. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- O'Keeffe ST. Thiamine deficiency in elderly people. Age Ageing. 2000;29:99–101. doi: 10.1093/ageing/29.2.99. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease. Brain. 2008a;131:1969–1978. doi: 10.1093/brain/awm318. [DOI] [PubMed] [Google Scholar]
- Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiology of disease. 2008b;32:16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Pannunzio P, Hazell AS, Pannunzio M, Rao KV, Butterworth RF. Thiamine deficiency results in metabolic acidosis and energy failure in cerebellar granule cells: an in vitro model for the study of cell death mechanisms in Wernicke's encephalopathy. Journal of neuroscience research. 2000;62:286–292. doi: 10.1002/1097-4547(20001015)62:2<286::AID-JNR13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Park LC, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE. Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy. Journal of neuroscience research. 2001;66:1028–1034. doi: 10.1002/jnr.10062. [DOI] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. The Journal of clinical investigation. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo JO, Jang MH, Kwon YK, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. The Journal of biological chemistry. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. The Journal of clinical investigation. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VL, Mousseau DD, Butterworth RF. Nitric oxide synthase activities are selectively decreased in vulnerable brain regions in thiamine deficiency. Neuroscience letters. 1996;208:17–20. doi: 10.1016/0304-3940(96)12541-6. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nature genetics. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Schwab C, Steele JC, McGeer PL. Neurofibrillary tangles of Guam parkinson-dementia are associated with reactive microglia and complement proteins. Brain research. 1996;707:196–205. doi: 10.1016/0006-8993(95)01257-5. [DOI] [PubMed] [Google Scholar]
- Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet neurology. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Tooze SA. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. The Journal of cell biology. 2009;186:773–782. doi: 10.1083/jcb.200907014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Coppola D, Matsushita N, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nature cell biology. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. The Journal of cell biology. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Todd K, Butterworth RF. Mechanisms of selective neuronal cell death due to thiamine deficiency. Annals of the New York Academy of Sciences. 1999;893:404–411. doi: 10.1111/j.1749-6632.1999.tb07866.x. [DOI] [PubMed] [Google Scholar]
- Trincheri NF, Follo C, Nicotra G, Peracchio C, Castino R, Isidoro C. Resveratrol-induced apoptosis depends on the lipid kinase activity of Vps34 and on the formation of autophagolysosomes. Carcinogenesis. 2008;29:381–389. doi: 10.1093/carcin/bgm271. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Johnston MV, Hess KM, Griffin JW, Price DL. Model of Wernicke's encephalopathy. Archives of neurology. 1981;38:350–354. doi: 10.1001/archneur.1981.00510060052007. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang B, Fan Z, Shi X, Ke ZJ, Luo J. Thiamine deficiency induces endoplasmic reticulum stress in neurons. Neuroscience. 2007;144:1045–1056. doi: 10.1016/j.neuroscience.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. The Journal of biological chemistry. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Molecular biology of the cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain pathology (Zurich, Switzerland) 2011;21:279–297. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nature cell biology. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. The Journal of cell biology. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Ding H, Chen H, Ye X, Li H, Lin X, Ke Z. Thiamine Nutritional Status and Depressive Symptoms Are Inversely Associated among Older Chinese Adults. J Nutr. 2013;143:53–58. doi: 10.3945/jn.112.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Ding H, Li W, Fan Z, Sun A, Luo J, Ke ZJ. Senescence accelerated mouse strain is sensitive to neurodegeneration induced by mild impairment of oxidative metabolism. Brain research. 2009;1264:111–118. doi: 10.1016/j.brainres.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, Ke ZJ. Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiology of aging. 2011;32:42–53. doi: 10.1016/j.neurobiolaging.2009.01.005. [DOI] [PubMed] [Google Scholar]